Summary
Amyotrophic lateral sclerosis (ALS) is a fatal neurological disorder characterized by selective degeneration of motor neurons throughout the central nervous systems. Non-cell autonomous damage induced by glial cells is linked to the selective susceptibility of motor neurons in ALS but the mechanisms underlying this phenomenon are not known. We found the expression of non-phosphorylated and phosphorylated forms (tyrosine residue 905, 1016, and 1062) of c-Ret, a member of the glial cell line-derived neurotrophic factor (GDNF) receptor, are altered in motor neurons of the lumbar spinal cord in ALS transgenic (G93A) mice and ALS (G93A) cell line models. Phosphorylated forms of c-Ret were colocalized with neurofilament aggregates in motor neurons of ALS mice. Consistent with the in vivo data, levels of non-phosphorylated and phosphorylated c-Ret (Tyr 905, 1016, and 1062) were decreased by oxidative stress in motor neuronal cells (NSC-34). Non-phosphorylated and phosphorylated forms of c-Ret immunoreactivity were markedly elevated in active microglia of ALS mice. Our findings suggest that constitutive oxidative stress modulates c-Ret function, thereby reducing GDNF signaling in motor neurons. Furthermore, the induction of c-Ret expression in microglia may contribute to non-cell autonomous cell death of motor neurons by depriving available GDNF in ALS.
Keywords: Amyotrophic lateral sclerosis, c-Ret, glial cell line-derived neurotrophic factor, motor neuron, microglia, astrocyte
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by selective degeneration of motor neurons located in the spinal cord, brain stem, and motor cortex, resulting in progressive atrophy and paralysis of limb, bulbar, and respiratory muscles (Rosen, 1993; Cleveland and Rothstein, 2001; Rowland & Shneider, 2001; Weiss et al., 2004). Mutations of the free radical scavenging enzyme superoxide dismutase-1 (SOD1) are a cause of familial ALS (Rosen et al., 1993). Transgenic mice expressing human mutant superoxide dismutase (mtSOD1) develop age-dependent clinical and pathological features closely mirroring those found in human ALS and thus provide a comprehensive model to study pathogenic mechanisms that may underlie the human disease (Gurney et al., 1994; Festoff et al., 2003; Rothstein, 2003; Ryu et al., 2005; Ilzecka et al., 2003). In ALS, oxidative damage of spinal cord proteins occurs, and motor neurons are particularly vulnerable to oxidative stress, a phenomena attributed to low levels of antioxidant enzymes, a high content of easily oxidized substrates, and an inherently high flux of ROS generated during energy metabolism (Rowland & Shneider, 2001; Rosen, 1993; Weiss et al., 2004). Another prominent feature of ALS pathology is the generation and migration of new cells, specifically microglia, within and around damaged regions (Barbeito et al., 2004). Microglia, the macrophages of the CNS, has been long suspected as central components in neurodegenerative diseases where their role may include secretion of trophic or toxic molecules. The interplay between motor neurons and glial cells is important in the clinical progression of both familial and sporadic motor neuron diseases. The release of reactive oxygen and nitrogen species or cytokines from microglia leads to the damage of motor neurons (Agar and Durham, 2003). This accumulating evidence suggests the contribution of microglia to non-cell-autonomous motor neuron death in ALS animal models.
The c-Ret, a membrane-associated receptor protein tyrosine kinase, has been shown to be a component of the glial cell line-derived neurotrophic factor (GDNF) receptor complex (Jing et al., 1996; Zhou et al., 2009). The GDNF signaling is mediated through the tyrosine kinase properties of c-Ret. It is essential for signal transduction to form the complex of GDNF, GDNFR-α and c-Ret and the phosphorylation of several tyrosine residues of c-Ret is a crucial step in the intracellular signaling pathway (Ohiwa et al., 1997). Tyr-1062 has been identified as a major docking site of the C-terminal regulatory domain in seven of potential auto-phosphorylation site (Liu et al., 1996). It is thought that the phosphorylation of Tyr-1062 is important for cell survival, growth and differentiation along with downstream signaling kinase cascades through such as Akt and IKKs (DeVita et al., 2000; Encinas et al., 2008). Yamamoto et al. (2001) reported that c-Ret is expressed in the specific subsets of neurons, including motor neurons, paralleling the distribution of GDNF-responsive neurons (Trupp et al., 1996). GDNF is considered as a potential therapeutic candidate for ALS as well as for other neuromuscular disorders (Klein et al., 2005; Suzuki et al., 2008). In this context, GDNF could rescue motor neurons from the neurodegenerative process in ALS.
Given that c-Ret mRNA and protein are preserved in ALS (Mitsuma et al., 1999; Yamamoto et al., 2001), it is important to determine how the expression and phosphorylation of c-Ret are regulated in motor neurons versus non-neuronal cells in the spinal cord of ALS. Moreover, the modulation of phosphorylation status of c-Ret in response to oxidative stress remains to be elucidated. We found that the expression of non-phosphorylated and phosphorylated forms (tyrosine residue 905, 1016, and 1062) of c-Ret is differentially expressed in motor neurons as well as in non-neuronal cells in the lumbar spinal cord of ALS transgenic (G93A) mice. We further found that oxidative stress reduces the level of non-phosphorylated and phosphorylated c-Ret in cell line models of ALS.
MATERIALS AND METHODS
Animals
Male transgenic ALS mice of the G93A H1 high-expresser strain (The Jackson Laboratory, Bar Harbor, ME, USA) were bred with females with a similar background (B6/SJLF1). Offspring were genotyped using a polymerase chain reaction (PCR) assay on tail DNA (Gurney et al, 1994). To ensure homogeneity of the cohorts tested, we have developed a standardized method to select mice. Body weights were taken at 20 days, and mice were equally distributed according to weight within each experimental cohort. Mice under 8 g at 20 days were excluded from the experiments (Ryu et al., 2005). These experiments were carried out in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by both the Veterans Administration and Boston University Animal Care Committees.
Cell culture
Motor neuron-like cells (NSC-34) transfected with pCI-neo expression vector containing human wild-type, hSOD1wt (NSC-34/hSOD1wt cells) and mutant hSOD1G93A (NSC-34/hSOD1G93A cells) were previously established (Cashman et al, 1992; Durham et al., 1993; Gomes et al., 2007; Gomes et al., 2008). Cell lines were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum (FBS), 100 units penicillin/ml and 0.1 mg streptomycin/ml. Cells were kept in a humidified incubator at 37°C under 5% CO2. Cells were subcultured in 60 mm dishes at a density of 1×106 cells per well. After 80% confluence, cells were treated with 100 µM H2O2 for 6 h. For immunostaining experiments, cells were seeded in 24-well plates containing 13 mm round cover slips at a density of 5×104 cells per well. For drug treatment, NSC-34/hSOD1wt cells and NSC-34/hSOD1G93A cells in 6-well plates were treated with GDNF (100 ng/ml) for 0, 10, 20, 30 and 60 min.
Small-interfering RNA experiment
NSC-34 cells were transiently transfected with 50 nM of Stealth control RNAi, c-Ret siRNA (GenePharma) using Lipofectamine RNAiMAX (Invitrogen Life Tech). After 48 hours, cells were treated with 100 µM H2O2 for 6 and 24 h. The sequences of siRNA are listed in the Supplementary Table 1.
