Abstract
The three-step conversion of d-quinotoxine into quinine, as originally reported by Rabe and Kindler in 1918, has been experimentally verified. This conversion serves to re-affirm the formal total synthesis of quinine reported by Woodward and Doering in 1944.
Keywords: quinine, quinotoxine, quininone, quinidinone, quinidine
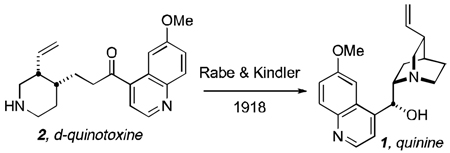
Robert Burns Woodward and William von Eggers Doering of Harvard University published a communication in 1944 and a full paper in 1945 in the Journal of the American Chemical Society both entitled: “The Total Synthesis of Quinine”.[1] This now famous paper has been the subject of much attention over the years, particularly recently. It is now well-understood, that the Woodward-Doering “total synthesis” was actually a “formal” total synthesis of quinine (1) that relied on a seminal 1918 publication by Paul Rabe and Karl Kindler, wherein d-quinotoxine (2), the final synthetic substance in the Woodward-Doering work, was converted into quinine by a three-step sequence (Scheme 1).[2] This sequence involved: (1) oxidation of d-quinotoxine with sodium hypobromite to produce “N-bromoquinotoxine” (3); (2) base-mediated cyclization of 3 to produce “quininone” (4); and (3) aluminum powder reduction of “quininone” to produce quinine (1) and quinidine (6, as a minor product). The 1918 paper, termed a “preliminary notice” by the authors, provided only a terse summary of this three-step process. This paper referenced Rabe’s 1911 conversion of cinchotoxine into cinchoninone and cinchonidinone (employing the analogous reactions for the quinotoxine to quininone and quinidinone conversion)[3] but subsequently in 1932, complete experimental details for the aluminium powder reduction protocol were published.[4]
Scheme 1.
Conversion of d-quinotoxine to quinine. a) NaOBr, NaOH, HCl (aq), Et2O, 55% crude; b) NaOEt, EtOH, 88% crude; c) Aluminum powder, NaOEt, EtOH, 5% (as the tartrate salt).
Despite the significant fanfare accompanying the publication of the Woodward-Doering paper during World War II, and the ensuing rich history of quinine and the Cinchona alkaloids in modern medicine,[5] it is surprising that apparently no one has reported efforts to simply attempt to repeat the Rabe-Kindler conversion of d-quinotoxine into quinine. This is particularly significant since concomitant with their relatively recent total synthesis of quinine,[6] Gilbert Stork raised some possible doubts about the validity of this conversion, referring to Woodward and Doering’s “ total synthesis” as a “widely believed myth.”[7] Consequently, the Woodward-Doering claim for a “total synthesis” albeit as a “formal” total synthesis by relay through d-quinotoxine based on the Rabe-Kindler sequence, has been besmirched.[7]
