Abstract
Thiol-ene photopolymerization offers a unique platform for the formation of peptide-functionalized poly(ethylene glycol) hydrogels and the encapsulation, culture and differentiation of cells. Specifically, this photoinitiated polymerization scheme occurs at neutral pH and can be controlled both spatially and temporally. Here, we have encapsulated human mesenchymal stem cells (hMSCs) in matrix metalloproteinase (MMP) degradable and cell-adhesive hydrogels using thiol-ene photopolymerization. We find that hMSCs survive equally well in this system, regardless of MMP-degradability. When hMSCs are encapsulated in these cell-degradable hydrogels, they survive and are able to proliferate. In classic hMSC differentiation medias, hMSCs locally remodel their microenvironment and take on characteristic morphologies; hMSCs cultured in growth or osteogenic differentiation media are less round, as measured by elliptical form factor, and are smaller than hMSCs cultured in chondrogenic or adipogenic differentiation media. In addition, hMSCs encapsulated in completely cell-degradable hydrogels and cultured in osteogenic, chondrogenic, or adipogenic differentiation media generally express increased levels of specific differentiation markers as compared to cells in hydrogels that are not cell-degradable. These studies demonstrate the ability to culture and differentiate hMSCs in MMP-degradable hydrogels polymerized via a thiol-ene reaction scheme and that increased cell-mediated hydrogel degradability facilitates directed differentiation of hMSCs.
1. Introduction
Covalently crosslinked poly(ethylene glycol) (PEG) hydrogels are a commonly used class of scaffolds for cell encapsulation and transplantation [1]. These bio-inert systems have no inherent cell-adhesive or biological activities; however, they can be easily modified to present biochemical cues [2, 3]. Recent advances in hydrogel engineering have resulted in development of two distinct reaction mechanisms for step-growth polymerization of cell-instructive PEG-peptide network structures using either Michael-type addition polymerization or thiol-ene photopolymerization [4, 5]. These step-growth hydrogel networks present uniform biological motifs, such as cell adhesive peptides or proteolytic degradation sites, and have improved mechanical properties when compared to chain-growth networks [5–10]. While both Michael-type addition and thiol-ene polymerization schemes create networks that can be used as scaffolds for cell encapsulation and transplantation, there are several distinct advantages to the later. Thiol-ene photopolymerization reactions occur at neutral pH and can be controlled both spatially and temporally, whereas Michael-type reactions occur spontaneously under slightly alkaline conditions [4, 5]. The alkaline conditions under which Michael-type reactions occur may lead to off-stoichiometric reaction of monomers [4]. In addition, the photochemically mediated thiol-ene reaction allows opportunities for spatiotemporal control of the gelation process, which can have benefits for in situ formation or advanced patterning methods.
Cell-degradable polymer-peptide hydrogels can be created under cytocompatible conditions via the thiol-ene photopolymerization scheme by a radically-mediated step-growth reaction between norbornene and thiol moieties [5]. Norbornene functionalized four-arm PEG macromers can be crosslinked with a cysteine flanked peptide, such as matrix metalloproteinase (MMP) degradable sequences, to create a hydrogel network that is degraded by cell-secreted MMPs [5, 6, 8–10]. In addition, cell adhesive peptides, such as RGD, can be incorporated into the network as pendant functionalities to allow cell attachment and spreading [5, 8, 9]. The resulting network is cell-adhesive, cell-degradable, and can be formed in a spatially and temporally controlled manner at neutral pH.
The behavior of various cell types has been observed in cell-degradable polymer-peptide hydrogels. For example, human dermal fibroblasts migrate through MMP-degradable scaffolds that have been modified with RGD [9]. Primary bovine chondrocytes have also been seen to survive and display increased deposition of extracellular matrix in MMP-degradable hydrogels [11]. In cell-degradable polymer-peptide hydrogels formed via thiol-norbornene photopolymerization, valvular interstitial cells spread, proliferate and migrate within the hydrogels [12]. These examples illustrate the benefits of a synthetic scaffold that is both cell-degradable and cell-adhesive, allowing cells to proliferate, spread and migrate in a physiologically relevant manner.
Here, we encapsulated human mesenchymal stem cells (i.e., hMSCs or marrow stromal cells) in thiol-ene gels to better understand how the hMSC microenvironment influences the differentiation of these multipotent cells. Because of this multipotency and their ability to self-renew, hMSCs could fulfill the demands of many applications in tissue engineering and regeneration. While hMSCs offer a promising source of stem cells for therapeutic purposes, several hurdles still exist. Primarily, the low frequency of hMSCs in bone marrow requires extensive in vitro expansion to obtain sufficient numbers for tissue regeneration [13, 14]. During in vitro expansion, hMSCs quickly exhibit decreased differentiation potential and proliferative capacity [15]. Recent literature also suggests that the fate of hMSCs is highly dependent on their culture environment. Engler et al., [16] demonstrated that matrix elasticity directs hMSCs lineage specification. While others have shown that cell shape and curvature influence cell fate decisions [17, 18]. Fewer studies have examined such behavior in three-dimensions (3D). For example, hMSCs differentiate down the chondrogenic lineage more readily when cultured in a 3D microenvironment where cell-cell contact is either present or mimicked [19, 20]. Thus, 3D, cell-degradable and cell-adhesive polymer-peptide hydrogels could provide a unique platform in which to expand and differentiate hMSCs, allowing cells to spread, proliferate and locally remodel their microenvironment in a manner that better mimics in vivo conditions.
Here, we characterize the culture and differentiation of human hMSCs in thiol-ene photopolymerized PEG-peptide hydrogels. PEG macromers were crosslinked with varying combinations of MMP-degradable peptide or non-degradable PEG monomer to observe how the ability to locally degrade the surrounding hydrogel network affects hMSC behavior. Cells were seeded in these hydrogels at low densities in order to monitor cell-material interactions. hMSCs were observed for survival, proliferation and cell spreading in this system. Encapsulated hMSCs were also incubated in media containing classic differentiation signals to evaluate osteogenic, chondrogenic and adipogenic differentiation in this system.
2. Materials and Methods
2.1 Reagents
All chemical reagents were obtained from Sigma-Aldrich, unless otherwise noted. All cell culture reagents were obtained from Invitrogen, unless otherwise noted.
2.2 Synthesis, purification, and characterization of macromers and peptides
4-arm PEG norbornene was synthesized by reacting 4-arm PEG hydroxyl (JenKem USA) with norbornene acid as described previously [5]. Briefly, norbornene acid was converted to norbornene anhydride in dichloromethane (DCM) using N,N′-diisopropylcarbodiimide (DIC) as a catalyst. Norbornene anhydride was reacted with 4-arm PEG hydroxyl, in the presence of 4-(dimethylamino)pyridine (DMAP) and pyridine, to yield PEG-norbornene. The product was precipitated in diethyl ether. The product was characterized with proton NMR and the degree of functionalization was at least 92%. PEG dithiol was synthesized, with modifications, as previously described [21].