Histopathological evaluation
Serially cut lumbar spinal cord tissue sections were immunostained for c-Ret (Santa Cruz Biotechnology; dilution 1:500) and phospho-Ret (Tyr1062) (Santa Cruz Biotechnology; dilution 1:500) using a previously reported conjugated secondary antibody method in spinal cord tissue samples (Ryu et al., 2005, Lee et al, 2009a, b, and c). Spinal cord was obtained at autopsy from patients with clinically and pathologically definite ALS and control patients who died of nonneurological diseases. Preabsorption with excess target proteins, omission of the primary antibodies, and omission of secondary antibodies were performed to determine the amount of background generated from the detection assay.
Immunofluorescence staining and confocal microscopy
Indirect labeling methods were used to identify c-Ret, phospho-Ret (Tyr905), phospho-Ret (Tyr1016), phospho-Ret (Tyr1062), GFAP, SMI32, choline acetyltransferase (ChAT), Iba-1, and APC, ubiquitin in NSC-34 cells and spinal cord sections from ALS mice as described previously (Ryu et al., 2005; Lee et al., 2009a, b, and c). For the confocal microscopy, the specimens were incubated for 1 h with DyLight 594 donkey anti-rabbit IgG antibody, DyLight 488 donkey anti-goat IgG antibody and DyLight 488 donkey anti-mouse IgG antibody (Jackson ImmunoResearch, Baltimore Pike, PA, USA; 1:400) after the incubation of primary antibody. Images were analyzed using an Olympus FluoView FV10i confocal microscope (Olympus, Tokyo, Japan). Preabsorbtion with excess target protein or omission of primary antibody was used to demonstrate antibody specificity and background generated from the detection assay.
Western blotting
Tissue lysates and subcellular fractions from wild-type and G93A mice were prepared using an ice-cold cell extraction buffer containing 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 2 mM EDTA; 1% Triton X-100; 1 mM PMSF; 10 mg/ml leupeptin; 1 mM pepstatin; 1 mM N-ethylmleimide; 2 mM Na3VO4; 20 mM sodium pyrophosphate; and 50 mM NaF) (Lee at al, 2009a, b, and c). Lysates were centrifuged at 15,000 rpm at 4°C for 30 min, and the supernatant fraction was kept. For extraction of detergent-insoluble protein, the pellet was suspended in ice-cold cell extraction buffer, sonicated three times for 10 sec and centrifuged twice at 3000 rpm at 4°C for 1 min to obtain Triton X-100-resistant pellets. The protein concentration was quantified, and the samples were boiled for 10 min with Laemmli buffer (100 mM Tris-HCl, pH 6.8; 4% SDS; 200 mM dithiothreitol; 20% glycerol; 2% SDS; 0.2% bromphenol blue; 10 mg/ml aprotinin; and 10 mg/ml leupeptin) at 100°C. In general, 30 mg of proteins was electrophoresed on 10% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. Membranes were blocked in 5% skim milk in TBST (Tris, pH 7.4; 150 mM NaCl; and 0.05% Tween 20) for 30 min at room temperature. Blots were probed with primary antibodies overnight at 4°C. The antibodies used were the following; c-Ret, phospho-Ret (Tyr 1062), Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:500), phospho-Ret (Tyr 905) (Cell Signaling Technology, Danvers, MA, USA; dilution 1:500), phospho-Ret (Tyr 1016) (Invitrogen, Camarillo, CA, USA; dilution 1:500), ubiquitin (Dako, Carpinteria, CA, USA; dilution 1:500), and β-actin (Sigma; dilution 1:2000). Horseradish perioxidase-conjugated secondary IgG anti-rabbit IgG (Bio-Rad Laboratories, Richmond, CA, USA) was used at 1:5000.
Immunoprecipitation analysis
Cell pellets were suspended in lysis buffer containing protease inhibitors, and lysates were centrifuged at 14,000 rpm for 10 min. Equal amounts of protein were precipitated with c-Ret antibody at 4°C for 6 hr on a rocker. Protein A/G agarose beads (Santa cruz) were added to each sample and incubated at 4°C for 1 hr. Immunoprecipitates were collected by centrifugation and then washed three times with the same buffer. The agarose beads were resuspended in 30 µl of 1 × SDS-PAGE sample buffer and incubated at 100°C for 10 min to release the proteins. After a pulse spin, the supernatants were loaded.
Quantitative RT-PCR analysis
Fifty nanograms of RNA were used as a template for quantitative RT-PCR amplification, using SYBR Green Real-time PCR Master Mix (TOYOBO, JAPAN). Primers were standardized in the linear range of cycle before onset of the plateau. The sequence of the primers was as follow: c-Ret forward: 5’-CAGGAGCCTGTCTATCTCAGA-3’; c-Ret reverse: 5’-CTGGCAGTTTTCCACACAGA-3’; GAPDH forward: 5’-TGTGTCCGTCGTGGATCTGA-3’; GAPDH reverse: 5’-CCTGCTTCACCACCTTCTTGA-3’. GAPDH was used as an internal control. Two-step PCR thermal cycling for DNA amplification and real-time data acquisition were performed with an ABI StepOnePlus™ Real-Time PCR System using the following cycle conditions: 95°C for 1min × 1 cycle, and 95°C for 15s followed by 60°C for 1min × 40 cycles. Fluorescence data were analyzed by the ABI StepOnePlus software and expressed as Ct, the number of cycles needed to generate a fluorescent signal above a predefined threshold. The ABI StepOnePlus software set baseline and threshold values.
Statistics
The data are expressed as means ± SEM. Statistical comparisons of data were performed using Student’s t test.
