Abstract
Background
It is unclear whether antiretroviral (ART) naive HIV-positive individuals with high CD4 counts have a raised mortality risk compared with the general population, but this is relevant for considering earlier initiation of antiretroviral therapy.
Methods
Pooling data from 23 European and North American cohorts, we calculated country-, age-, sex-, and year-standardised mortality ratios (SMRs), stratifying by risk group. Included patients had at least one pre-ART CD4 count above 350 cells/mm3. The association between CD4 count and death rate was evaluated using Poisson regression methods.
Findings
Of 40,830 patients contributing 80,682 person-years of follow up with CD4 count above 350 cells/mm3, 419 (1.0%) died. The SMRs (95% confidence interval) were 1.30 (1.06-1.58) in homosexual men, and 2.94 (2.28-3.73) and 9.37 (8.13-10.75) in the heterosexual and IDU risk groups respectively. CD4 count above 500 cells/mm3 was associated with a lower death rate than 350-499 cells/mm3: adjusted rate ratios (95% confidence intervals) for 500-699 cells/mm3 and above 700 cells/mm3 were 0.77 (0.61-0.95) and 0.66 (0.52-0.85) respectively.
Interpretation
In HIV-infected ART-naive patients with high CD4 counts, death rates were raised compared with the general population. In homosexual men this was modest, suggesting that a proportion of the increased risk in other groups is due to confounding by other factors. Even in this high CD4 count range, lower CD4 count was associated with raised mortality.
Introduction
Lower CD4 count is associated with an increased risk of death in HIV infection (1), and this trend has been observed even in the high CD4 count range (2;3). There is a growing understanding that the risk of non-AIDS death is associated with CD4 count, although not as strongly as for AIDS deaths (4). The optimal CD4 count at which to initiate antiretroviral therapy (ART) in HIV-positive individuals is, at present, unclear. Most current guidelines state that, in patients without a previous AIDS event, ART should be initiated when the CD4 count falls to 350 cells/mm3 (5;6). Evidence as to whether initiating ART at higher CD4 counts may be beneficial is currently restricted to observational studies (7;8), and to a sub-analysis of the SMART trial (9). The observational studies have attempted to mimic the comparison made in a randomized trial, comparing outcomes from immediate versus deferred ART initiation, and have generally concluded that earlier initiation is likely to lead to a lower risk of death, although there are inconsistencies in results (7;8). We sought to use observational data to address a more fundamental question concerning the potential for benefit of early use of ART: do ART-naïve patients with CD4 count above 350 cells/mm3 experience a higher risk of death than the general population? It appears that there would need to be such a raised risk for there to be a potential benefit of early ART initiation.
We compared the mortality rates observed in a large multinational collaborative cohort study with those expected in the general population, standardized by age, sex, country, and year. Furthermore, we considered whether death rates in such HIV-infected patients differ according to CD4 count.
Methods
Data
Twenty-three cohorts and cohort collaborations contributed data for this analysis: 18 cohorts were based in Europe and 5 in North America. Data were requested in a standardised format (10), and duplicate records were removed where patients appeared in more than one cohort. Data requested from participating cohorts included demographic information, CD4 counts, viral load measurements, hepatitis C co-infection status, smoking status, date of death and whether AIDS-related or not. This analysis was restricted to patients aged 20 to 59 who had at least one CD4 count above 350 cells/mm3 while ART naïve. All pre-ART CD4 counts above 350 cells/mm3 from January 1990 to December 2004 were included: the exclusion of CD4 counts after 2005 was in order to mitigate the effect of any delay in reporting of deaths. All CD4 counts measured during prospective follow-up, i.e., after enrolment to a cohort while ART naive, were included.
Statistical methods
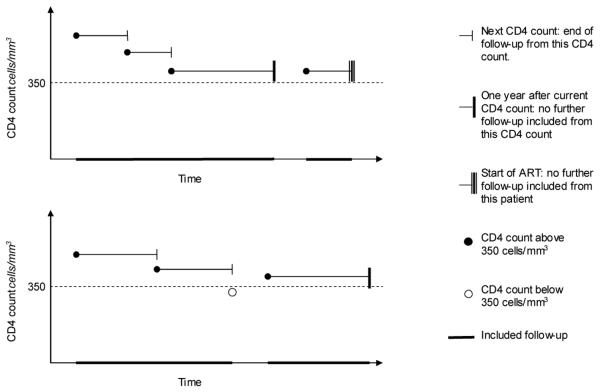
In each patient, included follow-up was counted from the date of each eligible CD4 count until the earliest of: next CD4 count; death; start of ART; or elapse of one year (Figure 1). Standardised mortality ratios were calculated by comparing observed death rates with those expected in the general population, standardised by age, sex, country, and year of CD4 count. The data used for calculation of the SMRs were restricted to those countries on which general population mortality data were available from the Human Mortality Database (11), and for which there was at least 2000 person-years of follow-up.
Figure 1.
Illustration of included follow-up for example patients.
