Abstract
Analogues of the tetrahydroisoquinoline family of antitumor antibiotics, 3-epi-jorumycin (3) and 3-epi-renieramycin G (4) in addition to their respective parent natural products (−)-jorumycin (1) and (−)-renieramycin G (2) were evaluated against both human colon (HCT-116) and human lung (A549) cancer cell lines. (−)-Jorumycin (1) displayed potent growth inhibition with GI50 values in the low nanomolar range (1.9–24.3 nM), while compounds 2–4 were found to be substantially less cytotoxic (GI50 0.6–14.0 µM).
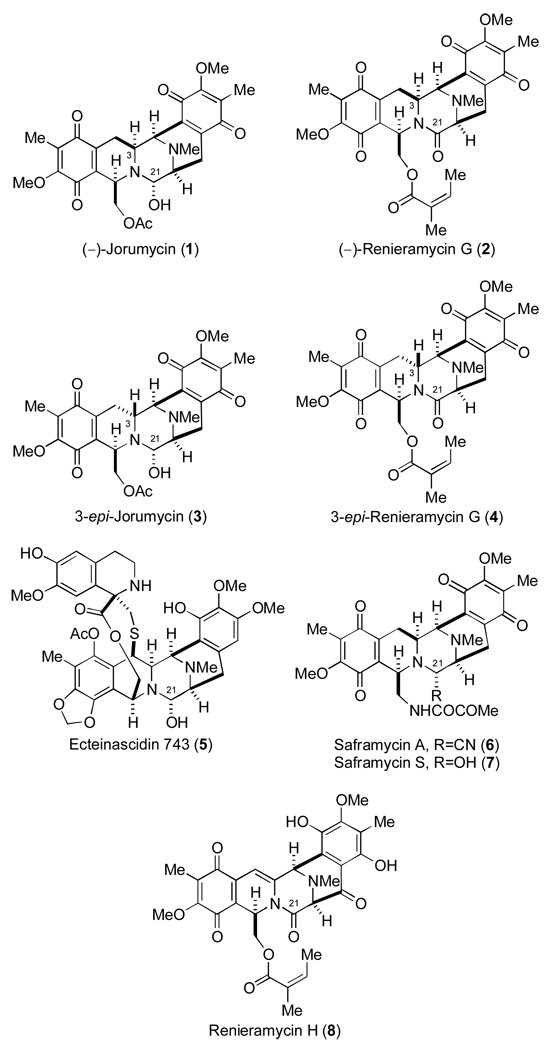
The tetrahydroisoquinoline family of alkaloids includes potent cytotoxic agents that display a range of biological properties such as antitumor and antimicrobial activities.1 (−)-Jorumycin (1) (Figure 1), a member of the family, was isolated from the mantle and mucus of the pacific nudibranch Jorunna funebris2 and is closely related to the saframycins, members of the renieramycin family, and, most notably, Ecteinascidin 743 (Et-743, 5) which was demonstrated to be a highly promising, exceedingly potent antitumor agent currently in phase II/III clinical trials.3 Consistent with other members in the group, (−)-jorumycin (1) has been shown to harbor potent biological activities.2,4 Fontana and co-workers, in the context of their original isolation studies, have demonstrated that 1 possesses activity against NIH 3T3 tumor cells (100% of inhibition at 50 ng/mL) in addition to P388, A549, HT29, and MEL28 tumor cell lines (IC50 12.5 ng/mL).2 Furthermore, 1 was shown to inhibit the growth of various Gram-positive bacteria (eg. Bacillus subtilis, Staphylococcus aureus) at a concentration lower than 50 ng/mL.2 Saito and co-workers, employing (−)-jorumycin (1) prepared semisynthetically from renieramycin M, have reported potent antiproliferative activity for 1 against HCT-116 (IC50 0.57 nM), QG56 (IC50 0.76 nM), and DU145 (IC50 0.49 nM) cell lines.4
Figure 1.