RESUTLS
The level of c-Ret is altered in the spinal cord of human and ALS (G93A) mice
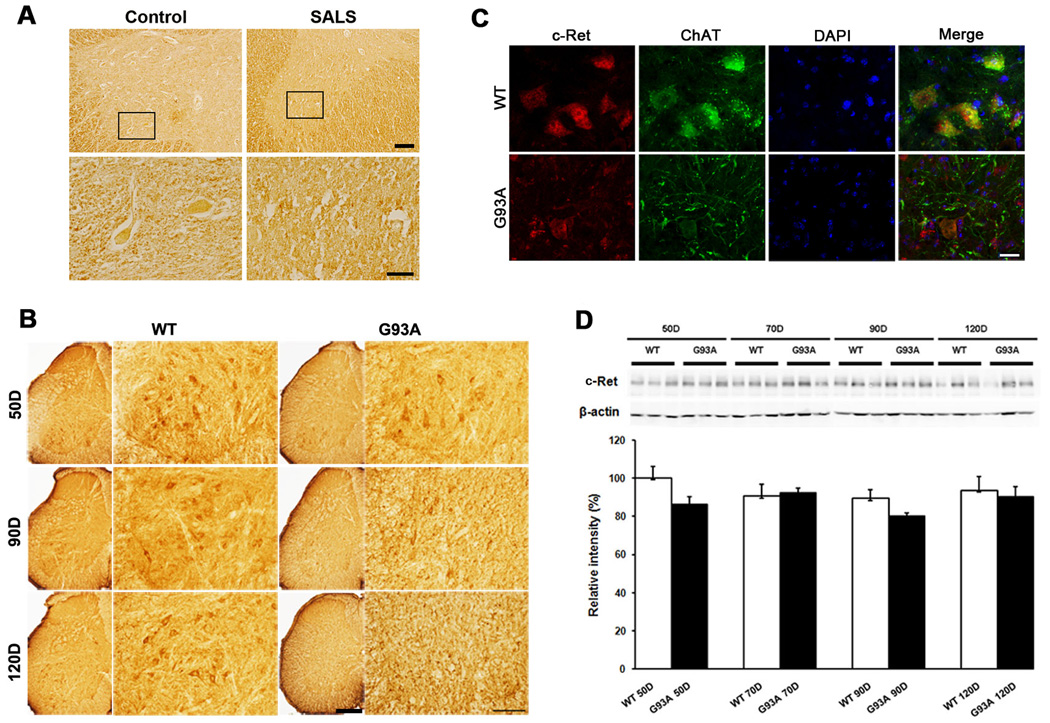
In the first series of experiment, we determined whether the level of c-Ret is changed between control and sporadic ALS (SALS) patient by immunohistochemistry. We found that the immunoreactivity of c-Ret is found in motor neurons of the ventral horn of spinal cord sections in control but not in sporadic ALS (SALS) patient (Figure 1A). A marked increase in the immunoreactivity of c-Ret was shown in non-neuronal cells (Figure 1A). Next, we analyzed the distribution of c-Ret in the ventral horn of lumbar spinal cord sections from wild-type (WT) littermate control mice and G93A mice when they reached at 50, 90, and 120 days of age (Figure 1B). Similarly to humans, c-Ret immunoreactivity was found mainly in cytoplasmic compartment of motor neurons in WT mice while its expression was elevated in non-neuronal cells in the spinal cord of G93A mice in an age-dependent manner. The increase of c-Ret immunoreactivity was detected in the atrophic motor neurons and other degenerating neurons in G93A mice from 90 days of age. A significant increase of c-Ret staining was seen in non-neuronal cells of G93A mice in comparison to control mice at 120 days of age (Figure 1C). Western blot analysis showed constant levels of c-Ret protein both in the spinal cords of WT mice and G93A mice at 50, 70, 90, and 120 days of age (Figure 1D). Immunohistochemistry data supported that the insignificant change of total c-Ret level in the lumbar spinal cord was due to c-Ret expression in non-neuronal cells despite the immunoreactivity of c-Ret was reduced in motor neurons (Figure 1B).
Figure 1.
The c-Ret is altered in ALS. A, c-Ret immunoreactivity was observed in motor neurons of control while its expression was observed in non-neuronal cells in the spinal cord of human SALS. Scale bars = top, 250 µm; bottom, 100 µm. B, c-Ret immunoreactivity in the ventral horn of lumbar spinal cord sections in WT and G93A ALS mice at 50, 90, and 120 days of age. c-Ret immunoreactivity was observed mainly in motor neurons of WT mice while its expression was elevated in non-neuronal cells in G93A mice in an age-dependent manner. Scale bars = left, 250 µm; right, 100 µm. C, Double immunofluorescence staining and confocal microscopy for c-Ret (red) and choline acetyltransferase (ChAT: a motor neuron marker) (green) in the spinal cord of WT and G93A mice at 120 days of age. c-Ret immunoreactivity was observed in non-neuronal (ChAT-negative) cells in the lumbar spinal cord of G93A mice. The nucleus was stained with DAPI. Scale bars (white): 20 mm. D, Western blot analysis of c-Ret in the spinal cords of WT and G93A mice at 50, 70, 90, and 120 days of age. c-Ret signals were normalized to β-actin signal. Band densities were analyzed using an image analyzer and are expressed as percentages of controls. Bars indicate means ± SD.
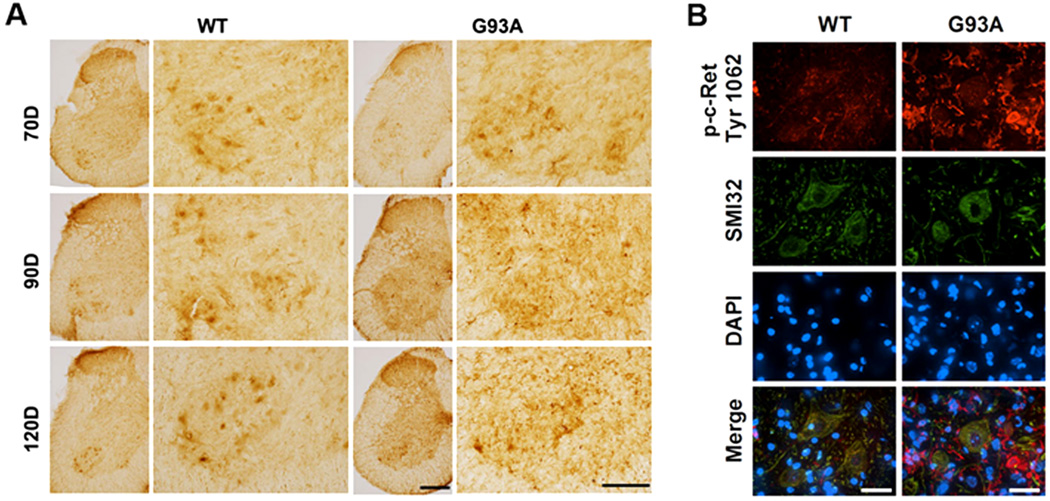
We further demonstrated an age-dependent increase of phosphorylated (p)-Ret (Tyr1062)-positive non-neuronal cells within the ventral horn of lumbar spinal cord sections from G93A mice as compared to WT mice (Figure 2). A significant increase in the immunoreactivity of p-Ret Tyr1062 in non-neuronal cells was observed at 90 and 120 days of age in comparison to control mice (Figure 2A). p-Ret (Tyr1062) immunoreactivity was observed in the nucleus of motor neurons in WT mice while p-Ret (Tyr 1062) was shown as punctate structures in the cytoplasm of motor neurons in G93A mice at 120 days of age. Confocal microscopy demonstrated that p-Ret (Tyr1062) and SMI-32, a motor neuron marker were not colocalized within the ventral horn of lumbar spinal cord G93A mice as compared with WT mice at 120 days of age (Figure 2B).
Figure 2.
Phosphorylated (p)-c-Ret is altered in ALS. A, The immunoreactivity of p-c-Ret (Tyr 1062) in the ventral horn of lumbar spinal cord sections in WT and G93A mice at 70, 90, and 120 days of age. p-c-Ret (Tyr 1062) immunoreactivity was observed in motor neurons of WT mice, while its expression was elevated in non-neuronal cells in G93A mice in an age-dependent manner. Scale bars (black); left, 250 µm; right, 100 µm. B, Double immunofluorescence staining and confocal microscopy for p-c-Ret (Tyr 1062) (red) and SMI32 (a motor neuron marker) (green) in the spinal cord of WT and G93A mice at 120 days of age. The immunoreactivity of p-c-Ret (Tyr 1062) was elevated in non-neuronal (SMI32-negative) cells in the lumbar spinal cord of G93A mice. The nucleus was stained with DAPI. Scale bars (white): 20 µm.
