Abstract
Rationale
Chronic exposure to ambient air-borne particulate matter <2.5 µm (PM2.5) increases cardiovascular risk. The mechanisms by which inhaled ambient particles are sensed and how these effects are systemically transduced remain elusive.
Objective
To investigate the molecular mechanisms by which PM2.5 mediates inflammatory responses in a mouse model of chronic exposure.
Methods and Results
Here we show that chronic exposure to ambient PM2.5 promotes Ly6Chigh inflammatory monocyte egress from bone-marrow and mediates their entry into tissue niches where they generate reactive oxygen species via NADPH oxidase. Toll-like receptor-4 (TLR4) and Nox2 (gp91phox) deficiency prevented monocyte NADPH oxidase activation in response to PM2.5 and was associated with restoration of systemic vascular dysfunction. TLR4 activation appeared to be a prerequisite for NAPDH oxidase activation as evidenced by reduced p47phox phosphorylation in TLR4 deficient animals. PM2.5 exposure markedly increased oxidized phospholipid derivatives of 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine (oxPAPC) in bronchioalveolar lavage fluid. Correspondingly, exposure of bone-marrow derived macrophages to oxPAPC but not PAPC recapitulated effects of chronic PM2.5 exposure while TLR4 deficiency attenuated this response.
Conclusions
Taken together, our findings suggest that PM2.5 triggers an increase in oxidized phospholipids in lungs that then mediates a systemic cellular inflammatory response through TLR4/NADPH oxidase dependent mechanisms.
Keywords: particulate matter, monocyte, Toll-like receptor 4, superoxide, oxidized phospholipids
Introduction
Particulate matter (PM) has been consistently linked to morbidity and mortality from ischemic cardiovascular events and reduced life expectancy in epidemiologic studies (1–4). Particles less than 2.5 µm (PM2.5) in diameter represent the size fraction that has been most consistently implicated in the pathogenesis of cardiovascular disease (5). Several new studies have also demonstrated that residing in locations with higher long-term average PM levels elevates the risk for cardiovascular (CV) morbidity and mortality with the risks associated with chronic exposure to PM2.5 (years) vastly exceeding the risks noted with short term exposure (days) (6, 7). PM2.5 air pollution has been linked with endothelial dysfunction, systemic inflammatory and oxidative stress responses and the progression of atherosclerosis (8, 9). Seaton and colleagues first proposed that deposition of particles in the lung provoked a low-grade alveolar inflammation with a secondary systemic inflammatory response (10). Since then, numerous studies have supported this inflammatory hypothesis and further expanded on it (11, 12). However, the precise mechanisms by which particulates are sensed and the responses transduced remain elusive. It is increasingly apparent that biologic systems commonly employ evolutionarily conserved mechanisms to sense a variety of environmental signals (13, 14). Toll-like receptors (TLRs) play a central role in the recognition of a broad diversity of environmental and pathogen associated molecular patterns (15, 16). We therefore hypothesized that PM2.5 either directly or via biologic intermediates modulates systemic effects via TLR4 pathways. Accordingly, we performed exposures to PM2.5 using a system that exposes animals chronically to 8–10 fold higher levels of ambient particulates (17, 18). Our findings suggest that TLR4 and Nox2 may be involved in PM2.5 induced systemic inflammation.
Material and Methods
An expanded methods section is available in the Online Data Supplement.
Briefly, This study utilized male mice at the age of six-weeks of following different strains: C57BL/6, Nox2−/− (C57BL/6 background), Balb/c (TLR4wt), Tlr4Lps-d (TLR4d, background strain BALB/cAnPt) and c-fmsYFP transgenic mice (FVB/N background).
Animal exposure and the monitoring of exposure atmosphere and ambient aerosol were performed as previously described using a versatile aerosol concentration enrichment system that was modified for long-term exposures (19). The Committee on Use and Care of Animals from the Ohio State University (OSU) approved all experimental procedures.
Results
PM2.5 concentration and exposure protocol
The exposure metrics are described in Supplementary Table I. Ambient mean daily PM2.5 concentration at the exposure facility was 10.7 ± 2.1 µg/m3. Mean concentration of PM2.5 in the chamber was 92.4 ± 2.1 µg/m3 (Supplementary Figure I). Because the mice were exposed for 6 hours a day, 5 days a week, the normalized PM2.5 concentration was 24.7 µg/m3 which is close to the annual average PM2.5 National Ambient Air Quality Standard (NAAQS) of 15 µg/m3 (20). The total PM2.5 dose inhaled during the exposure corresponded to 104 ± 20 µg assuming a ventilation rate of 105 breaths/minute and 0.2 cc/breath in mice (21). LPS levels in the serum and BAL did not differ between the FA and PM2.5 group (55.2 ± 19.4 pg/ml vs. 49.8 ± 13.6 pg/ml in the serum and 423 ± 233 pg/ml vs. 654 ± 262 pg/ml in the BAL, respectively).
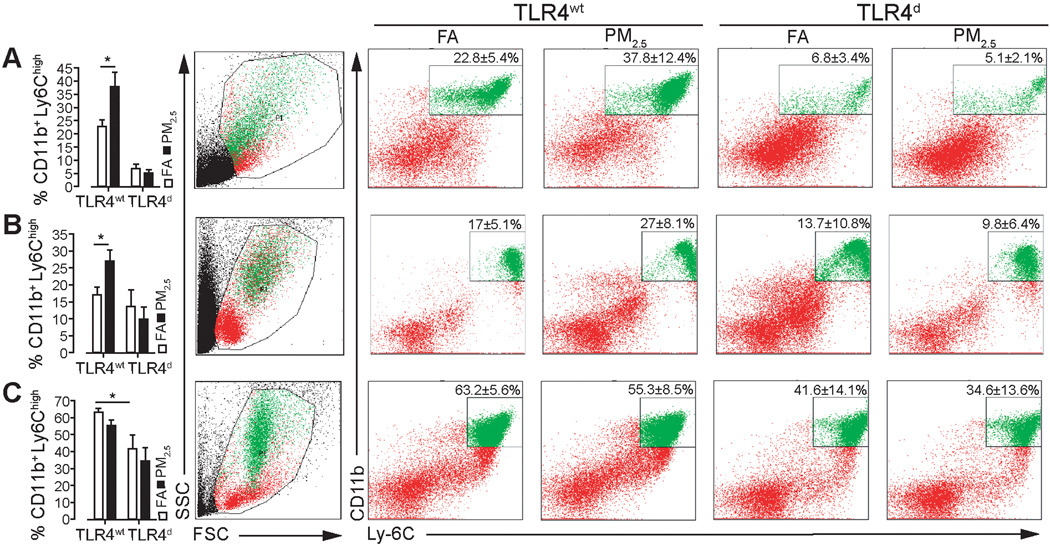
PM2.5 exposure promotes inflammatory monocyte egress from bone marrow to blood via TLR4 pathways
To determine whether chronic PM2.5 exposure had effects on monocyte sub-populations, we analyzed peripheral blood, bone marrow and splenic cell populations. PM2.5 increased inflammatory monocytes in the periphery in TLR4wt mice while deficiency of TLR4 diminished the effect (Figure 1B). The spleen and bone marrow played a differential role in contribution of inflammatory monocytes to the systemic circulation (Figure 1A and C).
