Abstract
Exposure to stress during puberty can lead to long-term behavioral alterations. Female mice of the inbred C57BL/6 strain have been shown to display lower levels of sexual receptivity in adulthood when exposed to shipping stress or to an immune challenge during puberty. The present study investigated whether this effect can be extended to CD1 outbred mice and examined a possible mechanism through which exposure to stressors could suppress sexual receptivity. The results revealed that CD1 mice injected with lipopolysaccharide (LPS) or exposed to shipping stress at six weeks old display lower levels of sexual receptivity in response to estradiol and progesterone in adulthood than control mice. Moreover, mice exposed to shipping stress at eight weeks old also displayed reduced sexual receptivity, but those injected with LPS at that time showed slightly reduced effects, suggesting that the sensitive pubertal period extends to eight weeks of age in this strain of mice. The examination of estrogen receptor-α (ER-α) expression revealed that mice exposed to shipping stress during the sensitive period (six weeks) display lower levels of ER-α expression in the medial preoptic area and the ventromedial nucleus and the arcuate nucleus of the hypothalamus than mice shipped at a younger age. These findings support the prediction that exposure to shipping stress or LPS during puberty decreases behavioral responsiveness to estradiol and progesterone in adulthood in an outbred strain of mice through enduring suppression of ER-α expression in some brain areas involved in the regulation of female sexual behavior.
Keywords: Puberty, Stress, Female sexual behavior, Estrogen receptor-α, CD1 mice
Early life experiences influence adult behavior, including sexual behavior. This behavior is sexually differentiated by exposure to steroid hormones during the perinatal period (Baum, 1979; Morris et al., 2004), which is considered to be the most sensitive period for sexual differentiation in rats and mice (Barraclough and Gorski, 1962; MacLusky and Naftolin, 1981; Goy and McEwen, 1980). Puberty, the transition from a non-reproductive to a reproductive state, is also a period of significant brain reorganization (Levitt, 2003; Sisk and Foster, 2004), as the brain undergoes further remodeling and reorganization by sex steroid hormones (Schulz et al., 2009).
During the pubertal period, changes in hypothalamic-pituitary-adrenal (HPA) axis responsiveness have been reported. For example, basal levels of stress-related hormones differ between pubertal and adult mice (Goel and Bale, 2007; Laviola et al., 2002). Furthermore, female mice exposed to an immobilization stressor during puberty display a persistent shift in the peak, as well as loss of daily corticosterone secretion rhythm (Paris and Ramaley, 1974). Moreover, exposure to stressors, such as heat, immobilization, or ether, during the prepubertal period has long-term negative effects on the reproductive capacity of female mice (Paris and Ramaley, 1973).
Shipping from animal suppliers is a multifactor stressor, in that animals are simultaneously exposed to a number of stressors such as noise, temperature changes, predator odors, circadian variation, and food and water deprivation. Shipped mice express increases in corticosterone levels for up to 24 hours, and behavioral changes such as increases in feeding behavior and aggression that last several days (Tuli et al., 1995). C57BL/6 female mice shipped during puberty display reduced sexual receptivity in adulthood following treatment with estradiol and progesterone contrasted with mice shipped earlier or later in development (Laroche et al., 2009a). Exposure to other stressors that increase plasma corticosterone levels, such as restraint stress, food deprivation or a multiple stressor regimen during puberty, fails to reduce behavioral responsiveness to estradiol and progesterone in adulthood (Laroche et al., 2009b). However, exposure of C57BL/6 mice to an immune stressor, the bacterial endotoxin, lipopolysaccharide (LPS), during puberty, results in a similar reduction in sexual receptivity in adulthood in response to estradiol and progesterone (Laroche et al., 2009b). Together, these findings suggest that exposure to particular stressors during puberty reduces behavioral responsiveness to estradiol and progesterone in adulthood.
Sexual behavior in females of some rodent species, including mice (Ring, 1944), is induced by the sequential presence of estradiol and progesterone (Blaustein, 2010). Estradiol regulates sexual behavior by activating estrogen receptors (ERs) in the brain (Blaustein, 2010). Of the two main estrogen receptor subtypes, estrogen receptor-alpha (ER-α) and -beta (ER-β), ER-α subtype plays a greater role in female sexual behavior (Ogawa et al., 1998, Rissman et al., 1997). ER-α is widely distributed throughout the brain of female mice, with greater concentration in areas involved in reproduction (Shughrue et al., 1997). Site specific knockdown of ER-α in the ventromedial nucleus of the hypothalamus (VMN) abolishes female sexual behavior (Musatov et al., 2006), confirming the importance of ER-α in this region in regulation of female sexual behavior. Moreover, several other brain areas have also been shown to be responsive to mating stimulation. These regions include the medial preoptic area (MPOA), the VMN, the arcuate nucleus (Arc) (Cameron and Erskine, 2003) and anteroventral periventricular nucleus (AVPV) (Patchev, et al., 2004).
Because earlier work on pubertal stressors and adult behavioral responsiveness to ovarian hormone was carried out in inbred C57BL/6 mice (Laroche et al., 2009a; Laroche et al., 2009b), the first objective of this study was to examine whether this effect can be seen in the outbred CD1 strain. We hypothesized that CD1 mice exposed to either LPS or a shipping stressor during puberty would display reduced sexual receptivity in response to estradiol and progesterone in adulthood, similar to C57BL/6 mice. In a follow-up experiment, we also examined a possible mechanism mediating the altered behavioral responsiveness to steroid hormones by investigating the effect of exposure to a stressor during puberty on later ER-α expression in brain regions important for female sexual behavior. We hypothesized that mice stressed during puberty would have reduced expression of ER-α in areas of the brain involved in female sexual behavior.
General Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst. CD1 female mice were purchased from Charles River Laboratories (Kingston, NY), and housed in an all-female colony room under controlled temperature (24 ± 2°C) and reversed 14-h light: 10-h dark cycle (lights off at 10:00am). Mice were housed in groups of four in clear polycarbonate cages with ad libitum access to food (Teklad 2014, phytoestrogen-reduced diet, Harlan Laboratories, Madison, WI) and water in glass bottles. Cage bottoms were lined with a combination of wood shavings and CareFRESH (International Absorbents, Inc., Ferndale, WA) bedding, and a Nestlet (Ancare Corp., Baltimore, NY). Testing occurred in the dark phase of the cycle under dim red light.
Bacterial endotoxin, lipopolysaccharide (LPS)
At six or eight weeks old, mice were injected at the end of the light cycle with either sterile saline or 1.5mg LPS/kg body weight (obtained from Escherichia coli serotype O26:B6; no. L3755; Sigma Chemical Co., St. Louis, MO) dissolved at a concentration of 0.1mg/ml in sterile saline. Mice were returned to their home cage immediately after injection. Sickness behavior was scored as described below, and animals were weighed 24, 48 and 72 hours following treatment to assess the effect of the LPS on body weight. The dose of LPS was chosen based on previous work (Laroche et al., 2009b) and on pilot work in which it caused only mild sickness lasting less than 48 hours in CD1 mice under our housing conditions.
