Abstract
Aims
The purpose of this study was to determine the clinical significance of detecting microbial footprints of ureaplasmas in amniotic fluid (AF) using specific primers for the polymerase chain reaction (PCR) in patients presenting with cervical insufficiency.
Methods
Amniocentesis was performed in 58 patients with acute cervical insufficiency (cervical dilatation, ≥1.5 cm) and intact membranes, and without regular contractions (gestational age, 16–29 weeks). AF was cultured for aerobic and anaerobic bacteria as well as genital mycoplasmas. Ureaplasmas (Ureaplasma urealyticum and Ureaplasma parvum) were detected by PCR using specific primers. Patients were divided into three groups according to the results of AF culture and PCR for ureaplasmas: those with a negative AF culture and a negative PCR (n=44), those with a negative AF culture and a positive PCR (n = 10), and those with a positive AF culture regardless of PCR result (n=4).
Results
1) Ureaplasmas were detected by PCR in 19.0% (11/58) of patients, by culture in 5.2% (3/58), and by culture and/or PCR in 22.4% (13/58); 2) Among the 11 patients with a positive PCR for ureaplasmas, the AF culture was negative in 91% (10/11); 3) Patients with a negative AF culture and a positive PCR for ureaplasmas had a significantly higher median AF matrix metalloproteinase-8 (MMP-8) concentration and white blood cell (WBC) count than those with a negative AF culture and a negative PCR (P<0.001 and P<0.05, respectively); 4) Patients with a positive PCR for ureaplasmas but a negative AF culture had a higher rate of spontaneous preterm birth within two weeks of amniocentesis than those with a negative AF culture and a negative PCR (P<0.05 after adjusting for gestational age at amniocentesis); 5) Of the patients who delivered within two weeks of amniocentesis, those with a positive PCR for ureaplasmas and a negative AF culture had higher rates of histologic amnionitis and funisitis than those with a negative AF culture and a negative PCR (P<0.05 after adjusting for gestational age at amniocentesis, for each); (6) However, no significant differences in the intensity of the intra-amniotic inflammatory response and perinatal outcome were found between patients with a positive AF culture and those with a negative AF culture and a positive PCR.
Conclusions
1) Cultivation techniques for ureaplasmas did not detect most cases of intra-amniotic infection caused by these microorganisms (91% of cases with cervical insufficiency and microbial footprints for ureaplasmas in the amniotic cavity had a negative AF culture); 2) Patients with a negative AF culture and a positive PCR assay were at risk for intra-amniotic and fetal inflammation as well as spontaneous preterm birth.
Keywords: Amniotic fluid (AF), cervical insufficiency, chorioamnionitis, intra-amniotic infection, intra-amniotic inflammation, polymerase chain reaction (PCR), pregnancy, preterm birth, spontaneous abortion, ureaplasmas
Introduction
Microbial invasion of the amniotic cavity is found frequently in patients with cervical insufficiency [24, 37, 40] and is a risk factor for failure of cerclage [28, 37, 40], chorioamnionitis [7, 37], preterm birth and adverse pregnancy outcome [37]. Ureaplasmas (Ureaplasma urealyticum and Ureaplasma parvum) are the organisms most frequently isolated from the amniotic fluid (AF) of patients with cervical insufficiency [24, 37]. However, the fastidious nature of these microorganisms requires special culture conditions for isolation. One limitation of cultivation in clinical medicine is that this procedure takes time, and results of the culture may not be available in time for clinical management decisions. Moreover, the lack of a rigid cell wall of ureaplasmas, as well as their small size, make these organisms non-detectable by Gram stain examination.
Recently, molecular microbiology techniques based upon the polymerase chain reaction (PCR) have emerged as a sensitive and rapid method for the detection of ureaplasmas (U. urealyticum and U. parvum) in clinical samples, including AF [12, 46, 49], secretions from the urogenital tract [5, 39], tracheal aspirates [8, 41] and cerebrospinal fluid of preterm neonates [42]. We previously demonstrated that a positive PCR assay for ureaplasmas, using specific primers (regardless of culture results), is a powerful predictor of impending preterm birth and adverse pregnancy outcome in patients with preterm premature rupture of membranes (PROM) [46] or preterm labor with intact membranes [49]. The purpose of this study was to determine the clinical significance of detecting microbial footprints for ureaplasmas by PCR in the AF of patients with cervical insufficiency.
