Abstract
Non-neuronal release of acetylcholine (ACh) has been proposed to play a role in urinary bladder function. These studies investigated the expression and function of the non-neuronal cholinergic system in cultured urothelial cells isolated from the rat urinary bladder. Our findings have revealed that urothelial cells express the high-affinity choline transporter (CHT1) and acetylcholine synthesizing enzymes, choline acetyltransferase (ChAT) and carnitine acetyltransferase (CarAT). In contrast to neurons, urothelial cells do not express the vesicular acetylcholine transporter (VAChT) but do express OCT3, a subtype of polyspecific organic cation transporter (OCT) that is thought to be involved in the release of acetylcholine from nonneuronal cells. Following exposure of cultured urothelial cells to 3H-choline, radioactivity was detected in the cells and increased release of radioactivity into the eternal media was evoked by mechanical stimulation (exposure of the cells to 50% hypotonic Krebs) or chemical stimulation of purinergic receptors by 100 μM ATP. The present experiments did not establish if the evoked release of radioactivity (termed 3H-ACh release in this paper) was due to release of acetylcholine or choline. 3H-ACh release was not evoked by application of acetylcholine alone, however pretreatment with the non-selective muscarinic receptor antagonist atropine prior to application of acetylcholine facilitated 3H-ACh release, suggesting that the acetylcholine released from urothelial cells may participate in a negative feedback mechanism by acting on muscarinic receptors to inhibit its own release in the urothelium. Brefeldin, an agent which disrupts vesicular exocytosis, did not block hypotonic-evoked 3H-ACh release. These observations indicate that acetylcholine release from urothelial cells is mediated by different mechanisms than those such as vesicular storage and exocytosis that underlie the release of neurotransmitters from nerves.
Keywords: Urothelium, non-neuronal cholinergic system, acetylcholine release, organic cationic transporters
Introduction
The luminal surface of the urinary bladder is covered by several layers of epithelial cells (the urothelium) which functions as a passive barrier to the passage of substances from the urine into the bladder wall. Recently it has been shown that the urothelium is also a responsive structure capable of releasing a number of signaling molecules including ATP, nitric oxide and prostaglandins in response to mechanical and chemical stimuli (Birder, 2005). A basal release of acetylcholine has been detected in bladder and this release was considerably decreased following removal of the bladder mucosa suggesting a portion of the release originates in the urothelium (Yoshida et al., 2006; Yoshida et al., 2004).
Thus the urothelium appears to be similar to a variety of other non-neuronal cells including keratinocytes, endothelial, glial, and bronchial epithelial cells which are capable of synthesizing and releasing acetylcholine (Wessler and Kirkpatrick, 2001; Wessler et al., 1998). Various functions of non-neuronal cholinergic systems have been considered including regulation of cell-cell contact and signaling, growth, proliferation and apoptosis (Wessler and Kirkpatrick, 2001; Wessler et al., 1998).
Release of acetylcholine from the urothelium could have a number of consequences, such as activation of cholinergic (nicotinic/muscarinic) receptors on nearby bladder nerves, smooth muscle and myofibroblasts as well as autocrine/paracrine function to activate cholinergic receptors on the urothelial cell surface (Hegde, 2006; Andersson and Yoshida, 2003). Acetylcholine release from bladder urothelium may also play a role in a number of pathologies including detrusor overactivity. In support of this idea is evidence that antimuscarinic drugs, a long established therapy for the management of overactive bladder, exhibit efficacy during the bladder storage phase when bladder efferent nerves are “silent”, suggesting that release of acetylcholine from non-neuronal sources (i.e the urothelium) plays a significant role in bladder dysfunction (Andersson and Yoshida, 2003). Further support for a role for urothelial-derived acetylcholine in bladder pathology was obtained from recent studies that documented increased acetylcholine content and release in bladder mucosa from aged patients who exhibit a greater incidence of bladder overactivity (Yoshida et al., 2004).
The present study examined: (1) the properties of the non-neuronal cholinergic system in rat urinary bladder urothelial cells, (2) the mechanism of release of acetylcholine from bladder urothelial cells and (3) the possible involvement of this system in bladder function.