Sickness behavior
Sickness behavior was scored 30 min, 4 hours, 24 hours and 48 hours following treatment by two observers, who were blind to the treatment conditions. Animals were evaluated with respect to the number of symptoms displayed (one symptom = score of 1; two symptoms = score of 2, three symptoms = score of 3). The symptoms examined were lethargy (diminished locomotion), huddling (curled body posture), and ptosis (drooping eyelids). This rating procedure correlates with other methods of sickness scoring, such as scoring the severity of each symptom independently on a four-point scale (Gandhi et al., 2007).
Ovariectomy
Mice were anesthetized with isoflurane (3% at one l/min, inhaled). Incisions were made to the dorsal skin and the muscle layer to locate the uterine horns and ovaries. Once located, the uterine horns were tied with absorbable suture, and the ovaries were removed. The muscle layer was sutured, and the skin incision was closed with wound clips. Mice were placed on a heating pad to recover until they were fully awake. Then, they were returned to their home cage with an additional water bottle containing a 3% solution of Children’s Tylenol (160 mg of acetaminophen/ 5 ml dissolved in tap water) for 48 hours.
Female Sexual Behavior Testing
Female mice were primed with a subcutaneous injection of estradiol benzoate (EB; 2 µg/100 µl sesame oil) 52 to 54 hours prior to the sexual behavior testing. They were then treated with a subcutaneous injection of progesterone (P; 100 µg/100 µl sesame oil containing 5% benzyl benzoate and 15% benzyl alcohol) four to six hours prior to the sexual behavior testing. A sexually vigorous male was placed in a test chamber for five minutes to habituate to the environment. The test chamber was made of Plexiglas (39 × 30 × 19 cm) and had a slanted mirror underneath allowing visualization of the ventral side of the mice. At the end of the habituation period, a female was placed into the chamber until she received 20 mounts with or without intromissions. A mount occurred when the male placed both forepaws on the female’s hind region. To assess sexual receptivity, lordosis quotient (LQ) was computed (number of intromissive bouts (defined by successful penetrations)/total number of mounts × 100).
Tissue collection
Mice were deeply anaesthetized with isoflurane (5% at one l/min, inhaled). They were decapitated, and the brain was rapidly removed from the skull and immersion-fixed in 5% acrolein in tris-buffered saline (TBS; pH 7.2) for four hours. Brains were stored in TBS (pH 7.2) containing 30% sucrose at 4° C until they were cut into 30µ thick sections using a cryostat. Sections were stored in cryoprotectant (Gréco et al., 2003) at −20°C until immunostained.
Immunocytochemistry
Free-floating tissue sections were washed for 15 minutes in TBS. Then, the sections were blocked for 20 minutes in a solution containing 2% normal goat serum and 1% bovine serum albumin. Sections were then incubated in a solution containing a primary antiserum specific to ER-α (C1355; gift from M. Shupnik, University of Virginia, Charlottesville, VA, USA; 1:30000) for 72 hours at 4°C. After washing unbound antibodies with TBS, tissue sections were incubated for 90 minutes at room temperature in a secondary goat anti-rabbit antiserum (2µg/ml; Vector Laboratories, Burlingame, CA, USA) and then in the ABC detection system (1:100; Vector Laboratories, Burlingame, CA, USA). The sections were again washed with TBS to remove unbound antibodies. Then, the sections were incubated in freshly prepared diaminobenzidine (DAB) solution (DAB kit; Vector Laboratories, Burlingame, CA, USA) for one minute. DAB-stained sections were rinsed three times in TBS and mounted on microscope slides, dehydrated in four solution baths containing increasing concentrations of alcohol, cleared in xylene, and coverslipped.
Image Analysis
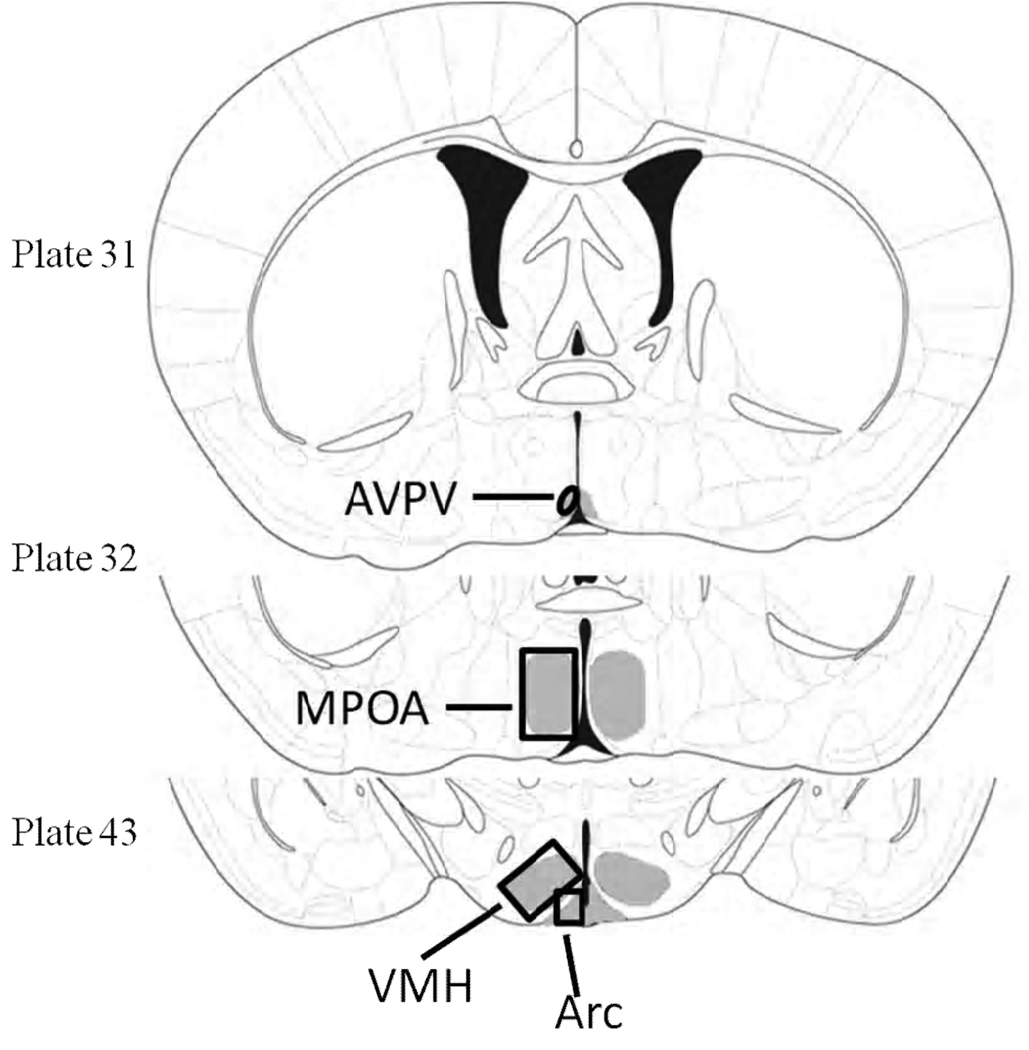
Immunoreactive cell counts were determined in optical density scaled images based on one best-matched section for each brain area for each mouse using a Nikon Optiphot-2 microscope connected to a QImaging Micropublisher RTV 5.0 digital color camera (Surrey, BC, Canada) and the Metamorph Image Analysis software (Molecular Devices, West Chester, PA, USA). The MPOA, VMN, PVN and Arc of the hypothalamus were analyzed (Figure 1). The identification of these regions was based on The Mouse Brain Atlas in Stereotaxic Coordinates (Franklin and Paxinos, 1997): AVPV (plate 31), MPOA (plate 32), VMN and Arc (plate 43).