To synthesize PEG-dithiol, linear PEG diol (Mn≈3.4 kDa) was dissolved in THF, heated to 40°C, and reacted with 1.5 molar equivalents NaH. Six molar equivalents allyl bromide was subsequently added to the solution and stirred overnight. The reaction mixture was filtered to remove NaBr, and the filtrand precipitated into cold ethyl ether. PEG allyl ether was dissolved in DCM, with 0.5 wt% I 651 and two molar equivalents thioacetic acid. Reaction was initiated with 365 nm light source (Omnicure) at an intensity of 10 mW/cm^2 for 45 minutes. PEG thioesther was precipated into ether and washed in DCM. The product was then desiccated and dissolved in degassed dI water and reacted with equivalent volume of 2 M NaOH, and neutralized with equivalent volume 2 M HCl.
All peptides were synthesized using standard Fmoc chemistry in a Tribute automatic peptide synthesizer (Protein Technologies). The cleaved peptides were purified in a reverse phase HPLC and characterized with MALDI-TOF.
2.3 Cell culture and encapsulation
Human mesenchymal stem cells (hMSCs) were isolated from human bone marrow aspirates (Lonza). Red blood cells were lysed per the manufacturer’s instructions using an Ammonium Chloride Solution (Stem Cell Technologies). Remaining cells were plated on tissue culture polystyrene in growth media (low-glucose DMEM, 10% fetal bovine serum (FBS), 1 ng/ml recombinant human fibroblast growth factor-basic (FGF-2, Peprotech), 50 U/ml each penicillin/streptomycin, 1 μg/ml Fungizone antimycotic). hMSCs were isolated as the adherent cell population. Cells were cultured for no more than two passages.
hMSCs were suspended, at a density of 5×106 cells/ml, in a stoichiometrically balanced (1 ene to 1 thiol) monomer solution comprised of 6 wt% PEG-norbornene dissolved in phosphate buffered saline, 0.05 wt% lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP photoinitiator)([22]), 1 mM CGRGDS and various combinations of MMP-degradable peptide and PEG-dithiol, as indicated. The cell-gel solution was exposed to UV light centered at 365 nm at 5 mW/cm2 for 2 min. Polymerized samples were transferred to indicated cell media and cultured at 37°C and 5% CO2.
Osteogenic differentiation media was comprised of high-glucose DMEM supplemented with 10% FBS, 50 U/ml each penicillin/streptomycin, 1 μg/ml Fungizone antimycotic, 100 nM dexamethasone, 50 μM ascorbic acid, and 20 mM β-glycerophosphate. Chondrogenic differentiation media was comprised of high-glucose DMEM supplemented with 10% FBS, 50 U/ml each penicillin/streptomycin, 1 μg/ml Fungizone antimycotic, 100 nM Dexamethasone, 150 μM ascorbic acid, 1 mM sodium pyruvate, 1X ITS+ premix (BD Biosciences) and 5 ng/ml TGF-β1 (Peprotech). The StemPro Adipogenesis Differentiation Kit (Invitrogen) was used as adipogenic differentiation media.
2.4 Cell density, proliferation and morphology analysis
hMSC density and morphology within MMP-degradable hydrogels was determined by analyzing images taken by confocal microscopy of live cells stained with Calcein AM and dead cells stained with Ethidium homodimer-1 (EthD). Live cell density per mm3 was assayed using Metamorph software (Molecular Devices, Inc.) by projecting a 51 μm z-stack (3 μm slices) into 2-D and counting the number of cells per image. Cell area and circularity (cell length/cell breadth) were also determined using Metamorph software.
Proliferation was assessed using the Click-iT EdU cell proliferation assay. Briefly, encapsulated cells were pulsed with 5-ethynyl-2′-deoxyuridine (EdU) for 24 hours and then fixed with 4% paraformaldehyde. EdU that was incorporated into the DNA of proliferating cells was labeled per the manufacturer’s instructions with AlexaFluor 594 azide and nuclei were counterstained with Hoescht 33342. Stained hydrogels were imaged by confocal microscopy.
2.5 Equilibrium Swelling Ratio
hMSCs were encapsulated in hydrogels with varying degrees of MMP-degradable crosslinker. Hydrogels were cultured in growth media for the indicated times. The equilibrium mass swelling ratio (q) was determined by measuring the wet weight of each hydrogel and then dividing by the dry weight. The equilibrium swelling ratio (Q) was derived from the equilibrium mass swelling ratio (q) using the corresponding densities of polymer and swelling solution.
2.6 Osteogenic differentiation assays
Alkaline phosphatase (ALP) activity was assayed as described previously [23]. Briefly, hydrogels with encapsulated cells were first incubated in media containing 10% Alamar Blue Reagent for 4 hours to determine relative cell numbers. Hydrogels were then rinsed with PBS, and cells were lysed with RIPA lysis buffer by manual homogenization. The supernatants were collected and reacted with p-nitrophenyl phosphate as a substrate for alkaline phosphatase. Normalized ALP activity was reported as the slope of the absorbance curve at 405 nm, read over a 10 min period, and divided by the alamar blue fluorescent signal.
Calcium deposition was assayed using Pointe Scientific, Inc Calcium Reagent Set, as described previously [24]. In hydrogels with encapsulated cells, calcium was solubilized overnight at 4°C with 0.6M HCl. The supernatant was collected and reacted in a 1:50 ratio with Calcium Reagent Solution (55 μM o-cresolphthalein complexone, 8.5 mM 8-hydroxyquinoline, 488 mM 2-amino-2-methyl-1-propanol, 1 mM potassium cyanide). Absorbance was determined at 570 nm. Calcium concentration for each hydrogel was determined by comparison to a standard curve of calcium chloride.
Calcium deposition was also assayed by staining 4% paraformaldehyde fixed cyrosections with Alizarin Red Dye.
2.7 Chondrogenic differentiation assays
Deposition of glycosaminoglycans was assayed as described previously [20, 25]. Samples were digested for 18 hours at 60°C with 125 μg/ml papain, 10 mM cysteine in phosphate buffer (pH 6.2). Sulfated glycosaminoglycans were detected with dimethylmethylene blue. Absorbance was determined at 520 nm. Glycosaminoglycan concentration was determined for each hydrogel by comparison to a standard curve of chondroitin sulfate.
Glycosaminoglycan deposition was also assayed by staining hydrogel cyrosections with safranin O and counterstaining with hematoxylin and fast green.