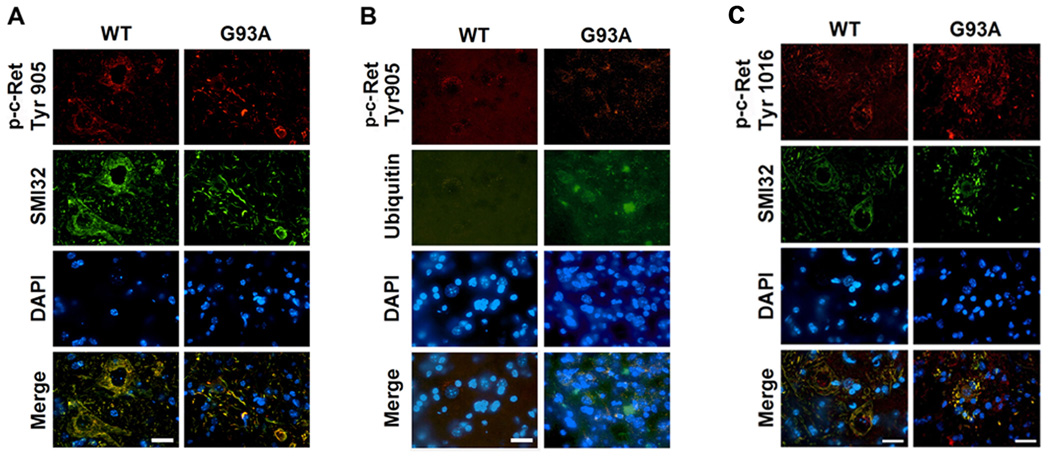
p-Ret (Tyr905) and SMI32 were colocalized in motor neurons both in WT and G93A mice (Figure 3A). Interestingly, p-Ret (Tyr905) immunoreactivity was observed in neurofilament aggregates in motor neurons of G93A mice while the punctate and diffuse pattern of p-Ret (Tyr905) immunoreactivity was mainly found in the cytoplasmic compartment in WT mice. In addition, p-Ret (Tyr905) was colocalized with ubiquitin in neurofilament aggregates within motor neurons of G93A mice (Figure 3B). p-Ret (Tyr1016) and SMI32 were also co-localized in motor neurons in both WT and G93A mice but p-Ret (Tyr1016) immunoreactivity was generally reduced and shown as punctate and granular patterns in the cytoplasm and nucleus of motor neurons in G93A mice (Figure 3C).
Figure 3.
Phosphorylated-c-Ret is found in punctate compartments of motor neurons. A, Double immunofluorescence staining and confocal microscopy for p-c-Ret (Tyr 905) and SMI32 in the spinal cord of WT and G93A ALS mice at 120 days of age. p-c-Ret (Tyr905) (red) and SMI32 (green) were colocalized in motor neurons in WT and G93A mice. The diffused staining of p-c-Ret (Tyr 905) immunoreactivity was found mainly in cell body and axonal arborization in WT mice. In contrast, the punctate structure of p-Ret (Tyr905) immunoreactivity was observed in the fibril tangles of G93A mice. B, Colocalization of p-Ret (Tyr905) and ubiquitin conjugates was elevated in the spinal cord of G93A mice in comparison to WT mice at 120 days of age. C, Double immunofluorescence staining and confocal microscopy for p-c-Ret (Tyr 1016) and SMI32 in the spinal cord of WT and G93A mice at 120 days of age. p-c-Ret (Tyr1062) and SMI32 were colocalized in motor neurons of WT and G93A mice. p-c-Ret (Tyr 1016) immunoreactivity (red) was observed in the cell body of SMI32-positive motor neurons (green) in WT mice. In contrast, p-c-Ret (Tyr 1016) immunoreactivity was found the cytosolic punctate compartment of motor neurons in the G93A mice. The nucleus was stained with DAPI. Scale bars (white): 20 µm.
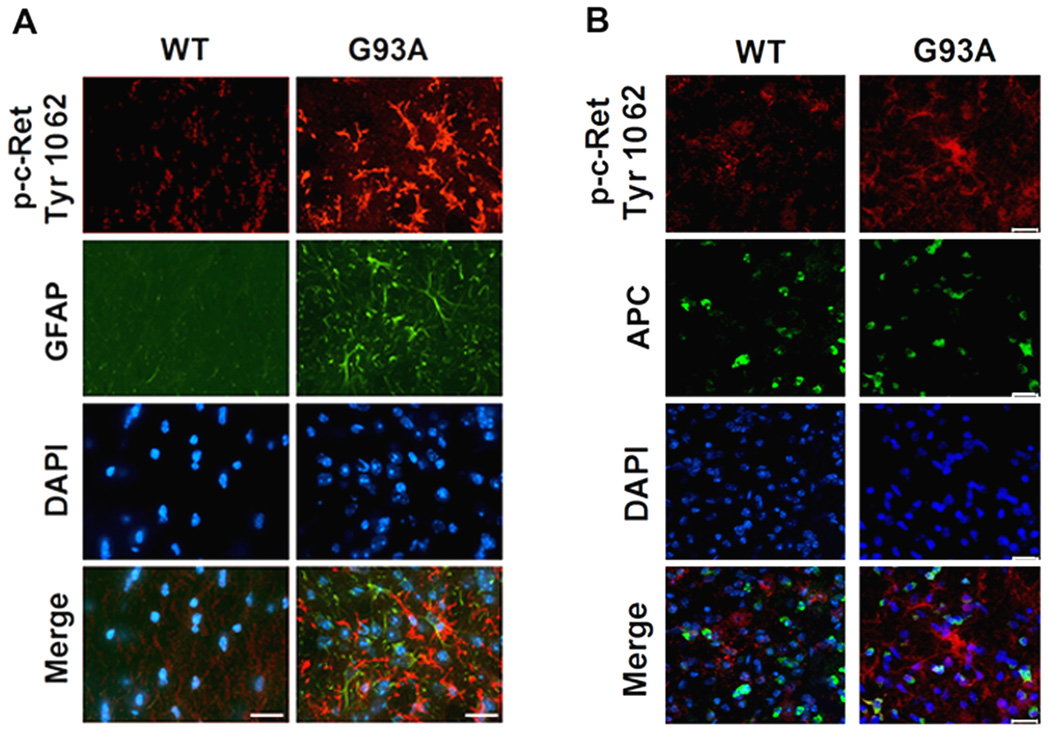
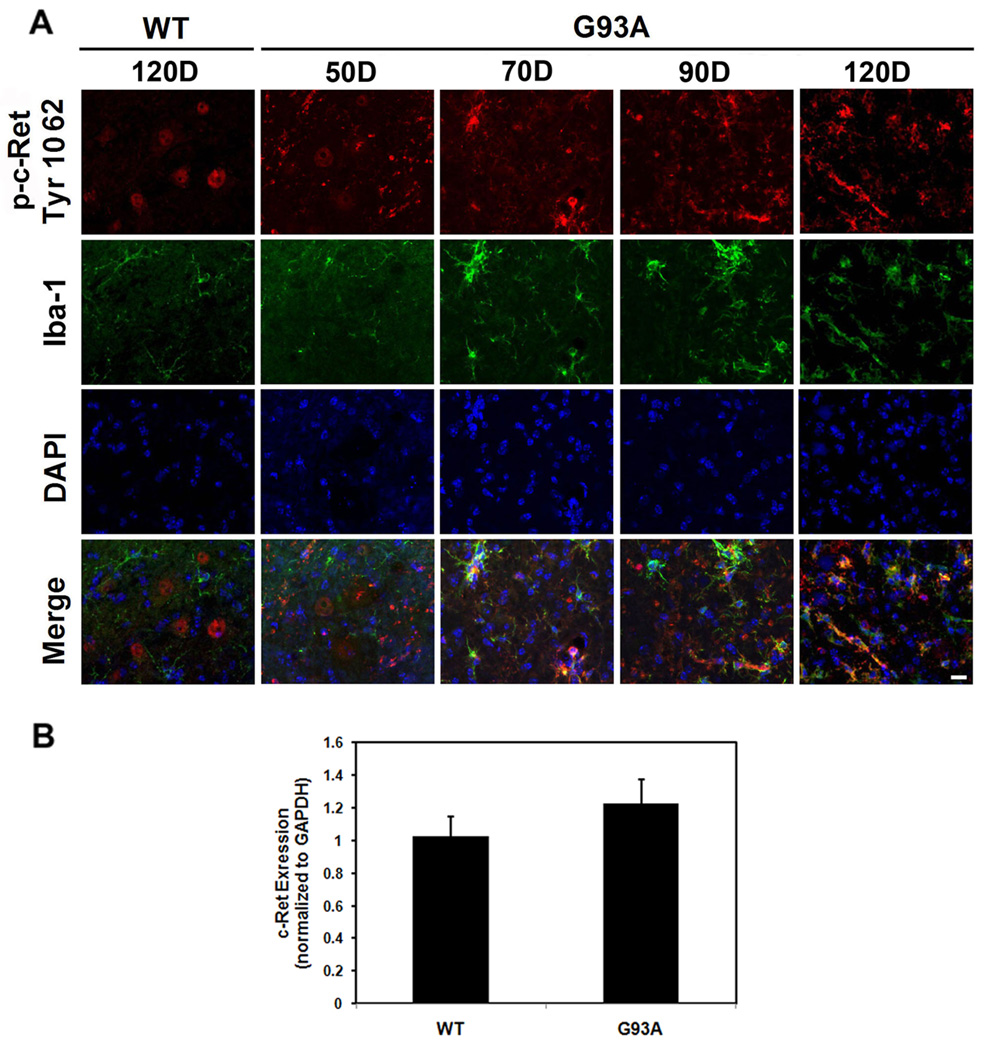
Since it appeared that p-Ret (Tyr1062) was also localized to non-neuronal cells, we performed double-immunoglobulin methods using p-Ret Tyr1062. p-Ret (Tyr1062) and GFAP, an astrocyte marker. We found that c-Ret and GFAP were not colocalized in either WT or G93A mice at 120 days of age (Figure 4A). In addition, p-Ret (Tyr1062) and APC, an oligodendrocyte marker, were not colocalized and, in fact, no