Figure 1.
Plates from the Franklin and Paxinos (1997) mouse brain atlas depicting brain regions analyzed (shaded in gray) for expression of ER-α. Medial preoptic area (MPOA), ventromedial nucleus (VMN), paraventricular nucleus (PVN) and arcuate nucleus (Arc) of the hypothalamus. Oval and rectangular frames were drawn to set the boundaries of these areas.
Statistical Analysis
Three-way repeated measures analyses of variance (ANOVAs) were used to examine the effects of treatment (saline or LPS), age at treatment (six or eight weeks) and time of measurements on the percent change in body weight from baseline (on the day of the treatment) and LQ following saline or LPS treatment. Sickness behavior was analyzed using the non-parametric Kruskal Wallis test to examine overall differences followed by Mann-Whitney tests to assess pairwise comparisons when appropriate. A Bonferroni correction was used to control for Type I error (Experiment 1) (Field, 2009). Two-way repeated measures ANOVAs were also used to examine the effect of shipping age and test trials on LQ and post hoc tests (least significant difference, LSD) were used to assess pairwise contrasts when appropriate (Experiment 2). Multivariate analysis was used to examine the number of immunoreactive cells in optical density scaled images (Experiment 3). All analyses were run in SPSS Inc. statistical package 11.5 (Chicago, Illinois). Outliers were eliminated using the Boxplot method (Reiman, Filzmoser & Garrett, 2005).
EXPERIMENT 1: Pubertal LPS injection on adult sexual receptivity
In order to determine if CD1 mice are also susceptible to the effects of LPS during puberty on adult behavioral response to ovarian hormones, 33 CD1 female mice were shipped at three weeks old. Mice were divided into two groups; those injected with either saline (n = 9) or LPS (n = 8) at six weeks old, and those injected with saline (n = 8) or LPS (n = 8) at eight weeks old. The latter group was included as a negative control group, since Laroche and colleagues (2009b) showed that LPS treatment at 8 weeks of age was without effect in C57BL/6 mice. Four weeks later, mice were ovariectomized. Following one week of recovery, mice were tested for sexual receptivity in five weekly tests.
EXPERIMENT 2: Pubertal shipping on adult sexual receptivity
The objective of this experiment is to examine whether CD1 mice are susceptible to the effects of pubertal shipping as are C57BL/6 mice, and if so, to determine the age of vulnerability. 40 CD1 female mice were shipped at three, four, six, eight or ten weeks old. At 12 weeks old, they were ovariectomized. Following one week of recovery, weekly female sexual behavior testing began for five weekly tests.
EXPERIMENT 3: Pubertal shipping stressor on adult sexual receptivity and ER-α expression
This experiment was conducted in order to determine if exposure to shipping stress during puberty reduces ER-α expression in brain areas involved in female sexual behavior. Sixteen CD1 female mice shipped at four weeks (prior to the age at which mice are vulnerable to the effects of shipping as shown in Experiment 1) or at six weeks (during the vulnerable period) old, were housed in groups of four. The group shipped at 4 weeks of age is the negative control group, because in Experiment 2 shipping stress did not affect sexual receptivity of mice shipped at that age. Six weeks after their arrival, they were ovariectomized and tested for female sexual receptivity in two weekly tests to confirm the results of Experiment 2 in this cohort of mice. One week later, they were euthanized, and their brains were collected and analyzed for ER-α expression.
Results
EXPERIMENT 1: Effect of pubertal LPS injection on adult sexual receptivity
As expected, female mice treated with LPS at either age displayed more sickness symptoms than saline-treated mice (Figure 2a). Kruskal-Wallis test revealed significant overall differences at 30 minutes (p < 0.05), 4 hours (p < 0.001) and 24 hours (p < 0.001).
Figure 2.
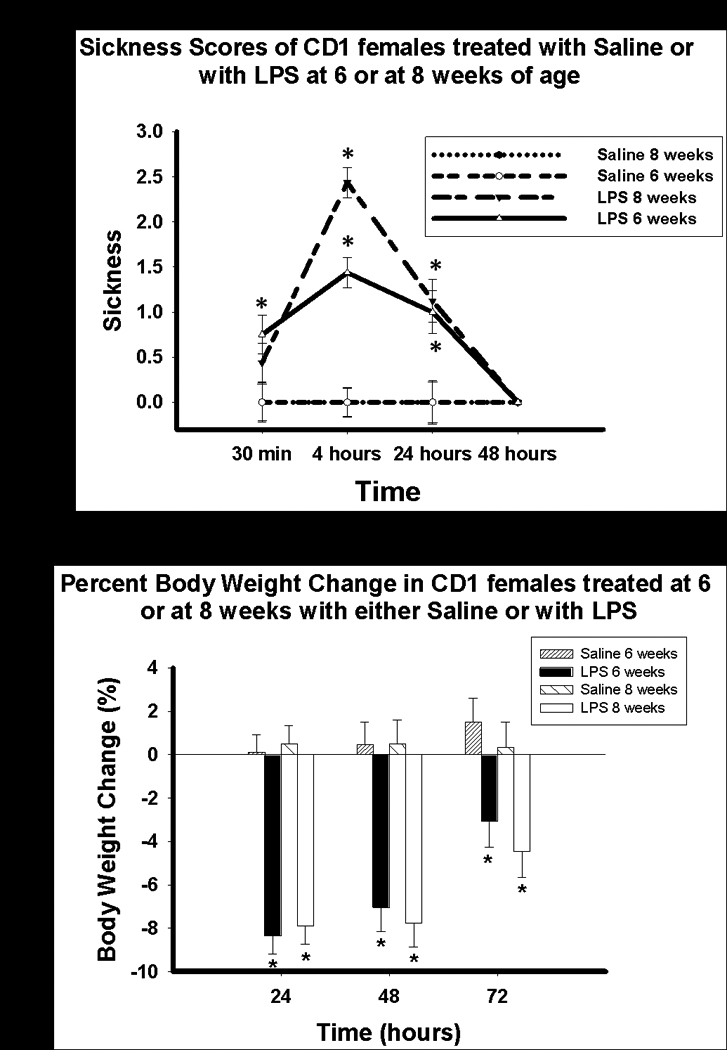
A) Sickness score (Mean ± SEM) and B) Percent body weight (Mean ±SEM) change in mice treated with either saline or LPS at six or eight weeks old. *p < 0.05
Mice treated with LPS at six or eight weeks old displayed significantly more sickness symptoms than saline-treated mice at four (p < 0.001, p < 0.001, respectively) and 24 hours (p = 0.01, p = 0.001, respectively) following treatment, but all sickness behaviors had ceased by 48 hours. No significant difference in sickness behavior was found between mice treated with LPS at six weeks and those treated with LPS at eight weeks of age 4 hours following treatment.