Collagen deposition was assayed using Masson’s trichrome technique; hydrogel cyrosections were stained with methyl blue and counterstained with celestin blue-hematoxylin and acid fuchsin.
2.8 Adipogenic differentiation assays
Expression of adipogenic markers was assayed by quantitative RT-PCR. mRNA was extracted per manufacturer’s instructions using Tri-Reagent. Hydrogels were manually disrupted using a pestle. cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). qRT-PCR was performed using TaqMan gene expression assays (Applied Biosystems) and a BioRad iCycler. Samples were standardized to GAPDH.
Lipid deposition was assayed by staining 4% paraformaldehyde fixed hydrogels with Oil Red O.
2.9 Statistical analysis
Data are presented as mean ± standard error of three samples, unless otherwise noted. Student’s T-test was used to compare data sets.
3. Results
3.1 hMSC survival and proliferation in MMP-degradable hydrogels
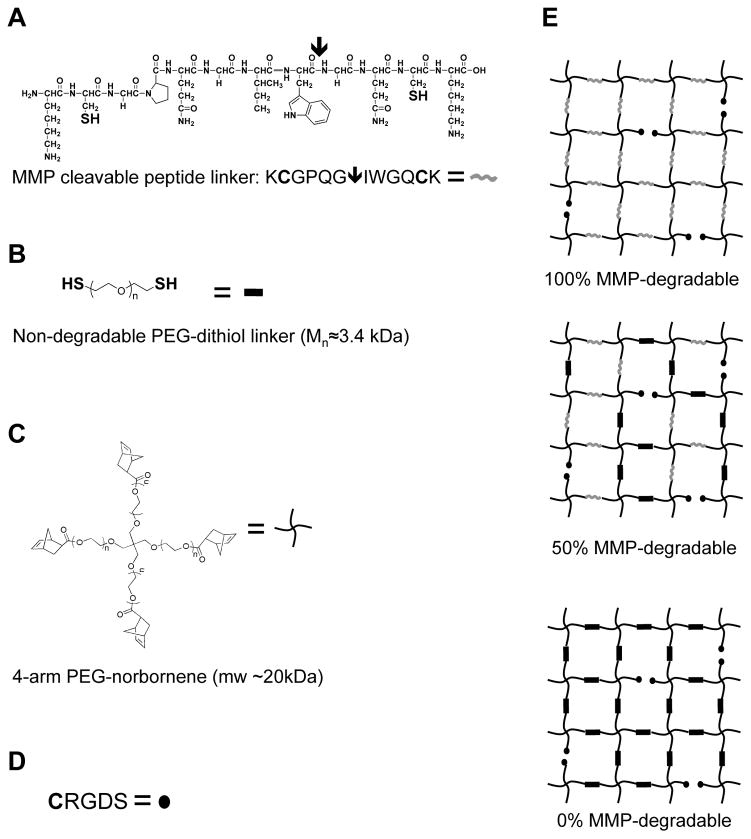
MMP-degradable PEG-peptide hydrogels create a versatile platform in which to culture and differentiate cells. These step-growth hydrogel networks offer the versatility to incorporate enzymatic degradation sequences and cell-adhesive peptide ligands. PEG-peptide hydrogels with variable cell-directed degradabilities were synthesized by altering the peptide crosslinker within the hydrogel networks (Fig 1). Hydrogels were rendered 100% cell-degradable by incorporation of the MMP-degradable peptide sequence KCGPQG↓IWGQCK (arrow indicates enzymatic cleavage site), and then 0% or 50% cell-degradable by varying the ratio of MMP-degradable peptide to a non-degradable PEG-dithiol linker. Cell adhesivity was added to the hydrogel matrix by incorporation of a pendant fibronectin-binding CGRGDS sequence. The initial shear elastic modulus (G′) for these hydrogels was approximately 3.8 kPa (data not shown).
Figure 1.
Schematic of thiol-ene hydrogels with variable degradability
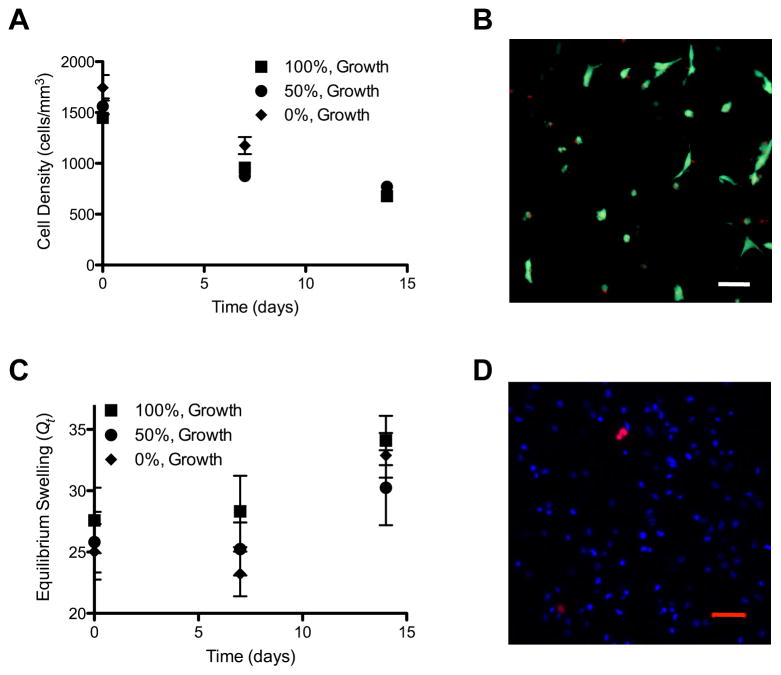
hMSCs were encapsulated in 6 wt% PEG-peptide hydrogels with varying degrees of degradability and 1 mM pendant CGRGDS peptide. Cell survival was monitored over time by staining live cells with Calcein AM and dead cells with EthD and then determining cell density within the hydrogel by counting the number of live cells per mm3 using confocal microscopy (Fig 2A). Cells survived equally well in each hydrogel condition tested, indicating that gel degradability does not affect cell-survival. A representative image of cells encapsulated in 100% degradable gels for 14 days and then stained with EthD demonstrates that cell death was minimal (Fig 2B). Changes in cell density reflect both loss of cell viability, differences in cell proliferation and hydrogel volume changes as a result of degradation induced swelling; therefore, the equilibrium swelling ratio (Q) was also monitored over time (Fig 2C). Note that over the 14 day culture period the gel volume expands ~25% while the cell density decreases ~55%, indicating that a large proportion of the decrease in cell density could be accounted for by the increase in the gel volume.