overlap was observed in WT mice, (Figure 4B). However, p-Ret (Tyr1062) and APC were partially colocalized in some oligodendrocytes of G93A mice. Importantly, p-Ret (Tyr1062) was significantly colocalized in Iba1-positive activated microglial cells in the lumbar spinal cord of G93A mice at 120 days of age while p-Ret (Tyr1062) was not colocalized with Iba-1 positive cells in WT mice (Figure 5A). Notably, non-phosphorylated and phosphorylated forms of c-Ret immunoreactivities were markedly elevated in activated microglia of the lumbar spinal cord of ALS mice in an age-dependent manner. Otherwise, quantitative RT-PCR analysis showed G93A mice (n=6) express c-Ret mRNA at slightly higher levels than WT mice (n=6) (Figure 5B). The increase of c-Ret mRNA was not statistically significant. Double immunofluorescence staining data provided evidence that the increased c-Ret mRNA is derived from microglial cells despite the level of c-Ret was reduced in motor neurons (Figure 5A).
Figure 4.
p-c-Ret is increased in non-neuronal cells of ALS mice. A, Double immunofluorescence staining and confocal microscopy for p-c-Ret (Tyr1062) and GFAP (an active astrocyte marker) in WT and G93A mice at 120 days of age. p-c-Ret (Tyr1062) immunoreactivity (red) was not colocalized with GFAP (green) in the lumbar spinal cord of WT nor in G93A mice. B, Double immunofluorescence staining and confocal microscopy for the p-c-Ret (Tyr1062) and APC (an oligodendrocyte marker) in WT and G93A mice at 120 days of age. p-c-Ret (Tyr1062) immunoreactivity (red) was not found in APC-positive cells (oligodendrocytes) (green) in the spinal cord of WT mice but p-Ret Tyr1062 was colocalized partially in the oligodendrocytes (APC-positive) cells in G93A mice. Scale bars (white): 20 µm.
Figure 5.
P-c-Ret is highly expressed in microglial cells in ALS mice. A, Double immunofluorescence staining and confocal microscopy for the p-c-Ret (Tyr1062) and Iba-1 (a microglia marker) in WT and G93A mice at 50, 70, 90, and 120 days. p-c-Ret (Tyr1062) immunoreactivity (red) was found in Iba-1-positive (microglial) cells (green) in the spinal cord of G93A mice but not in WT mice. The immunoreactivity of p-c-Ret (Tyr1062) was elevated in microglial cells in G93A mice in an age-dependent manner. Scale bars (white): 20 µm. B, Quantitative RT-PCR analysis of c-Ret mRNA expression. Total RNA samples were extracted from spinal cord of WT littermate control and G93A ALS mice (n=6 each). G93A mice express c-Ret mRNA at slightly higher levels than WT mice. Error bars represent SEM.