Figure 1.
PM2.5 exposure promotes inflammatory monocyte egress from bone marrow to blood via TLR4 pathways. Inflammatory monocyte population of TLR4wt and TLR4d mice (A) in spleen (B) in peripheral blood, (C) in bone marrow. Data are mean ± SD. (n=4–6/group; *p<0.05). Exposure duration of 20 weeks.
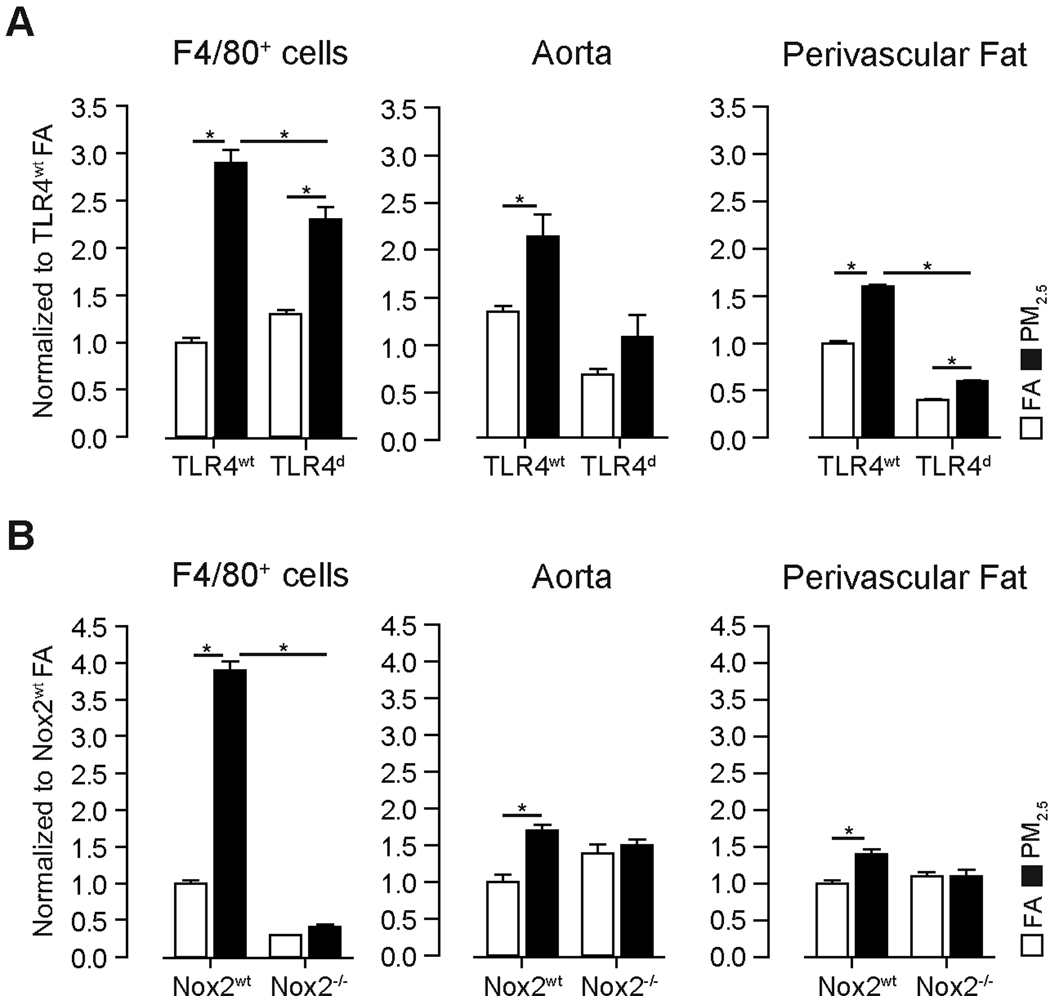
Chronic PM2.5 exposure increases NADPH oxidase derived superoxide (O2·−) in monocytes, aortic tissue and perivascular fat through TLR4
We investigated the systemic effects of PM2.5 exposure. Figure 2 depicts NADPH oxidase derived O2·− generation in F4/80+ monocytes and systemic vasculature. NADPH oxidase derived O2·− production increased in aorta and perivascular fat in response to PM2.5. TLR4 deficiency attenuated but did not abolish the effects of PM2.5 (Figure 2A). We then utilized mice deficient in Nox2 (gp91phox−/−), to address the relative role of this sub-unit in up-regulated NADPH oxidase activity in response to PM2.5. Nox2−/− mice were exposed for 20 weeks to either FA or PM2.5. NADPH oxidase derived O2·− production was decreased in Nox2−/− mice in F4/80 cells but not in aorta or perivascular fat of FA exposed animals. Nox2 deficiency abrogated the effects of PM2.5 on O2·− production in F4/80+ cells, aorta and perivascular fat (Figure 2B). In contrast TLR4d abolished the increase in F4/80 cells in response to PM2.5 in the peri-vascular fat in the aorta. These findings suggest that incursion into the perivascular space by monocytes may contribute to increased O2·− in response to PM2.5 and support a functional role for Nox2 in PM2.5 mediated effects.
Figure 2.
PM2.5 exposure increases NADPH oxidase derived O2·− production in monocytes, aortic tissue and perivascular fat in wildtype mice. (A) O2·− production in response to PM2.5 exposure in bone marrow derived F4/80+ cells, aortic and perivascular tissue from TLRwt and TLR4d mice. (B) O2·− production in response to PM2.5 exposure in F4/80+ cells, aortic and perivascular tissue from Nox2wt and Nox2−/− mice after 20 weeks of exposure. Data are mean ± SD. (n=5, *p<0.05).