Figure 2.
hMSCs encapsulated in MMP-degradable hydrogels survive and proliferate. hMSCs were encapsulated in MMP-degradable PEG-hydrogels, with varying degrees of degradability (percentages indicate extent of hydrogel linkers that are MMP-degradable). (A) Cell density was determined as the number of cells per mm3, at indicated times. (B) Representative image of hMSCs stained as live (Calcein AM/Green) or dead (EthD/Red) after 14 days of culture in growth media while encapsulated in 100% MMP-degradable PEG-hydrogels. (C) Equilibrium swelling ratio at indicated times (Qt). (D) Representative image of hMSCs encapsulated in 100% MMP-degradable PEG-hydrogels for 48 hours and labeled with Click-iT EdU cell proliferation marker (Red) and Hoescht DNA marker (Blue). Scale bars represent 50 μm.
hMSCs lose the ability to proliferate and differentiate when cultured on tissue culture polystyrene (TCPS) for multiple passages [15]. While direct evidence that substrate stiffness affects hMSC proliferation and maintenance of stem-like phenotype has not been presented, this is true for other cells types such as muscle satellite cells and non-transformed fibroblasts [26, 27]. A tailorable hydrogel platform in which cells could proliferate may offer an attractive culture environment for maintaining hMSC proliferation and differentiation potential; therefore, hMSC proliferation was also monitored in 100% cell-degradable hydrogels by EdU staining (Fig 2D). Proliferating cells were visible within the hydrogel, indicating that hMSCs are capable of dividing in this culture platform.
3.2 hMSC morphology in MMP-degradable hydrogels
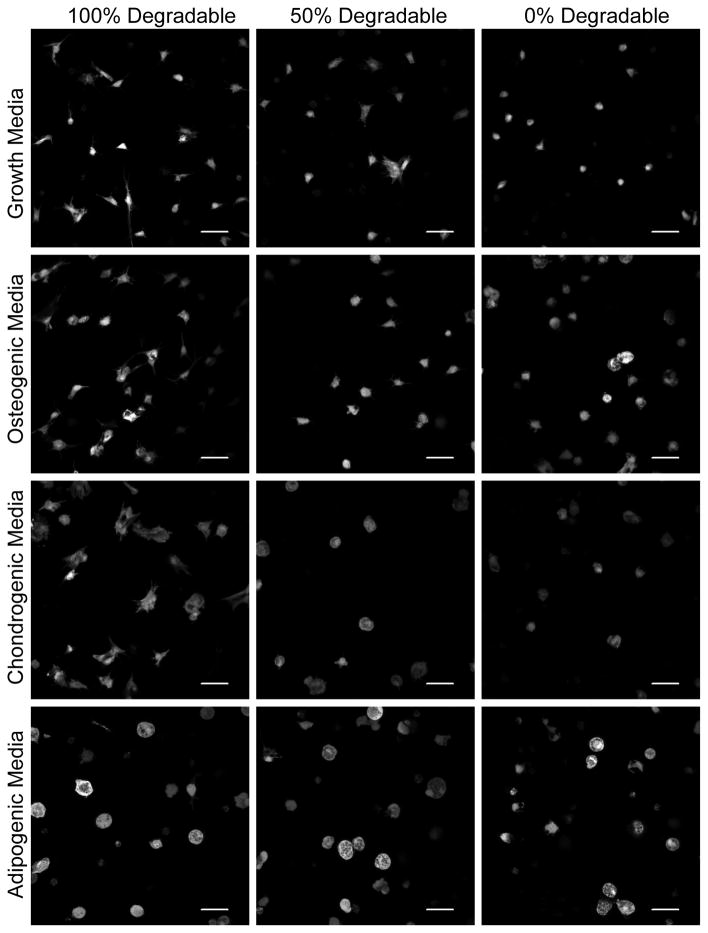
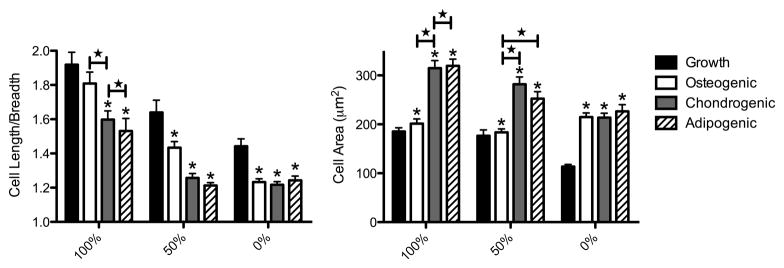
Cell shape has been implicated in the direction of hMSC differentiation in vitro [17, 18]. While hMSC phenotype has been observed in conditions where cells were forced into a specific cell-shape by a surrounding matrix, little work has been done to investigate how hMSC morphology varies in three-dimensions when cells are capable of locally remodeling the culture environment under various differentiation conditions. We have cultured hMSCs in PEG-peptide hydrogels polymerized with varying degrees of MMP-degradable crosslinkers and observed the morphology of cells in growth, osteogenic, chondrogenic and adipogenic media (Fig 3). Cell morphology varied dramatically with hydrogel degradability and culture conditions. While all cells spread less in hydrogels with fewer MMP-degradable crosslinkers, cells spread into various shapes in the 100% cell-degradable hydrogels. Cells cultured in growth media spread into a spindle-shaped morphology, while hMSCs cultured in osteogenic or chondrogenic media produced branching protrusions, but remained more rounded than in growth media. Cells in adipogenic media remained rounded despite the ability to locally degrade the hydrogel, as evidenced by an increase in cell area. These results were quantified in Figure 4 by assaying cell circularity and area. hMSCs encapsulated in 100% cell-degradable hydrogels and cultured in growth or osteogenic media were significantly less circular (i.e., more spread) then cells cultured in chondrogenic or adipogenic media. In contrast, cells cultured in chondrogenic or adipogenic media were significantly larger than cells cultured in growth or osteogenic media.
Figure 3.
Morphology of hMSCs encapsulated in MMP-degradable hydrogels. hMSCs encapsulated in MMP-degradable PEG-hydrogels, with varying degrees of degradability (percentages indicate extent of hydrogel linkers that are MMP-degradable), were stained with Calcein AM after 14 days of culture in indicated media and then imaged by confocal microscopy. Scale bar represents 50 μm.
Figure 4.
hMSC circularity and spreading vary with hydrogel degradability and culture conditions. hMSCs encapsulated in MMP-degradable PEG-hydrogels, with varying degrees of degradability (percentages indicate extent of hydrogel linkers that are MMP-degradable), were stained with Calcein AM after 14 days of culture in indicated media and then imaged by confocal microscopy. Cell length divided by cell breadth was calculated to estimate circularity of cells, where 1.0 equals completely circular. Cell area was calculated to estimate the degree of spreading in hydrogels. * indicates significantly different from growth (p< 0.04). ★ indicates significantly different from each other (p<0.03). n>80.