This fascinating saga, spanning more than eighty years, was meticulously researched very recently by Seeman whose 2007 publication entitled: “The Woodward-Doering/Rabe-Kindler Total Synthesis of Quinine: Setting the Record Straight”,[8] helped kindle our own interest in this story. In his analysis of all the available data in the literature and in archival materials, Dr. Seeman stated: “I conclude that Paul Rabe and Karl Kindler did convert d-quinotoxine into quinine as they reported in 1918.” and: “I therefore also conclude that the Woodward-Doering/Rabe-Kindler total synthesis of quinine is a valid achievement.” This insightful and perhaps even bold conclusion, based on a painstaking and meticulous review of a large body of published and unpublished material, still lacked unambiguous experimental data to back up the purported d-quinotoxine to quinine conversion originally reported in 1918 and so significantly relied upon by Woodward and Doering.[8]
In connection with our laboratory’s work on a novel C3–C4 intramolecular SN2´ approach to the total synthesis of quinine and the related Cinchona alkaloids,[9] we became very interested in this controversial, historically potent, yet from the perspective of the 21st century, straightforward semi-synthesis of quinine from d-quinotoxine. We have carefully examined this conversion as described by Rabe and Kindler in 1918[2] and in associated papers by Rabe and coworkers published in 1911,[3] 1932[4] and 1939.[10] We report herein the successful conversion of d-quinotoxine into quinine deploying the experimental protocols originally described by Rabe and his co-workers. This further serves to re-affirm the (formal) total synthesis claimed by Woodward and Doering in 1944.[1]
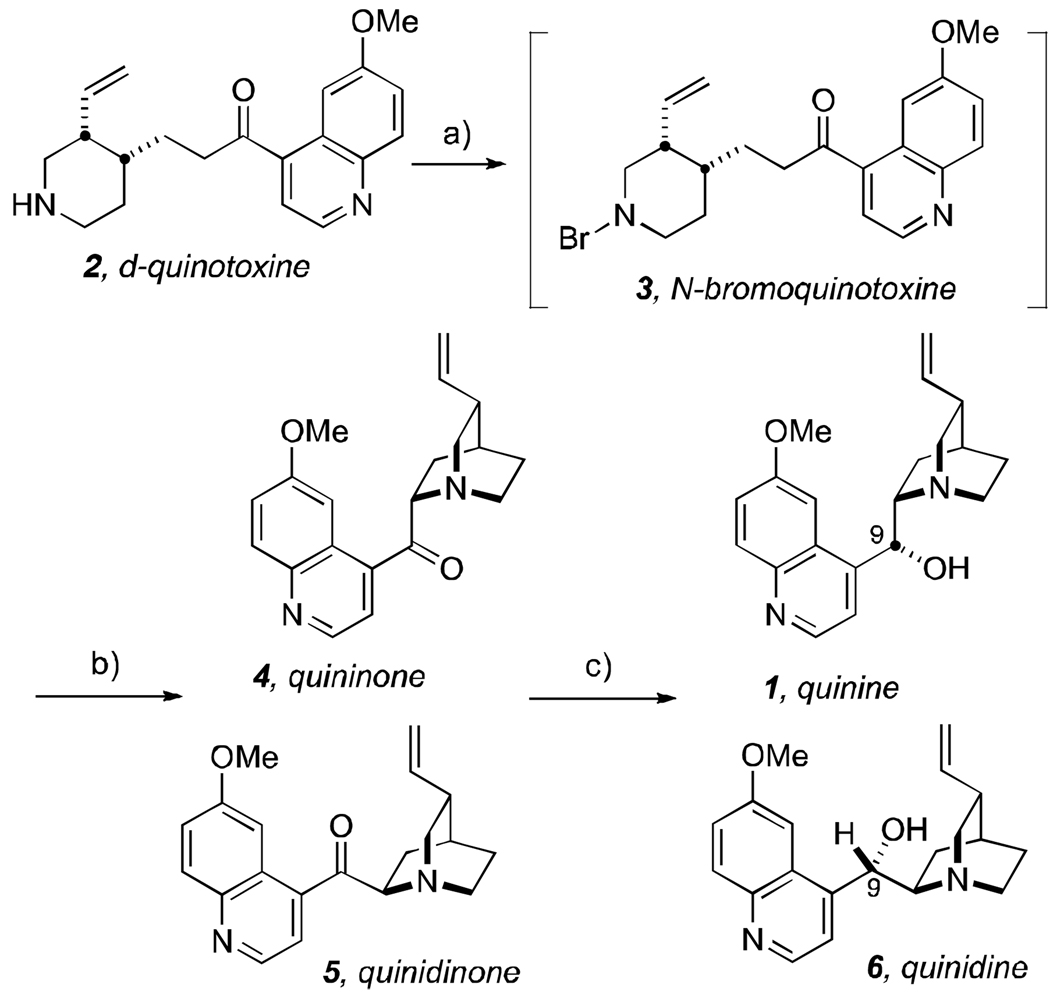
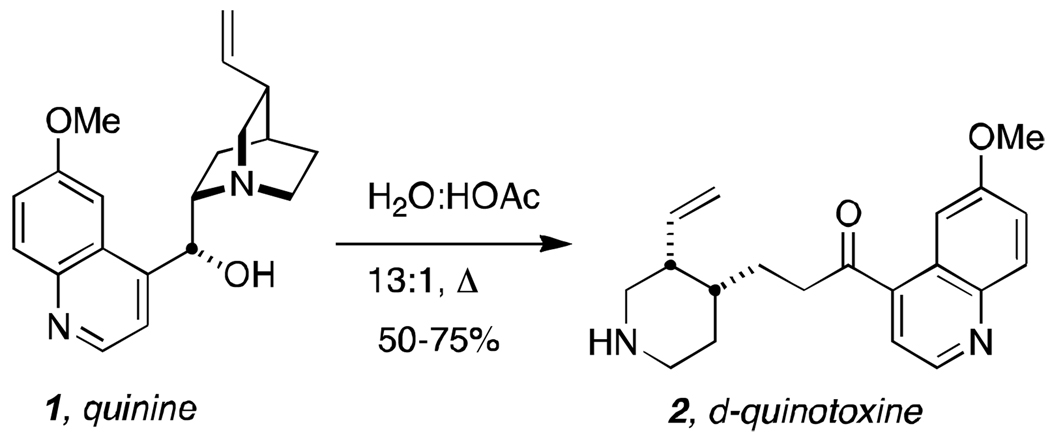
We prepared our d-quinotoxine (2) on a 29 gram scale from commercially available quinine (Aldrich, 90%) as described by Biddle in 1912 (Scheme 2).[11] Heating quinine in H2O-acetic acid (13:1) at 100 °C for 35 hours provided between 50–75% yield of pure d-quinotoxine. Thus, according to Rabe and Kindler,[2] d-quinotoxine (2) was treated with a solution of freshly-made sodium hypobromite (Scheme 2). The resultant product, “N-bromoquinotoxine” (3), proved to be an unstable substance recalcitrant to purification and was immediately treated with sodium ethoxide in ethanol under the same conditions described by Rabe and Kindler to effect quinuclidine cyclization and provided a mixture of quininone (4) + quinidinone (5). As documented in the literature, the product that Rabe and Kindler assumed to be quininone, was in fact its less-soluble epimer, quinidinone (5).[6,8,12,13] Quinidinone, when dissolved, spontaneously epimerizes α- to the ketone moiety at C-8 immediately establishing an equilibrium mixture of quininone (4) and quinidinone (5).[12] The mixture thus obtained above, was treated with newly purchased aluminum powder (Aldrich)[14] in a solution of sodium ethoxide and ethanol at reflux temperature to effect reduction of the carbonyl moiety but only provided quinine (1) in trace amount as detected by the appropriate signatures in the 1H nmr spectrum.