As expected, female mice treated with LPS also lost more body weight over the three days following treatment than saline-treated ones (Figure 2b). Statistical analyses revealed significant main effects of time (F(2, 58) = 23.49, p < 0.001), and treatment (F(1, 29) =54.57, p < 0.001). The analyses also showed a significant time × treatment interaction (F(2, 58) = 13.69, p < 0.001).
Pairwise comparisons revealed that mice treated with LPS at six weeks and eight weeks old displayed significant body weight loss at 24 (p < 0.001, p < 0.001, respectively), 48 (p < 0.001, p < 0.001, respectively) and 72 hours (p < 0.01, p < 0.01, respectively) following treatment contrasted with saline-treated ones. There was no difference in body weight loss between mice treated with LPS at six and eight weeks old.
Mice treated with LPS at six weeks old expressed lower levels of sexual receptivity than those treated with saline at six weeks old and those treated with saline or LPS at eight weeks old (Figure 3). Statistical analyses revealed significant main effects of test trial (F(4, 116) = 37.3, p < 0.001), age at treatment (F(1, 29) = 4.5, p < 0.05), and treatment (F(1, 29) = 12.19, p < 0.005). The analyses also showed significant test × treatment (F(4, 116) = 3.8, p < 0.01) and test × age × treatment interactions (F(4, 116) = 4.59, p < 0.005).
Figure 3.
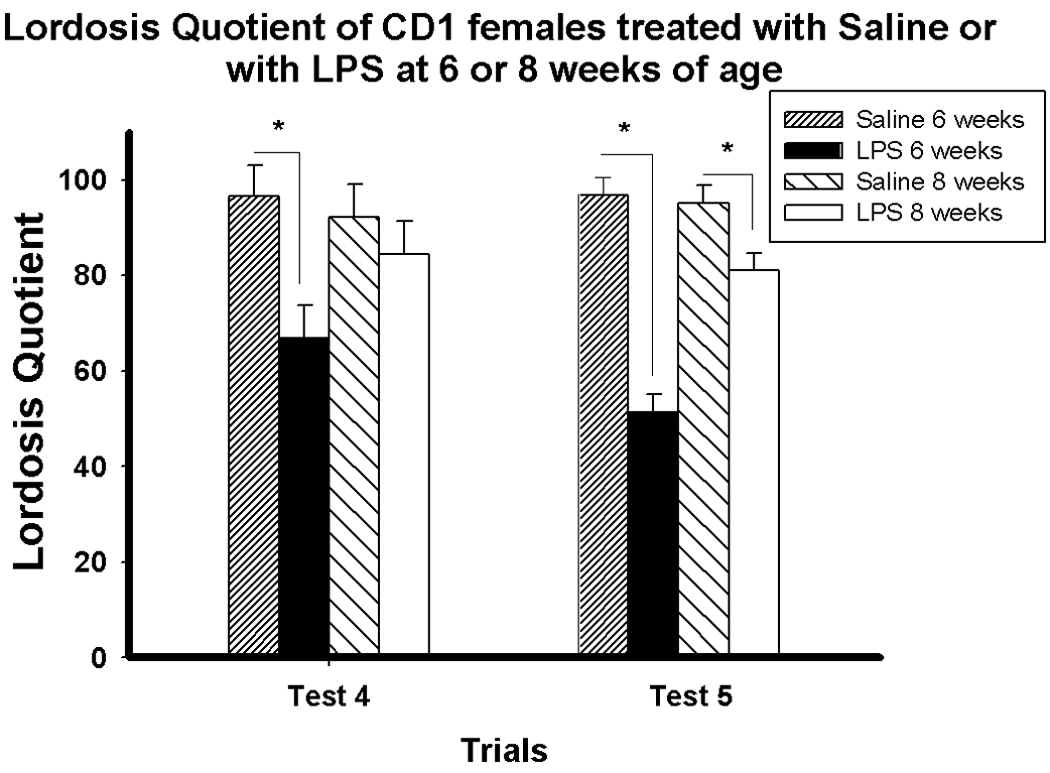
Lordosis quotient (Mean ± SEM) on weekly Test 4 and Test 5 of mice treated with either saline or LPS at six or eight weeks old. *p < 0.05
Pairwise comparisons at each trial revealed that mice treated with LPS at six weeks old displayed a lower LQ than saline-treated mice on Trials 2 – 5 (p < 0.05, p < 0.05, p < 0.005, p < 0.001, respectively). In contrast, mice treated with LPS at eight weeks old displayed a slightly lower LQ than saline-treated mice only on Trial 5 (p < 0.05).
EXPERIMENT 2: Effect of pubertal shipping on adult sexual receptivity
Mice shipped at six or eight weeks old displayed lower levels of sexual receptivity (LQ) than those shipped at three, four, and ten weeks old. Statistical analyses revealed significant main effects of test (F(4, 132) = 45.30, p < 0.001) and shipping age (F(1, 33) = 22.84, p < 0.001). There was no test × shipping age interaction (Figure 4).
Figure 4.