Material and methods
Study design
The study population consisted of 58 patients who were admitted to the participating institution between January 1993 and December 2007 with the diagnosis of cervical insufficiency (painless cervical dilatation) and singleton gestation, who underwent amniocentesis for the assessment of microbiologic status of AF. The diagnosis of cervical insufficiency was made in patients who met the following criteria: (1) progressive painless cervical dilation (≥1.5 cm), (2) intact membranes, and (3) the absence of regular uterine contractions. Gestational age ranged between 16 and 29 weeks. Cervical dilation was determined by visual evaluation of the sterile speculum examination. Retrieval of AF was performed after obtaining written informed consent. The Institutional Review Board of the participating institution approved the collection and use of these samples and information for research purposes. This University Hospital has a Federal Wide Assurance with the Office for Human Research Protection of the Department of Health and Human Services of the USA.
Amniotic fluid studies
AF was cultured for genital mycoplasmas (ureaplasmas [U. urealyticum and U. parvum] and M. hominis) as well as aerobic and anaerobic bacteria. An aliquot of AF was examined in a hemocytometer chamber to determine the white blood cell (WBC) count. The remaining fluid was stored at −70°C until assayed. The stored AF was analyzed for matrix metalloproteinase-8 (MMP-8), which was measured with a commercially available enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech, Inc, Bucks, UK), as previously reported [24, 34]. The sensitivity of the test was 0.3 ng/mL. Intra- and inter-assay coefficients for each were <10%.
PCR assay for ureaplasmas in AF
PCR assay for ureaplasmas (U. urealyticum and U. parvum) was performed with stored AF according to methods previously described [46, 49]. Briefly, after thawing, a 50 μL aliquot of AF was centrifuged (25,000×g for 10 min at 4°C). The pellet was resuspended in 15 μL deionized water, and the solution was boiled for 10 min and quickly cooled on ice. After centrifuge (25,000×g for 10 min at 4°C) of the solution, 5 μL of the supernatants were taken for PCR assay. The primers chosen were in the urease gene: 5′-CAA TCT GCT CGT GAA GTA TTA C-3′ (U5, forward) and 5′-ACG ACG TCC ATA AGC AAC T-3 (U4, reverse) and the amplified product size was 429 base pair DNA fragments of all serotypes of ureaplasmas (U. parvum and U. urealyticum) [2, 5, 41]. PCR assay was performed in a total volume of 25 μL containing 0.6 pmol of each primer, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.16 mM dNTP, 1 unit Taq polymerase (Takara Bio Healthcare, Shiga, Japan) and 5 μL extracted DNA template. The thermal cycling (PTC-200, MJ research, Waltham, MA, USA) profile consisted of 94°C for 5 min, 35 cycles of denaturation at 94°C for 1 min, annealing at 58°C for 1 min and elongation at 72°C for 1 min, and an extension at 72°C for 5 min. The amplified products were separated by electrophoresis in 1.5% agarose gel (SeaKem LE agarose, Cambrex BioScience Rockland, Rockland, ME, USA) at 150 V for 30 min.
Placental histologic examination and diagnosis of neonatal morbidity
Choriodeciduitis and amnionitis were defined in the presence of acute inflammatory changes in chorion-decidua and amnion, respectively; funisitis was diagnosed in the presence of neutrophil infiltration into umbilical vessel walls or Wharton's jelly with the use of criteria previously published [44]. Significant neonatal morbidity was defined as the presence of any of the following conditions: proven congenital neonatal sepsis, respiratory distress syndrome (RDS), congenital pneumonia, bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (grade ≥II), and necrotizing enterocolitis. These conditions were diagnosed according to definitions previously described in detail [47].
Statistical analysis
Proportions were compared with the Fisher's exact test. The Kruskal-Wallis analysis of variance test was used for comparison of continuous variables among groups. Multiple comparisons between groups were performed with the Mann-Whitney U-test. Logistic regression analysis was performed to examine the relationship between positive PCR results and the outcome of interest after adjusting for the effect of gestational age. Statistical significance was defined as P<0.05.