Poisson regression methods were used to investigate the relationship between death rate and CD4 count. In addition to CD4 count, factors included in the multivariable model were sex, risk group, age, current calendar year, and most recent viral load measurement (no earlier than six months before the date of the included CD4 count). Additionally, hepatitis C co-infection and smoking status were considered as factors in models restricted to data from cohorts able to provide such data.
Three sensitivity analyses were performed. In the first of these, follow-up was censored at six months after each CD4 count instead of at one year: this was to test the assumption implicit in the main analysis that the most recent CD4 count was valid for up to one year. In the second, the main analysis was repeated but only follow-up after CD4 counts above 500 cells/mm3 was included: this was to determine whether there was a raised risk of death in this higher CD4 count range. In the third sensitivity analysis, to assess the impact of any possible underascertainment of deaths, the SMRs were calculated using data from only those cohorts which are known to be linked to national death registers.
All p-values are two sided. Analyses were performed using SAS version 9.1., Cary, North Carolina, United States.
Role of the funding source
The study sponsors had no role in the study design, analysis, interpretation of data, writing of the report or in the decision to submit the paper for publication. Final responsibility for the decision to submit for publication was made by the Analysis and Writing Committee.
Results
Included patients and follow-up
A total of 40,830 patients were included in the analysis, contributing 201,620 CD4 counts and 80,682 person-years of follow-up, with a median of 3 CD4 counts per patient (IQR: 1-6). The number of patients for whom follow-up was censored due to ART initiation was 11713 (28.7%; 5.8% of CD4 episodes). The distribution of follow-up according to patient characteristics is described in Table 1. Most follow-up was from male patients (59,774 person-years, 74.1%), approximately half of follow-up was from homosexual men (39,732 person-years, 49.2%), and approximately half of follow-up was from patients aged between 30 and 39 (38,112 person-years, 47.2%). The amount of follow-up with CD4 count above 500 cells/mm3 was 50,357 person-years (62.4%). The most recent viral load was available for 48,487 person-years of follow up (60.1%). Around one third of follow-up was from patients within cohorts linked to national death registers (27,206 person-years, 33.7%). Data on hepatitis C co-infection status were available from 16 of the 23 participating cohorts (73,322 person-years; 90.9% of total follow-up) and on smoking from 9 cohorts (30,332 person-years; 37.6% of total follow-up).
Table 1.
Characteristics of follow-up of the 40,830 included patients contributing 80,682 person-years of follow-up
| Characteristics of follow-up | Person-years | % of follow-up | |
|---|---|---|---|
| Sex | Male | 59774 | 74.1 |
| Female | 20908 | 25.9 | |
| Risk group | Homosexual men | 39732 | 49.2 |
| Heterosexual | 18955 | 23.5 | |
| IDU | 17543 | 21.7 | |
| Other / Unknown | 4452 | 5.5 | |
| Age years |
20-29 | 22312 | 27.7 |
| 30-39 | 38112 | 47.2 | |
| 40-49 | 15759 | 19.5 | |
| 50-59 | 4500 | 5.6 | |
| Calendar year | 1990-1994 | 20857 | 25.9 |
| 1995-1999 | 28325 | 35.1 | |
| 2000-2004 | 31501 | 39.0 | |
| CD4 count cells/mm3 |
350-499 | 30325 | 37.6 |
| 500-699 | 28565 | 35.4 | |
| 700- | 21792 | 27.0 | |
| Viral load log10 copies/ml |
−2.99 | 9479 | 11.7 |
| 3.00-3.99 | 15586 | 19.3 | |
| 4.00-4.99 | 19220 | 23.8 | |
| 5.00- | 4202 | 5.2 | |
| Unknown | 32195 | 39.9 | |
| Hepatitis C | No previous infection | 35313 | 48.2 |
| co-infection1 | Current or previous infection | 13991 | 19.1 |
| Unknown | 24018 | 32.8 | |
| Smoking status2 | Never smoked | 6005 | 19.8 |
| Ever smoked | 16783 | 55.3 | |
| Unknown | 7544 | 24.9 | |
Participating cohorts who were able to provide hepatitis data contributed a total of 73,322 person-years of follow up.
Participating cohorts who were able to provide smoking data contributed a total of 30,332 person-years of follow up.
Deaths
A total of 419 (1.0%) patients died during follow-up, giving an overall death rate of 5.2 per 1000 person-years (95% CI: 4.7-5.7). Of these, 61 (14.6%) deaths were categorised as AIDS-related, 188 (44.9%) were categorised as non-AIDS-related, and the cause was unknown for 170 (40.6%) of deaths.