Scheme 2.
Preparation of d-quinotoxine from natural quinine.[11]
We were struck by the initial low yield of quinine obtained from the aluminum powder reduction and the trace material which we were able to isolate mandated the use of silica gel chromatography, a purification technique not yet discovered at the time of the Rabe-Kindler work nor available in 1944 to Woodward and Doering. It is of course impossible now to determine where Rabe and Kindler had obtained their aluminum powder, or the level of purity of the reagent that they had employed in their work. We thus set out to examine other reduction methods of the quinidinone/quininone mixture into quinine, to establish the robustness of this general transformation before returning to the issue of the quality of the aluminum powder. This was achieved through a relay synthesis of quinidinone (formed through oxidation of quinine)[13,15] to provide adequate quantities of pure material to study the reduction step.
As shown in Table 1, in 1973 Uskokovic and co-workers, had reported that quininone could be reduced to quinine through the agency of diisobutylaluminum hydride (entry 1).[12] This protocol provided a mixture of quinine + quinidine in 72% isolated yield (33% quinine as calculated by 1H nmr). Sodium borohydride also effects this reduction, but in much lower yield (entry 2). These “control” experiments provided us with authentic mixtures of quinine + quinidine for use as comparison standards to evaluate the aluminum powder reductions in more detail.
Table 1.
Conditions for reducing quinidinone/quininone to quinine.
| Entry | Reducing conditions | Temp. (°C) |
Isolated yield of quinine/quinidine |
yield of quinine[f] |
|---|---|---|---|---|
| 1[a] | DIBAL-H benzene | 20 | 72% | 33% |
| 2[a] | NaBH4, EtOH | 0 | 11% | 4% |
| 3 | Al powder (new)[c] NaOEt, EtOH | reflux | trace | trace |
| 4 | Al powder (new)[d] NaOEt, EtOH | reflux | 30% (1.1:1) | 16% |
| 5 | Al powder + Al2O3 NaOEt, EtOH | reflux | 26% (1.1:1) | 14% |
| 6 | Al powder (aerated)[c] NaOEt, EtOH | reflux | 24% (1.1:1) | 13% |
| 7 | Al powder MeOH, NaOMe | reflux | 8% (1.2:1) | 4% |
| 8 | Al powder (sonication) NaOEt, EtOH | reflux | 22% (1.1:1) | 12% |
| 9 | Al powder, Na(OiPr), i-PrOH | reflux | 32% (1:1.2) | 15% |
| 10 | Al(Oi-Pr)3, iPrOH | reflux | 28% | 16% |
| 11 | LiAlH4, ether | −78 | 45% | trace |
| 12 | LiAlH4, ether | 0 | 59% | trace |
| 13 | LiAlH4, ether | 20 | 56% | trace |
| 14 | LiAlH4, ether [e] | 0 | 40% (1:1.5) | 16% |
experiment from ref 11.