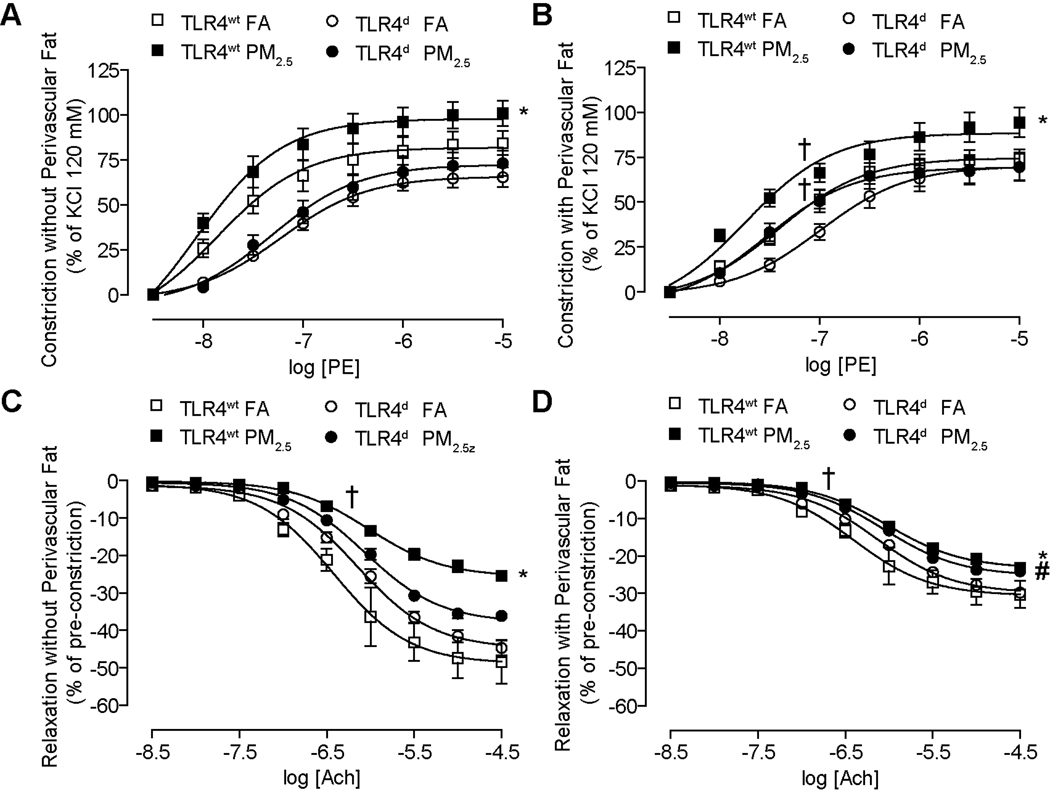
PM2.5 impairs macrovascular tonal responses and influences leukocyte trafficking in the microvasculature
As perivascular fat is an important portal of entry for inflammatory cells and has been shown to influence vascular tone (22), we investigated vascular responses in the presence and absence of perivascular fat. Segments with and without fat had different levels of tension following application of a pre-constrictor dose of phenylephrine (prior to acetylcholine) and are depicted separately in Figure 3. Chronically PM2.5 exposed TLR4wt mice demonstrated increased constriction to phenylephrine in the presence of perivascular fat (Figure 3A), an effect that was noted in the absence of fat as well. (Figure 3B). TLR4 deficiency normalized the heightened constriction in response to PM2.5. PM2.5 exposed TLR4wt mice demonstrated attenuation of peak relaxation and increased EC50 dose to acetylcholine compared with FA mice; findings that were also seen in segments without perivascular fat. TLR4 deficiency attenuated the effects of PM2.5 exposure (Figure 3C, 3D. Supplementary Figure II allows the interpretation of the delta change in the relaxation and constriction maxima in segments with and without fat. We studied the vasomotor response in mice with and without intact Nox2. Supplementary Table III depicts the responses in vascular segments from these mice. These results show a significantly higher constriction in Nox2wt mice in response to PM2.5, while deficiency of Nox2 abrogated this effect. These results suggest that both TLR4 and NADPH oxidase mediate the systemic vascular effects of PM2.5 exposure.
Figure 3.
PM2.5 impairs macrovascular tonal responses with chronic exposure to PM2.5 through TLR4 pathways (20 weeks of exposure). (A) Constriction of aortic rings without perivascular fat in response to increasing dosages of phenylephrine. (B) Constriction of aortic rings with perivascular fat in response to increasing dosages of phenylephrine. (C) Relaxation of aortic rings without perivascular fat in response to increasing dosages of acetylcholine. (D) Relaxation of aortic rings with perivascular fat in response to increasing dosages of acetylcholine. (n=8–10/group; *p<0.01 vs. TLR4wt FA; #p<0.05 vs. TLR4d FA; †logEC50 vs. same group FA).
To test the effect of PM2.5 on leukocyte trafficking in the microvasculature, we performed in-vivo intravital microscopic experiments on the cremasteric muscle as a surrogate for the perivascular microcirculatory environment. Here, we found a significant increase of leukocyte adherence (Supplementary Figure III) in response to chronic exposure to PM2.5.
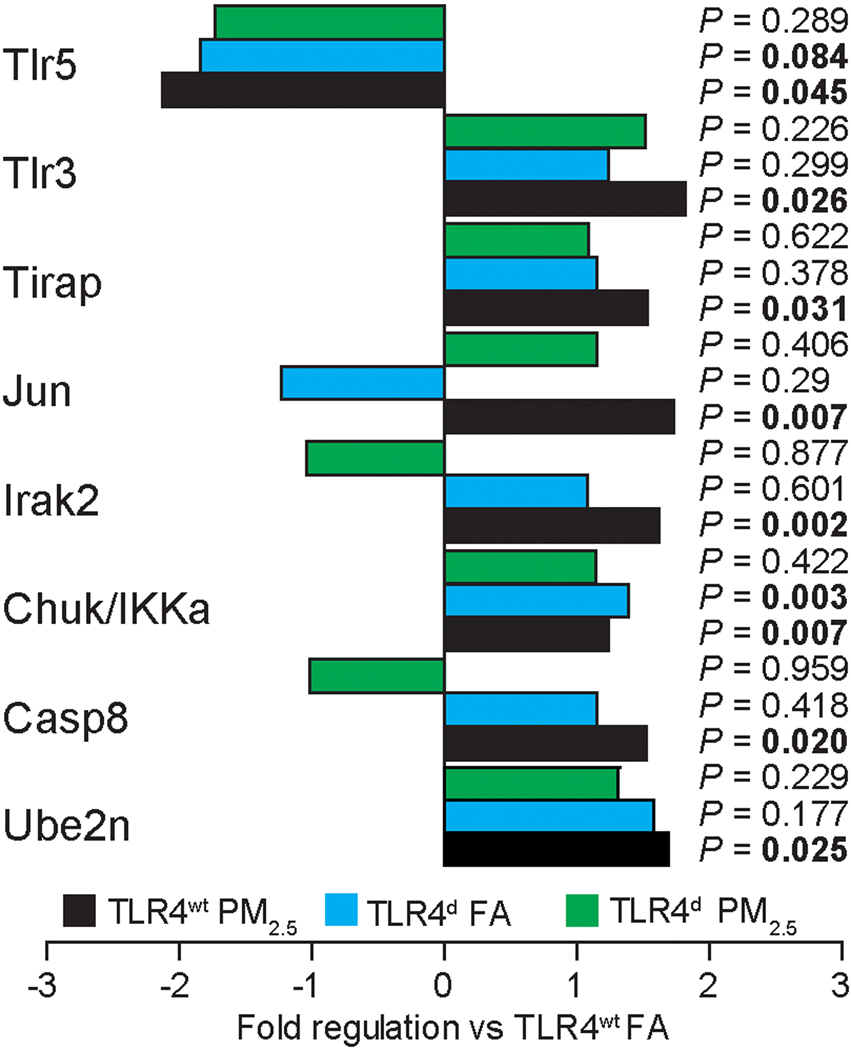
PM2.5 exposure increases Toll-like receptor dependent gene expression in aortic tissue in TLR4wt mice
To determine the molecular basis of PM2.5 exposure effects in the vasculature, we examined a panel of genes involved in TLR signaling in aortic tissue. We utilized a PCR array, profiling the expression of genes related to TLR-mediated signal transduction. While in TLR4wt mice 8 genes significantly changed, this induction was abolished in TLR4d mice (Figure 4).