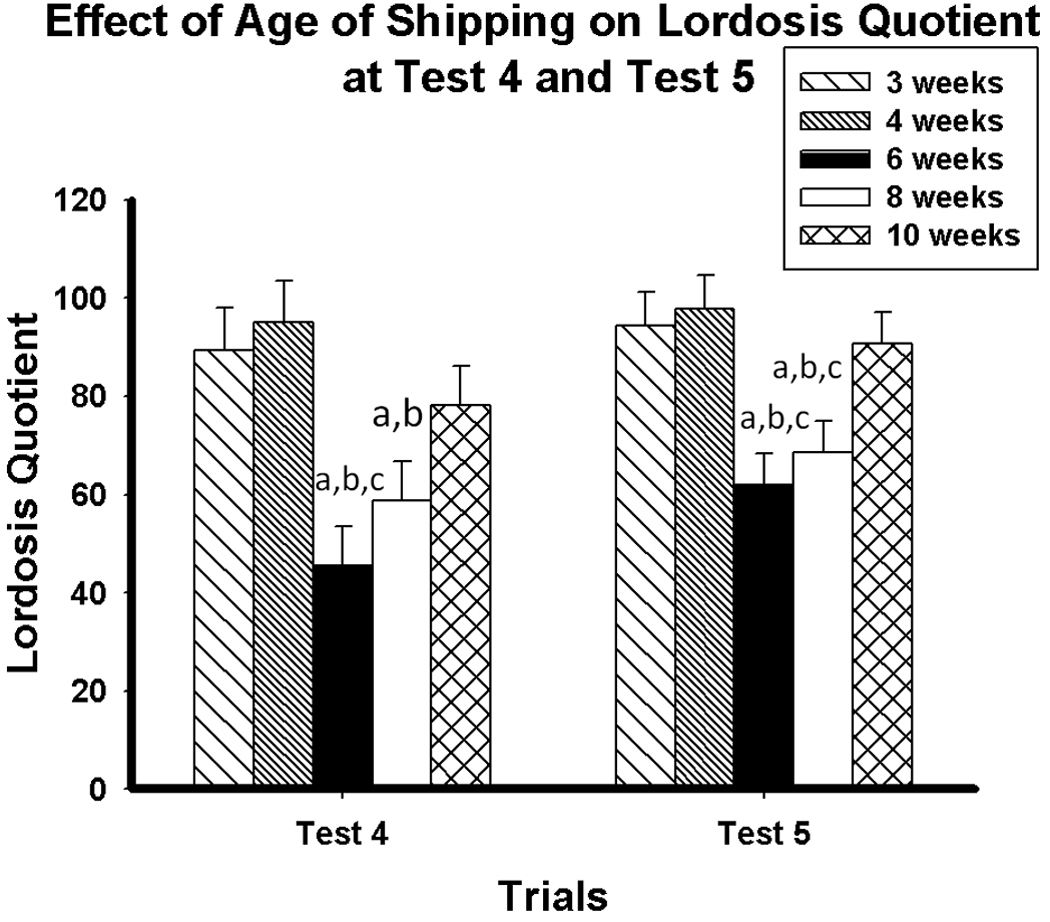
Lordosis quotient (Mean ± SEM) of mice shipped at 3, 4, 6, 8 and 10 weeks old on Test 4 and Test 5. a: lordosis quotient significantly lower than females shipped at three weeks old (p < 0.05). b: significantly lower than females shipped at four weeks old (p < 0.05). c: significantly lower than females shipped at 10 weeks old (p < 0.05).
Pairwise comparisons showed that mice shipped at six or eight weeks old expressed lower LQs than mice shipped at three weeks old on Trial 1 (p < 0.005, p < 0.05, respectively), Trial 2 (p < 0.001, p < 0.001, respectively), Trial 3 (p < 0.001, p < 0.005, respectively), Trial 4 (p < 0.001, p < 0.05, respectively) and trial 5 (p < 0.005, p < 0.01, respectively). Mice shipped at six or eight weeks old also expressed lower LQs than mice shipped at four weeks old on Trial 1 (p < 0.005, p < 0.05,respectively), Trial 2 (p < 0.001, p < 0.001, respectively), Trial 3 (p < 0.001, p < 0.005, respectively), Trial 4 (p < 0.001, p < 0.005, respectively) and Trial 5 (p < 0.005, p < 0.005, respectively). Mice shipped at six weeks old also displayed lower LQs than those shipped at ten weeks old on Trial 3, Trial 4 and Trial 5 (p < 0.001, p < 0.01, p < 0.005, respectively). Mice shipped at eight weeks old displayed lower LQs than those shipped at ten weeks old on Trial 3 and Trial 5(p < 0.005, p < 0.05, respectively).
EXPERIMENT 3: Effect of pubertal shipping stressor on adult sexual receptivity and ER-α expression
As in Experiment 1, females shipped at six weeks old expressed a significantly lower LQ than those shipped at four weeks old at Test 2 (p < 0.05) (Figure 5). Testing for sexual receptivity was not continued further, as hormone injections and testing would have interfered with the immunocytochemical analysis of ER-α expression.
Figure 5.
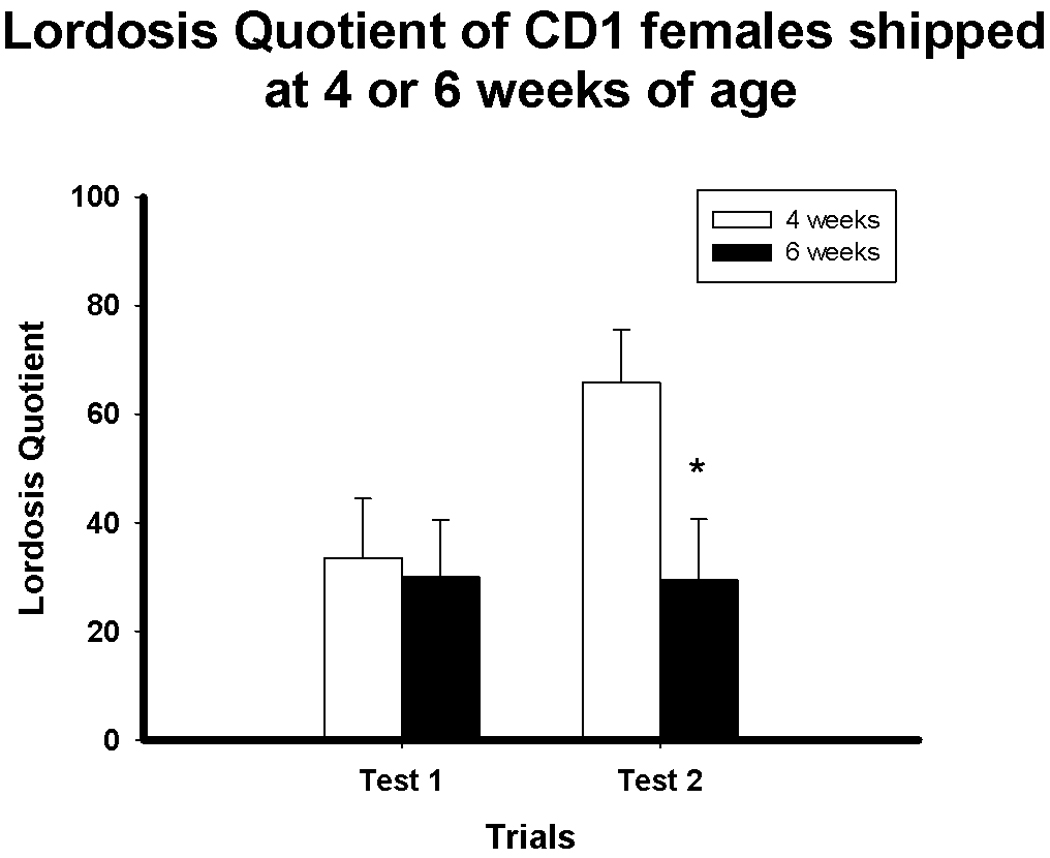
Lordosis quotient (Mean ± SEM) on Test 1 and Test 2 of mice shipped at four or six weeks old. *p < 0.05
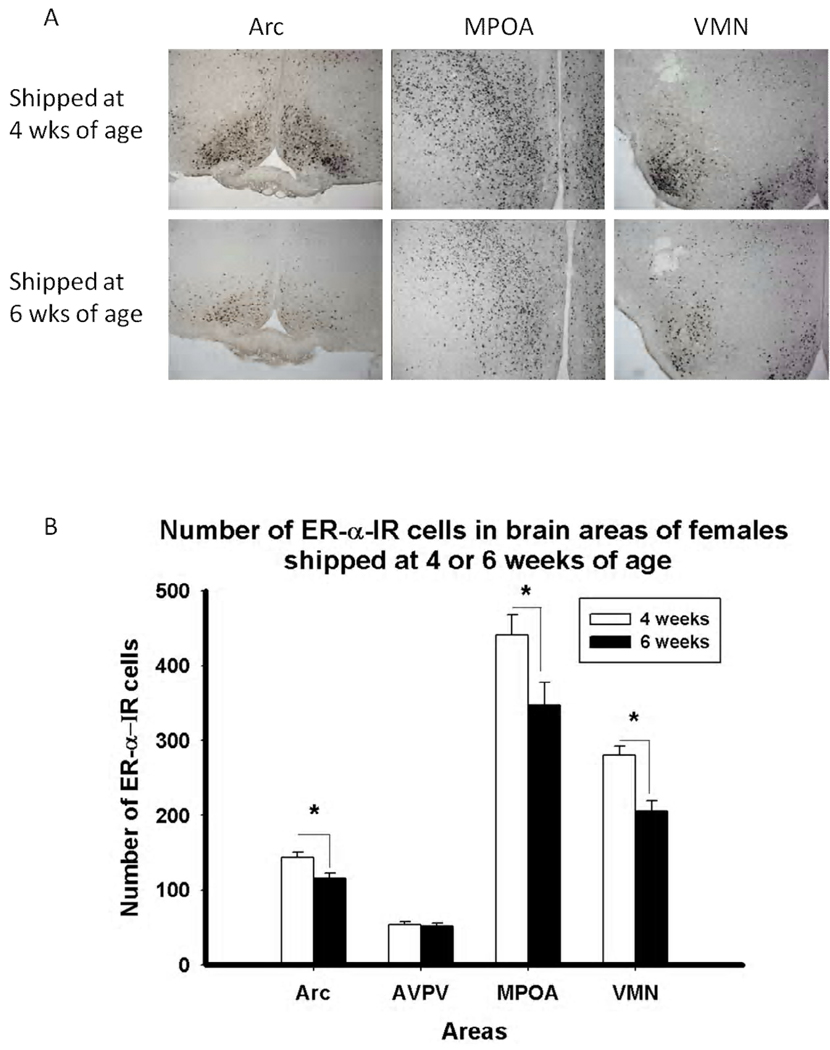
Consistent with the predictions, based on the decreased response of mice shipped during puberty, females shipped at six weeks old displayed a reduced number of ERα-ir cells contrasted with females shipped at four weeks old in the Arc (F(1, 11) = 8.95, p < 0.05), MPOA (F(1, 11) = 5.18, p < 0.05 and VMN (F(1, 11) = 17.91, p < 0.01). However, no difference in ER-α-ir was found in the AVPV (F(1, 12) = 0.143, p > 0.05) (Figure 6).