The levels of c-Ret and p-c-Ret (Tyr 905, 1016, and 1062) are altered in the ALS (G93A) cell line model
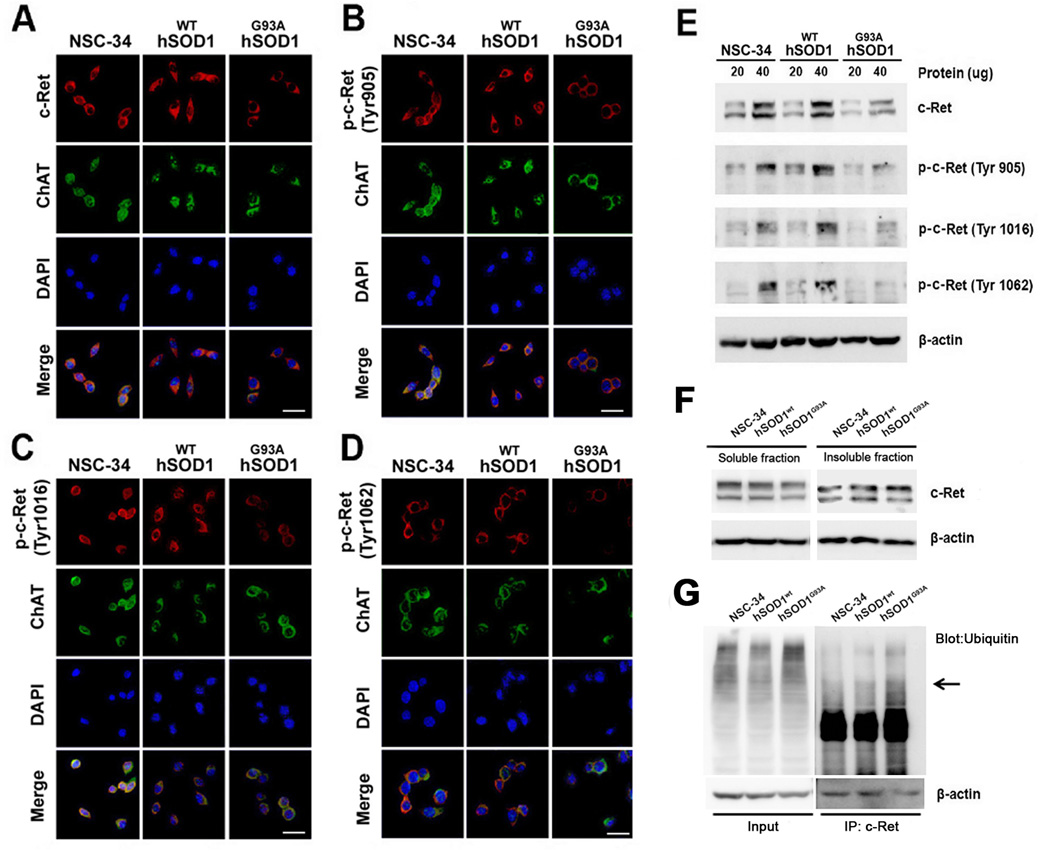
NSC-34 cells stably transfected with mutant WT hSOD1 and mutant hSOD1G93A have been used as a cellular model of ALS for studying numerous pathological characteristics of the disease (Cashman et al, 1992; Durham et al., 1993; Kirby et al., 2005; Sathasivam et al., 2005; Gomes et al., 2007; Gomes et al., 2008). We found that non-phosphorylated and phosphorylated forms of c-Ret (Try 905, 1016, and 1062) immunoreactivities were decreased in NSC-34/hSOD1G93A cells in comparison to Mock NSC-34 and WT-hSOD1 cells (Figure 6A–D). Concurrently with the immunofluorescence staining results, the Western blot showed a reduced protein level of non-phosphorylated and phosphorylated forms of c-Ret in NSC-34/hSOD1G93A cells in comparison to NSC34 and NSC-34/hSOD1wt in the soluble fraction, while an increased protein level of c-Ret is found in the insoluble fraction (Figure 6E, F). In addition, we examined whether in c-Ret is ubiquitinated in motor neurons under ALS condition. By means of immunoprecipitation of NSC-34, NSC-34/hSOD1wt and NSC-34/hSOD1G93A cells lysate with c-Ret antibody followed by a Western blot probing with ubiquitin antibody, we confirmed that an increase of c-Ret ubiquitination in NSC-34/hSOD1G93A cells in comparison to NSC-34 and NSC-34/hSOD1wt cells. This data indicates that oxidative stress caused by mutant SOD1 contributes to an increase of c-Ret ubiquitination and aggregation in the insoluble fraction of NSC-34/hSOD1G93A (Figure 6G).
Figure 6.
c-Ret and p-c-Ret (Tyr 905, 1016, and 1062) are altered in ChAT-positive NSC-34 cells. The constitutive expression of c-Ret (A) and p-c-Ret (Tyr 905) (B), p-c-Ret (Tyr 1016) (C), and p-c-Ret (Tyr 1062) protein (D) were found ChAT-positive NSC-34, NSC-34/hSOD1wt and NSC-34/hSOD1G93A cells. The immunoreactivity of c-Ret, p-c-Ret (Tyr 905), (Tyr 1016), and (Tyr 1062) were decreased in NSC-34/hSOD1G93A cells in comparison to NSC-34 and NSC-34/hSOD1wt cells. The nucleus was stained with DAPI. Scale bars (white): 20 µm. E, c-Ret and p-c-Ret were reduced in NSC-34/hSOD1G93A in comparison to NSC-34 and NSC-34/hSOD1wt cells. F, The level of c-Ret was reduced in the soluble fraction of NSC-34/hSOD1G93A in comparison to NSC34 and NSC-34/hSOD1wt cells but increased in the insoluble fraction. G, Immunoprecipitation assay of NSC-34, NSC-34/hSOD1wt and NSC-34/hSOD1G93A cells lysate with c-Ret antibody followed by a Western blot probing with ubiquitin. An increase level of ubiquitinated c-Ret was found in NSC-34/hSOD1G93A cells in comparison to NSC-34 and NSC-34/hSOD1wt cells.
Oxidative stress modulates the level of c-Ret and p-c-Ret (Tyr 905, 1016, and 1062) and GDNF-dependent c-Ret signaling is altered in the ALS (G93A) cell line model
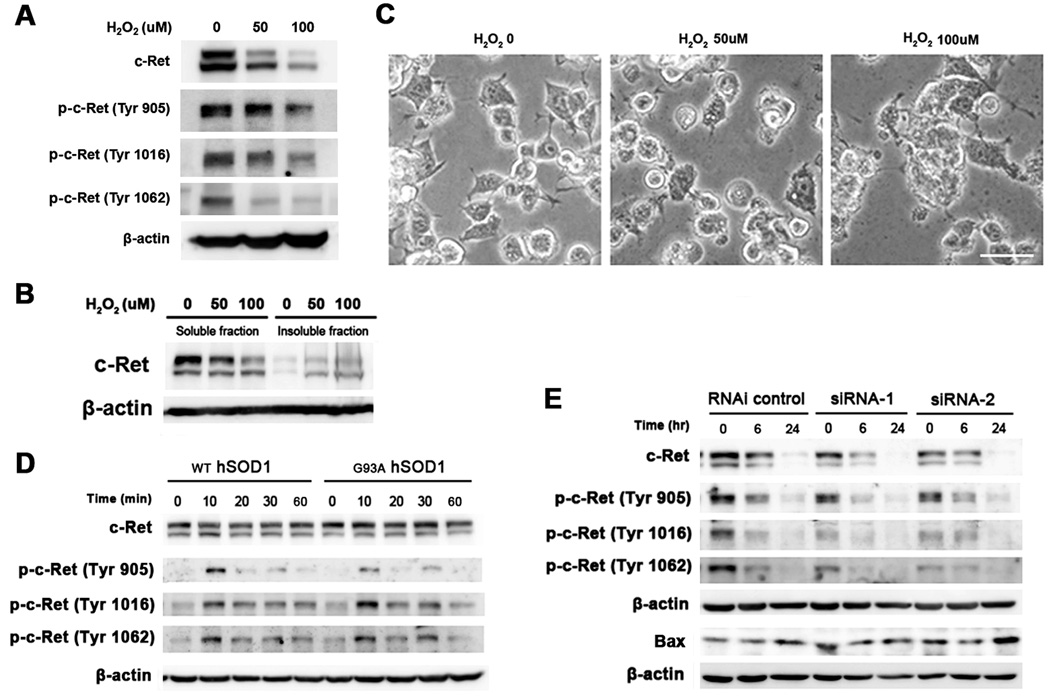
To examine whether c-Ret expression is induced in motor neuron-like cells (NSC-34) in response to oxidative stress, we exposed cells to hydrogen peroxide (H2O2). As we expected, the level of non-phosphorylated and phosphorylated c-Ret (Tyr 905, 1016, and 1062) were decreased by oxidative stress in NSC-34 cells (Figure 7A). H2O2 reduced the protein level of c-Ret dose dependently in the soluble fraction, while it increased protein levels of c-Ret in the insoluble fraction (Figure 7B). Exposure of NSC-34 cells to 50 and 100 µM of H2O2 for 6 hours resulted in altered cell morphology, i.e., either extensive membrane blebbing or shrunken morphology with distinct nuclear condensation (Figure 7C). At this time point, expression of non-phosphorylated and phosphorylated c-Ret (Tyr 905, 1016, and 1062) protein was markedly decreased by oxidative stress in a dose dependent manner. In addition, NSC-34 cells exposed to 50 and 100 µM of H2O2 demonstrated cell death after 12 hours. Our results indicate that oxidative stress directly affects the protein level of c-Ret and the morphology of NSC-34 cells.