3.3 Osteogenic differentiation of hMSCs in MMP-degradable hydrogels
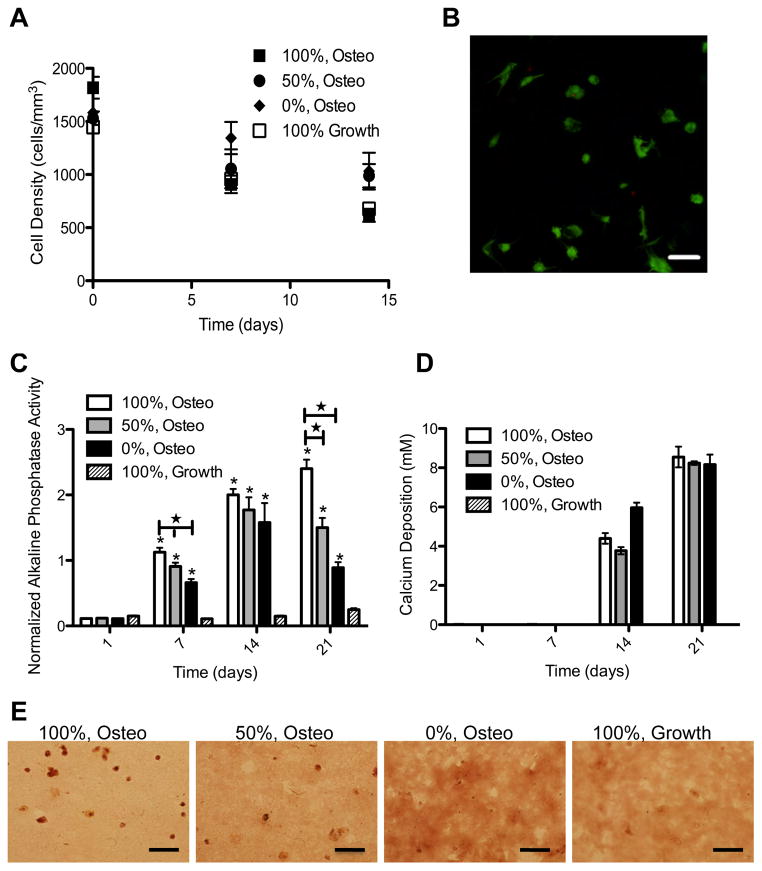
Platforms in which hMSCs can be directed to differentiate down the osteogenic lineage are advantageous for fundamental studies of osteogenic lineage commitment, as well as more applied applications such as bone fracture and critical size bone defect repair. A cell-degradable matrix would allow matrix deposition and scaffold degradation to occur in parallel and on an appropriate time-scale. We encapsulated hMSCs in MMP-degradable hydrogels and cultured the constructs in osteogenic media. Cell survival was monitored over time by determining the number of cells per mm3 (Fig 5A). hMSCs survive equally well in the hydrogel constructs regardless of the media condition or degradability. A representative image of hMSCs cultured for 14 days in osteogenic media following encapsulation in 100% degradable gels is presented in Figure 5B. Typical measures of ostegenesis were used to monitor osteogenic differentiation, including alkaline phosphatase activity and mineralized matrix deposition. Alkaline phosphatase activity increased under osteogenic conditions and with hydrogel degradability (Fig 5C). After 7 or 14 days in culture, 100% cell-degradable hydrogels in osteogenic media had significantly more alkaline phosphatase activity than 50% or 0% cell-degradable hydrogels. hMSCs in cell-degradable hydrogels were also capable of depositing mineralized matrix, as assayed by calcium concentration (Fig 5D) and alizarin red staining of fixed hydrogel sections (Fig 5D).
Figure 5.
hMSCs survive and express osteogenic markers in MMP-degradable hydrogels. hMSCs were encapsulated in PEG-hydrogels, with varying degrees of degradability (percentages indicate extent of hydrogel linkers that are MMP-degradable), and cultured in growth or osteogenic (Osteo) media. (A) Cell density was determined by staining encapsulated hMSCs with calcein AM and assaying cell density as the number of viable cells per mm3. (B) Representative image of hMSCs stained as live (Calcein AM/Green) or dead (EthD/Red) after 14 days of culture in osteogenic media while encapsulated in 100% MMP-degradable PEG-hydrogels. (C) Induction of alkaline phosphatase activity was normalized to alamar blue. * indicates significantly different from 100%, Growth condition (p<0.02). ★ indicates significantly different from each other (p<0.05). Deposition of mineralized matrix was assayed by (D) calcium chloride concentration and (E) alizarin red staining of fixed hydrogel cytrosections. Scale bars represent 50 μm.
3.4 Chondrogenic differentiation of hMSCs in MMP-degradable hydrogels
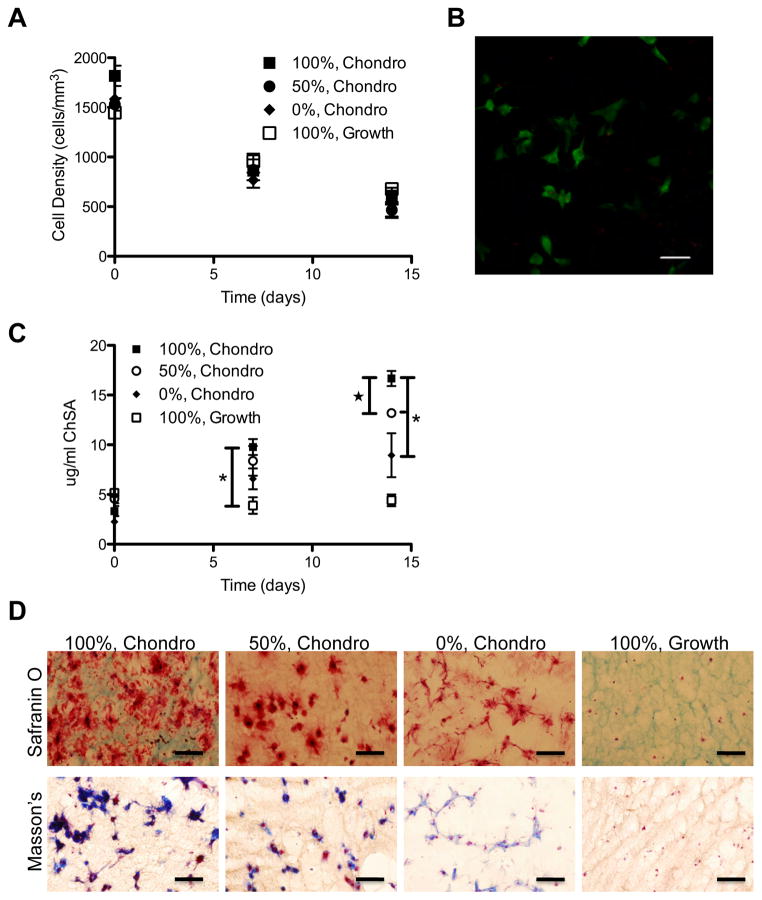
hMSCs require cell-cell contact or a scaffold that mimics an extracellular matrix to differentiate down the chondrogenic lineage [19, 20]. A scaffold that provides both cell-adhesive ligands and cell-mediated degradation could provide a useful platform for chondrogenic differentiation, in that hMSCs would receive cues to differentiate down the chondrogenic lineage and the scaffold would eventually degrade to allow nascent matrix deposition and cell-matrix interactions. We encapsulated hMSCs in cell-degradable hydrogels and cultured the constructs in chondrogenic media. Cell survival and expression of chondrogenic markers were monitored over time. hMSCs survived equally well in growth and chondrogenic media, regardless of gel degradability (Fig 6A,B). Typical markers of chondrogenic differentiation include glycosaminoglycan (GAGs) and collagen deposition. Similar to the trend seen in osteogenic media, hMSCs secreted more GAGs and collagen when encapsulated in cell-degradable hydrogels, as compared to hydrogels without MMP-degradable linkers. GAG secretion, as measured by the biochemical DMMB assay and staining of hydrogel sections with Safranin O Dye, increased significantly with increased gel degradability (Fig 6C,D). Collagen deposition, as demonstrated by Masson’s trichrome staining of hydrogel sections, also increased with gel degradability (Fig 6D).