Results
Amniotic fluid culture and PCR for ureaplasmas
The prevalence of a positive AF culture was 6.9% (4/58). Ureaplasmas were detected in 19.0% (11/58) by PCR, in 5.2% (3/58) by culture and in 22.4% (13/58) by culture and/or PCR. Other microorganisms isolated by culture included Enterococcus sp. (n = 1), Staphylococcus aureus (n = 1), and Streptococcus mitis (n = 1). One patient had three organisms that were isolated from AF (Ureaplasma sp, Enterococcus sp, and S. aureus). Table 1 shows the results of AF culture and PCR assay. Among the 11 patients with a positive PCR for ureaplasmas, AF culture was negative in 90.9% (10/11).
Table 1.
Comparison of amniotic fluid (AF) culture and polymerase chain reaction (PCR) assay for ureaplasmas.
| PCR result for ureaplasmas | AF culture for ureaplasmas | AF culture for all microorganisms | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Positive (n=11) | 1 | 10 | 1 | 10 |
| Negative (n=47) | 2 | 45 | 3 | 44 |
| Total (n=58) | 3 | 55 | 4 | 54 |
Table 2 describes the clinical characteristics of the study population. There were no significant differences in the clinical characteristics including age, parity, median gestational age at amniocentesis, and the use of antenatal antibiotics and corticosteroids among the three groups.
Table 2.
Characteristics of the study population according to the results of amniotic fluid (AF) culture and polymerase chain reaction (PCR) assay for ureaplasmas.
| Characteristics | Negative AF culture | Positive AF culture | ||||
|---|---|---|---|---|---|---|
| Negative PCR (group 1; n=44) |
Pa | Positive PCR (group 2; n=10) |
Pb | Group 3 (n=4) |
Pc | |
| Maternal age (years)d | 31 (22–39) | NS | 32 (26–39) | NS | 30 (28–34) | NS |
| Nulliparity (n) | 19 (43%) | NS | 5 (50%) | NS | 1 (25%) | NS |
| Gestational age at amniocentesis (weeks)d | 23.2 (17.1–28.9) | NS | 25.0 (16.9–27.0) | NS | 23.4 (22.3–25.1) | NS |
| Bag bulging (n)e | 41/43 (95%) | NS | 9 (90%) | NS | 4 (100%) | NS |
| Cerclage (n) | 15 (34%) | NS | 4 (40%) | NS | 0 | NS |
| Antibiotics (n) | 31 (71%) | NS | 9 (90%) | NS | 4 (100%) | NS |
| Antenatal corticosteroid (n/N)f | 16/23 (70%) | NS | 5/6 (83%) | NS | 1/3 (33%) | NS |
Comparison between groups 1 and 2.
Comparison between groups 2 and 3.
Comparison between groups 3 and 1.
Values are given as median (range).
Defined as the presence of bulging membranes at the level of the external cervical os. One patient was excluded from the analysis because the presence or absence of bag bulging was not described in her medical record.
Administration of antenatal corticosteroid was considered when gestational age reached at least 23 weeks. Therefore, 32 patients with a gestational age at amniocentesis of >23 weeks were included for the analysis.
NS = not significant.
Intra-amniotic inflammatory response
Figure 1 displays the differences in AF MMP-8 concentrations and WBC counts according to the results of AF culture and PCR for ureaplasmas. Patients with a negative AF culture and a positive PCR (group 2) had a significantly higher median AF MMP-8 concentration and WBC count than those with a negative AF culture and negative PCR (group 1) (P<0.05 and <0.001, respectively). However, there were no significant differences in the median AF MMP-8 concentration and WBC count between patients with a negative AF culture and a positive PCR (group 2) and those with a positive AF culture regardless of the PCR result (group 3) (P>0.1 for each).
Figure 1.