Figure 6.
A) Photomicrographs and B) Number of ER-α IR cells in females shipped at four or six weeks old in the arcuate nucleus (Arc), anteroventral paraventricular nucleus (AVPV), medial preoptic area (MPOA) and ventromedial nucleus of the hypothalamus (VMN).
Discussion
Exposure to shipping stress or an immune challenge during puberty decreases behavioral responsiveness to estradiol and progesterone in adults of the inbred C57Bl/6 strain of mice (Laroche et al., 2009a; Laroche et al., 2009b). We examined whether this finding could be extended to outbred CD1 mice, because strain differences have been reported in the behavioral responsiveness to steroid hormones. For example, ovariectomized female mice from the C57BL/6 inbred strain of mice are more responsive to exogenous estradiol and progesterone treatment than Swiss-Webster outbred mice (Thompson and Edwards, 1971). Moreover, while 5α-dihydroprogesterone is effective at increasing the frequency of lordosis in the CD1 strain, it is ineffective in the Swiss-Webster strain of mice (Gorzalka and Whalen, 1976). There are also strain differences in immune resistance. For instance, DBA/2, C57Bl/6, BALB/cBy and CBA inbred mice are more resistant to immune challenges and infections than the Swiss OF1 outbred mice (Duchet-Suchaux et al., 1990).
In the current study, we extended the earlier findings of a deleterious effects of some stressors on adult behavioral response to estradiol and progesterone in C57BL/6 mice (Laroche et al., 2009a; Laroche et al., 2009b) to the CD1 outbred strain of mice. This suggests that the effect of a pubertal stressor on adult behavioral response to estradiol and progesterone is robust, and it is not limited to a single strain of mice. Furthermore, the data suggest differences in the age of maximal vulnerability to the stressors.
It is interesting to note that the vulnerable period appears to be shifted slightly later in CD1 mice. Although not an indicator of puberty, defined as reproductive capability, vaginal opening of CD1 mice in our laboratory occurs at approximately 30 days old, while it occurs at approximately 25 days old in C57BL/6 mice (Ismail and Blaustein, unpublished observation). It is probable that the onset of puberty in CD1 mice is later than in C57BL/6 mice.