Figure 7.
Oxidative stress modulates the levels of non-phosphorylated and phosphorylated forms (Tyr 905, 1016, and 1062) of c-Ret in NSC-34 cells. A, Western blot analysis of c-Ret and p-c-Ret (Tyr 905, 1016, and 1062) protein levels in NSC-34 cells treated with hydrogen peroxide (H2O2) (50 and 100 µM) for 6 hours. H2O2 reduced the protein level of c-Ret dose dependently. β-actin was blotted as a protein loading control. B, H2O2 reduced the protein level of c-Ret in the soluble fraction but increased protein levels of c-Ret in the insoluble fraction in a dose dependent manner. C, H2O2 induced morphological changes of NSC-34 cells. Scale bar (white): 20 µm. D, Western blot analysis of c-Ret and p-c-Ret (Tyr 905, 1016, and 1062) in NSC-34/hSOD1wt and NSC-34/hSOD1G93A cells treated with GDNF (100 ng/ml) for various time points (0, 10, 20, 30, 60 min). E, c-Ret siRNAs (siRNA-1 and siRNA-2) down regulated the levels of c-Ret and p-c-Ret (Tyr 905, 1016, and 1062) in NSC-34 cells. Oxidative stress (100 µM of H2O2) additively increased Bax expression under c-Ret knock-down condition.
In order to determine whether GDNF-dependent signaling is impaired in cell line model of ALS, we treated NSC-34/hSOD1wt and NSC-34/hSOD1G93A cells with GDNF (100 ng/ml) for various time points (0, 10, 20, 30, 60 min) (Figure 7D). Interestingly, the phosphorylation status of c-Ret at Tyr 1016 and 1062 in NSC-34/hSOD1G93A cells was not lasting longer in response to GDNF than in NSC-34/hSOD1wt cells. This data indicates that GDNF signaling via c-Ret phosphorylation is impaired in NSC-34/hSOD1G93A cells. The shorter phosphorylation period of c-Ret by GDNF may not be sufficient enough to trigger a sequential amplification of normal signal transduction via c-Ret in NSC-34/hSOD1G93A cells. To further examine whether the knockdown of c-Ret affects alterations of c-Ret phosphorylation-dependent signaling pathway and induction of Bax, a mitodchondrial pro-death protein, NSC-34 cells were transiently transfected with c-Ret siRNA and the outcome was determined by Western blotting. We confirmed that protein levels of non-phosphorylated and phosphorylated form of c-Ret are down regulated by c-Ret siRNA but not by control siRNA (Figure 7E). Interestingly, protein level of Bax was elevated in NSC-34 cells transfected with c-Ret siRNAs (Figure 7E). Moreover, loss-of-function of c-Ret by siRNA resulted an additive induction of cell death protein (Bax) in response to oxidative stress (Figure 7E).
Discussion
This study demonstrates that the expression of non-phosphorylated and phosphorylated forms (tyrosine residue 905, 1016, and 1062) of c-Ret is altered in motor neurons of the lumbar spinal cord in ALS transgenic (G93A) mice. The immunoreactivity of phosphorylated forms of c-Ret was colocalized with neurofilament aggregates in motor neurons of ALS mice but not in WT control mice. Consistent with the in vivo data, both the level of non-phosphorylated and phosphorylated c-Ret (Tyr 905, 1016, and 1062) were decreased by oxidative stress in the cell line model of ALS (NSC-34/hSOD1G93A).
c-Ret expression has been studied in spinal cord motor neurons of patients with ALS using in situ hybridization and immunohistochemistry (Duberley et al., 1998; Mitsuma et al., 1999). c-Ret is a major GDNF ligand-receptor family members that mediate a GDNF signaling pathway in motor neurons (Jing et al, 1996). In general, the C-Ret protein in motor neuron cell bodies is derived from newly synthesized component and retrogradely transported component from nerve terminals. Therefore, the dysregulation of c-Ret protein level in motor neurons of ALS could be due to an impairment of transcription or a abnormal retrograde transport of Ret/GDNF complex in ALS (Navelihan et al., 1997, Mitsuma et al., 1999). GDNF is proposed to act in different manners to c-Ret bearing motor neurons, particularly in a pathological state; target-derived mode from muscle, paracrine mode from glial cells, and autocrine mode within neurons (Yamamoto et al., 1996). In this context, the increased CSF levels of GDNF and increased expression of GDNF mRNA in muscle in ALS indicates that the abnormal increase of GDNF may trigger c-Ret pathways in motor neurons that may play a compensatory role for preventing motor neuron degeneration of ALS (Grundström et al., 2000). However, the disparity between the decreased level of c-Ret and the increased level of GDNF could subsequently result in a different mechanism being involved in neurodegeneration process in motor neurons in later stage of ALS. The details of such a multicomponent signaling pathway remain to be investigated. P-c-Ret Tyr 1062 immunoreactivity has been observed in the nuclei of motor neurons in ALS (Yamamoto et al., 2001). We identified p-c-Ret Tyr 1062 immunoreactivity in motor neurons nuclei in WT mice and found that expression was elevated in microglial cells in the G93A spinal cord in an age-dependent manner. It has been previously shown that c-Ret and p-Ret Tyr 1062 are expressed in microglia in human brain tissue with Parkinson’s disease (Walker et al., 1998) suggesting that c-Ret expression in active microglial cells might be an important marker of neurodegeneration.
Multiple lines of evidence suggest that non-neuronal cell types modulate the pathogenesis of motor neuronal death in mutant SOD1-mediated neurodegeneration. It is interesting that mutant SOD1 expression in motor neurons influences the onset of disease, but does not influence its progression (Beers et al., 2006; Boillee et al., 2006; Wang et al., 2009. Mutant SOD1 toxicity is non-cell autonomous (Ilieva et al., 2009), that is, non-neuronal cells contribute greatly to the pathogenesis of motor neuron damage (Clement et al., 2003; Lino et al., 2002; Monk and Shaw, 2006; Pramatarova et al., 2001). The role of inflammation and the activation of microglia in ALS are thought to play a significant role (Monk and Shaw, 2006).
GDNF enhances the function of motor neurons and rescues them from neurodegeneration in the early stages of ALS. The phosphorylation status of c-Ret and its tyrosine kinase activity may be important to this process. Oxidative stress may decrease c-Ret levels and its phosphorylation leading to c-Ret aggregation and the disruption of GDNF signaling in motor neurons. Oxidative stress-induced expression of c-Ret in microglia may also exacerbate neuronal damage by diverting GDNF from motor neurons or by triggering toxicity through inflammatory pathways as the disease progresses. The regulation of c-Ret expression and its role in microglia remains to be fully determined but our data suggest that microglial c-Ret expression may be a biomarker of the non-cell autonomous motor neuronal death in ALS (Ilieva et al., 2009). Furthermore, our findings suggest that effects on both neurons and microglia must be considered in the context of therapeutic modulation via c-Ret signaling pathway (Ryu et al., 2007, Lee et al., 2009c).