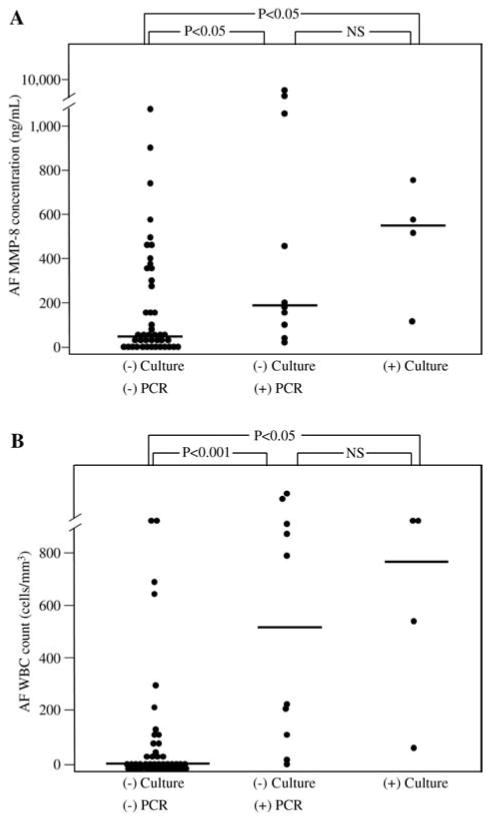
Amniotic fluid (AF) matrix metalloproteinase-8 (MMP-8) concentrations and white blood cell (WBC) counts according to the results of AF culture and polymerase chain reaction (PCR) assay for ureaplasmas.
(A) AF MMP-8 concentrations (group 1: median, 51.4 ng/mL [range, 0.5–1801.4 ng/mL]; group 2: median, 178.4 ng/mL [range, 45.7–6142.6 ng/mL]; group 3: median, 547.3 ng/mL [range, 129.1–770.6 ng/mL]; P<0.01 by Kruskal-Wallis analysis of variance test) and (B) AF WBC counts (group 1: median, 3 cells/mm3 [range, 0–>1000 cells/mm3]; group 2: median, 501 cells/mm3 [range, 2–>1000 cells/mm3]; group 3: median, 775 cells/mm3 [range, 62–>1000 cells/mm3]; P <0.001 by Kruskal-Wallis analysis of variance test).
Pregnancy outcome
Tables 3 and 4 show the pregnancy and neonatal outcomes according to the results of AF culture and PCR for ureaplasmas. Patients in group 2 had a significantly higher rate of spontaneous delivery within two weeks of amniocentesis than those in group 1 (P <0.05 after adjusting for gestational age at amniocentesis). Of the patients who delivered within two weeks of amniocentesis, patients in group 2 had higher rates of histologic amnionitis and funisitis than those in group 1 (P <0.05 after adjusting for gestational age at amniocentesis, for each). However, there were no significant differences in pregnancy and neonatal outcomes between patients in groups 2 and 3 (P>0.1).
Table 3.
Pregnancy outcome of the study population according to the results of amniotic fluid (AF) culture and polymerase chain reaction (PCR) assay for ureaplasmas.
| Pregnancy outcome | Negative AF culture | Positive AF culture | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative PCR (group 1; n = 44) |
Pa | Positive PCR (group 2; n = 10) |
Pb | Group 3 (n = 4) |
Pc | |||
| Unadjusted | Adjustedd | Unadjusted | Unadjusted | Adjustedd | ||||
| Gestational age at delivery (weeks)e | 26.0 (17.6–40.1) | NS | – | 26.5 (17.4–34.0) | NS | 23.6 (22.4–25.9) | NS | – |
| Amniocentesis to spontaneous delivery interval (n/N)f | ||||||||
| <48 h | 8/41 (20%) | NS | NS | 1/10 (10%) | NS | 2/4 (50%) | NS | NS |
| <7 days | 13/38 (34%) | NS | NS | 4/10 (40%) | NS | 3/3 (100%) | <0.1 | NS |
| <2 weeks | 16/36 (44%) | <0.05 | <0.05 | 7/8 (88%) | NS | 3/3 (100%) | NS | NS |
| Spontaneous preterm delivery (n/N)f | ||||||||
| ≤28 weeks | 20/36 (56%) | NS | NS | 7/8 (88%) | NS | 3/3 (100%) | NS | NS |
| ≤34 weeks | 23/33 (70%) | NS | NS | 7/7 (100%) | NS | 3/3 (100%) | NS | NS |
| Choriodeciduitis (n/N) | 15/24 (63%) | NS | NS | 4/6 (67%) | NS | 2/2 (100%) | NS | NS |
| Amnionitis (n/N) | 7/23 (30%) | NS | NS | 4/6 (67%) | NS | 1/2 (50%) | NS | NS |
| Funisitis (n/N) | 6/24 (25%) | NS | NS | 4/6 (67%) | NS | 1/2 (50%) | NS | NS |
| Choriodeciduitis: delivered <2 weeks (n/N)g | 6/10 (60%) | NS | NS | 4/5 (80%) | NS | 2/2 (100%) | NS | NS |
| Amnionitis: delivered <2 weeks (n/N)g | 2/10 (20%) | <0.1 | <0.05 | 4/5 (80%) | NS | 1/2 (50%) | NS | NS |
| Funisitis: delivered <2 weeks (n/N)g | 2/10 (20%) | <0.1 | <0.05 | 4/5 (80%) | NS | 1/2 (50%) | NS | NS |
Comparison between groups 1 and 2.