Laroche and colleagues (Laroche et al., 2009a; Laroche et al., 2009b) reported that in C57BL/6 mice, the effects of LPS and shipping on adult behavioral response to ovarian hormones were most robust at five to six weeks old with a lesser effect seen in mice exposed at four weeks old. C57BL/6 females shipped or injected with LPS later than six weeks old were insensitive to the long-term effects of the stressors on response to ovarian hormones. In contrast, in CD1 mice, the effects of shipping at puberty resulted in decreased sexual receptivity in adulthood in females shipped at six weeks old and also those shipped at eight weeks old, but not in those shipped at ten weeks old. In contrast, immune challenge at six weeks old resulted in a decrease in sexual receptivity in adult mice, but LPS injection at eight weeks old had only a minor effect, decreasing sexual receptivity only on the last trial. These findings suggest that the vulnerable pubertal period during which exposure to a stressor decreases behavioral responsiveness to steroid hormones in adulthood extends until at least eight weeks old in the CD1 strain, although the effects with LPS are not as robust in the older mice.
The present study also shows that the difference in lordosis quotient between mice treated with LPS at six and eight weeks old is not due to a differential response to the LPS. The sickness results revealed only a small and non-significant increase in sickness symptoms four hours following treatment in mice treated with LPS at eight weeks old compared to those treated at six weeks old. This difference was not maintained at other observed time points and both groups recovered by 48 hours following treatment. Furthermore, there was no difference in LPS-induced body weight loss between mice treated at six and eight weeks old. Therefore, it is unlikely that the difference in lordosis quotient between mice treated with LPS at six and eight weeks old is due to a differential response to the LPS.
The dose of LPS used in this study was based on previous work (Laroche et al., 2009b) that examined the effect of exposure to varying doses of LPS (0.1mg/kg to 1.5mg/kg) during puberty in C57BL/6 mice on sexual receptivity in response to estradiol and progesterone priming in adulthood. Exposure to doses lower than 0.5mg/kg during puberty did not significantly reduce sexual receptivity in adulthood in comparison to control mice. However, exposure to 0.5, 1.0 and 1.5mg/kg of LPS at that time decreased behavioral responsiveness (as indicated by sexual receptivity) to estradiol and progesterone in adulthood. Since the behavioral effects were most consistent following treatment with 1.5mg/kg of LPS during puberty (Laroche et al., 2009b), this dose was selected for the present study. Although this dose is considered to be a high dose, it is important to note that mice treated with this dose of LPS during puberty only display mild sickness and have fully recovered by 48 hours following treatment (Experiment 1).
Mice shipped during the pubertal period expressed lower levels of ER-α expression in the MPOA, VMN and Arc, but not in the AVPV, contrasted with those shipped at a younger age. It is important to note that this work used a standard cell counting procedure to examine ER-α immunoreactive cells rather than unbiased stereological assessments. Therefore, it is not clear whether the reduction in ER-α expression relates to potential volumetric changes in the brain areas analyzed. Nonetheless, these findings suggest a mechanism by which the pubertal stressors cause long-term neuroendocrine changes. During puberty, the brain undergoes further remodeling and reorganization by steroid hormones (Schulz et al., 2009; Schulz et al., 2004; Schulz and Sisk, 2006). For example, when male Syrian hamsters are castrated before puberty, testosterone treatment in adulthood fails to induce typical levels of male sexual behavior (Schulz et al., 2004). Moreover, female rats ovariectomized pubertally express increased testosterone-induced mounting behavior and female-oriented sexual preference compared to intact females, suggesting a masculinization of sexual behavior (de Jonge et al., 1988). In the present work, exposure to particular stressors during puberty causes long-term disruption of neuroendocrine mechanisms involved in the expression of female sexual behavior (e.g., ER-α expression).
Shipping and LPS may activate similar systems in mice. For example, both stressors activate the HPA axis and increase corticosterone release in mice (Tuli et al., 1995; Laroche et al., 2009b). However, it is clear now that the reduced behavioral responsiveness to estradiol and progesterone in adulthood caused by pubertal exposure to these stressors is not due to mere activation of the HPA axis; pubertal exposure to restraint stress, food deprivation, or combined restraint, light and heat also activate the HPA axis and increase corticosterone release but fail to decrease behavioral responsiveness to estradiol and progesterone in adulthood (Laroche et al., 2009b). Shipping and LPS also activate the immune system (Landi et al., 1982; Lu et al., 1999) and disrupt circadian rhythms (Syversen et al., 2008; Marpegan et al., 2005). Thus, it is possible that activation of the immune system or disruptions in circadian rhythms during puberty alter behavioral responsiveness to estradiol and progesterone in adulthood.
The present findings could also be due to long-term changes in HPA axis regulation in mice stressed during puberty, which in turn may decrease the expression of sexual behavior. Female mice shipped at six weeks old display slightly reduced levels of corticosterone 15–20 minutes following the start of the sexual behavior test contrasted with females shipped in adulthood (Laroche et al., 2009a). This suggests that exposure to a stressor during puberty has long-term effects on the response to stressors later in life, although it is not clear how this slight alteration in regulation of corticosterone secretion could result in decreased response to ovarian hormones, nor in the decrease in ER-α in some neural areas.
It is important to mention that in order to maintain a constant time interval across groups between the administration of the treatment and ovariectomy, the animals differed in age at the time of testing. However, because the animals were ovariectomized in adulthood, it is unlikely that the age at testing was responsible for the behavioral differences observed. In fact, in our earlier work, C57BL/6 mice were shipped at six weeks old and ovariectomized either one or seven weeks later. Regardless of the age at ovariectomy or testing, mice shipped at six weeks old displayed reduced behavioral responsiveness to estradiol and progesterone contrasted with mice shipped in adulthood (Laroche et al., 2009a). These findings suggest that age at ovariectomy or testing does not contribute to behavioral differences between groups.
There are several limitations to this study. All mice were treated with the same dose of estradiol and progesterone, so it is not known whether the pubertally stressed mice are less sensitive to estradiol and/or progesterone, or if their maximal response is lower. Also, since the sexual behavior tests were terminated after five weeks of testing, it is not known if the pubertally stressed mice would have responded eventually if the weekly testing had continued, a result that would still be consistent with a long-term altered response to estradiol and progesterone. Finally, these experiments do not shed light on how long into adulthood the decreased behavioral responsiveness to estradiol and progesterone lasts. However, in our previous work, we have seen enduring effects at least until 17 weeks old (Laroche et al., 2009b).
Taken together, these findings support the prediction that exposure to LPS or shipping stress during puberty decreases behavioral responsiveness to estradiol and progesterone in a second, in this case outbred, strain of mice. The enduring alteration in the expression of ER-α in some brain areas involved in the regulation of female sexual behavior also provides a possible mechanism by which these effects are mediated.