Methods
Cell culture
Preparation of urothelial cultures was carried out as previously described (Birder et al., 2003; Birder et al., 2002). Briefly, bladders were excised from adult female Sprague-Dawley rats (250–350 g), killed by inhalation of medical grade CO2 followed by thoracotomy and cardiac puncture. The bladders were cut open, gently stretched and pinned with the urothelial side up. Following an overnight incubation in minimal essential medium containing antibiotic and 2.5 mg/ml dispase (Gibco), the urothelium was gently scraped from underlying tissue, treated with 0.25% trypsin and resuspended in keratinocyte medium (Gibco). The cells were plated at a density of 50,000–70,000 cells/ml on collagen-coated cover slips. Immunocytochemistry using cytokeratin antibody (1:200, Dako) was performed on samples from each culture to confirm that the cells were epithelial. Purity of the cultures was confirmed by the absence of vimentin-positive staining (a marker for fibroblasts), using monoclonal anti-vimentin (1:200, Sigma).
Reverse transcription-PCR
RNA was extracted from cultured cells at the time of confluency (2–3 days in culture) by homogenization in Trizol (Life Technologies) and was reverse transcribed with an oligo-dT primer, using Superscript II (Life Technologies). RT− controls were performed for each sample by omission of the enzyme. PCR amplification was conducted in the presence of 1.25 units of platinum Taq DNA Polymerase (Life Technologies) using primers for the following: rat organic cation transporters (rOCT) 1, 2 and 3, the acetylcholine-synthesizing enzyme choline acetyltransferase (ChAT); the vesicular acetylcholine transporter (VAChT); glyceraldehyde-3-phosphate dehydrogenase (GAPDH); the high-affinity choline transporter (CHT1) and carnitine acetyltransferase (CarAT), which also exhibits choline acetyltransferase activity. PCR was initially conducted with a temperature gradient to determine ideal annealing temperatures for the primers used.
Amplification primer sequences were as follows: rOCT1:5'- catctgtgtccggtgtgc -3' and 5'- cttcaggtcagcaggagg-3'; rOCT2:5'- gcctcctgatcctggctg -3' and 5'- ggtgtcaggttctgaagagag -3'; rOCT3:5'- ttcggcgttggcatcacg -3' and 5'-ctgtaactgtgatctctgag-3'; GAPDH: 5'- cgtcttcaccaccatggaga-3' and 5'- cggccatcacgccacagctt-3' (Lips et al., 2005); CHAT: 5'-ctagacgaaccccagttcca-3' and 5'- cacccacgttttctgatcct-3'; CarAT: 5'-ccaagcaggacttcatggat -3' and 5'- tgtgtgggtggtttctttga -3'; CHT1: 5'-ctgtctcctcagggcaaaag-3' and 5'-ctaaagctggggctgctatg-3';VAChT : 5'-tggtcattctgcaagagcac -3' and 5'- ccatttccccaatgaatacg-3'. Amplification products were visualized on a 1.3% agarose gel with ethidium bromide. Purity of the RNA isolation was confirmed by empty RT− lanes.
Acetylcholine release
Acetylcholine was measured with a radiolabelled method (H3-choline) used in previous studies to examine acetylcholine release from nerves in the urinary bladder (Somogyi and de Groat., 1999). Urothelial cells were incubated in Krebs solution containing 10μCi.ml−1[3H]-choline (specific activity 83.0Ci.mmol−1) for 60min. In order to remove non-selective bound radioactivity, following incubation with [3H]-choline, urothelial cells were superfused with oxygenated Krebs solution using a peristaltic pump (Dynamax® Model RP-1; Rainin Instrument Co. Inc.), at a rate of 0.3 ml. min−1 for 30 minutes. At t=30 min, 100μl samples were taken every minute for a total of five minutes to assess control 3H-ACh release.