Figure 4.
Chronic PM2.5 exposure increases Toll-like receptor dependent gene expression in perivascular tissue in TLR4wt mice. 8 of 82 analyzed genes found to have a significant change vs. the TLR4wt FA control group are depicted. (n=4/group).
PM2.5 exposure increases monocyte homing and adherence in tissue niches
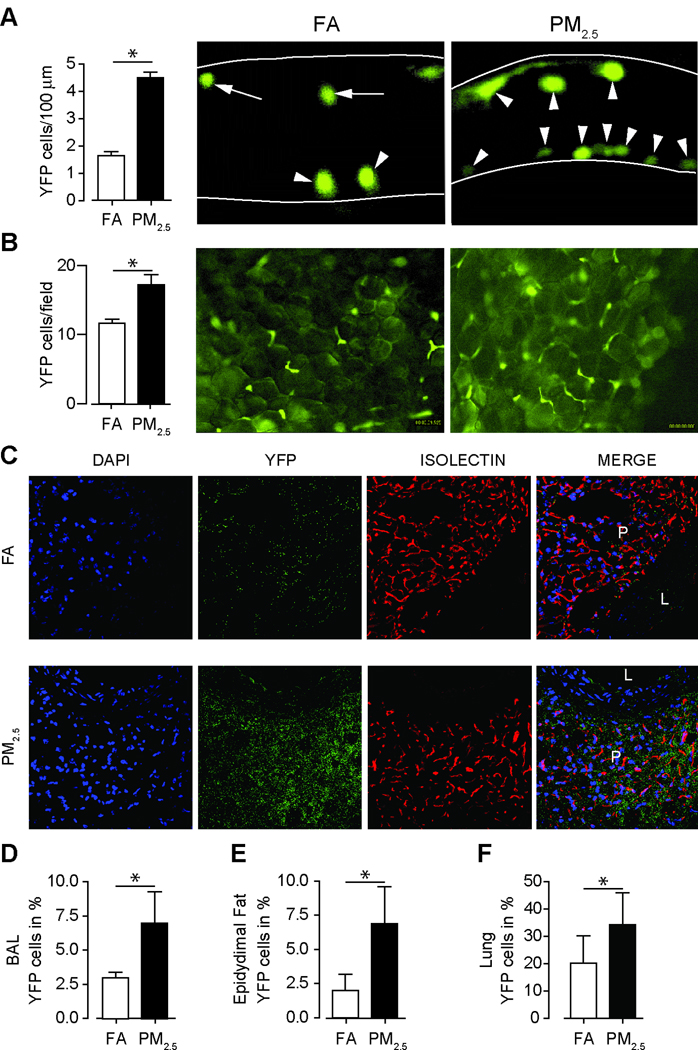
To provide additional evidence of recruitment of monocytes with chronic PM2.5 exposure in tissue niches, we used a transgenic reporter mouse model expressing YFP under the control of a c-fms promoter (c-fmsYFP) that were exposed to FA or PM2.5 initially over a duration of 20-weeks. We first examined the number of adherent YFP+ cells in the cremasteric and mesenteric adipose tissue. Significantly more adherent YFP+ cells were found in the cremasteric endothelial wall in response to chronic PM2.5 (Figure 5A). Figure 5B indicates similar findings in the mesenteric adipose tissue; representative images of mesenteric tissue from c-fmsYFP mice exposed to either FA or PM2.5, respectively are depicted in Supplementary Video I–IV. Impairment in contractile properties of isolated thoracic aortic rings with and without perivascular fat in c-fmsYFP mice are shown in Supplementary Figure IV in response to PM2.5 exposure. An increase of YFP+ cell-infiltration into the perivascular adipose tissue was observed in response to PM2.5 (Figure 5C). YFP+ cells in the BAL, lung and epididymal fat were noted in response to PM2.5 (Figure 5D, E and F). In additional experiments we exposed c-fmsYFP mice over a shorter 12 week duration to PM2.5 or FA. Flow-cytometric analysis of YFP+ cells co-expressing CCR2, CCR5 and CXCR3 in the bone marrow and spleen revealed a significant increase in YFP+CCR2+ cells in response to PM2.5 exposure in bone marrow (Supplementary Table IV). In light of increased YFP+ monocytes and F4/80 macrophages in the lung we hypothesized that the inflammatory milieu in the lung may provide a substrate for the generation of mediators that in turn could participate in TLR signaling and NADPH oxidase activation.
Figure 5.
Chronic PM2.5 exposure over 20 weeks increases monocyte adherence within microvasculature and tissue niches in c-fmsYFP mice (FVB/N background). (A) Representative images and quantification of adherent YFP cells in the cremasteric venular endothelium (open arrow heads adherent monocytes; arrow heads with tail, rolling monocytes). (Original magnification 400×, n=5). (B) Representative images and quantification of adherent YFP cells in the mesenteric adipose tissue. (Original magnification 200×, n=5). (C) Immunohistochemical staining for YFP positive monocyte infiltration into perivascular fat tissue in c-fmsYFP mice exposed to FA or PM2.5. Perivascular fat tissue from mice that express a yellow fluorescent protein (c-fmsYFP, yellow) was stained with DAPI (blue) and isolectin (red) and visualized by confocal microscopy. (L=lumen; P=perivascular fat). (Original magnification 400×, n=3). Quantification of YFP positive cells in BAL fluid (D), in epidydimal fat (E) and in lung tissue (F) (n=4–6). Exposure time was about 20 weeks. Data are mean ± SD. (*p<0.05).