general reaction conditions here,
bottle “#1”;
bottle “#2”;
after epimerization;
calculated by 1H nmr analysis
When a new bottle of aluminum powder (bottle “#1”)[14] was employed in the Rabe-Kindler protocol, quinine was visible in trace amounts by analysis of the 1H nmr spectrum of the crude mixture after work up (entry 3). When a different batch of fresh aluminum powder was examined (bottle “#2”, entry 4),[14] we observed a 30% combined yield of quinine + quinidine, that contained ~16% of quinine by 1H nmr analysis. We reasoned that it was possible that the material used by Rabe and Kindler in 1918 may have contained significant Al(III) impurities, either as a consequence of the manufacture of elemental aluminum in the early 1900’s, or as a consequence of their bulk reagent becoming “stale” through exposure to air. To test this speculative hypothesis, we added Al2O3 to the aluminum powder reduction using “bottle #1” and observed a significant improvement in the yield (26% yield combined of quinine + quinidine; ~14% quinine, entry 5).
Next, we took our initial batch of aluminum powder (“bottle #1”), and aerated this material for 72 hours by passing a stream of air over a beaker containing the metal. Re-subjecting the quinidinone (5) to the “aerated” aluminum powder yielded quinine in ~ 15% yield (entry 6). Several variations on these reductions were also employed and provided similar results (entries 7–9). These results clearly establish that the “aluminum powder” reagent that is deployed in these reductions, must contain some Al(III) impurities to give significant conversion of the ketone substrates to the secondary alcohol products. A further inference from these studies, is that the Rabe-Kindler aluminum powder reduction, may be viewed as an early, “activated” progenitor of the Meerwein-Ponndorf-Verley (MPV) reduction.[16] We also conducted a classical MPV reduction[13,16] (entry 10) to compare the reactivity of that system to that of the aluminum powder. The MPV reaction did provide quinine (16%), but the reaction took 48 hours compared to just 2 h for the other conditions employed.
Finally, lithium aluminum hydride was investigated (entries 10–13). When pure quinidinone (5) was utilized as the substrate, LiAlH4 provided quinidine (6) as the major reduction product in most cases with only a trace amount of quinine detectable by 1H NMR (entries 11–13). This shows that minimal epimerization of quinidinone takes place under these reaction conditions. When epimerization of quinidinone is effected prior to exposure to LiAlH4, and a mixture of quininone:quinidinone is employed as the substrate mixture, quinine was formed in 16% yield which comports with that obtained with the Rabe-Kindler aluminum powder reduction conditions. Therefore, we can speculate that, had Woodward and Doering attempted to repeat the Rabe-Kindler reduction protocol and experienced difficulties (ie., due to the absence of sufficient Al(III) impurities in their reagent), they could have (and in our view, would have) reasonably turned to other reducing agents available in 1944; lithium aluminum hydride being one such reasonable alternative and the MPV reaction[13,16] as another that, as demonstrated here, provides quinine (see below).
