Abstract
Purpose
Surgical resection provides the only possibility of cure for pancreas cancer. A standard adjuvant approach has not been established. We tested the safety and efficacy of a granulocyte-macrophage colony-stimulating factor (GM-CSF)-based immunotherapy administered in patients with resected pancreatic adenocarcinoma.
Patients and Methods
A single institution phase II study of 60 patients with resected pancreatic adenocarcinoma was performed. Each immunotherapy treatment consisted of a total of 5 × 108 GM-CSF-secreting cells distributed equally among 3 lymph node regions. The first immunotherapy treatment was administered 8 to 10 weeks after surgical resection. Subsequently, patients received 5-FU based chemoradiation. Patients who remained disease-free after completion of chemoradiotherapy received treatments 2 to 4, each 1 month apart. A fifth and final booster was administered 6 months after the fourth immunotherapy. The primary endpoint was disease free survival and secondary endpoints were overall survival and toxicity, and the induction of mesothelin-specific T cell responses.
Results
The median disease-free survival is 17.3 months (95% CI, 14.6–22.8) with median survival of 24.8 months (95% CI, 21.2–31.6). The administration of immunotherapy was well tolerated. In addition, the postimmunotherapy induction of mesothelin-specific CD8+ T cells in HLA-A1+ and HLA-A2+ patients correlates with disease-free survival.
Conclusions
An immunotherapy approach integrated with chemoradiation is safe and demonstrates an overall survival that compares favorably with published data for resected pancreas cancer. These data suggest additional boost immunotherapies given at regular intervals beyond 1 year postsurgery should be tested in future studies, and provide the rationale for conducting a multicenter phase II study.
Pancreatic cancer remains the fourth leading cause of cancer related death in the United States.1 Surgical resection provides the only possibility of cure. However, the historical 5-year survival postresection remains approximately 15% to 20%, with 1 and 2-year survival of 63% and 42% respectively.2 A standard adjuvant treatment approach for patients with resected disease has not yet been determined.2–11
We developed irradiated GM-CSF transfected allogeneic whole cell tumor lines for pancreas ductal adenocarcinoma immunotherapy.12 We previously reported that this immunotherapy treatment administered intradermally in sequence with chemoradiotherapy to patients with resected pancreatic adenocarcinoma induced posttreatment delayed type hypersensitivity (DTH) responses to autologous tumor cells in 1 of 3 participants receiving 1 × 108 cells and in 2 of 5 participants receiving 5 × 108 vaccine cells. The 3 DTH responders are the only participants who remain disease-free at 10+ years. Importantly, immunized lymphocytes from the DTH-responders were used to screen a number of differentially expressed genes in pancreatic cancer. All 3 DTH responders demonstrated a postimmunotherapy T cell response to mesothelin, a glycosyl-phosphatidylinositol (GPI)-linked cell surface protein that likely serves as an adhesion molecule that promotes metastases.13–16
We now report the results of a single institution 60 patient phase II study testing the highest immunotherapy dose of GM-CSF secreting pancreatic tumor cells found to be safe and bioactive in phase I testing. This study was designed to estimate the disease-free and overall survival rates and the prevalence of mesothelin-specific T cell responses that are associated with immunotherapy treatment.
PATIENTS AND METHODS
Study Design
This was a single institution study of 60 patients with pancreatic adenocarcinoma who underwent pancreaticoduodenectomy (PD) at the Johns Hopkins Hospital (Baltimore, Maryland) between December, 2001 and November, 2004. Eligible patients received 5 × 108 vaccine cells at specified intervals integrated with adjuvant chemoradiotherapy and chemotherapy. The study was approved by the Johns Hopkins IRB and the FDA Center for Biologics Evaluation and Research.
Patient Selection
Main eligibility requirements included: histologic diagnosis of pancreatic ductal adenocarcinoma after R0 or R1 resection; enrollment within 10 weeks of surgery; no known second malignancies within 5 years of diagnosis of pancreatic cancer (other than carcinoma-in-situ of the cervix, superficial skin cancer, or superficial bladder cancer); Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; no clinical evidence of metastases; no serious autoimmune or allergic disease requiring treatment with systemic corticosteroids; no systemic corticosteroids within 1 month before receiving the immunotherapy; adequate hematologic, hepatic, and renal function; and human immunodeficiency virus-negative status.