Comparison of death rates with those expected from the general population
The SMR analysis for pre-ART CD4 counts above 350 cells/mm3 included 38,997 patients (95.5% of the total: the SMR analysis was restricted to those countries on which data were available from the Human Mortality Database (11), and for which there was at least 2000 person-years of follow-up), with 77,936 person-years of follow-up (96.6% of the total) and 401 deaths (95.7% of the total). The observed death rates and age-, sex-, country-, and year-standardised SMRs for each risk group are displayed in Table 2. For homosexual men, the SMR was 1.30 (95% CI: 1.06-1.58). The SMRs in the other risk groups were greater in value, at 2.94 (95% CI: 2.28-3.73), 9.37 (95% CI: 8.13-10.75) and 4.57 (95% CI: 3.09-6.53) for the heterosexual, IDU and other/unknown risk groups respectively. When the analysis was restricted to CD4 counts above 500 cells/mm3, the resulting SMRs were slightly lower at 2.83 (95% CI: 2.03-3.87), 7.97 (95% CI: 6.55-9.60) and 4.47 (95% CI: 2.61-7.16) for the heterosexual, IDU and other/unknown risk groups. For homosexual men, however, the SMR was 1.03 (95% CI: 0.76-1.37). Again considering CD4 counts above 350 cells/mm3 but with follow-up censored at 6 months after each CD4 count instead of at 1 year, the SMRs were slightly lower than in the main analysis at 1.14 (95% CI: 0.90-1.43) for homosexual men, 2.74 (95% CI: 2.05-3.60) for the heterosexual risk group, 9.35 (95% CI: 7.93-10.94) for IDUs and 3.30 (95% CI: 1.96-5.22) for the other/unknown risk group. Restricting the analysis to follow-up from patients within cohorts linked to national death registers resulted in slightly higher SMRs than in the main analysis, at 1.52 (95% CI: 1.10-2.04) for homosexual men, 3.29 (95% CI: 2.09-4.94) for the heterosexual risk group, 15.85 (95% CI: 11.27-21.67) for IDUs and 6.99 (95% CI: 3.99-11.35) for the other/unknown risk group.
Table 2.
Observed death rates and standardized mortality ratios based on 77,936 person-years of follow-up from 38,997 patients
| Risk group | Deaths observed |
Follow-up person-years |
Death rate (95% CI) per 1000 person-years |
Deaths expected |
SMR (95% CI) |
|---|---|---|---|---|---|
| Homosexual men | 100 | 38764 | 2.58 (2.07-3.09) | 76.79 | 1.30 (1.06-1.58) |
| Heterosexual | 68 | 18311 | 3.71 (2.83-4.60) | 23.13 | 2.94 (2.28-3.73) |
| IDU | 203 | 16725 | 12.14 (10.47-13.81) | 21.77 | 9.37 (8.13-10.75) |
| Other / Unknown | 30 | 4137 | 7.25 (4.66-9.85) | 6.56 | 4.57 (3.09-6.53) |
Association between CD4 count and risk of death
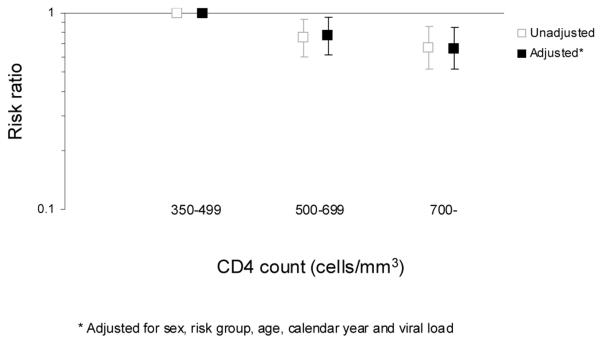
In a Poisson regression model fitted to the full dataset, a CD4 count above 500 cells/mm3 was found to be associated with a lower risk of death than CD4 count between 350 and 499 cells/mm3 (Figure 2). The unadjusted risk ratios were 0.75 (95% CI: 0.60-0.93) and 0.67 (95% CI: 0.52-0.86) for CD4 counts 500-699 cells/mm3 and 700- cells/mm3 respectively, compared to 350-499 cells/mm3 (Table 3). Factors found in univariable analyses to be significant predictors of death were risk group and age. Sex and calendar year of CD4 count were not associated with the rate of death in univariable analyses, but were significant factors in the multivariable model. Viral load, which was only available for 60.1% of total follow-up, was not found to be associated with death rate. Following adjustment for the other factors in the model, the rate ratios for the CD4 count remained largely unchanged at 0.77 (95% CI: 0.61-0.95) and 0.66 (95% CI: 0.52-0.85) for CD4 counts 500-699 cells/mm3 and 700-cells/mm3 respectively, compared to 350-499 cells/mm3.
Figure 2.
Unadjusted and adjusted risk ratios from a Poisson regression model for the risk of death according to CD4 count. The number of patients was 40,830, contributing 80,682 person-years of follow-up and with 419 deaths.
Table 3.