The effect of mechanical or chemical stimulation was investigated by changing the superfusate medium to one of a lower osmolality or containing the drug; samples were taken every minute for 5 minutes during the test phase. Following a 10-minute washout phase in Krebs, five samples were taken at one minute intervals. Superfusate fractions (100 μl), were added to scintillation vials followed by the addition of 4ml of scintillation fluid (ScintiSafe™ 30%; Fischer Scientific) to each sample. At the end of each experiment the cells were lysed with a 5M Urea solution containing 0.02% Triton® X-100 (Sigma) and the total volume of lysate was collected. This count was added to the cumulative counts from all fractions collected during the course of the experiment (control, test and washout phases), in order to estimate the `total releasable' 3H-ACh. Counts were estimated as a percent of this pool (% fractional release). Radioactive counts were measured with a liquid scintillation counter (LS 6500, Beckman Coulter).
Drugs and radiochemicals
All chemicals and drugs were obtained from Sigma-Aldrich Corporation USA unless otherwise noted. These include: Acetylcholine chloride, Atropine sulphate, Brefeldin A and ATP. methyl-[3H]-Choline chloride was supplied by GE Healthcare, Amersham, UK. Hypotonic Krebs was prepared by a 50% reduction in [NaCl].
Results
PCR data
Using RNA extracted from urothelial cells (2–3 days in culture), RT-PCR studies were carried out to obtain evidence for an acetylcholine synthesis/storage mechanism in the epithelial cells. Primers for the high affinity choline transporter (CHT1), choline acetyltransferase (ChAT) and carnitine acetyltransferase (CarAT) produced positive bands following gel electrophoresis of the PCR products. However, failure to produce a PCR product using the VAChT primer in all UT cell cultures suggests that acetylcholine is not stored in synaptic vesicles. RT-PCR experiments, using RNA extracted from rat DRG cultures (2DIV), were carried out to validate the adequacy of the VAChT primers; positive bands were evident following gel electrophoresis of the PCR products. In addition the demonstration that the cells express OCT3, a polyspecific cation transporter, suggests that membrane transport rather than a vesicular exocytosis may be the mode of secretion of the acetylcholine from these cells.
Functional Study
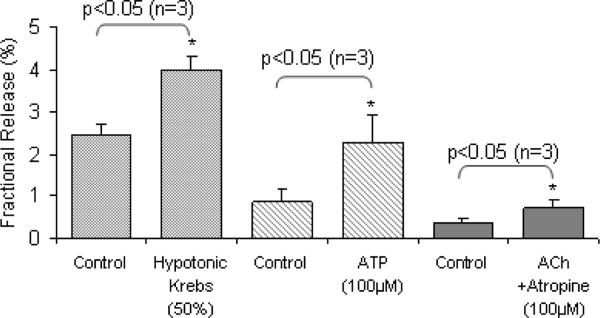
Cells exhibited control levels of 3H-ACh hereafter termed radioactivity released in all protocols; values ranged from 0.2 −2.0% of total radioactivity released over the various protocols used. Exposure of cells to mechanical stimulation, in this instance cellular swelling in 50% hypotonic Krebs (Fig.1), caused a significant increase in radioactivity release (p<0.05; paired Students t-test; n=4). This was noted within the first minute of exposure to hypotonicity. Following washout, (return to isotonic Krebs superfusate), radioactivity release was not statistically different from values (control) recorded prior to exposure of the cells to the stimulus.
Fig 1.
Mechanical (50% hypotonic Krebs) and chemical (ATP 100μM) stimuli evoke significant (p< 0.05; Students paired t-test) increase in radioactivity release from cultured urothelial cells. Stimulated neurotransmitter release was of the order of two-fold that of the preceding control level in the respective groups of experiments. While acetylcholine alone did not evoke release of radioactivity, acetylcholine (100 μM) administered following preincubation of UT cells with the non-specific muscarinic receptor antagonist, atropine (100 μM) evoked a significant increase in release of radioactivity (p<0.05; Student's paired t-test). Values are mean radioactivity release per minute averaged over the 5-min control and test periods.