Comparison between groups 2 and 3.
Comparison between groups 3 and 1.
Adjusted for gestational age at amniocentesis (logistic regression).
Values are given as median (range).
Cases with delivery for maternal or fetal indication were excluded.
To preserve a meaningful temporal relationship between the results of AF study and histologic findings of the placenta and umbilical cord at delivery, cases who were delivered after 14 days of amniocentesis were excluded for the analysis.
NS = not significant.
Table 4.
Neonatal outcome of the study population according to the results of amniotic fluid (AF) culture and polymerase chain reaction (PCR) assay for ureaplasmas.
| Characteristics | Negative AF culture | Positive AF culture | ||||
|---|---|---|---|---|---|---|
| Negative PCR (group 1; n=44) |
Pa | Positive PCR (group 2; n=10) |
Pb | Group 3 (n=4) |
Pc | |
| Birth weight (g)d | 871 (130–3860) | NS | 760 (160–2000) | NS | 650 (500–768) | NS |
| 1-min Apgar score <4 (n/N) | 24/39 (62%) | NS | 5/8 (63%) | NS | 4/4 (100%) | NS |
| 5-min Apgar score <7 (n/N) | 27/39 (69%) | NS | 6/8 (75%) | NS | 4/4 (100%) | NS |
| Deaths (n) | ||||||
| Shortly after birth, <1 day | 18 (41%) | NS | 5 (50%) | NS | 3 (75%) | NS |
| Perinatal period, <28 days (n) | 22 (50%) | NS | 6 (60%) | NS | 4 (100%) | NS |
| Admission to neonatal intensive care unit (n/N) | 16/26 (62%) | NS | 5/5 (100%) | NS | 1/1 (100%) | NS |
| Significant morbidities (n/N)e | 11/26 (42%) | NS | 4/5 (80%) | NS | 1/1 (100%) | NS |
Comparison between groups 1 and 2.
Comparison between groups 2 and 3.
Comparison between groups 3 and 1.
Values are given as median (range).
Defined as the presence of any of the following conditions: proven congenital neonatal sepsis, respiratory distress syndrome, congenital pneumonia, bronchopulmonary dysplasia, intraventricular hemorrhage (≥grade II), and necrotizing enterocolitis. Twenty-six neonates who were not actively resuscitated at birth or who died in the delivery room, despite intensive resuscitative efforts, as a result of extreme prematurity were excluded from the analysis because they could not be evaluated with respect to the presence or absence of morbidities.
NS = not significant.
Patients with a negative culture but a positive PCR for ureaplasmas
Ten patients had a positive PCR for ureaplasmas but a negative AF culture. All patients except one case delivered at 28 weeks of gestation or less and within two weeks of amniocentesis. Serial amniocenteses revealed decreases in the AF WBC count (from 35 to 2 cells/mm3) and MMP-8 concentration (from 140.1 to 13.4 ng/mL) after the administration of antibiotics for two weeks in that case. The AF WBC counts and MMP-8 concentrations were elevated in all but one case, which had an AF WBC count of 2 cells/mm3, but an elevated AF MMP-8 concentration of 107.1 ng/mL. The patient had a cerclage after the amniocentesis, but subsequently ruptured her membranes and delivered prematurely.