Procedures and Treatment
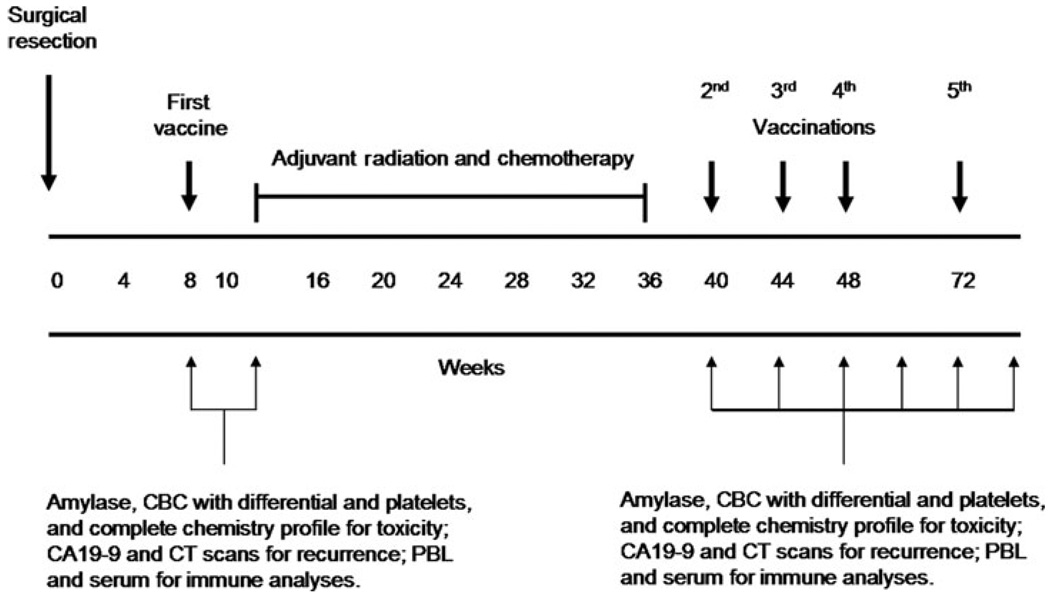
After obtaining informed consent, baseline studies were performed for tumor assessment [computed tomography (CT) scan and CA19–9 serum marker level measurements] and toxicity evaluation (complete blood counts with differential and platelets, complete chemistry profile, absolute eosinophil count, and serum amylase). The intervention and data collection schedules are diagrammed in Figure 1. Patients received the first immunotherapy 8 to 10 weeks after pancreaticoduodenectomy. All patients underwent weekly toxicity monitoring that included a complete blood count with differential, chemistry profile, and serum amylase. Patients were assessed by CT scan (every 2–3 months) and measurement of CA 19–9 levels (monthly). Patients were subsequently treated with adjuvant radiation and 5-flurouracil (5-FU) chemotherapy as per the standard arm of RTOG 9704.9 Patients who were still disease-free by CT criteria 4 to 8 weeks after completion of adjuvant chemoradiation were treated with up to 3 additional immunotherapies given 1 month apart and a final boost 6 months after completing the fourth immunotherapy treatment. Patients receiving the fifth immunotherapy were also asked to consider providing peripheral blood leukocytes by leukapheresis as per protocol. Two pancreas cancer cell lines (PANC 10.05 and PANC 6.03) were combined and tested in this study and have previously been described.12,17,18
FIGURE 1.
Intervention and data collection schedule.
ASSESSMENTS
Toxicities
Toxicities were graded using the National Cancer Institute’s cancer clinical trials common toxicity criteria CTCV2.0. Toxicities were identified by medical history, physical examination, and review of the laboratory studies performed.
IMMUNE MONITORING STUDIES
The methodology for the synthesis of peptides, ELISA assay for identifying reactive mesothelin peptides, mesothelin-specific T cell assays, enzyme-linked immunosorbent spot (ELISPOT) assays have previously been described.12,16,17
Statistical Considerations
Disease-free survival (DFS) is the primary endpoint and is defined as time from surgery until clinical evidence of disease (eg, CT scan) or death due to any cause. Individuals are censored with respect to DFS at the date of the last follow-up with documented disease status if they withdraw from the study, they have no evidence of disease at the time of analysis, or they are lost to follow-up. To obtain a conservative estimate of DFS, an individual is also censored at the date of last follow-up with a documented disease status if death occurs and the disease status is unknown. Overall survival is defined as the time from surgery until death, regardless of cause. At the time of trial design, the 1-year cumulative incidence of cancer recurrence or death in patients not treated with vaccine was 79.8%. We assumed that the failure rates would follow an exponential distribution and that the accrual and minimum follow-up periods would be 12 and 18 months, respectively. On the basis of these assumptions, a sample size of 60 patients was chosen to achieve 91% power to detect an increase in the percentage of individuals surviving at 1-year from 20.2% to 34.3% with a 2-sided type 1 error rate of 10%.19 This is equivalent to an increase in the median progression free survival from 5.2 to 7.8 months, a 50% improvement.
Statistical analyses were performed using R,20 version 2.5.1. Descriptive statistics (means, ranges, counts, percentages) and plots (eg, bargraphs) were used to describe the characteristics of the study population and the immunologic endpoints. Postimmunotherapy responses were compared to pretreatment responses using 2-tailed Wilcoxon sign-rank tests. T cell repertoires were compared using logistic regression.