Tetrahydroisoquinoline alkaloids and C-3-epi analogues.
(−)-Renieramycin G (2) (Figure 1) was isolated from the marine sponge Xestospongia caycedoi by Davidson in 1992.5 Despite having an amide carbonyl residue at C-21, 2 was reported to retain cytotoxicity against human cancer cells with MIC values of 0.5 µg/mL and 1.0 µg/mL against KB and LoVo cell lines, respectively.5 These results are surprising in light of the fact that virtually all biologically active members of this family of tetrahydroisoquinoline alkaloids possess a carbinolamine or cyano function at C-21 that permits the formation of a potent, electrophilic iminium ion species that has been implicated in the formation of covalent bonds to ds-DNA and possibly, other biomacromolecules at this position.1
Recently, we reported the asymmetric total syntheses of (−)-jorumycin (1) and (−)-renieramycin G (2).6 However, en route to 1 and 2, a serendipitous discovery afforded a method to efficiently and selectively access two related natural product analogues, 3-epi-jorumycin (3) and 3-epi-renieramycin G (4). Preliminary biological evaluation of 3-epi-jorumycin (3), in addition to relevant synthetic intermediates, revealed low micromolar growth inhibition profiles for all compounds tested in the context of two human cancer cell lines (HCT-116 and A549).6 At the time of our report, despite the wealth of elegant investigations directed toward synthesizing potent and more readily accessible analogues of the tetrahydroisoquinoline family bearing the pentacyclic skeleton characteristic of the saframycins and ecteinascidins,1,7–8 structure activity relationship investigations had not yet addressed the cytotoxicity profiles of stereoisomeric members of this family. Therefore, this preliminary biological evaluation constituted the basis for a new investigation of this class of antitumor antibiotics with a focus on the effect of stereochemical changes at C-3.
Herein, we report a significant extension of our initial investigation on the cytotoxicity of jorumycin, renieramycin and analogs.6 Specifically, in an effort to accurately determine the influence of the stereochemistry at C-3 on the biological activity of (−)-jorumycin (1) and (−)-renieramycin G (2), we endeavoured to evaluate the cytotoxicity of 3-epi-jorumycin (3) and 3-epi-renieramycin G (4) in conjunction with the natural materials. In accord with these goals, compounds 1–4 were prepared through our previously reported procedure6,9 and were subsequently examined, simultaneously, for their antiproliferative activities against human colon (HCT-116) and human lung (A549) cancer cell lines.
HCT-116 and A549 cells were plated in triplicate at 1500 and 5000 cells per well, respectively, in a flat-bottomed 96 well tissue culture plate. Cells were allowed to adhere and grow for at least one doubling and then exposed to the test compound at a dose range of 0.01 to 20 uM for 48 h. When the GI50 of a given test compound was very low on the dosing range, the experiment was repeated with an adjusted dosing scale to more accurately identify the true GI50. Cell proliferation was quantified by measuring fluorescence using an Fmax plate reader after incubating with a flourogenic redox indicator, Alamar Blue, for 3–4 h. Statistical analysis was performed using XLfit made by IDBS.
Of the four compounds evaluated, (−)-jorumycin (1), harbouring a carbinolamine function at C-21, displayed the most impressive cytotoxic activity with GI50 values in the low nanomolar range for both HCT-116 and A549 cell lines (Table 1). With respect to the HCT-116 cell line, data obtained for 1 from this study (GI50 1.9–2.4 nM) was in relatively good agreement with that recently reported by Saito and co-workers (IC50 0.57 nM) on a sample prepared through semi-synthetic methods.4 As well, our data acquired for 1 in the context of the A549 cell line (GI50 19.2–24.3 nM) substantially agreed with that reported by Fontana and co-workers (IC50 12.5 ng/mL (23.7 nM)).2
Table 1.