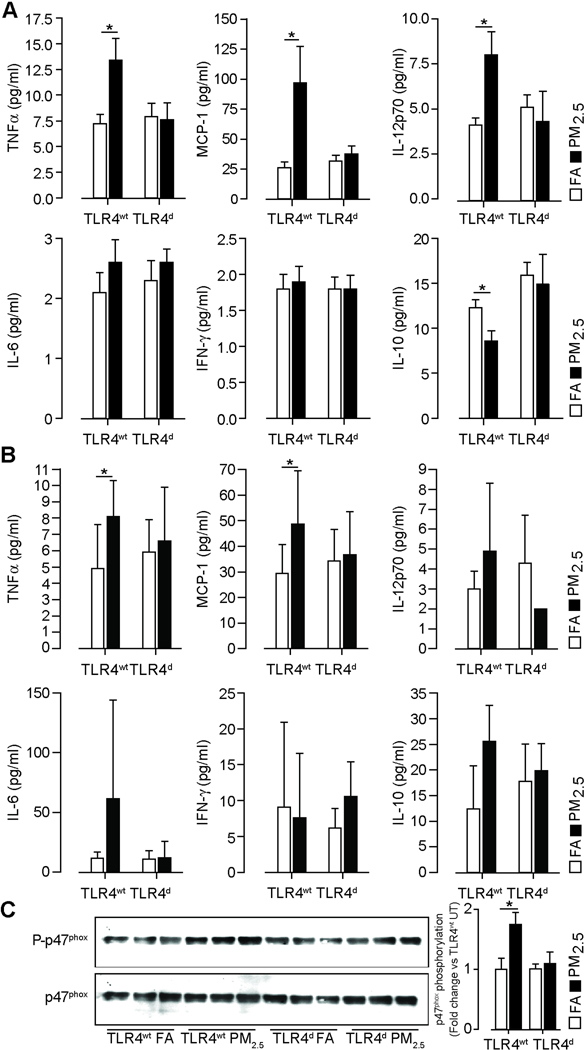
TLR4 deficiency normalizes inflammatory cytokine and MCP-1 release in response to PM2.5
TLR4wt mice demonstrated an increase in TNFα, MCP-1 and IL12p70 and a decrease of IL-10 levels in the lung (Figure 6A). TLR4 deficiency attenuated TNFα, MCP-1 and IL-12p70 levels in response to PM2.5. Corresponding plasma measurements demonstrated an increase in TNFα and MCP-1 with PM2.5 exposure with normalization in TLR4d (Figure 6B). We then investigated if TLR4 activation by PM2.5 leads to downstream NADPH oxidase activation (23–25). We chose phosphorylation of the p47phox subunit in the lung as an index of NADPH oxidase activation in response to PM2.5 (12, 26). TLR4wt showed a significant increase in p47phox phosphorylation in lung following PM2.5 exposure, compared to FA mice, while TLR4 deficiency prevented this effect (Figure 6C).
Figure 6.
TLR4 deficiency normalizes inflammatory cytokine release and prevents p47phox phosphorylation in response to PM2.5 exposure over 20 weeks. (A) Cytokine analysis of lung homogenates (500 µg/ml protein) in TLR4wt and TLR4d mice measured by a cytokine bead array. (n=8–10/group). (B) Cytokine analysis of plasma samples in TLR4wt and TLR4d mice measured by a cytokine bead array. (n=8–10/group) (C) Immunoblots demonstrating increased p47phox expression in response to PM2.5 exposure compared to FA in TLR4wt and normalization of p47phox phosphorylation in TLR4d mice. Lung homogenates from TLR4wt and TLR4d mice were immunoblotted for p47phox and phospho-p47phox (left). The figure on the right represents the photodensitometric quantification of the blots. (n=5/group). Data are mean ± SD. (*p<0.05).
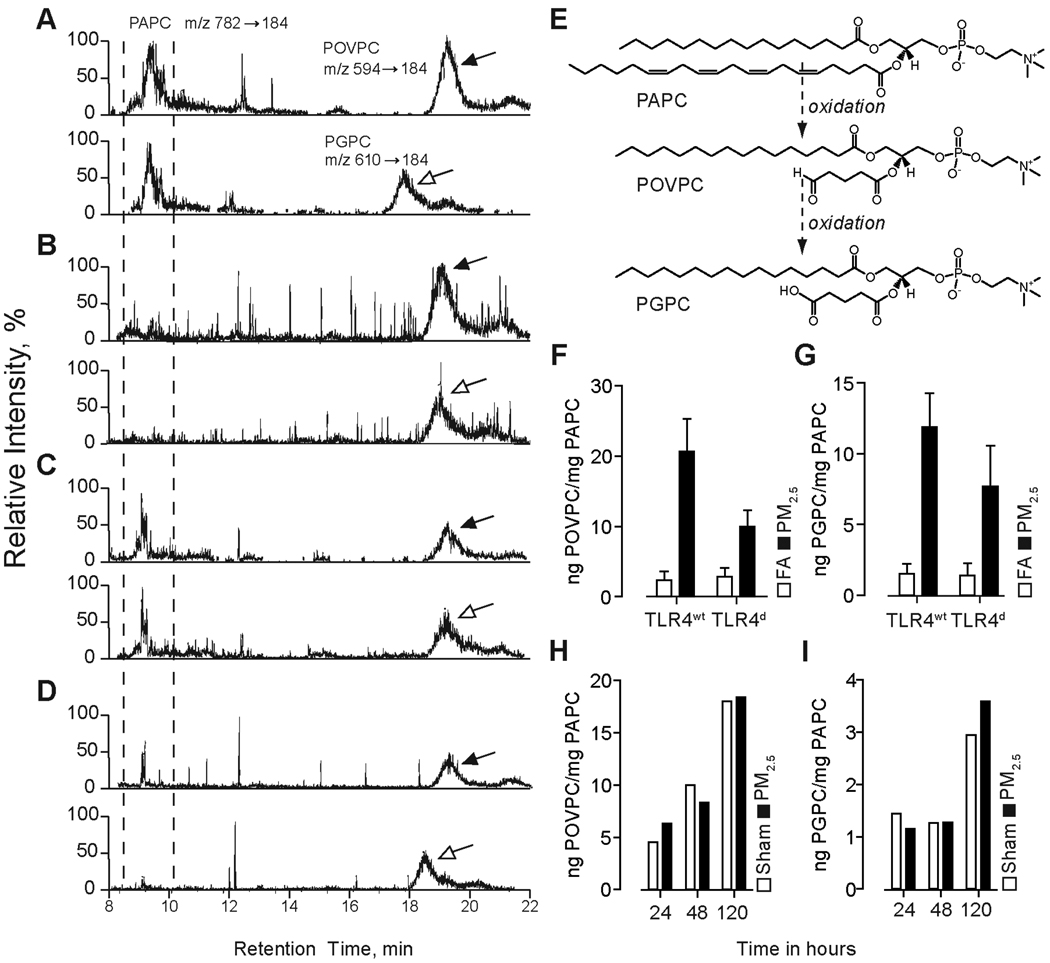
Airborne particulate matter causes increased levels of two oxidized PAPC derivatives in BAL fluid of PM2.5 exposed mice
Prior studies have indicated a role for surfactant phospholipids in mitigating inflammatory responses while generation of oxidized derivatives of surfactant phospholipids has been shown to potentiate inflammatory signaling (14, 27, 28). At first, we analyzed 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC), the dominant phospholipid in BAL and its oxidized derivatives by means of liquid chromatography-electrospray mass spectrometry. Representative LC-MS images are shown in Figure 7A (TLR4wt FA), 7B (TLR4wt PM2.5), 7C (TLR4d FA) and 7D (TLR4d PM2.5) and depict a peak shift from PAPC to the oxidized derivatives in response to PM2.5 exposure. Figure 7E illustrates the oxidation steps from PAPC through POVPC (1-palmitoyl-2-(5'-oxo-valeroyl)-sn-glycero-3-phosphocholine) to PGPC (1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine). Mice deficient of TLR4 had lower amounts of oxidized phospholipids in response to PM2.5 (Figure 7F and G). To address if the oxidation products were a consequence of oxidative chemistry relating to PM2.5 constituents or related to cellular oxidative end-products, we incubated PAPC with or without added PM2.5 at 37°C for duration of 24–120 h. Our data demonstrates that PM2.5 alone is not the primary cause of oxidation. Thus, we have found no difference in the degree of oxidation products of PAPC between PM2.5 group and control group. The increasing levels of these products over time in both groups suggest PAPC auto-oxidation (Figure 7H and I).