Comment
Principal findings of this study
1) In patients with the clinical diagnosis of cervical insufficiency, ureaplasmas were detected in 19.0% of patients by PCR alone, in 5.2% by culture alone, and 22.4% by either PCR and/or culture; 2) Cultivation techniques for genital mycoplasmas missed 91% of cases with microbial invasion of the amniotic cavity with ureaplasmas demonstrated by molecular microbiology techniques; 3) Patients with a positive PCR for ureaplasmas but a negative AF culture had a more intense intra-amniotic inflammatory response reflected by the AF WBC count and MMP-8 concentration than those with a negative culture and a negative PCR; 4) Spontaneous preterm delivery within two weeks of amniocentesis occurred in 88% of patients with a positive PCR for ureaplasmas but a negative culture; 5) Collectively, this evidence indicates that the presence of microbial footprints for ureaplasmas in the absence of a positive culture is a poor prognostic sign.
PCR assay for detecting ureaplasmas
The observation that microbial footprints for ureaplasmas are detected (using molecular microbiology techniques) more frequently than the isolation of these organisms using cultivation techniques is in keeping with those of other investigators [8, 26, 39, 46, 49]. The advantages of a PCR-based assay for the detection of ureaplasmas include its sensitivity as well as speed. The results of culture techniques may take several days, but the results of PCR assays can be available within hours.
One potential problem of PCR-based assays is the difficulty of false-positive results (positive PCR but negative culture). A false-positive result may be the consequence of a low sensitivity of cultivation techniques or, alternatively, amplification of DNA from dead bacteria or contamination. Contamination at the time of amniocentesis is unlikely, because ureaplasmas are not normally found in the skin.
Our study demonstrated the importance of the detection of microbial footprints, even if enough ureaplasmas are not present to yield a positive culture. This may occur not only because the number of viable bacteria are small, but also because the bacteria present may have limited viability as a consequence of attack by the innate or adaptive immune system. Indeed, heat-inactivated Ureaplasma can stimulate human and rat macrophage cell lines to produce pro-inflammatory cytokines [25]. An in vitro study demonstrated that microbial products of Ureaplasma can stimulate the production of the inflammatory mediators by explants of choriodecidual tissue [1]. We believe that such observations provide a link between the detection of microbial footprints (despite negative cultures) and adverse pregnancy outcome.
The results of the present study indicate that intra-amniotic infection with ureaplasmas determined by PCR assay is associated with intra-amniotic/placental inflammation and adverse pregnancy outcome, despite the failure of culture techniques to detect the presence of the microorganism in patients with cervical insufficiency. Only one of ten patients with a positive PCR but a negative AF culture delivered after 28 weeks of gestation and had an amniocentesis-to-delivery interval of >2 weeks in the current study. However, we observed decreases in AF WBC count and MMP-8 concentration with the use of antibiotics over the following two weeks in that case. These observations are consistent with those reported by Hassan et al. [17], indicating that antibiotic administration can eradicate ureaplasmas detected by culture.
Intra-amniotic infection with ureaplasmas in cervical insufficiency
The prevalence of ureaplasmas by AF culture and/or PCR assay was 22.4% (13/58) in patients with cervical insufficiency. One small study [6] reported that the detection rate of this microorganism using PCR was 33.3% (5/15) in patients with cervical insufficiency. Romero et al. [37] found an 18.1% (6/33) prevalence of ureaplasmas using culture techniques in patients with cervical dilatation ≥2 cm with gestational age from 14 to 24 weeks. These findings suggested that about a quarter of patients with cervical insufficiency have intra-amniotic infection with ureaplasmas. This is somewhat higher than the prevalence of ureaplasmas in other obstetrical conditions, such as preterm labor with intact membranes [36, 49], a short cervix [17] and normal pregnancy at the time of genetic amniocentesis [12, 15, 35].
Whether ureaplasmas are present and lead to cervical insufficiency, or alternatively, whether the organisms gain access to the amniotic cavity after the cervix is dilated and the membranes exposed, remains to be determined.