Supplementary Material
Acknowledgments
Les Turner ALS Foundation Grant (J.L.) and VA Merit Award (J.L. and N.W.K.) supported this study. The WCU Neurocytomics Program Grant (800-20080848) (H.R.) through SNU from KOSEF supported this study, in part.
REFERENCES
- Agar J, Durham H. Relevance of oxidative injury in the pathogenesis of motor neuron diseases. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:232–242. doi: 10.1080/14660820310011278. [DOI] [PubMed] [Google Scholar]
- Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma × spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: Deciphering selective motor neuron death in ALS. Nat Neurosci Rev. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A, Billaud M, Fusco A, Tsichlis PN, Santoro M. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 2006;60:3727–3731. [PubMed] [Google Scholar]
- Duberley RM, Johnson IP, Martin JE, Anand P. RET-like immunostaining of spinal motoneurons in amyotrophic lateral sclerosis. Brain Res. 1998;789:351–354. doi: 10.1016/s0006-8993(97)01570-9. [DOI] [PubMed] [Google Scholar]
- Durham HD, Dahrouge S, Cashman NR. Evaluation of the spinal cord neuron X neuroblastoma hybrid cell line NSC-34 as a model for neurotoxicity testing. Neurotoxicology. 1993;14:387–395. [PubMed] [Google Scholar]
- Encinas M, Rozen EJ, Dolcet X, Jain S, Comella JX, Milbrandt J, Johnson EM., Jr Analysis of Ret knockin mice reveals a critical role for IKKs, but not PI 3-K, in neurotrophic factor-induced survival of sympathetic neurons. Cell Death Differ. 2008;15:1510–1521. doi: 10.1038/cdd.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festoff BW, Suo Z, Citron BA. Prospects for the pharmacotherapy of amyotrophic lateral sclerosis: old strategies and new paradigms for the third millennium. CNS Drugs. 2003;17:699–717. doi: 10.2165/00023210-200317100-00002. [DOI] [PubMed] [Google Scholar]
- Gomes C, Keller S, Altevogt P, Costa J. Evidence for secretion of Cu, Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett. 2007;428:43–46. doi: 10.1016/j.neulet.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Gomes C, Palma AS, Almeida R, Regalla M, McCluskey LF, Trojanowski JQ, Costa J. Establishment of a cell model of ALS disease: Golgi apparatus disruption occurs independently from apoptosis. Biotechnol Lett. 2008;30:603–610. doi: 10.1007/s10529-007-9595-z. [DOI] [PubMed] [Google Scholar]
- Grundström E, Lindholm D, Johansson A, Blennow K, Askmark H. GDNF but not BDNF is increased in cerebrospinal fluid in amyotrophic lateral sclerosis. Neuroreport. 2000;11:1781–1783. doi: 10.1097/00001756-200006050-00037. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilzecka J, Stelmasiak Z, Solski J, Wawrzycki S, Szpetnar M. Plasma amino acids concentration in amyotrophic lateral sclerosis patients. Amino acids. 2003;25:69–73. doi: 10.1007/s00726-002-0352-2. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, SvendsenCN CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- Lee J, Kannagi M, Ferrante RJ, Kowall NW, Ryu H. Activation of Ets-2 by oxidative stress induces Bcl-xL expression and accounts for glial survival in amyotrophic lateral sclerosis. FASEB Journal. 2009a;23:1739–1749. doi: 10.1096/fj.08-121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ryu H, Kowall NW. Motor neuronal protection by L-arginine prolongs survival of mutant SOD1 (G93A) ALS mice. Biochem Biophys Res Commun. 2009b;384:524–529. doi: 10.1016/j.bbrc.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ryu H, Kowall NW. Differential regulation of neuronal and inducible nitric oxide synthase (NOS) in the spinal cord of mutant SOD1 (G93A) ALS mice. Biochem. Biophys. Res. Commun. 2009c;387:202–206. doi: 10.1016/j.bbrc.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Vega QC, Decker RA, Pandey A, Worby CA, Dixon JE. Oncogenic RET receptors display different autophosphorylation sites and substrate binding specificities. J Biol Chem. 1996;271:5309–5312. doi: 10.1074/jbc.271.10.5309. [DOI] [PubMed] [Google Scholar]
- Mitsuma N, Yamamoto M, Li M, Ito Y, Mitsuma T, Mutoh T, Takahashi M, Sobue G. Expression of GDNF receptor (RET and GDNFR-alpha) mRNAs in the spinal cord of patients with amyotrophic lateral sclerosis. Brain Res. 1999;820:77–85. doi: 10.1016/s0006-8993(98)01344-4. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptor Ret and GDNFR-α after sciatic nerve lesion in the mouse. Eu J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Of mice and men: reconciling preclinical ALS mouse studies and human clinical trials. Ann Neurol. 2003;53:423–426. doi: 10.1002/ana.10561. [DOI] [PubMed] [Google Scholar]
- Rowland L, Shneider N. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Ryu H, Ferrante RJ. Translational therapeutic strategies in amyotrophic lateral sclerosis. Mini Rev Med Chem. 2007;7:141–150. doi: 10.2174/138955707779802570. [DOI] [PubMed] [Google Scholar]
- Ryu H, Smith K, Camelo SI, Carreras I, Lee J, Iglesias AH, Dangond F, Cormier KA, Cudkowicz ME, Brown RH, Jr, Ferrante RJ. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J Neurochem. 2005;93:1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, Aebischer P, Svendsen CN. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16:2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Beach TG, Xu R, Lile J, Beck KD, McGeer EG, McGeer PL. Expression of the proto-oncogene Ret, a component of the GDNF receptor complex, persists in human substantia nigra neurons in Parkinson's disease. Brain Res. 1998;792:207–217. doi: 10.1016/s0006-8993(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Weiss MD, Weydt P, Carter GT. Current pharmacological management of amyotrophic lateral sclerosis and a role for rational polypharmacy. Expert Opin Pharmacother. 2004;5:735–746. doi: 10.1517/14656566.5.4.735. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Expression of glial cell line-derived growth factor mRNA in the spinal cord and muscle in amyotrophic lateral sclerosis. Neurosci Lett. 1996;204:117–120. doi: 10.1016/0304-3940(96)12342-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Li M, Mitsuma N, Ito S, Kato M, Takahashi M, Sobue G. Preserved phosphorylation of RET receptor protein in spinal motor neurons of patients with amyotrophic lateral sclerosis: an immunohistochemical study by a phosphorylation-specific antibody at tyrosine 1062. Brain Res. 2001;912:89–94. doi: 10.1016/s0006-8993(01)02542-2. [DOI] [PubMed] [Google Scholar]
- Zhou R, Niwa S, Homma N, Takei Y, Hirokawa N. KIF26A is an unconventional kinesin and regulates GDNF-Ret signaling in enteric neuronal development. Cell. 2009;139:802–813. doi: 10.1016/j.cell.2009.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.