Incidence rate ratios of death at CD4 count above 350 cells/mm3 while ART näve, according to CD4 count and other patient characteristics. Results were obtained from Poisson regression models: there were 419 deaths in 80,682 person-years
| Univariable analyses | Multivariable analysis | ||||
|---|---|---|---|---|---|
| Characteristics | Risk ratio (95% CI) |
p-value | Risk ratio (95% CI) |
p-value | |
| CD4 count cells/mm3 | |||||
| 350-499 | 1 | 0.0019 | 1 | 0.0023 | |
| 500-699 | 0.75 (0.60-0.93) | 0.77 (0.61-0.95) | |||
| 700- | 0.67 (0.52-0.86) | 0.66 (0.52-0.85) | |||
| Sex | |||||
| Male | 1 | 0.78 | 1 | 0.015 | |
| Female | 1.03 (0.83-1.28) | 0.74 (0.57-0.94) | |||
| Risk group | |||||
| Homosexual men | 1 | <0.0001 | 1 | <0.0001 | |
| Heterosexual | 1.37 (1.01-1.85) | 1.83 (1.29-2.59) | |||
| IDU | 4.58 (3.63-5.78) | 5.85 (4.54-7.53) | |||
| Other / Unknown | 2.53 (1.69-3.79) | 2.97 (1.96-4.50) | |||
| Age years | |||||
| 20-29 | 1 | <0.0001 | 1 | <0.0001 | |
| 30-39 | 1.38 (1.07-1.79) | 1.41 (1.08-1.83) | |||
| 40-49 | 1.76 (1.32-2.35) | 2.09 (1.55-2.82) | |||
| 50-59 | 2.21 (1.50-3.25) | 3.24 (2.17-4.83) | |||
| Calendar year | |||||
| 1990-1994 | 1 | 0.091 | 1 | 0.035 | |
| 1995-1999 | 0.84 (0.66-1.06) | 0.71 (0.54-0.94) | |||
| 2000-2004 | 0.77 (0.61-0.98) | 0.67 (0.48-0.93) | |||
| Viral load log10 copies/ml | |||||
| −2.99 | 1 | 0.34 | 1 | 0.28 | |
| 3.00-3.99 | 0.76 (0.53-1.10) | 0.86 (0.59-1.23) | |||
| 4.00-4.99 | 1.00 (0.71-1.39) | 1.20 (0.85-1.68) | |||
| 5.00- | 0.97 (0.59-1.60) | 1.16 (0.70-1.91) | |||
| Unknown | 1.03 (0.76-1.41) | 0.96 (0.68-1.37) | |||
In the two models fitted to subsets of the data to include hepatitis C and smoking covariates respectively, the associations between CD4 count and death rate were consistent with that in the main model.
In a sensitivity analysis where follow-up was censored at 6 months after each CD4 count instead of at 1 year, the association between CD4 count and the risk of death was similar. Compared to 350-499 cells/mm3, the adjusted rate ratio for CD4 count 500-699 cells/mm3 was 0.71 (95% CI: 0.55-0.92), and for CD4 count 700-cells/mm3 was 0.67 (95% CI: 0.50-0.89). When the analysis was restricted to CD4 counts above 500 cells/mm3, there was no significant difference in the risk of death between the CD4 count categories (adjusted risk ratio for CD4 count 700-cells/mm3 compared to 500-699 cells/mm3: 0.84, 95% CI: 0.65-1.10, p=0.22).
Discussion
In this large collaborative analysis, using data from industrialised countries, we found that death rates in ART-naïve patients with CD4 count above 350 cells/mm3 tend to be raised compared to the general population. However, the extent of the increase in risk varied markedly with risk group, being substantial in IDU and heterosexuals but relatively small in homosexual men. This suggests that much of the raised risk in the former two risk groups is likely to be due to confounding by socio-economic and lifestyle factors (12;13), rather than due to effects of HIV infection itself. Consistent with this, an increased risk of death has been observed in the siblings of people with HIV compared with the siblings of a control population without HIV (14). We also observed that a higher CD4 count was associated with a lower risk of death, even in this high CD4 count range, a trend that has previously been observed in patients on ART (2;3). Taken together, it would seem that there is a risk of death due to HIV in antiretroviral-naïve people with high CD4 count, but this appears to be of modest magnitude. This is despite the very low risk of AIDS diseases at this CD4 count (15,16), and appears to be consistent with the hypothesis that in people with high CD4 count, HIV causes some appreciable increased mortality risk (2-4;17;18).
We observed a raised risk of death in men compared with women, consistent with the general population. When considering the association between CD4 count and risk of death, in multivariable models fitted to subsets of our data, we were able to adjust for two potential confounding factors (hepatitis C co-infection and smoking history): we found that the relationship between death rate and CD4 count remained. Interestingly, we did not observe an association between plasma viral load level and risk of death in the subset of patients with this information available. This appears inconsistent with data suggesting an association between viral suppression and risk of non-AIDS diseases (4), although here we are comparing people with various levels of unsuppressed viral load.