Intra-amniotic inflammation without evidence of intra-amniotic infection in cervical insufficiency
Intra-amniotic inflammation (regardless of the presence or absence of proven intra-amniotic infection) is associated with adverse pregnancy outcome [9, 23, 38, 48]. Recent studies revealed that various chemokines [11, 14, 16, 18, 21, 29, 30], as well as traditional proinflammatory cytokines such as the interleukin family [3, 13, 19, 45] and MMPs [4, 10, 27, 32], play a role in the pathogenesis of intra-amniotic infection/inflammation and preterm parturition. Our previous study [24] demonstrated a high prevalence of intra-amniotic inflammation (defined as an elevated MMP-8 concentration) in cases of cervical insufficiency, and its relationship with adverse pregnancy outcome. Other investigators [20] reported significantly increased concentrations of AF inflammatory cytokines regardless of proven AF infection in patients with a short cervix (≤5 mm). In the present study, we were able to demonstrate that two-thirds of cases with a negative AF culture and a negative PCR have histologic choriodeciduitis, which is not different from that of cases with proven intra-amniotic infection (Table 3). This may be due to infections that escape detection with standard cultivation techniques, or alternatively, that intra-amniotic infections develop after the amniocenteses were performed. Another possibility is that extra-amniotic inflammation without intra-amniotic infection plays a role in the occurrence of cervical insufficiency. Other investigators [31] showed that extra-amniotic infection is much more frequent than intra-amniotic infection. However, more studies are needed to clarify the association between extra-amniotic inflammation and cervical insufficiency.
In contrast to the high prevalence of choriodeciduitis, amnionitis and funisitis were found in only 20% of patients without evidence of intra-amniotic infection (by culture and PCR) that is lower than the prevalence of 80% in those with a positive PCR but a negative culture (Table 3). The amnion is the final barrier to reach the amniotic cavity and funisitis is a marker of fetal inflammation. Involvement of the amnion and the umbilical cord is an indicator of a fetal inflammatory response [33]. It is consistent with the finding of our previous study [22] that patients with documented AF infection had a more intense fetal inflammatory response than women with intra-amniotic inflammation without AF infection. Such findings suggest that intra-amniotic infection with ureaplasmas, detected by PCR assay, may be more frequently associated with a fetal inflammatory response syndrome and adverse neonatal outcome than intra-amniotic inflammation with a negative culture and PCR in patients with cervical insufficiency.
Neonatal outcome
Ureaplasmas have been implicated in the genesis of neonatal disease, such as BPD, congenital pneumonia, bacteremia and meningitis [43]. In the present study, significant neonatal morbidity was present in 80% of neonates with a positive PCR, but a negative culture, but only in 42% of those with a negative PCR and a negative AF culture. This trend did not reach statistical significance (Table 4); however, the power to detect a difference of 38% between the two groups was only 27% in the present study, and therefore, this may be the result of a type II error.
Acknowledgments
This research was supported in part by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Education, Science and Technology) (No. 2009-0080429) and in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
The authors stated that there are no conflicts of interest regarding the publication of this article.
References
- 1.Aaltonen R, Heikkinen J, Vahlberg T, Jensen JS, Alanen A. Local inflammatory response in choriodecidua induced by ureaplasma urealyticum. Br J Obstet Gynecol. 2007;114:1432–5. doi: 10.1111/j.1471-0528.2007.01410.x. [DOI] [PubMed] [Google Scholar]
- 2.Abele-Horn M, Wolff C, Dressel P, Zimmermann A, Vahlensieck W, Pfaff F, et al. Polymerase chain reaction versus culture for detection of ureaplasma urealyticum and mycoplasma hominis in the urogenital tract of adults and the respiratory tract of newborns. Eur J Clin Microbiol Infect Dis. 1996;15:595–8. doi: 10.1007/BF01709369. [DOI] [PubMed] [Google Scholar]
- 3.Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. 1999;78:379–82. [PubMed] [Google Scholar]
- 4.Biggio JR, Jr, Ramsey PS, Cliver SP, Lyon MD, Goldenberg RL, Wenstrom KD. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:109–13. doi: 10.1016/j.ajog.2004.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17(Suppl 1):S148–53. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 6.Bujold E, Morency AM, Rallu F, Ferland S, Tetu A, Duperron L, et al. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can. 2008;30:882–7. doi: 10.1016/S1701-2163(16)32967-X. [DOI] [PubMed] [Google Scholar]
- 7.Charles D, Edwards WR. Infectious complications of cervical cerclage. Am J Obstet Gynecol. 1981;141:1065–71. doi: 10.1016/s0002-9378(16)32698-9. [DOI] [PubMed] [Google Scholar]
- 8.Cunliffe NA, Fergusson S, Davidson F, Lyon A, Ross PW. Comparison of culture with the polymerase chain reaction for detection of ureaplasma urealyticum in endotracheal aspirates of preterm infants. J Med Microbiol. 1996;45:27–30. doi: 10.1099/00222615-45-1-27. [DOI] [PubMed] [Google Scholar]