The bulk of the experiments that we performed to ascertain the molecular validity of the Rabe-Kindler d-quinotoxine into quinine conversion, relied on the utilization of all of the modern separation, analytical and spectroscopic techniques available to us today. We of course realize, that these powerful tools were not available to Rabe and Kindler in 1918 and that most of these tools were likewise still not available to Woodward and Doering in 1944. It then remained for us to repeat the Rabe-Kindler work under conditions and using techniques that would have been available in 1944 to reasonably validate the relay conversion of their synthetic d-quinotoxine into quinine, had Woodward and Doering chosen to do so. In our hands, the oxidation of quinotoxine with sodium hypobromite was performed on a multi-gram scale and the crude N-bromoquinotoxine product was directly used for the subsequent steps without purification. This substance proved to be somewhat unstable to handling and we were unable to crystallize this material as reported.[2] This notwithstanding, the crude N-bromoquinotoxine was successfully converted into a mixture of quininone + quinidinone through the action of sodium ethoxide in ethanol.[2] Rabe and Kindler reported that: “The N-bromoquinotoxine, prepared in the same way as the bromo compound obtained from cinchotoxine, crystallizes from ether as colorless needles with m.p. 123°. The quininone obtained from it with m.p. 108° is in all respects identical to quininone obtained from quinine.”[2] Unfortunately in our hands, and despite extensive efforts, neither quininone nor its epimer quinidinone could be purified from the aforementioned reaction mixture using crystallization techniques.[17] We nevertheless continued to carry the crude material forward as a mixture of quininone + quinidinone which also contained several, unidentified impurities. In the event, treatment of the crude ketone mixture with aluminum powder[14] in the presence of sodium ethoxide in ethanol at reflux temperature according to the protocol described by Rabe and Kindler,[2] delivered a diastereomeric mixture of alcohols from which pure quinine tartrate (5% isolated yield; see Figure 1) could be crystallized as described below.
Figure 1.
Crystals of quinine tartrate obtained directly from quinotoxine according to the Rabe-Kindler protocol[2] without the use of any modern isolation, chromatographic or analytical techniques.
Thus, the entire three-step sequence originally reported by Rabe and Kindler in 1918, was validated from d-quinotoxine to quinine without the need to purify any intermediates, nor resorting to any modern separation, purification or analytical technologies as a guide or aid. This sequence was found to be consistently reproducible and was done under laboratory conditions that existed in 1944.
The most difficult step of the Rabe-Kindler protocol in our hands proved to be the isolation of pure quinine from the aluminum powder reduction reaction mixture. We were able to readily isolate a mixture of quinine and quinidine from the aluminum powder reductions by silica gel chromatography as the crude reaction mixture, which consisted of at least four products: quinine, quinidine, 9-epi-quinine and 9-epi-quinidine, was complex as evidenced in the crude 1H nmr spectrum. Identification of quinine in the reduction reaction mixture served to validate the Rabe-Kindler conversion, and our isolation of this material by silica gel chromatography provided further corroboration. Rabe and Kindler state in their 1918 paper: “16.3 g synthetic quininone when treated with the aforementioned reducing mixture yielded, besides 0.9 g quinidine, 2 g of analytically pure quinine. Quinine melted as required at 177° and had an optical rotation in absolute alcohol of (c=2.1432 at 20°C) while Rabe for the natural alkaloid had found (c=2.1362 at 15°C).”[2,8] Based on these experimental disclosures, Rabe and Kindler reported obtaining a 12.3% isolated yield of analytically pure quinine from the aluminum powder reduction of quininone (now known to be quinidinone[6,8,12,13]). Due to the low and variable yields of the final reduction step in our hands, which we conclude is a function of the quality of the aluminum powder used, Rabe and Kindler may have found it difficult to isolate pure quinine from the reduction reaction mixture in a reproducible manner. This would be particularly true if a new batch of aluminum powder were used that contained less Al(III) impurities. The reduction conditions used in our studies deployed aluminum powder that had been left open to the air as well as newly opened bottles that were not exposed to the air; the quality of the reagent apparently varying significantly from batch-to-batch.
We were able to obtain pure quinine from the crude aluminum powder reduction reaction mixture through the use of the selective crystallization protocol first described by Rabe in 1939[10] and successfully employed by Doering in 1947.[18] The crude aluminum reduction mixture, constituted of quinine and the corresponding C9 and quinidine diastereomers, was purified by selective formation of the di-quinine L-tartaric acid monohydrate salt from 95% ethanol in 5% yield (923 milligrams of white needles were isolated from 13.7 grams of the crude quininone:quinidinone mixture). The quinine tartrate thus obtained, had a melting point of 212–214 °C (recryst. 95% ethanol; lit.[6,12] m.p. 211–212.5 °C; see Figure 1) and had an optical rotation of (c = 0.90, MeOH) (lit.[12] , c = 0.97, MeOH).