Although, to our knowledge, there are no substantive studies restricted to ART naïve people, several studies have compared the death rate in HIV-positive individuals, including those on ART, with the general population, matched for factors such as age and sex (19-22). Although all such studies have demonstrated an excess risk of death in people with HIV, it has been observed that there are some successfully treated subgroups of patients for whom the death rate has been found to approach that of the general population. A study combining data from two French cohorts found that the death rate in patients with a CD4 count above 500 cells/mm3 reached that of the general population by the sixth year after starting ART (19), while a study on a collaboration of HIV seroconverter cohorts observed that, in the most recent period of follow-up (where 73% of person-time was on ART), there was no excess mortality during the first five years after seroconversion in patients infected sexually when compared to the general population (22).
Patients included in this analysis had been diagnosed earlier than most HIV-positive people in these settings. Of patients presenting with HIV from 1996 to 2006 at selected clinics in the UK, 39% had an initial CD4 count above 350 cells/mm3 (23). Patients who are diagnosed earlier may differ from patients diagnosed later with respect to their attitudes towards health and access to healthcare services.
Although several included cohorts are linked to national death registers, a further limitation is that possible under-ascertainment of deaths may have resulted in death rates being underestimated: this is supported by the slightly higher SMRs seen in the sensitivity analysis restricted to data from cohorts linked to national death registers. It may be the case that the decrease in risk of death over calendar time observed in the multivariable model (Table 3) is due, at least in part, to a delay in reporting of deaths.
Patients included in this study were under care at clinics linked to cohort studies and collaborations in Europe and North America. Results from this study may not be generalisable to all settings, either in clinics in these regions without research links, or in resource limited settings.
In conclusion, these data suggest that people with HIV who have not taken ART and have CD4 count above 350 cells/mm3 have a raised risk of death compared with the general uninfected population, although this increased risk seems to be of modest magnitude. As ART may well reduce the risk of death in such patients, these findings support the need for ongoing studies (such as the START trial (24) and further exploration of existing observational databases) of the risks and benefits of initiation of ART at CD4 counts higher than 350 cells/mm3.
Supplementary Material
Acknowledgements
Study Group on Death Rates at High CD4 Count in Antiretroviral Naïve Patients: Analysis and Writing Committee: Rebecca K. Lodwick, Caroline A. Sabin (UK CHIC), Kholoud Porter (CASCADE), Bruno Ledergerber (Swiss HIV Cohort Study), Ard van Sighem (Athena), Alessandro Cozzi-Lepri (ICONA), Pavel Khaykin (Frankfurt HIV Cohort), Amanda Mocroft (EuroSIDA), Lisa Jacobson (MACS), Stephane De Wit (Brussels St Pierre), Niels Obel (Danish HIV Cohort Study), Antonella Castagna (IDD-HSR), Jan-Christian Wasmuth (Cologne-Bonn), John Gill (Southern Alberta Cohort), Marina B. Klein (Montreal Chest Immunodeficiency Cohort), Stephen Gange (WIHS), Melchor Riera (PISCIS), Cristina Mussini (Modena), Félix Gutiérrez (CoRIS and CoRIS-MD), Giota Touloumi (AMACS), Patrizia Carrieri (MANIF 2000), Jodie L. Guest (HAVACS), Norbert H. Brockmeyer (KOMPNET), Andrew N. Phillips.
All members of the Analysis and Writing Committee participated in discussions on the design of the study, the choice of statistical analyses and interpretation of the findings, and were involved in the preparation and review of the final manuscript for submission. In addition, Rebecca Lodwick and Andrew Phillips are responsible for performing all analyses; Rebecca Lodwick acts as guarantor for the analyses and has full access to the dataset.
This project was supported by European AIDS Treatment Network (NEAT) contract FP6/03757.
AMACS
Steering Committee: Antoniadou A., Gargalianos-Kakolyris P., Katsarou O., Kordossis T., Lazanas M., Panos G., Paparizos V., Paraskevis D., Petrikkos G., Sambatakou H., Skoutelis A., Touloumi G. (Chair). Coordinating Center: Department of Hygiene, Epidemiology and Medical Statistics, Athens University Medical School, Greece (Touloumi G., Paraskevis D., Pantazis N., Bakoyannis G, Gioukari V.)
ATHENA
The ATHENA database is supported by a grant from the Dutch Health Minister and was set up and is maintained by the Stichting HIV Monitoring. Director: F. de Wolf; data analysis: D.O. Bezemer, L.A.J. Gras, A.M. Kesselring, A.I. van Sighem, C. Smit, S. Zhang, S. Zaheri; site-coordinating physicians: J.M. Prins, G. Schreij, B. Bravenboer, M.E. van der Ende, R.H. Kauffmann, R.W. ten Kate, F.P. Kroon, W. Bronsveld, R. Vriesendorp, D. van Houte, C.H.H. ten Napel, K. Brinkman, A. van Eeden, J.W. Mulder, J.R. Juttmann, J. Veenstra, P.P. Koopmans, H.G. Sprenger, I.M. Hoepelman, S.A. Danner, C. Richter, A.A. Tanis.