Figure 6.
hMSCs survive and express chondrogenic markers in MMP-degradable hydrogels. hMSCs were encapsulated in PEG-hydrogels, with varying degrees of degradability (percentages indicate extent of hydrogel linkers that are MMP-degradable), and cultured in growth or chondrogenic (Chondro) media. (A) Cell density was determined by staining encapsulated hMSCs with calcein AM and assaying cell density as measured by the number of viable cells per mm3. (B) Representative image of hMSCs stained as live (Calcein AM/Green) or dead (EthD/Red) after 14 days of culture in chondrogenic media while encapsulated in 100% MMP-degradable PEG-hydrogels. (C) Deposition of chondrogenic glycosaminoglycans was determined by DMMB assay as determined against a chondroitin sulfate standard. (D) Safranin O staining of hydrogel cryosections demonstrates GAGs in red, cytoplasm and background hydrogel staining in green and nuclie in black. Collagen deposition was demonstrated by Masson’s trichrome stain where collagen is stained blue, cytoplasm is stained red and nuclei are stained blue-black. * indicates significantly different from each other. ★ indicates significantly different from day 14 100%, Growth. (p<0.02). Scale bars represent 50 μm.
3.5 Adipogenic differentiation of hMSCs in MMP-degradable hydrogels
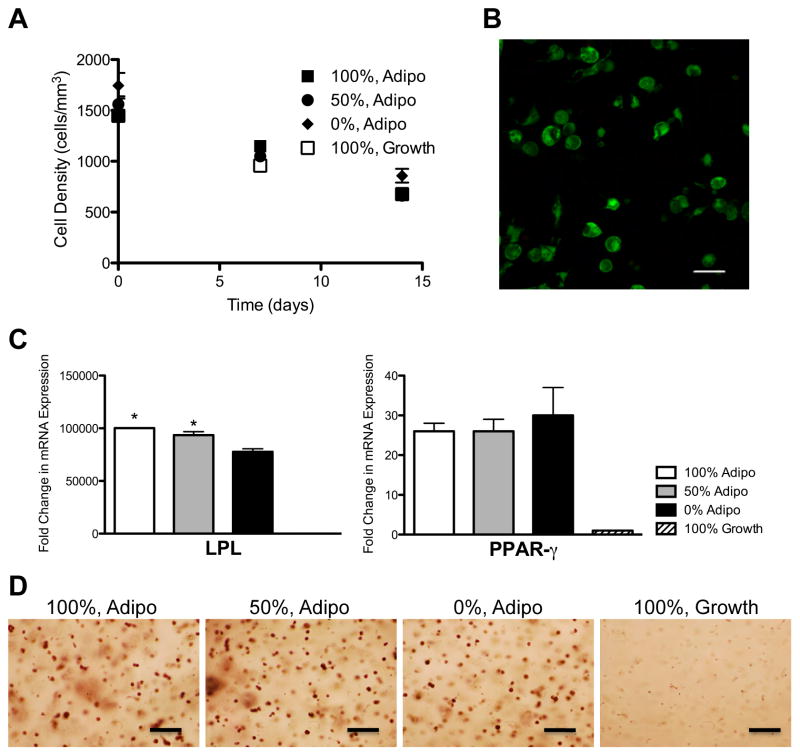
Recently, attention has focused on the use of hMSCs as a source of adipocytes for soft-tissue engineering of adipose tissue [28]. Directed differentiation of hMSCs down the adipogenic lineage can provide a plentiful source of adipocytes for soft-tissue reconstruction. Encapsulation of these cells in a degradable scaffold could provide the structure to maintain graft shape until the adipogenic phenotype has stabilized. We encapsulated hMSCs in cell-degradable hydrogels and cultured them in growth or adipogenic media. Cell survival and expression of adipogenic markers were monitored over time. hMSCs survived equally well in growth and adipogenic media, regardless of gel degradability (Fig 7A,B). In adipogenic conditions, hMSCs expressed the typical adipocyte markers lipoprotein lipase (LPL) and peroxisome proliferator activated receptor gamma (PPAR-γ) (Fig 7C). hMSCs encapsulated in 100% or 50% cell-degradable hydrogels and cultured in adipogenic media expressed significantly more LPL than cells encapsulated in 0% cell-degradable hydrogels cultured in adipogenic media or cells cultured in growth media albeit the differences were not as dramatic as under osteogenic (Fig 4) or chondrogenic (Fig 5) differentiation conditions. Adipogenic differentiation of hMSCs was confirmed by staining hydrogel sections for lipids with Oil Red O dye (Fig 7D).
Figure 7.
hMSCs survive and express adipogenic markers in MMP-degradable hydrogels. hMSCs were encapsulated in PEG-hydrogels, with varying degrees of degradability (percentages indicate extent of hydrogel linkers that are MMP-degradable), and cultured in growth or adipogenic (Adipo) media. (A) Cell density was determined by staining encapsulated hMSCs with calcein AM and assaying cell density as measured by the number of viable cells per mm3. (B) Representative image of hMSCs stained as live (Calcein AM/Green) or dead (EthD/Red) after 14 days of culture in growth media while encapsulated in 100% MMP-degradable PEG-hydrogels. (C) Expression of the adipogenic markers lipoprotein lipase (LPL) and peroxisome proliferator activated receptor gamma (PPAR-γ) were determined by qRT-PCR. * Indicates significantly different from 0%, Adipo (p<0.02). (D) Lipids were stained in fixed hydrogel cryosections using Oil Red O. Scale bars represent 50 μm.