Pure quinine could then be prepared from these crystals of the tartrate salt by simple aqueous base extraction. The quinine thus obtained, had a melting point of 178 °C (recryst. benzene; lit.[2] m.p. = 177 °C) and an optical rotation of (c = 0.95, ethanol; lit.[19] , c= 1.05, ethanol; lit.[6] , c = 0.995, ethanol) and thus matches the data for the natural alkaloid. To further corroborate this procedure, we asked two additional co-workers (see Acknowledgement) to repeat and check this sequence in its entirety as just described (the optimal conditions were employed using entry 6, Table 1; see Supporting Information) starting with 9~15 grams of quinotoxine and culminating with the isolation of pure, crystalline quinine tartrate. In the event, both individuals were able to successfully repeat and confirm the Rabe-Kindler conversion of quinotoxine into quinine.
In conclusion, we have demonstrated that the originally reported conversion of quinotoxine to quinine as described by Rabe and Kindler in 1918[2] is readily reproducible and can be conducted under laboratory conditions and with literature available to Woodward and Doering in the early 1940’s[2,3,4,10] without the use of any modern separation, purification, analytical or spectroscopic methods or techniques. The entire sequence can be conducted on crude material with analytically pure quinine being isolable from the final reaction mixture by selective crystallization of the corresponding tartrate salt; an isolation protocol disclosed by Rabe in 1939[10] and readily available to Woodward and Doering in 1944. We have discovered that the aluminum powder reduction, when fresh, non-aerated reagent is employed, typically gives only trace amounts of quinine and that “synthetically meaningful” yields are apparently only obtainable when “aged” aluminum powder is utilized that contains Al(III) surface impurities. We note that the quality of commercial grade aluminum powder varies in substantial ways with respect to the Rabe-Kindler protocol from batch-to-batch and in some instances, freshly opened bottles of aluminum powder work satisfactorily while others do not provide sufficient quinine for selective crystallization-based isolation. Further, we have provided solid experimental support for the notion that, had Woodward and Doering chosen to follow the Rabe and Kindler protocol and had difficulty reproducing the reported 12.3% isolated yield of quinine from this last step, that other reducing agents known at the time (1944), such as lithium aluminum hydride or the MPV reduction, could have been successfully deployed to reach quinine as an alternative. Indeed, Woodward and co-workers actually published the reduction of quininone under MPV conditions to provide quinine (30% yield) + quinidine (60%) in a full paper in 1945.[13] Woodward states in this paper: “The only previous successful reduction of the carbonyl group of quininone was that of Rabe3 [ref. 2 here] who obtained 12% of quinine and 6% of quinidine by reducing the ketone with aluminum and ethanol in the presence of sodium ethoxide. It is worthy of note that this reaction constitutes the last step in the total synthesis of the cinchona alkaloid [ref. to the 1945 paper, ref. 1b here], which has thus been noticeably improved.”
Finally, the conclusions reached by Seeman[8] on the validity of the Rabe-Kindler work now have firm experimental support which vanquishes any resilient doubts initially raised by Stork in a letter to Woodward in 1944[8] (apparently unanswered)[8] where he queried if the Rabe-Kindler procedure had been repeated at Harvard; these concerns were then made more visible in his series of publications in 2000 and 2001[6,7] questioning the 1918 Rabe-Kindler publication[2] and the ensuing Woodward-Doering (formal) total synthesis.[1] The Woodward and Doering paper concludes unambiguously: “In view of the established conversion of quinotoxine to quinine, with the synthesis of quinotoxine (emphasis ours) the total synthesis of quinine was complete.”[1] The experimental facts reported herein reaffirm that assertion. Validation herein, of a formal total synthesis of quinine as originally reported by Woodward and Doering in 1944[1] should serve to remove the blemish asserted[6,7] on the reputations of Rabe and Kindler a well as that of Woodward and Doering. The Supporting Information addendum to the present work, provides the complete experimental details to the chemical literature of the Rabe-Kindler d-quinotoxine into quinine conversion in both a modern experimental setting as well as a pre-1944 setting; may Paul Rabe and Karl Kindler requiescant in pace.