Brussels St Pierre
The members of the Brussels Saint Pierre Cohort are N. Clumeck (Head of Unit), S. De Wit (Cohort Coordinator), M. Delforge (Biostatistician), C. Necsoi (Data Manager), R. Demeester, A-F. Gennotte, M. Gerard, M-P Guillaume, P. Hermans, K. Kabeya, D. Konopnicki, C. Martin, A. Libois, M-C Payen, P. Semaille, Y. Van Laethem.
CASCADE
Steering Committee: Julia Del Amo (Chair), Laurence Meyer (Vice Chair), Heiner C. Bucher, Geneviève Chêne, Deenan Pillay, Maria Prins, Magda Rosinska, Caroline Sabin, Giota Touloumi. Co-ordinating Centre: Kholoud Porter (Project Leader), Sara Lodi, Kate Coughlin, Sarah Walker, Abdel Babiker. Clinical Advisory Board: Heiner Bucher, Andrea de Luca, Martin Fisher, Roberto Muga.
Cologne-Bonn
Gerd Fätkenheuer, Jürgen Rockstroh, Jan-Christian Wasmuth. Data management: Janne Vehreschild and Caroline Hertenstein.
CoRIS and CoRIS-MD
Red de Investigación en SIDA-ISCIII-RETIC-RD06/0006. Steering commitee: Juan Berenguer, Julia del Amo, Federico García, Félix Gutiérrez, Pablo Labarga, Santiago Moreno y María Ángeles Muñoz. Field work, data management and statistical analyses: Ana M. Caro-Murillo, Paz Sobrino, Santiago Pérez-Cachafeiro, Inmaculada Jarrín, Belén Alejos. BioBank: María Ángeles Muñoz, Isabel García. Hospitals: Hospital Universitario de Canarias (Santa Cruz de Tenerife), Hospital Carlos III (Madrid), Hospital Doce de Octubre (Madrid), Hospital Donostia (San Sebastián), Hospital Universitario de Elche (Elche), Hospital Gregorio Marañón (Madrid), Hospital Universitari Joan XXIII (Tarragona), Hospital de la Princesa (Madrid), Hospital San Pedro (Logroño), Hospital de Navarra (Pamplona), Hospital Ramón y Cajal (Madrid), Hospital San Cecilio (Granada).
Danish HIV Cohort Study
Steering commitee and members of The Danish HIV Cohort Study are N. Obel, J Gerstoft, G. Kronborg, B. Røge, C.S.Larsen, G. Pedersen, A.L. Laursen, L. Nielsen, J. Jensen.
EuroSIDA
Primary support for EuroSIDA is provided by the European Commission BIOMED 1 (CT94-1637), BIOMED 2 (CT97-2713), the 5th Framework (QLK2-2000-00773) and the 6th Framework (LSHP-CT-2006-018632) programs. Current support also includes unrestricted grants by Gilead, Pfizer, and Merck and Co. The participation of centres from Switzerland was supported by a grant from the Swiss Federal Office for Education and Science.
Frankfurt HIV Cohort
Babacan E. (data management), Bickel M., Bodtländer A., Brodt H-R., Carlebach A., Gute P., Haberl A., Helm E., Khaykin P., Klauke S., Knecht G., Lennemann T., Locher L., Lutz T., Mösch M., Müller A., Nisius G., Staszewski S., Stephan C., Stürmer M. (Virology), von Hentig N., Wolf T.
HAVACS
The members of the HIV Atlanta VA Cohort Study (HAVACS) are: Guest JL, Rimland D, Moanna A, Moorfield M, Dorsey M, Desilva KE, Schlueter Wirtz S, Mindley R, Dozier R, Robinson Y, Brown P.
ICONA
Governing body: M Moroni (Chair), G Carosi, R Cauda, F Chiodo, A d’Arminio Monforte, G Di Perri, M Galli, R Iardino, G Ippolito, A Lazzarin, R Panebianco, G Pastore, CF Perno. Steering committee: A Ammassari, A Antinori, C Arici, C Balotta, P Bonfanti, MR Capobianchi, A Castagna, F Ceccherini-Silberstein, A Cozzi-Lepri, A d’Arminio Monforte, A De Luca, C Gervasoni, E Girardi, S Lo Caputo, R Murri, C Mussini, M Puoti, C Torti. Funding: The Icona Foundation Study is supported by unrestricted educational grants from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Pfizer and Janssen-Cilag.
IDD-HSR
Representative of the cohort: Antonella Castagna. Data Managers: Stefania Salpietro, Mario Marangione. Statistician: Laura Galli. Scientific commmittee: Adriano Lazzarin (representative of the institution) Nicola Gianotti, Francesca Cossarini, Vincenzo Spagnuolo.