- 9.Erez O, Romero R, Hoppensteadt D, Fareed J, Chaiworapongsa T, Kusanovic JP, et al. Premature labor: a state of platelet activation? J Perinat Med. 2008;36:377–87. doi: 10.1515/JPM.2008.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortunato SJ, Menon R. Screening of novel matrix metalloproteinases (MMPs) in human fetal membranes. J Assist Reprod Genet. 2002;19:483–6. doi: 10.1023/A:1020362519981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35:385–93. doi: 10.1515/JPM.2007.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 13.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529–47. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–16. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of ureaplasma urealyticum. Prenat Diagn. 1992;12:111–7. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 16.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36:217–27. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–9. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst RM, Laurini R, Jacobsson B, Samuelsson E, Savman K, Doverhag C, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med. 2007;20:885–93. doi: 10.1080/14767050701752601. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Nikolaitchouk N, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–31. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 20.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intra-amniotic inflammation? Am J Obstet Gynecol. 2009;200:374.e1–5. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 21.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–16. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intra-amniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294.e1–6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Lee SE, Han BD, Park IS, Romero R, Yoon BH. Evidence supporting proteolytic cleavage of insulin-like growth factor binding protein-1 (IGFBP-1) protein in amniotic fluid. J Perinat Med. 2008;36:316–23. doi: 10.1515/JPM.2008.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633.e1–8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 25.Li YH, Brauner A, Jonsson B, van der Ploeg I, Soder O, Holst M, et al. Ureaplasma urealyticum-induced production of proinflammatory cytokines by macrophages. Pediatr Res. 2000;48:114–9. doi: 10.1203/00006450-200007000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Luki N, Lebel P, Boucher M, Doray B, Turgeon J, Brousseau R. Comparison of polymerase chain reaction assay with culture for detection of genital mycoplasmas in perinatal infections. Eur J Clin Microbiol Infect Dis. 1998;17:255–63. doi: 10.1007/BF01699982. [DOI] [PubMed] [Google Scholar]
- 27.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 28.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652–5. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 29.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008;36:485–96. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21:763–75. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onderdonk AB, Hecht JL, McElrath TF, Delaney ML, Allred EN, Leviton A. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol. 2008;199:52.e1–10. doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36:497–502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CW, Moon KC, Park JS, Jun JK, Romero R, Yoon BH. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta. 2009;30:56–61. doi: 10.1016/j.placenta.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–61. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 35.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and ureaplasma urea-lyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–6. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–91. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 38.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 39.Teng K, Li M, Yu W, Li H, Shen D, Liu D. Comparison of PCR with culture for detection of ureaplasma urealyticum in clinical samples from patients with urogenital infections. J Clin Microbiol. 1994;32:2232–4. doi: 10.1128/jcm.32.9.2232-2234.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treadwell MC, Bronsteen RA, Bottoms SF. Prognostic factors and complication rates for cervical cerclage: a review of 482 cases. Am J Obstet Gynecol. 1991;165:555–8. doi: 10.1016/0002-9378(91)90283-w. [DOI] [PubMed] [Google Scholar]
- 41.Viscardi RM, Manimtim WM, Sun CC, Duffy L, Cassell GH. Lung pathology in premature infants with ureaplasma urealyticum infection. Pediatr Dev Pathol. 2002;5:141–50. doi: 10.1007/s10024001-0134-y. [DOI] [PubMed] [Google Scholar]
- 42.Viscardi RM, Hashmi N, Gross GW, Sun CC, Rodriguez A, Fairchild KD. Incidence of invasive ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J Perinatol. 2008;28:759–65. doi: 10.1038/jp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–89. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 45.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 46.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–7. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 47.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 48.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 49.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, et al. The clinical significance of detecting ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–24. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]