Antiproliferative activity of tetrahydroisoquinolines 1–4.a
| Cell Line | ||
|---|---|---|
| Compound | A549 | HCT-116 |
| 1 | 0.0192 (± 0.0009) | 0.0019 (± 0.0002) |
| 2 | 12.9 | 3.87 (± 0.28) |
| 3 | 4.64 (± 1.73) | 0.61 (± 0.04) |
| 4 | 10.1 (± 1.6) | 1.40 (± 0.46) |
Values reported are GI50 in µM.
Although (−)-jorumycin (1) displayed a nanomolar growth inhibition profile, the corresponding epimer, 3-epi-jorumycin (3), proved substantially less cytotoxic. Indeed, the low micromolar GI50 values obtained for 3 in these studies were in good agreement with those observed in our prior investigation.6 (−)-Renieramycin G (2) and the corresponding epimer, 3-epi-renieramycin G (4), both of which possess an amide carbonyl group at C-21, also revealed low micromolar growth inhibition profiles. Importantly, this study constitutes the first cytotoxic evaluation of 2 in the context of the HCT-116 and A549 cell lines as minimal antiproliferative activity data continues to persist for members of the tetrahydroisoquinoline family possessing a C-21 amide carbonyl group relative to their respective C-21 cyano- or carbinolamine-containing relatives.1,10
From these studies, a number of important observations were made concerning the influence of the stereochemical configuration at C-3 on the antiproliferative activity of 1 and 2, both representative members of the tetrahydroisoquinoline family of antitumor agents. (−)-Jorumycin (1), like Et-743 (5), is presumably capable of alkylating ds-DNA through an incipient iminium species generated through dehydration of the C-21 carbinolamine group.1,2 Indeed, the low nanomolar growth inhibition values obtained for 1 in these studies are consistent with other related members including saframycin A (6), saframycin S (7) and analogues.1 However, the low micromolar growth inhibition profile observed for 3-epi-jorumycin (3) is not consistent with members of the tetrahydroisoquinoline family bearing a leaving group at C-21. In fact, the data for 3 is more akin to that obtained for (−)-renieramycin G (2) as well as 3-epi-renieramycin G (4). Interestingly, in stark contrast to the differences in cytotoxicity observed between 1 and 3, compounds 2 and 4 proved essentially equipotent in these studies, apparently uninfluenced by the configuration at C-3.
It has been conjectured that (−)-renieramycin G (2) and the closely related natural product renieramycin H (cribrostatin 4) (8), both of which possess amide carbonyl groups at C-21, in comparison to their relatives bearing a leaving group at C-21, owe their diminished cytotoxicity, to the apparent absence of a viable C-21 iminium ion precursor.5,11 Moreover, although the notion has not yet been proven, the absence of such a structural feature would perhaps significantly compromise if not entirely subvert the capacity of amide-containing tetrahydroisoquinoline members to form covalent bonds with ds-DNA. The inability to form covalent drug-DNA adducts would certainly account for a significant portion of the approximate two to three orders of magnitude difference in cytotoxicity1,10 between these two classes of substrates. Based on the fact that 3-epi-jorumycin (3) displays a low micromolar cytotoxicity profile similar to that of 2 and 4 in the context of the cell lines tested, we hypothesize that 3, like the amide-containing substrates, may perhaps exhibit a reduced capacity for forming covalent bonds to DNA, irrespective of its possession of a C-21 iminium ion precursor. Furthermore, based on the aforementioned theories, the similar behavior of 2 and 4 in these studies suggests that these amide-containing substrates are capable of exerting cytotoxic effects through alternative mechanisms,1 which remain fully intact irrespective of the C-3 stereochemical configuration.