KOMPNET
Steering Committee: Gabriele Arendt(Speaker of the Clinical Science Board), Norbert H. Brockmeyer (Chair KompNet), Stefan Esser (Representative of the Sites), Annette Haberl(Speaker of the Gender & Pediatric Studies Board), Hans Jäger (Representative of the Sites), Siegfried Schwarze (Representative of the Patients Council), Matthias Stoll (Speaker of the Social Sciences & Public Health Board), Hans Wolf (Speaker of the Basic & Translational Research Board), Klaus Jansen (Cohort Manager), Claudia Michalik (Data Manager), Adriane Skaletz-Rorowski (Scientific Coordinator) Children Cohort: Christoph Königs (Speaker); Pregnant Women Cohort: Andrea Gingelmaier (Speaker) The KompNet is supproted by the Federal Ministry of Education and Research and the Ruhr-Universität Bochum.
MACS
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at The Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick, Lisa P. Jacobson), Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair, Steven M. Wolinsky), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles R. Rinaldo). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute. UO1-AI-35042, 5-MO1-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041.
Website located at http://www.statepi.jhsph.edu/macs/macs.html
MANIF 2000
Cohort Management Team: C. Boirot, AD. Bouhnik, MP. Carrieri, JP. Cassuto, M. Chesney, J. Cohen, P. Dellamonica, P. Dujardin, H. Gallais, JA. Gastaut, P. Kurkdji, G. Lepeu, D. Mechali, JP. Moatti, J. Moreau, M. Nègre, Y. Obadia, I. Poizot-Martin, C. Pradier, M. Préau, D. Rey, P. Roux, C. Rouzioux, A. Sobel, B. Spire, F. Trémolières, V. Villes, E. Vincent, D. Vlahov.
Modena
Cohort representative: Cristina Mussini. Data management: Vanni Borghi.
Montreal Chest Immunodeficiency Cohort
The cohort was founded by Richard Lalonde and has received infrastructure funding from the Fonds de recherche en santé du Québec, Réseau SIDA/maladies infectieuses (FRSQ). Marina Klein is supported by a Chercheur-Boursier clinicien senior career award from the FRSQ.
PISCIS
Coordinators: J. Casabona (CEEISCAT-Autonomous Universitat Autònoma de Barcelona –UAB-) Jose M. Miró (Hospital Clínic-Idibaps, Universitat de Barcelona – UB-). Scientific committee: JM Gatell, M López-Dieguez-Puerta (Hospital Clínic-Idibaps, -UB-), C. Tural, B. Clotet (Hospital Universitari Germans Trias i Pujol, -UAB-), D Podzamczer E. Ferrer (Hospital de Bellvitge, Hospitalet), M. Riera, J. Murillas (Hospital Son Dureta, Mallorca), F. Segura, G. Navarro (Corporació Parc Taulí, Sabadell), L. Force (Hospital de Mataró), J. Vilaró (Hospital de Vic), A. Masabeu (Hospital de Palamós), I. García (Hospital General d’Hospitalet), M.Guadarrama (Hospital Alt Penedès, Vilafranca), Alvaro J. Betancourt, A. Romero, C. Agustí (CEEISCAT), for the PISCIS cohort.
Southern Alberta Cohort
Cohort representative: John Gill.
Swiss HIV Cohort Study
Battegay M, Bernasconi E, Böni J, Bucher HC, Bürgisser P, Calmy A, Cavassini M, Dubs R, Egger M, Elzi L, Fischer M, Flepp M, Fontana A, Francioli P, Furrer H, Fux CA, Gorgievski M, Günthard HF, Hirsch HH, Hirschel B, Hösli I, Kahlert C, Kaiser L, Karrer U, Kind C, Klimkait T, Ledergerber B, Martinetti G, Martinez B, Müller N, Nadal D, Paccaud F, Pantaleo G, Rauch A, Regenass S, Rickenbach M, Rudin C, Schmid P,Schultze D, Schöni F, Schüpbach J, Speck R, Taffé P, Telenti A, Trkola A, Vernazza P, Weber R, Yerly S.
UK CHIC
Steering Committee: Jonathan Ainsworth, Jane Anderson, Abdel Babiker, Valerie Delpech, David Dunn, Philippa Easterbrook, Martin Fisher, Brian Gazzard, Richard Gilson, Mark Gompels, Teresa Hill, Margaret Johnson, Clifford Leen, Mark Nelson, Chloe Orkin, Adrian Palfreeman, Andrew Phillips, Deenan Pillay, Kholoud Porter, Frank Post, Caroline Sabin, Achim Schwenk, John Walsh. Central Co-ordination: UCL Medical School, London (Caroline Sabin, Loveleen Bansi, Teresa Hill, Andrew Phillips, Susie Huntington); Medical Research Council Clinical Trials Unit (MRC CTU), London (David Dunn, Adam Glabay, Kholoud Porter). UK CHIC is funded by the Medical Research Council, UK.
WIHS
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) located at: New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Analysis Center, Johns Hopkins University (Stephen Gange). The WIHS is funded by the National Institutes of Health.
Footnotes
Conflict of interest statement
No member of the Analysis and Writing Committee has any financial or personal relationships with people or organizations that could inappropriately influence this work, although most members of the group have, at some stage in the past, received funding from a variety of pharmaceutical companies for research, travel grants, speaking engagements or consultancy fees.