Urothelial cells exhibited a significant increase in radioactivity release above control levels (p<0.05; paired Students t-test; n=3) upon exposure to ATP (100μM) (Fig 1). The response was again rapid, seen within the first minute of exposure to the chemical stimulus. Following washout, release returned to the low levels recorded during the initial control phase.
In contrast, application of acetylcholine (100μM) did not alter control radioactivity release. However, following pre- incubation with the non-specific muscarinic receptor antagonist, atropine (100μM) and in its continued presence, acetylcholine (100μM) evoked an increase in radioactivity release (data not shown).
Cells incubated with brefeldin (10μM) for 1 hour prior to the test phase exhibited a response to mechanical stimulation in the continued presence of the agent. After changing the superfusate to 50% hypotonic Krebs, the cells exhibited a rapid and significant increase in radioactivity release with values increasing almost two-fold above the control values (p<0.05; paired Students t-test; n=3).This response was reversed after changing the bath to isotonic Krebs.
Discussion
The present experiments, which were carried out to investigate the properties of a non-neuronal cholinergic system in cultured urothelial cells from the rat urinary bladder, revealed that urothelial cells express the mechanisms for the synthesis of acetylcholine including the high-affinity choline transporter (CHT1) and the acetylcholine synthesizing enzymes choline acetyltransferase (ChAT) and carnitine acetyltransferase (CarAT). The experiments also provided evidence that 3H-ACh release from urothelial cells is mediated by a mechanism that does not depend on vesicular storage of acetylcholine.
In urothelial cells as in neurons, the synthesis of acetylcholine is likely to involve several steps including the uptake of choline from the extracellular compartment and then formation of acetylcholine via cytoplasmic enzymes. The transporter for choline uptake (CHT1) and the enzymes for acetylcholine synthesis (CHAT and CarAT) were identified in urothelial cells. On the other hand, it seems unlikely that acetylcholine is stored in vesicles in urothelial cells because the VAChT was not found in these cells and brefeldin, a chemical that blocks vesicle formation, did not block hypotonic-induced release of 3H-ACh from urothelial cells. Thus, our findings which are consistent with those of Wessler and others (Lips et al., 2005;Wessler et al., 2001) indicate that release of 3H-ACh in urothelial cells does not occur via an exocytotic mechanism. As it has been shown that ATP release from urothelial cells is reduced by brefeldin (Knight et al., 2002), it is likely that ATP and acetylcholine are released by different mechanisms from urothelial cells.
It has been recently reported that acetylcholine released from human placental epithelial cells is mediated by electrogenic polyspecific organic cationic transporters (OCTs)(Wessler et al., 2001). It has also been shown that OCT1 and OCT2 isoforms are localized at the apical membrane of ciliated airway epithelial cells while the OCT3 isoform is present at the basalateral membrane in a number of non-neuronal cell types including human bronchial epithelium (Lips et al., 2005;Koepsell et al., 2003). Consistent with these findings, the present experiments revealed that bladder urothelial cells express the organic cationic transporter isoform OCT3.
It has been reported that nicotinic and muscarinic cholinergic receptors are widely expressed on non-neuronal cells including intestinal, skin and urinary bladder epithelial cells, immune cells as well as myofibroblasts (Wessler and Kirkpatrick, 2001; Hegde, 2006; Beckel et al., 2005; Mukerji et al., 2006). In the present study, 3H-ACh evoked release was unmasked by atropine suggesting this was mediated by activation of nicotinic receptors. In addition, similar to that reported in other types of non-neuronal cells (Wessler and Kirkpatrick, 2001), stimulation of acetylcholine-receptors in bladder urothelial cells results in a release of nitric oxide, prostanoids (unpublished observations) and ATP (Birder et al., 2003).