4. Discussion
A cell-degradable PEG-peptide hydrogel platform was evaluated as a 3D culture and differentiation system for hMSCs. The hydrogel platform consists of four-arm PEG chains linked together by MMP-degradable peptides and modified with integrin binding peptides for cell-adhesivity. The chemistry used to form these hydrogels is unique in that gel polymerization can be controlled spatially and temporally using light at a neutral pH. We find that hMSCs survive, proliferate, spread and are capable of undergoing osteogenic, chondrogenic and adipogenic differentiation in this unique system. We propose that this purely synthetic, photopolymerizable and cell-degradable platform could be useful for further fundmanetal studies of hMSC behavior in 3D culture, as well as translational studies as a cell delivery system.
Analysis of cell density within hydrogel constructs revealed that hMSCs survive equally well regardless of the degradability of the gel (Fig 1). These results agree with previous studies in which hMSCs survive well in either non-degradable or completely cell-degradable systems, indicating that gel degradability does not influence cell survival [20, 29]. In this study, we have taken a unique approach to evaluating cell survival by assaying the density of cells within the hydrogel, as measured by the number of live cells per mm3 throughout the course of the experiment. Because this technique analyzes confocal images of cells within the hydrogel network, it eliminates errors due to cells attached to the outside of the hydrogel that could artificially increase apparent cell survival as measured by DNA content or metabolic activity of hydrogel discs. This analysis also eliminates errors in assaying percentage of live cells, which captures only the live and dead cells from the recent past, but does not consider the cells lost over time. While the density of live cells at the end of our studies is approximately 55% of the beginning density, we believe this is a more accurate representation of cell behavior within a hydrogel and does not contradict previous work that demonstrated close to 80% viability as assayed by the ratio of live cells divided by the total number of live plus dead cells at any given time [20]. In agreement with previous work, we observed very small numbers of dead cells stained with EthD at each time point [30], indicating a high percentage of live cells within the hydrogels, and suggesting that the decrease in cell density is due to both low numbers of cells dying over time and swelling of the hydrogel matrix. We observe an increase in equilibrium swelling ratio of approximately 25% over the course of the experiment, correlating well with the approximately 30% decrease in cell density we see compared to previous work.
In addition to explaining the decrease in cell density that we observe, the equilibrium swelling data presented here (Fig 2) raises an interesting point regarding local degradation of the hydrogel matrix. We did not observe a significant difference in swelling ratios between hydrogels with varying degrees of cell-degradability. In contrast, we see significant changes in cell spreading and cell morphology in these hydrogels. This suggests that when cells are seeded at this low of a density (1×106 cells/ml), cell-mediated degradation does not significantly contribute to bulk degradation of the material. The fact that the equilibrium swelling ratio does not accurately reflect changes in local microenvironment points towards inadequacies in our abilities to measure changes at this scale. Further work is needed to develop techniques that precisely determine changes in the local mechanical environment within a dynamically degrading hydrogel.
The ability of hMSCs to self-renew within cell-degradable PEG-peptide hydrogel systems, as demonstrated here (Fig 2), could be advantageous for controlled expansion of hMSCs. Proliferation of hMSCs indicates that terminal differentiation has not been induced. This expands the utility of the PEG-peptide hydrogel system, allowing for its potential use as a tool for either (i) expansion of the population for downstream uses or (ii) observation of cell behavior in a matrix where local degradation is sufficient to allow for either proliferation or differentiation.
The hydrogel system presented here is degradable by cell-secreted MMPs. The specific MMP-degradable linker used in this study has been shown to be susceptible to cleavage by MMP-1, MMP-2, MMP-3, MMP-7, MMP-8 and MMP-9, all of which are expressed by hMSCs [31–36]. The ability of hMSCs to locally degrade the hydrogel matrix in an MMP-dependent manner is demonstrated here, as measured by cell spreading within gels (Fig 3 and 4). This ability to locally remodel their environment could be advantageous for directed differentiation of hMSCs, as it is well established that cell shape and local microenvironment play a role in differentiation [17, 18]. In the system presented here, hMSC shape within the 3D matrix changes with media conditions and hydrogel degradability. As expected, hMSCs are more circular and spread less into the hydrogel matrix as degradability of the gel decreases. hMSCs in growth media extend more processes into the 3D matrix and are less round than cells in other conditions, appearing similar in morphology to the typical MSC on tissue culture polystyrene [19]. hMSCs cultured in osteogenic, chondrogenic or adipogenic media are increasingly more round, but also larger, as measured by cell area. Decreased expression or activity of the MMPs responsible for gel degradation could explain the increased circularity observed here; however, this would not account for the increased cell area we also observe. Additionally, previous studies have reported increased MMP expression and activity with chondrogenic and adipogenic differentiation, and while some reports have noted a decrease in MMP expression under osteogenic conditions, preliminary work from our group suggests the opposite for hMSC culture in PEG-based gels (data not shown)[34, 36–38]. Thus, hMSCs locally remodel their microenvironment and change shape in a manner dependent on MMP-degradation of the hydrogel, suggesting that cell shape may be dictated more by cell phenotype and culture conditions and less by physical constraints imposed by a 3D matrix. This ability for cell phenotype to dictate cell shape offers advantages over other culture conditions in which cell shape is dictated by either a 2D surface or a hydrogel environment that is not cell-degradable.
The osteogenic hMSC lineage is the most widely studied differentiation pathway for hMSCs. Multiple approaches have been taken to create 3D scaffolds with the appropriate mechanical strength, biochemical cues and degradation kinetics to promote osteogenic differentiation [39]. Although not the focus of the current study, the biochemical properties of the thiol-norbornene gels can be locally modified in the presence of cells to alter cell fate processes [5]. Our findings suggest that cell-degradability of the hydrogel network increases alkaline phosphatase (ALP) activity (Fig 5), suggesting that hMSCs are more likely to undergo osteogenic differentiation in cell-degradable hydrogels.
Chondrogenic differentiation of hMSCs in a supportive 3D scaffold could be a useful tool for asking basic questions about chondrogenic differentiation of hMSCs as a function of cell density and shape. We find that despite the lack of cell-cell contact that hMSCs normally require for chondrogenic differentiation, hMSCs are capable of expressing chondrogenic glycosaminoglycans in our system (Fig 6). In addition, increased cell-degradability of the hydrogel results in increased glycosaminoglycan and collagen deposition, suggesting that hMSCs undergo chondrogenic differentiation more effectively in cell-degradable hydrogels.