References
- (1).Phillips AN, Elford J, Sabin C, Bofill M, Janossy G, Lee CA. Immunodeficiency and the Risk of Death in Hiv-Infection. JAMA. 1992;268(19):2662–6. [PubMed] [Google Scholar]
- (2).Weber R. Liver-related deaths in persons infected with the human immunodeficiency virus - The D : A : D study. Archives of Internal Medicine. 2006;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- (3).Smit C, Geskus R, Walker S, Sabin C, Coutinho R, Porter K, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20(5):741–9. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- (4).Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22(18):2409–18. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Clumeck N, Pozniak A, Raffi F. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of HIV-infected adults. HIV Medicine. 2008;9(2):65–71. doi: 10.1111/j.1468-1293.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- (6).Hammer SM, Eron JJ, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection - 2008 recommendations of the International AIDS Society USA panel. JAMA. 2008;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- (7).Sterne JAC, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of Early versus Deferred Antiretroviral Therapy for HIV on Survival. New England Journal of Medicine. 2009;360(18):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. Journal of Infectious Diseases. 2008;197(8):1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- (10).Kjaer J, Ledergerber B. HIV cohort collaborations: proposal for harmonization of data exchange. Antiviral Therapy. 2004;9(4):631–3. [PubMed] [Google Scholar]
- (11).Max Planck Institute for Demographic Research (Germany) Human Mortality Database. University of California; Berkeley (USA): Available at www.mortality.org or www.humanmortality.de. Data downloaded on 20 May 2009. [Google Scholar]
- (12).Lloyd-Smith E, Brodkin E, Wood E, Kerr T, Tyndall MW, Montaner JSG, et al. Impact of HAART and injection drug use on life expectancy of two HIV-positive cohorts in British Columbia. AIDS. 2006;20(3):445–50. doi: 10.1097/01.aids.0000206508.32030.92. [DOI] [PubMed] [Google Scholar]
- (13).Lampe FC, Smith CJ, Madge S, Kinloch-de Loes S, Tyrer M, Sabin CA, et al. Success of Clinical Care for Human Immunodeficiency Virus Infection According to Demographic Group Among Sexually Infected Patients in a Routine Clinic Population, 1999 to 2004. Arch Int Med. 2007;167(7):692–700. doi: 10.1001/archinte.167.7.692. [DOI] [PubMed] [Google Scholar]
- (14).Hansen ABE, Gerstoft J, Kronborg G, Pedersen C, Sorensen HT, Obel N. Mortality in siblings of patients coinfected with HIV and hepatitis C virus. Journal of Infectious Diseases. 2007;195(2):230–5. doi: 10.1086/510246. [DOI] [PubMed] [Google Scholar]
- (15).CASCADE Collaboration Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS. 2004;18(1):51–8. doi: 10.1097/00002030-200401020-00006. [DOI] [PubMed] [Google Scholar]
- (16).Phillips AN, Gazzard B, Gilson R, Easterbrook P, Johnson M, Walsh J, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21(13):1717–21. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- (17).Neaton JD, Grund B. Earlier initiation of antiretroviral therapy in treatmentnaive patients: implications of results of treatment interruption trials. Current Opinion in HIV and AIDS. 2008;3(2) doi: 10.1097/COH.0b013e3282f3808b. [DOI] [PubMed] [Google Scholar]
- (18).Marin B, Thiebaut R, Bucher H, Rondeau V, Costagliola D, Dorrucci M, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–53. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lewden C, Chene G, Morlat P, Raffi F, Dupon M, Dellamonica P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. JAIDS. 2007;46(1):72–7. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- (20).van Sighem A, Danner S, Ghani AC, Gras L, Anderson RM, de Wolf F. Mortality in patients with successful initial response to highly active antiretroviral therapy is still higher than in non-HIV-Infected individuals. JAIDS. 2005;40(2):212–8. doi: 10.1097/01.qai.0000165911.97085.d0. [DOI] [PubMed] [Google Scholar]
- (21).Jaggy C, von Overbeck J, Ledergerber B, Schwarz C, Egger M, Rickenbach M, et al. Mortality in the Swiss HIV Cohort Study (SHCS) and the Swiss general population. Lancet. 2003;362(9387):877–8. doi: 10.1016/S0140-6736(03)14307-3. [DOI] [PubMed] [Google Scholar]
- (22).Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300(1):51–9. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- (23).Sabin CA, Schwenk A, Johnson MA, Gazzard B, Fisher M, Walsh J, et al. Late diagnosis in the HAART era: proposed common definitions and associations with mortality. AIDS. 2010;24(5):723–727. doi: 10.1097/QAD.0b013e328333fa0f. [DOI] [PubMed] [Google Scholar]
- (24).INSIGHT [Accessed 4 June 2009];Strategic Timing of Antiretroviral Treatment (START) http://insight.ccbr.umn.edu/start.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.