Supplementary Material
Lordosis quotient (Mean± SEM) of mice treated with either saline or LPS at six or eight weeks old during five weekly tests beginning one week after ovariectomy. *p < 0.05
Lordosis quotient (Mean± SEM) of adult mice shipped at three, four, six, eight or ten weeks of age during five weekly tests beginning one week after ovariectomy. * p < 0.05, significantly lower than females shipped at 3 or 4 weeks old. Along with mice shipped at 6 or 8 weeks of age, those shipped at 10 weeks of age also displayed significantly lower LQ than mice shipped at 3 or 4 weeks of age on trials 1 and 2. We have often seen variable results in the first few sexual behavior tests. This is why we typically focus on the more reliable results of the later sexual behavior tests to draw conclusions.
Acknowledgements
The authors would like to thank Ryan Rogan for technical assistance during behavioral testing and Dr. Kristin Olesen, Emily Merchasin and Hanna King for helpful discussions. This work was supported by NIH NS019327-20 and an Isis grant from the Study of Women’s Health Research to JDB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barraclough CA, Gorski RA. Studies on mating behavior in the androgen-sterilized female rat in relation to the hypothalamic regulation of sexual behavior. J. Endocrinol. 1962;25:175–182. doi: 10.1677/joe.0.0250175. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Differentiation of coital behavior in mammals: a comparative analysis. Neurosci. Biobehav. Rev. 1979;3:265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Hormones and female sexual behavior. In: Koob GF, Moal MLe, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Oxford: Academic Press; 2010. pp. 49–56. [Google Scholar]
- Cameron N, Erskine MS. c-Fos expression in the forebrain after mating in the female rat is altered by adrenalectomy. Neuroendocrinology. 2003;77:305–313. doi: 10.1159/000070283. [DOI] [PubMed] [Google Scholar]
- De Jonge FH, Muntjewerff J-W, Louwerse AL, van de Poll NE. Sexual behavior and sexual orientation of the female rat after hormonal treatment during various stages of development. Horm. Behav. 1988;22:100–115. doi: 10.1016/0018-506x(88)90034-7. [DOI] [PubMed] [Google Scholar]
- Duchet-Suchaux M, Le Maitre C, Bertin A. Differences in susceptibility of inbred and outbred infant mice to enterotoxigenic Escherichia coli of bovine, porcine and human origin. J. Med. Microbiol. 1990;31:185–190. doi: 10.1099/00222615-31-3-185. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering Statistics using SPSS. 3rd edition. London: Sage Publications; 2009. [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain. Behav. Immun. 2007;21:477–489. doi: 10.1016/j.bbi.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL. Identifying early behavioral and molecular markers of future stress sensitivity. Endocrinology. 2007;148:4585–4591. doi: 10.1210/en.2007-0479. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Whalen RE. Effects of genotype on differential behavioral responsiveness to progesterone and 5α-dihydroprogesterone in mice. Behav. Genet. 1976;1:7–15. doi: 10.1007/BF01065674. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sex differences in behavior: Rodents, birds, and primates. In: Goy RW, McEwen BS, editors. Sexual differentiation of the brain: Based on a work session of the Neurosciences Research Program. Cambridge: MIT press; 1980. [Google Scholar]
- Gréco B, Blasberg ME, Kosinski EC, Blaustein JD. Responses of ER-α-IR and ER-β-IR cells in the forebrain of female rats to mating stimuli. Horm. Behav. 2003;43:444–453. doi: 10.1016/s0018-506x(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Landi MS, Kreider JW, Lang CM, Bullock LP. Effects of shipping on the immune function in mice. Am. J. Vet. Res. 1982;43:1654–1657. [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Morley-Fletcher S, Terranova ML. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: Evidence of sex differences. Behav. Brain Res. 2002;130:117–125. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. J. Pediatr. 2003;143:s35–s45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Lu ZW, Hayley S, Ravindran AV, Merali Z, Anisman H. Influence of psychosocial, psychogenic and neurogenic stressors on several aspects of immune functioning in mice. Stress. 1999;3(1):55–70. doi: 10.3109/10253899909001112. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Marpegan L, Bekinschtein TA, Costas MA, Golombek DA. Circadian responses to endotoxin treatment in mice. J. Neuroimmunol. 2005;160:102–109. doi: 10.1016/j.jneuroim.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor-α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc. Natl. Acad. Sci. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Paris A, Ramaley JA. Effects on short-term stress upon fertility. I. Before puberty. Fertil Steril. 1973;24:540–545. doi: 10.1016/s0015-0282(16)39795-3. [DOI] [PubMed] [Google Scholar]
- Paris AL, Ramaley JA. Adrenal-gonadal relations and fertility: The effects of repeated stress upon the adrenal rhythm. Neuroendocrinology. 1974;15:126–136. doi: 10.1159/000122301. [DOI] [PubMed] [Google Scholar]
- Patchev AV, Götz F, Rohde W. Differential role of estrogen receptor isoforms in sex-specific brain organization. FASEB. 2004;18:1568–1570. doi: 10.1096/fj.04-1959fje. [DOI] [PubMed] [Google Scholar]
- Reimann C, Filzmoser P, Garrett RG. Background and threshold: critical comparison of methods of determination. Sci. Total Environ. 2005;346:1–16. doi: 10.1016/j.scitotenv.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Ring JR. The estrogen-progesterone induction of sexual receptivity in the spayed female mouse. Endocrinology. 1944;34:269–275. [Google Scholar]
- Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm. Behav. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol. Cell. Endocrinol. 2006:254–255. 120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and –β mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neurobiology of puberty and adolescence. Nat. Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Syversen E, Pineda FJ, Watson J. Temperature variations recorded during interinstitutional air shipments of laboratory mice. J. Am. Assoc. Lab. Anim. 2008;47:31–36. [PMC free article] [PubMed] [Google Scholar]
- Thompson ML, Edwards DA. Experiential and strain determinants of the estrogen-progesterone induction of sexual receptivity in spayed female mice. Horm. Behav. 1971;2:299–305. [Google Scholar]
- Tuli JS, Smith JA, Morton DB. Stress measurements in mice after transportation. Lab. Anim. 1995;29:132–138. doi: 10.1258/002367795780740249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lordosis quotient (Mean± SEM) of mice treated with either saline or LPS at six or eight weeks old during five weekly tests beginning one week after ovariectomy. *p < 0.05
Lordosis quotient (Mean± SEM) of adult mice shipped at three, four, six, eight or ten weeks of age during five weekly tests beginning one week after ovariectomy. * p < 0.05, significantly lower than females shipped at 3 or 4 weeks old. Along with mice shipped at 6 or 8 weeks of age, those shipped at 10 weeks of age also displayed significantly lower LQ than mice shipped at 3 or 4 weeks of age on trials 1 and 2. We have often seen variable results in the first few sexual behavior tests. This is why we typically focus on the more reliable results of the later sexual behavior tests to draw conclusions.