Figure 7.
Airborne particulate matter causes increased levels of two oxidized PAPC derivatives in BAL fluid of PM2.5 exposed mice. Lipid extracts from BAL fluid of TLR4wt and TLR4d mice exposed for 20 weeks to FA or PM2.5 were analyzed by HPLC with positive electrospray ionization mass-spectrometry operating in multiple reaction monitoring mode. Parent PAPC and oxidized derivatives (POVPC and PGPC) ion pairs were monitored by their characteristic retention time and daughter ions. Corresponding chromatograms were post-processed by extraction of POVPC and PGPC ions for quantitative analysis. Representative LC-MS chromatograms are shown for (A) TLR4wt FA, (B) TLR4wt PM2.5, (C) TLR4d FA, (D) TLR4d PM2.5. (E) Chemical structures of monitored phospholipids. Quantitative analysis of levels of (F) POVPC and (G) PGPC against PAPC with an exaggerated level of oxidation in the PM2.5 exposed mice over 20 weeks. In-vitro incubation of PAPC in the presence of PM2.5 or with PBS was performed in time-dependent manner followed by quantification of levels of (H) POVPC and (I) PGPC by LC/MS-MS. BAL fluid of 5 mice per group were pooled for these experiments with extraction of the lipid content. The amount of oxidized phospholipid is set in ratio to non-oxidized phospholipid in order to compare the different groups.
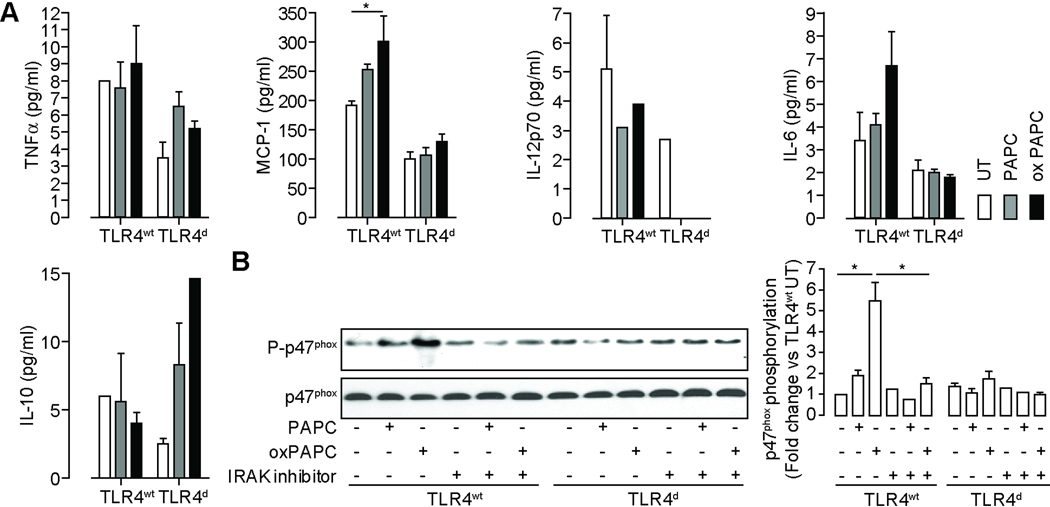
TLR4 triggers inflammatory gene expression, cytokine release and promotes IRAK modulated p47phox phosphorylation in response to oxidized phospholipids treatment in BMDM
To test the hypothesis that oxidized phospholipids may activate inflammatory pathways via TLR4, we treated BMDM with and without intact TLR4 to oxidized PAPC derivatives. Supplemental figure V shows the gene expression profile of cells treated with oxidized PAPC derivatives. oxPAPC strongly induced the expression of TNFα, various components of the NADPH oxidase including Nox2 and p67phox and the homing receptors CCR1, CCR2 and CCR5. The cytokine profile of BMDM treated with oxidized and non-oxidized PAPC is outlined in Figure 8A. TLR4wt BMDM exhibited a significant increase of TNFα and MCP-1 but a marginal increase of IL-6 in response to oxPAPC treatment. Figure 8B shows that oxidized PAPC phosphorylates the p47phox subunit of NADPH oxidase. This response was abrogated by an Interleukin-1 Receptor-associated kinase (IRAK) inhibitor. TLR4 deficiency in BMDM abolished the effects of oxPAPC.
Figure 8.
TLR4 triggers inflammatory cytokine release and promotes IRAK modulated p47phox phosphorylation in response to oxidized phospholipid treatment in BMDM derived from TLR4wt and TLR4d mice. (A) BMDM were treated with PAPC and oxPAPC and inflammatory cytokine levels in the supernatant were determined. (n=3/group; *p<0.05) (B) These blots show p47phox expression and phosphorylation in response to PAPC and oxidized PAPC treatment. Lysates from bone marrow derived monocytes isolated from TLR4wt and TLR4d mice were immunoblotted for p47phox and phospho-p47phox (left). A subset of experiments was performed in presence of an IRAK inhibitor. The figure on the right represents the photodensitometric quantification of the blots. (n=3) Data are mean ± SD. (*p<0.05).
Discussion
The inhalation of airborne pollutants is linked to lung and systemic inflammation. The mechanisms by which dust is recognized and how chronic inflammatory diseases are triggered are poorly understood. Here we show that: 1. Chronic PM2.5 exposure promotes monocyte egress from bone marrow into the systemic circulation. 2. Increased presence of inflammatory monocytes in the circulation corresponds to ingress of these cells into vascularized tissue niches such as perivascular fat and visceral adipose. 3. Alteration in macrovascular and microvascular function with PM2.5 is related to vascular infiltration in the perivascular fat and superoxide generation by monocytes. 4. Activation of TLR4 and NADPH oxidase in monocyte/macrophages by oxidized phospholipids may represent one potential mechanism by which PM2.5 mediates systemic inflammation.