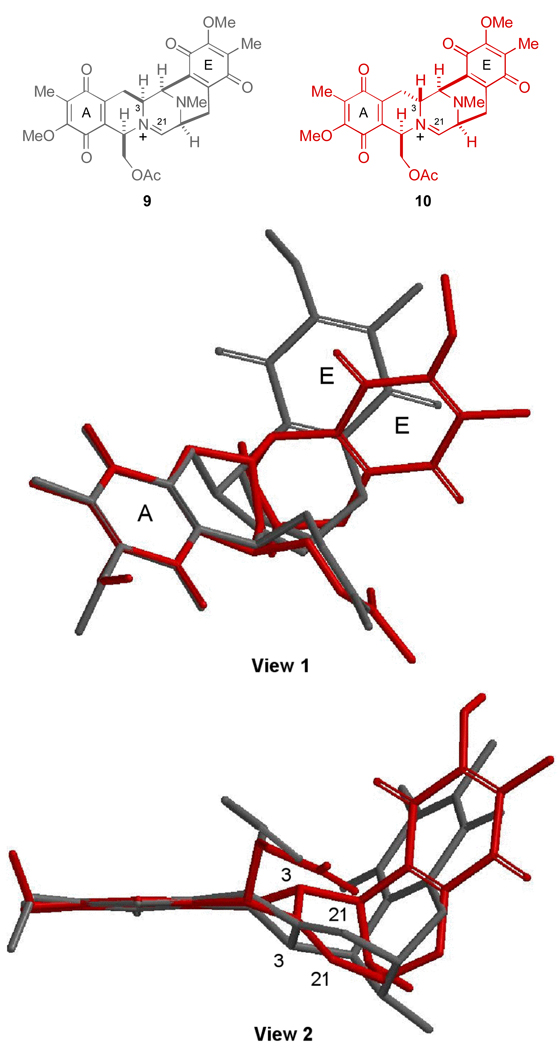
As discussed in our initial disclosure, the stereochemical configuration at C-3 was found to significantly influence the conformation of the two diastereomeric pentacyclic amide intermediates but remarkably displayed the C-21 carbon atom in a similar region of space. Similarly, the proposed reactive iminium ion intermediates generated through the dehydration of (−)-jorumycin (1) and 3-epi-jorumycin (3), 9 and 10, respectively, also display significant conformational differences, particularly with respect to the orientation of the E-ring as illustrated in the overlays presented in Figure 2.12
Figure 2.
Views (1 and 2) of an overlay of iminium ion intermediates 9 (gray) and 10 (red). Low-energy conformations of 9 and 10 were identified by systematic conformational searching (conformer distribution) using molecular mechanics (MMFF94). Geometry optimization of the resulting low-energy conformations of 9 (eight) and 10 (seven) obtained from the conformational search was performed at the semi-empirical PM3 level of theory. Further refinement of the lowest energy conformation of 9 and 10 (HF/6-31G*) provided the structures shown. These molecules were then aligned at the left-hand quinone (A-ring).
In light of the structural differences between 9 and 10, which are evidenced by the spatial distribution of the right-hand E ring systems when the left-hand A ring systems of these substances are superimposed (Figure 2), the possibility exists that the unique conformational features associated with 10, arising from the inversion of configuration at C-3, ultimately compromise the ability of 3-epi-jorumycin (3) to form stable covalent adducts with DNA. This notion seems perhaps counterintuitive since the overall spatial arrangements of the C-21 carbon atoms in both 9 and 10, C-21 being the well-accepted site of DNA alkylation in the saframycin-ecteinascidin family of antitumor agents, are similar in the context of this illustration (Figure 2).6 Significantly, a network of hydrogen bonds has been proposed to assist in stabilizing the saframycin-ecteinascidin class of drugs in the minor groove of ds-DNA.13,14 Therefore, it seems reasonable that perhaps other more distant structural perturbations, could possibly affect this delicate relationship and result in a drug-DNA adduct of diminished stability.
A complete explanation for the diminished activity of 3-epi-jorumycin (3) is not available at this juncture. Nonetheless, these results could potentially, in part, rationalize the significantly diminished activity that has been observed in related analogues, which possess conformational deviations in comparison to the natural pentacyclic scaffolds contained within 1 and other members.8 Indeed, an SAR profile of the core of these natural products would certainly prove useful in guiding efforts toward the construction of more potent tetrahydroisoquinoline agents. Efforts to unambiguously establish the capacity of jorumycins 1 and 3 in addition to renieramycins 2 and 4 to alkylate DNA are presently under study and will be the subject of a separate study.