Supplementary Material
Footnotes
We are grateful to the National Institutes of Health for financial support (GM068011). Mass Spectra were obtained on instruments supported by the NIH Shared Instrument Grant GM49631. We are particularly grateful to Professor William von Eggers Doering of Harvard University for insightful and provocative discussions. We are indebted to Dr. Jeffrey I. Seeman, for many thoughtful discussions and encouragement. We thank our co-workers, Dr. Thomas J. Greshock and Brandon English, for independently checking and repeating our best determined experimental conditions using the Rabe-Kindler conversion of d-quinotoxine into quinine procedure.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Woodward RB, Doering WE. J. Am. Chem. Soc. 1944;66:849. [Google Scholar]; b) Woodward RB, Doering WE. J. Am. Chem. Soc. 1945;67:860–874. [Google Scholar]
- 2.Rabe P, Kindler K. Ber. Dtsch. Chem. Ges. 1918;51:466–467. [Google Scholar]
- 3.Rabe P. Ber. Dtsch. Chem. Ges. 1911;44:2088–2091. [Google Scholar]
- 4.Rabe P. Justus Liebigs Ann. Chem. 1932;492:242–266. [Google Scholar]
- 5.a) Turner RB, Woodward RB. The Chemistry of the Cinchona Alkaloids. In: Manske RHF, editor. The Alkaloids. Vol. 3. New York: Academic Press; 1953. Chap. 16. [Google Scholar]; b) Uskokovic´ MR, Grethe G. The Cinchona Alkaloids. In: Manske RHF, editor. The Alkaloids. Vol. 14. New York: Academic Press; 1973. p. 181. [Google Scholar]; c) Kaufman TS, Rfflveda EA. Angew. Chem. Int. Ed. 2005;44:854–885. doi: 10.1002/anie.200400663. [DOI] [PubMed] [Google Scholar]
- 6.Stork G, Niu D, Fujimoto A, Koft ER, Balkovec JM, Tata JR, Duke GR. J. Am. Chem. Soc. 2001;123:3239–3242. doi: 10.1021/ja004325r. [DOI] [PubMed] [Google Scholar]
- 7.As reported in a C&E News article (M. Rouhi, Chem. Eng. News 2001, 79 (May 7) 54–56): “The literature shows that these accolades [to Woodward and Doering] were ‘in part based on wishful thinking,’ Stork says… The final steps that Woodward and Doering assumed would take that intermediate to quinine likely would not have worked had they tried them.”; see also letters by Stork to C&E News 2001, 79, pg. 8 and C&E News 2000, 78, pg. 8.
- 8.Seeman JI. Angew. Chem. Int. Ed. 2007;46:1378–1413. doi: 10.1002/anie.200601551. [DOI] [PubMed] [Google Scholar]
- 9.Johns DM, Mori M, Williams RM. Org. Lett. 2006;8:4051–4054. doi: 10.1021/ol061524s. [DOI] [PubMed] [Google Scholar]
- 10.Rabe P, Kindler K. Ber. Dtsch. Chem. Ges. B. 1939;72:263–264. [Google Scholar]
- 11.a) Biddle HC. J. Am. Chem. Soc. 1912;34:500–515. [Google Scholar]; b) Pasteur L, C R. Hebd. Seances Acad. Sci. 1853;37:162. [Google Scholar]
- 12.Gutzwiller J, Uskokovic MR. Helv. Chim. Acta. 1973;56:1494–1503. [Google Scholar]
- 13.Woodward RB, Wendler NL, Brutschy FJ. J. Am. Chem. Soc. 1945;67:1425–1429. [Google Scholar]
- 14.The aluminum powder used in our studies was Aldrich catalog #21,4752, >75 micron, 99%, 25 gm bottle and 500 gm bottle.
- 15.Hutchison DR, Khau VV, Martinelli MJ, Nayyar NK, Peterson BC, Sullivan KA. Org. Synth. 1998;75:223–234. [Google Scholar]
- 16.a) Meerwein H, Schmidt R. Ann. 1925;444:221. [Google Scholar]; b) Ponndorf W. Angew. Chem. 1926;39:138. [Google Scholar]; c) Verley A. Bull. Soc. Chim. France. 1925;37(4):537–871. [Google Scholar]
- 17.We must despair that mastery of crystallization techniques by those skilled in the early part of the 20th century, certainly surpasses that routinely practiced today.
- 18.Doering WE, Cortes G, Knox LH. J. Am. Chem. Soc. 1947;69:1700–1710. doi: 10.1021/ja01199a039. [DOI] [PubMed] [Google Scholar]
- 19.Gutzwiller J, Uskokovic MR. J. Am. Chem. Soc. 1978;100:576–581. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.