Chronic PM2.5 promotes monocyte egress from systemic reservoirs
Our results add to the growing body of evidence that chronic PM2.5 exposure modulates systemic inflammatory effects and provides important insights into the mechanisms and mediators of these responses (9, 11, 29, 30). Prior cohort and panel studies have postulated an effect of PM on the bone marrow to enhance the systemic release of inflammatory cells (31, 32). A few controlled exposure studies have corroborated a cellular pro-inflammatory response, manifested as increase in circulating white blood cell counts or immune cell infiltration (33, 34). Our results provide mechanistic proof of the existence of a significant contribution of the bone marrow and potentially spleen in response to chronic PM2.5 exposure (35, 36). Blood monocytes are heterogenous and comprise of distinct subsets with specific migratory properties (35, 37). In the mouse, monocyte subsets can be distinguished on the basis the Ly6C antigen expression. Ly6Chigh cells (F4/80+, CD11b+, CD115+) cells, originally called "inflammatory” are recruited to tissue niches in response to chemokine signals. In contrast, Ly6Clow monocytes represent resident cells with lower recruitment to sites of inflammation (36–38). Prior studies have suggested an important role for the corresponding ligands in promoting the egress of monocytes into the peripheral circulation (39, 40). In keeping with these results there was increase in lung and circulating levels of MCP-1 (CCL2). Increase in MCP-1 could represent one mechanism for the enhanced flux of monocytes from with PM2.5. However it is possible that other chemokines or mechanisms may be responsible, as other studies have reported that MCP-1 does not contribute to macrophage infiltration into adipose tissue (50). We hypothesize that other mechanism might drive those inflammatory monocytes to adipose tissue niches. The results with the c-fmsYFP mouse model provide additional evidence that monocytes mediate tissue infiltration in response to chronic PM2.5 exposure and suggest that MCP-1 release may indeed result in homing of CCR2+ cells from the bone-marrow. Ly6Chigh cells almost always expressed YFP in this study. The results in the YFP model are partially supported by additional exposure studies where there was an increase in CCR2+ monocytes with exposure durations as brief as 10-weeks.
TLR4 activation is critical to the transduction of PM2.5 systemic response
The up-regulation of multiple genes involved in TLR4 signaling in the vasculature in response to PM2.5 and abrogation in TLR4d mice is suggestive of a specific interaction of PM2.5 with this pattern recognition receptor. Our data also seem to suggest an important contribution of the lung as MCP-1 and TNFα levels are elevated. These changes in lung cytokine content were accompanied by increased phosphorylation of p47phox subunit of NADPH oxidase in the lung and heighted circulating levels of MCP-1 and TNFα. MCP-1 may then potentially lead to the egress of subsets of monocytes from the bone marrow through CCR2 dependent mechanisms.
Role of the NADPH oxidase in PM2.5 effects
Our findings may have important consequences for regulation of vascular tone. Although many studies have demonstrated an important role for vascular NADPH oxidase in maladaptive responses in the vessel wall; the contribution of NADPH oxidase in inflammatory cells, versus those of resident vascular cells has only been recently appreciated (41–44). Importantly, it has been shown that infiltrating macrophages in the peri-adventitial fat may release cytokines and O2·− in a Nox2 dependent manner, which by itself, or via activation of Nox1/Nox2 in vascular cells may then contribute to adverse tonal responses (44–46). We believe that our studies provide evidence that monocyte NADPH oxidase derived O2·− in response to PM2.5 may alter redox balance and promote vasoconstrictive responses. While p47phox phosphorylation is required for the coordinate generation of superoxide by both Nox1 (present only in vascular cells) and Nox2 (present in myeloid cells and vascular cells), it is likely that upstream TLR4 activation in monocytes/macrophages was contributing to Nox2 activation in these cells, rather than Nox1 (41). We base this conclusion on the fact that ablation of Nox2 virtually abolished the O2·− in monocytes both in response to PM2.5 as well as in the FA group. These results in conjunction with monocyte infiltration in perivascular fat are highly suggestive of a monocyte source for ROS generation in response to PM2.5. The magnitude of O2·− production was equivalent in the perivascular fat compared to the actual vessel wall (data not shown) and is further supportive of the concept that the vaso-vasorum coursing through the perivascular fat may represent important entry points for inflammatory cells (44, 46).
Role of NADPH oxidase in TLR4 mediated responses
Our results suggest that NADPH oxidase activation occurs in the lung as well in the systemic circulation. Based on in-vitro studies in cultured macrophages, inhibition of IRAK, prevented phosphorylation of the p47phox subunit of NADPH oxidase, suggesting that TLR4 activation occurs upstream of NADPH oxidase. Pacquelet et al demonstrated that NADPH oxidase is activated as a result of the phosphorylation of p47phox by IRAK4 and identified the residues of p47phox as targets of IRAK4 phosphorylation (23). In our in-vivo experiments IRAK2 (but not IRAK4) was up regulated transcriptionally in the aorta. Both IRAK1 and IRAK4 are active serine/threonine kinases, and phosphorylation of IRAK1 or IRAK2 is crucial for their activation during TLR/MyD88 signaling. Although IRAK1 is the dominant kinase, shown by many studies to be involved in TLR4 signaling, our results suggest that IRAK2 may be important in PM2.5 exposure. The up-regulation of caspase-8 may represent a non-apoptotic role of this protein as it has been shown that TLR4 activation results in the recruitment of caspase-8 to a complex containing IKK (47). The ubiquitin-conjugating enzyme E2N (Ube2N) may represent a general homeostatic response to inflammatory injury as has been previously demonstrated with other toxins (48).
Exposure considerations and limitations
The exposures used in this protocol are broadly relevant to human health as they mimic “real-world” ambient air at doses that are commonly encountered in many parts of the world without requiring invasive methods or the generation of artificial particles (49).. Our results provide mechanistic rationale for inflammatory effects of PM2.5 exposure and why even relatively low levels of particle exposure (when compared to cigarette smoking for instance) results in relatively robust systemic inflammatory effects (3). Several important limitations must be acknowledged. Although we did not test for the involvement of other pattern recognition receptors, it is conceivable that the Nalp3 inflammasome and other TLR receptors such as TLR3 pathways may also be involved (13). Although we have shown an involvement of oxidized PAPC, it is conceivable that alternate/additional TLR4 ligands may participate such as modified matrix components, hyaluronan, or even HMGB1. We have not provided definitive evidence that non-MyD88 pathways are not involved (TRAF6/TRIF). Another limitation stems from the fact that we performed exposures in different strains. It is well known that different strains may have widely different responses to inflammatory stimuli. Thus we cannot conclude that these pathways are conserved and identical across strains. A final limitation is that although we have tried to characterize the precise locus of activation of inflammatory cells in response to PM2.5, we have not been able to definitively conclude that the lung is integral to this response. Our findings reveal that PM2.5 may represent a chronic inflammatory stimulus and may contribute to the pathogenesis of cardiometabolic disease.