In conclusion, two tetrahydroisoquinoline analogues 3-epi-jorumycin (3) and 3-epi-renieramycin G (4) in addition to their corresponding parent natural products, (−)-jorumycin (1) and (−)- renieramycin G (2), respectively, were examined side-by-side for their antiproliferative activities against human colon (HCT-116) and human lung (A549) cancer cell lines. As anticipated, (−)-jorumycin (1) displayed potent growth inhibition with GI50 values in the low nanomolar range. Conversely, 3-epi-jorumycin (3) displayed a low micromolar cytotoxicity profile similar to that of compounds 2 and 4. This somewhat unexpected result is currently under further investigation.
Acknowledgements
We thank the National Institutes of Health for financial support (Grant CA85419) and a postdoctoral research fellowship to J.W.L. (Grant CA105898). Dr. Stephen Chamberland is thanked for his generous assistance in the molecular modeling studies.
References and notes
- 1.For a review, see: Scott JD, Williams RM. Chem. Rev. 2002;102:1669–1730. doi: 10.1021/cr010212u.
- 2.Fontana A, Cavaliere P, Wahidulla S, Naik CG, Cimino G. Tetrahedron. 2000;56:7305–7308. [Google Scholar]
- 3.Aune GJ, Furuta T, Pommier Y. Anti-Cancer Drugs. 2002;13:545–555. doi: 10.1097/00001813-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Saito N, Tanaka C, Koizumi Y-i, Suwanborirux K, Amnuoypol S, Pummangura S, Kubo A. Tetrahedron. 2004;60:3873–3881. [Google Scholar]
- 5.Davidson BS. Tetrahedron Lett. 1992;33:3721–3725. [Google Scholar]
- 6.Lane JW, Chen Y, Williams RM. J. Am. Chem. Soc. 2005;27:12684–12690. doi: 10.1021/ja0535918. [DOI] [PubMed] [Google Scholar]
- 7.Myers AG, Lanman BA. J. Am. Chem. Soc. 2002;124:12969–12971. doi: 10.1021/ja027729n. [DOI] [PubMed] [Google Scholar]
- 8.Ong WO, Chang YA, Wu J-y, Cheng C-c. Tetrahedron. 2003;59:8245–8249. [Google Scholar]
- 9.Compounds 1–4 are amenable to chromatographic purification utilizing flash chromatography with either normal-phase silica gel or florisil followed by reverse-phase HPLC according to the reported procedure.6 Long-term storage (i.e. several weeks or more) of compounds 1–4 requires refrigeration (−80 °C) in order to avoid decomposition.
- 10.Pettit GR, Knight JC, Collins JC, Herald DL, Pettit RK, Boyd MR, Young VG. J. Nat. Prod. 2000;63:793–798. doi: 10.1021/np990618q. [DOI] [PubMed] [Google Scholar]
- 11.Chan C, Heid R, Zheng S, Guo J, Zhou B, Furuuchi T, Danishefsky SJ. J. Am. Chem. Soc. 2005;127:4596–4598. doi: 10.1021/ja050203t. [DOI] [PubMed] [Google Scholar]
- 12.All calculations were performed and graphical representations were generated using Spartan ’04 for Macintosh, Wavefunction, Inc., Irvine, CA.
- 13.Hill GC, Remers WA. J Med. Chem. 1991;34:1990–1998. doi: 10.1021/jm00111a011. [DOI] [PubMed] [Google Scholar]
- 14.Zewail-Foote M, Li V-s, Kohn H, Bearss D, Guzman M, Hurley LH. Chem. Biol. 2001;135:1. doi: 10.1016/s1074-5521(01)00071-0. [DOI] [PubMed] [Google Scholar]