What are the possible functions of acetylcholine release from urothelial cells? Taken together, these observations suggest multiple lines of cell-cell communication with non-neuronal acetylcholine release acting to stimulate cholinergic receptors (nicotinic/muscarinic) expressed on nearby bladder nerves, myofibroblasts as well as smooth muscle. In addition, acetylcholine released from urothelial cells or efferent nerves could lead (via paracrine or autocrine signaling) to release of modulators such as ATP, nitric oxide or prostanoids from urothelial cells which in turn, could alter neural excitability. In the present study hypotonic stimuli as well as ATP elicited release of 3H-ACh from urothelial cells. The finding that mechanical stretch, due to hypotonic swelling, evoked 3H-ACh release may also indicate that acetylcholine plays an important autocrine role in the physiological homeostasis of these cells.
A number of studies have provided evidence for prejunctional autoreceptors on bladder nerve terminals (D'Agostino et al., 2000; Somogyi and de Groat, 1999). These receptors which are known to inhibit or facilitate the release of acetylcholine have also been localized in nonneuronal tissues including the tracheal epithelium (Vlahos et al., 2000). In epithelium-intact tracheal preparations, acetylcholine efflux was enhanced in the presence of the muscarinic antagonist, atropine raising the possibility that the increased release of acetylcholine was due to blockade of prejunctional muscarinic autoreceptors on nerve terminals. In the present study, we showed that administration of atropine, enhanced the acetylcholine-evoked release of 3H-ACh from cultured urothelial cells. These observations suggest that acetylcholine acts in an autofeedback manner on muscarinic receptors on the urothelial cells to suppress its own release.
Cholinergic mechanisms in the urinary bladder activated by acetylcholine arising in non-neuronal tissues may play an important role in the initiation of bladder disorders such as overactive bladder (OAB) (Hegde, 2006;Andersson and Yoshida, 2003;Kumar et al., 2005). Thus, urothelial cholinergic receptors and release of acetylcholine from urothelial cells are attractive targets to treat these disorders. Antimuscarinic drugs are the cornerstone in the treatment regimen for bladder overactivity. These drugs are used to block muscarinic receptors in bladder smooth muscle as well as other sites including bladder nerves. Previous studies revealed that activation of muscarinic receptors in the urothelium triggers the release of ATP and nitric oxide which could lead to activation of afferent pathways. Thus blockade of urothelial muscarinic receptors could act indirectly to reduce afferent nerve activation and therefore decrease OAB symptoms. However, it is also possible that antagonism of inhibitory muscarinic receptors in the urothelium could lead to additional acetylcholine release from the urothelium and a corresponding enhancement in afferent nerve activity. In bladder efferent nerves, different subtypes of muscarinic receptors mediate inhibitory (M2-M4) and facilitatory effects (M1) on transmitter release. It will be important in future experiments to determine if different subtypes of muscarinic receptors exert facilitatory or inhibitory effects on transmitter release in the urothelium. Because of the possibility of mixed effects, subtype selective receptor antagonists might be used to suppress excitatory responses without affecting inhibitory responses.
Injection of botulinum toxin into the bladder wall has recently been used to treat bladder disorders that are resistant to other therapies such as neurogenic detrusor overactivity and interstitial cystitis (Ho et al., 2005;Chancellor, 2002). The inhibition of detrusor muscle contractions is due to the ability of the toxin to block exocytosis and the release of transmitters such as acetylcholine and ATP from bladder nerves. Recent studies have also shown that botulinum toxins can also block ATP release from bladder urothelium (Smith et al., 2005). In light of the present observations it would appear that botulinum toxins could prevent the release of purines but not acetylcholine, from bladder urothelium. Thus, botulinum toxins could be utilized experimentally in future studies to discriminate between purinergic and cholinergic release mechanisms in bladder urothelium and to confirm our preliminary results indicating that ATP and acetylcholine are released by different mechanisms in urothelial cells.
Conclusion
The present results coupled with previous descriptions of cholinergic mechanisms in the urothelium indicate that acetylcholine released from urothelial cells or from nerves in the vicinity of urothelial cells may activate multiple types of receptors (nicotinic and muscarinic) to facilitate or inhibit the initiation of afferent activity in the bladder. Targeting of urothelial acetylcholine receptors/ion channels or acetylcholine release mechanisms may lead to development of new strategies for the clinical management of bladder disorders.