Similar to the results found in osteogenic and chondrogenic conditions, we find that adipogenic differentiation is also possible in our system. While PPAR-γ expression and Oil Red O staining are substantial, independent of gel-degradability, LPL expression is significantly increased in more cell-degradable hydrogels (Fig 7). While strong adipogenic differentiation is observed across all gel compositions, this cell-degradable platform could be useful for further studies of spatially directed differentiation of hMSCs, as the chemistry and mechanics of the thiol-ene gels can be selectively modified in both space and time.
In the cases of osteogenic, chondrogenic and adipogenic differentiation of hMSCs in cell-degradable hydrogels, we have observed a correlation between cell-degradability and differentiation. This observation suggests that restricting morphology reduces differentiation capacity. While determining the mechanism of this restriction is beyond the scope of this study, further work is needed to determine whether this data contradicts previous observations suggesting that restricting cells to specific shapes in 2D directs differentiation down specific lineages [17, 18]. The versatility of the thiol-ene hydrogel system would allow these questions to be asked in a precise manner. For instance, the ability of cells to adhere within the hydrogel network could be controlled in 3D to create shapes that limit hMSC spreading, thus allowing investigation of lineage specification in a 3D environment where cell shape is dictated.
In this study, we have focused on the interactions between individual cells and a cell-degradable matrix. In general, cell differentiation was enhanced in hydrogels in which hMSCs could locally remodel their microenvironment. Future work will investigate how this ability to locally degrade the hydrogel system facilitates cell proliferation and differentiation at more physiologically relevant cell densities where both cell-hydrogel and cell-cell interactions contribute to matrix deposition and character. In addition, this thiol-ene system can be precisely tuned with varying mechanics, degradation kinetics and pendant peptides to control cell shape, proliferation and differentiation.
5. Conclusions
We have introduced a cell-degradable hydrogel scaffold for culture and differentiation of hMSCs. This PEG based hydrogel is polymerized via a thiol-ene photopolymerization scheme that occurs at neutral pH, in less than 2 min, and can be modified with bioactive pendant peptide and enzymatically cleavage linkers. We find that hMSCs survive and proliferate in this system. In addition, hMSC shape and area varies with culture conditions and gel-degradability. hMSCs express osteogenic, chondrogenic and adipogenic markers in this system under the appropriate culture conditions, and the expression of these markers tends to increase with gel-degradability.
Acknowledgments
The authors thank April Kloxin and Peter Mariner for input on manuscript preparation. We are grateful to Joshua McCall for providing PEG-dithiol. Funding for these studies was provided by the National Institutes of Health (5RO1DE016523) and Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370–7. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salinas CN, Anseth KS. Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells. J Biomed Mater Res A. 2009;90:456–64. doi: 10.1002/jbm.a.32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by michael-type addition. Biomacromolecules. 2003;4:713–22. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 5.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mat. 2009;21:5005–10. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1998;32:241–4. [Google Scholar]
- 7.Malkoch M, Vestberg R, Gupta N, Mespouille L, Dubois P, Mason AF, et al. Synthesis of well-defined hydrogel networks using click chemistry. Chem Commun (Camb) 2006:2774–6. doi: 10.1039/b603438a. [DOI] [PubMed] [Google Scholar]
- 8.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. P Natl Acad Sci USA. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutolf MP, Raeber GP, Zisch AH, Tirelli N, Hubbell JA. Cell-responsive synthetic hydrogels. Adv Mat. 2003;15:888–92. [Google Scholar]
- 10.Schwartz MP, Fairbanks BD, Rogers RE, Rangarajan R, Zaman MH, Anseth KS. A synthetic strategy for mimicking the extracellular matrix provides new insight about tumor cell migration. Integr Biol. 2010;2:32–40. doi: 10.1039/b912438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10:515–22. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 12.Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30:6593–603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedenstein AJ, Latzinik NW, Grosheva AG, Gorskaya UF. Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges. Exp Hematol. 1982;10:217–27. [PubMed] [Google Scholar]
- 14.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–74. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 15.Bonab M, Alimoghaddam K, Talebian F, Ghaffari S, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biology. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28:564–72. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 19.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20.Salinas CN, Cole BB, Kasko AM, Anseth KS. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007;13:1025–34. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 21.Hiemstra C, van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Novel in situ forming, degradable dextran hydrogels by michael addition chemistry: Synthesis, rheology, and degradation. Macromolecules. 2007;40:1165–73. [Google Scholar]
- 22.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials. 2009;30:6702–7. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CC, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNF-alpha. Biomaterials. 2009;30:4907–14. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benoit DS, Nuttelman CR, Collins SD, Anseth KS. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102–10. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 26.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–50. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanzi MC, Fare S. Adipose tissue engineering: State of the art, recent advances and innovative approaches. Expert Rev Med Devices. 2009;6:533–51. doi: 10.1586/erd.09.37. [DOI] [PubMed] [Google Scholar]
- 29.Adelow C, Segura T, Hubbell JA, Frey P. The effect of enzymatically degradable poly(ethylene glycol) hydrogels on smooth muscle cell phenotype. Biomaterials. 2008;29:314–26. doi: 10.1016/j.biomaterials.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 30.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hmsc viability. Matrix Biol. 2005;24:208–18. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Nagase H, Fields GB. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Biopolymers. 1996;40:399–416. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C399::AID-BIP5%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 32.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–45. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 33.Ho IA, Chan KY, Ng WH, Guo CM, Hui KM, Cheang P, et al. Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells. 2009;27:1366–75. doi: 10.1002/stem.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20:53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 35.Parikka V, Vaananen A, Risteli J, Salo T, Sorsa T, Vaananen HK, et al. Human mesenchymal stem cell derived osteoblasts degrade organic bone matrix in vitro by matrix metalloproteinases. Matrix Biol. 2005;24:438–47. doi: 10.1016/j.matbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Djouad F, Delorme B, Maurice M, Bony C, Apparailly F, Louis-Plence P, et al. Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res Ther. 2007;9:R33. doi: 10.1186/ar2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauney J, Volloch V. Adult human bone marrow stromal cells regulate expression of their MMPs and TIMPs in differentiation type-specific manner. Matrix Biology. 2010;29:3–8. doi: 10.1016/j.matbio.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider RK, Puellen A, Kramann R, Raupach K, Bornemann J, Knuechel R, et al. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2010;31:467–80. doi: 10.1016/j.biomaterials.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 39.Panetta NJ, Gupta DM, Quarto N, Longaker MT. Mesenchymal cells for skeletal tissue engineering. Panminerva Med. 2009;51:25–41. [PubMed] [Google Scholar]