Hypothetical Model
This work emphasizes on the role of TLR4 in mediating systemic inflammation in response to PM2.5 exposure. Supplementary Figure VI shows the hypothetical model of TLR4/ NADPH oxidase interaction.
Supplementary Material
Acknowledgements
The authors acknowledge Uwe and Roland Kampfrath, Dresden, Germany for the generous provision and development of the software OptiTest (Version 1.4.1.0) and the exposure team Aixia Wang and Terry R. Williams. We also would like to acknowledge DHLRI core facilities for providing mass spectrometry instrumentation for this work.
Sources of Funding
This study was supported by grants from National Institutes of Health (NIH) R01ES013406 and R01ES015146 to Dr. Rajagopalan. Dr. Sun is supported by NIH KO1ES016588.
Non-standard Abbreviations and Acronyms
- BALF
broncheolar alveolar lavage fluid
- d
deficient
- PAPC
1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine
- PGPC
1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine
- PM2.5
particulate matter <2.5 µm
- POVPC
1-palmitoyl-2-(5'-oxo-valeroyl)-sn-glycero-3-phosphocholine
- TLR4
Toll-like receptor 4
- wt
wildtype
- YFP
Yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
None.
References
- 1.Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 2.Peters A, Pope CA., 3rd Cardiopulmonary mortality and air pollution. Lancet. 2002;360:1184–1185. doi: 10.1016/S0140-6736(02)11289-X. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA, 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RDRS, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux A, Holguin F, Hong Y, Luepker R, Mittleman M, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD. Particulate Matter Air Pollution and Cardiovascular Disease. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 6.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 7.Puett RC, Schwartz J, Hart JE, Yanosky JD, Speizer FE, Suh H, Paciorek CJ, Neas LM, Laden F. Chronic particulate exposure, mortality, and coronary heart disease in the nurses' health study. Am J Epidemiol. 2008;168:1161–1168. doi: 10.1093/aje/kwn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 9.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 10.Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, Watt M, Agius R, Stout R. Particulate air pollution and the blood. Thorax. 1999;54:1027–1032. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants PM(10) Am J Respir Crit Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 12.Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznadjar JI, Budinger GR. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117:2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Hohmdahl R, Nicholls J, Jiang C, Binder CJ, Peninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2007;Chapter 14(Unit 14):12. doi: 10.1002/0471142735.im1412s77. [DOI] [PubMed] [Google Scholar]
- 17.Chen LC, Zhong M, Narciso S, Kleinman M, Nadziejko C, Lippmann M. Methods for Exposing Rodents and Cells to Concentrated Ambient PM using a VACES; American Association for Aerosol Research - Particulate Matter Conference; Pittsburgh, PA; 2003. [Google Scholar]
- 18.Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol. 2005;17:189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- 19.Kampfrath T, Deiuliis JA, Moffatt-Bruce SD, Anderson J, Sun Q, Wood K, Ostrowski MC, Rajagopalan S. A mouse model of yellow fluorescent protein (YFP) expression in hematopoietic cells to assess leukocyte-endothelial interactions in the microcirculation. Microvasc Res. 2009;78:294–300. doi: 10.1016/j.mvr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anonymous. Washington, DC.: U.S. Environmental Protection Agency; 2006. Air quality criteria for particulate matter. http://www.epa.gov/ttn/naaqs/pm/pm25_index.html. [Google Scholar]
- 21.Chen BT, Yordanov AT, Johnson GA. Ventilation-synchronous magnetic resonance microscopy of pulmonary structure and ventilation in mice. Magn Reson Med. 2005;53:69–75. doi: 10.1002/mrm.20307. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Ruch S, Weintraub EL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacquelet S, Johnson JL, Ellis BA, Brzezinska AA, Lane WS, Munafo DB, Catz SD. Cross-talk between IRAK-4 and the NADPH oxidase. Biochem J. 2007;403:451–461. doi: 10.1042/BJ20061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 25.Laroux FS, Romero X, Wetzler, Engel P, Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J Immunol. 2005;175:5596–5600. doi: 10.4049/jimmunol.175.9.5596. [DOI] [PubMed] [Google Scholar]
- 26.Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51:1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowther JE, Kutala VK, Kuppusamy P, Ferguson JS, Beharka AA, Zweier JL, McCormack FX, Schlesinger LS. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;172:6866–6874. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- 28.Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 2008;180:7847–7858. doi: 10.4049/jimmunol.180.12.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345:176–178. doi: 10.1016/s0140-6736(95)90173-6. [see comments] [DOI] [PubMed] [Google Scholar]
- 30.Peretz A, Peck EC, Bammler TK, Beyer RP, Sullivan JH, Trenga CA, Srinouanprachnah S, Farin FM, Kaufman JD. Diesel exhaust inhalation and assessment of peripheral blood mononuclear cell gene transcription effects: an exploratory study of healthy human volunteers. Inhal Toxicol. 2007;19:1107–1119. doi: 10.1080/08958370701665384. [DOI] [PubMed] [Google Scholar]
- 31.Tan WC, Qiu D, Liam BL, Ng TP, Lee SH, van Eeden SF, D'Yachkova Y, Hogg JC. The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med. 2000;161:1213–1217. doi: 10.1164/ajrccm.161.4.9904084. [DOI] [PubMed] [Google Scholar]
- 32.van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:61–67. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 33.Ghio AJ, Hall A, Bassett MA, Cascio WE, and Devlin RB. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhal Toxicol. 2003;15:1465–1478. doi: 10.1080/08958370390249111. [DOI] [PubMed] [Google Scholar]
- 34.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 38.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Weisberg S, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lassegue B, Griendling KK. Reactive oxygen species in hypertension; An update. Am J Hypertens. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Siow RC, Churchman AT. Adventitial growth factor signalling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovasc Res. 2007;75:659–668. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, Ohashi PS, Jurisicova A, Lenardo M, Hakem R, Hakem A. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 2007;282:7416–7423. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Wang H, Qiu P, Luo H. Proteomic profiling of proteins associated with methamphetamine-induced neurotoxicity in different regions of rat brain. Neurochem Int. 2008;52:256–264. doi: 10.1016/j.neuint.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. 50. [DOI] [PubMed] [Google Scholar]
- 50.Kirk EA, Sagawa ZK, McDonald TO, O'Brien KD, Heinecke JW. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue. Diabetes. 2008;57:1254–1261. doi: 10.2337/db07-1061. [corrected] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.