Acknowledgments
This study was supported by National Institute of Health Diabetes and Digestive and Kidney Diseases Grant R01-DK54824 (to L. Birder).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson KE, Yoshida M. Antimuscarinics and the overactive detrusor-which is the main mechanism of action? European Urology. 2003;43:1–5. doi: 10.1016/s0302-2838(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Kanai A, Lee SJ, De Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. American Journal of Physiology. 2005;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. American Journal of Physiology. 2005;289:F489–F495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- Birder LA, Barrick SR, Roppolo JR, Kanai AJ, De Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. American Journal of Physiology. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- Chancellor MB. New frontiers in the treatment of overactive bladder and incontinence. Reviews in Urology. 2002;4:S50–S60. [PMC free article] [PubMed] [Google Scholar]
- D'agostino G, Bolognesi ML, Lucchelli A, Vicini D, Balestra B, Spelta V, Melchiorre C, Tonini M. Prejunctional muscarinic inhibitory control of acetylcholine release in the human isolated detrusor: involvement of the M4 receptor subtype. British Journal of Pharmacology. 2000;129:493–500. doi: 10.1038/sj.bjp.0703080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS. Muscarinic receptors in the bladder: from basic research to therapeutics. British Journal of Pharmacology. 2006;147:S80–S87. doi: 10.1038/sj.bjp.0706560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MH, Lin LL, Haessler AL, Bhatia NN. Intravesical injection of botulinum toxin for the treatment of overactive bladder. Current Opinion Obstetrics and Gynecology. 2005;17:512–518. doi: 10.1097/01.gco.0000180659.09320.3e. [DOI] [PubMed] [Google Scholar]
- Knight GE, Boden P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. American Journal of Physiology. 2002;282:F282–288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Reviews Physiology Biochemical Pharmacology. 2003;150:36–90. doi: 10.1007/s10254-003-0017-x. [DOI] [PubMed] [Google Scholar]
- Kumar V, Cross RL, Chess-Williams R, Chapple CR. Recent advances in basic science for overactive bladder. Current Opinion in Urology. 2005;15:222–226. doi: 10.1097/01.mou.0000172393.52857.92. [DOI] [PubMed] [Google Scholar]
- Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, Ermert L, Kummer W, Koepsell H. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. American Journal of Respiratory Cell Molecular Biology. 2005;33:79–88. doi: 10.1165/rcmb.2004-0363OC. [DOI] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Grogono J, Underwood J, Agarwal SK, Khullar V, Anand P. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. Journal of Urology. 2006;176:367–373. doi: 10.1016/S0022-5347(06)00563-5. [DOI] [PubMed] [Google Scholar]
- Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochemical International. 2005;47:291–297. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Somogyi GT, De Groat WC. Function, signal transduction mechanisms and plasticity of presynaptic muscarinic receptors in the urinary bladder. Life Sciences. 1999;64:411–418. doi: 10.1016/s0024-3205(98)00580-3. [DOI] [PubMed] [Google Scholar]
- Vlahos R, Fabiani ME, Story DF. Influence of the epithelium on acetylcholine release from parasympathetic nerves of the rat trachea. Journal of Autonomic Pharmacology. 2000;20:237–251. doi: 10.1046/j.1365-2680.2000.00187.x. [DOI] [PubMed] [Google Scholar]
- Wessler IK, Kirkpatrick CJ. The non-neuronal cholinergic system: an emerging drug target in the airways. Pulmonary Pharmacology & Therapeutics. 2001;14:423–434. doi: 10.1006/pupt.2001.0313. [DOI] [PubMed] [Google Scholar]
- Wessler IK, Kirkpatrick CJ, Racke K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacology Therapeutics. 1998;77:59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- Wessler IK, Roth E, Deutsch C, Brockerhoff P, Bittinger F, Kirkpatrick CJ, Kilbinger H. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. British Journal of Pharmacology. 2001;134:951–956. doi: 10.1038/sj.bjp.0704335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, Murakami S. Non-neuronal cholinergic system in human bladder urothelium. Urology. 2006;67:425–430. doi: 10.1016/j.urology.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]