We also compared our clinical outcomes to a recent historical cohort of individuals who underwent surgery followed by adjuvant chemoradiation therapy (CRT) at Johns Hopkins University (JHU) between 1994 and 2005. Comparisons between the overall survival of the active treatment arm and the control group are made using Kaplan-Meier techniques and a log-rank test.
RESULTS
Patient Characteristics
Sixty patients were enrolled of a total of 83 screened (Table 1). The most common cause for screening failure was disease progression (n = 17). As shown in Table 2, the median age of patients enrolled onto the study was 62.5 years (range, 40–83 years). The majority of patients enrolled had moderate/poorly differentiated pancreatic adenocarcinoma (59/60 with at least Grade 2) and lymph node positive resections (53/60 patients or 88%). In addition, 32 of the 60 had carcinomas more than 3 cm in diameter, 18 of the 60 patients had positive margins, and 10 of the 60 patients had postoperation CA19–9 levels above 90 U/mL. Thirty-six of the 60 patients were still disease-free based on clinical and radiographic evaluation 1 month after completing adjuvant radiation and chemotherapy and were therefore able to continue on study. One participant developed a new melanoma that was diagnosed after the second immunotherapy and was taken off study. This participant eventually died of metastatic melanoma. Another participant with a history of heart disease had a cardiac event 2.1 months after completing the first immunization, which resulted in death. Thirty-six patients received the second immunotherapy, 33 received the third immunotherapy, and 28 received the fourth immunotherapy before demonstrating clinical or radiographic evidence of pancreatic cancer progression. Of the remaining 28 patients, 13 remained disease-free 6 months after receiving the fourth immunotherapy. These participants were eligible for and received a fifth and final immunotherapy boost.
GM-CSF secreting cell lines as immunotherapy is feasible and safe to administer to patients with resected pancreatic cancer.
TABLE 1.
Summary of Patient Screening
| Screening Results | N |
|---|---|
| Enrollments | 60 |
| Screening failures | 23 |
| Disease recurrence | 17 |
| Elevated liver enzymes | 2 |
| Other* | 4 |
A single screening failure occurred for each of the following reasons: anemia, a second primary cancer developed, inadequate wound healing, withdrew consent during an assessment of a mass at pancreatic bed.
TABLE 2.
Characteristics of the Sixty Enrolled Participants
| Characteristics | Summary Statistics |
|---|---|
| Age | |
| Median (Range) | 62.5 (40.0–83.0) |
| Over 65 | 27 (45%) |
| Sex | |
| Male | 37 (62%) |
| Female | 23 (38%) |
| Race | |
| White | 53 (88%) |
| Nonwhite | 7 (12%) |
| Preoperative diabetes | |
| Yes | 14 (23%) |
| No | 46 (77%) |
| Surgery type | |
| Classic PD | 6 (10%) |
| Pylorus Preserving PD | 52 (87%) |
| Total pancreatectomy | 2 (3%) |
| Tumor diameter (cm) | |
| Median (Range) | 3.0 (1.3–6.0) |
| Over 3 cm | 32 (53%) |
| Lymph node status | |
| Positive | 53 (88%) |
| Negative | 7 (12%) |
| Margin status | |
| Positive | 18 (30%) |
| Negative | 42 (70%) |
| Histologic grading | |
| G1 well differentiated | 1 (2%) |
| G2 moderately differentiated | 37 (61%) |
| G3 poorly differentiated | 22 (37%) |
| DTH material | |
| Yes | 8 (13%) |
| No | 52 (87%) |
| Postoperative CA 19–9 | |
| ≤ 90 U/ml | 50 (83%) |
| > 90 U/ml | 10 (17%) |
Continuous variables are summarized using medians and ranges. Categorical variables are summarized with counts and percentages.
No local or systemic dose-limiting toxicities were observed. A summary of all treatment related adverse events are described in Table 3. The most common adverse events included erythema/induration or pain/soreness at the vaccine sites after each immunotherapy. These reactions were expected and self-limiting, lasting up to 1 week. These reactions increased in intensity but not duration in the majority of patients who received repetitive immunotherapy treatments. There was no evidence of autoimmunity or pancreatitis associated with repetitive immunotherapies. In addition, there was no evidence of enhanced or new toxicities associated with the chemoradiation given after vaccination.
Disease-free and overall survival associated with the GM-CSF secreting cell lines as immunotherapy integrated with chemoradiation following pancreaticoduodenectomy.
TABLE 3.
Summary of Treatment Related Adverse Events for All Immunotherapy Treatment Cycles
| All Grades N (%) | Grade 3 or 4 N (%) | |
|---|---|---|
| Local at vaccine sites | ||
| Erythema/induration | 170 (100) | 0 (0) |
| Pruritus | 149 (88) | 0 (0) |
| Pain, soreness, tenderness | 142 (84) | 0 (0) |
| Recall induration | 14 (8) | 0 (0) |
| Blister formation | 4 (2) | 0 (0) |
| Hyperpigmentation | 1 (0.5) | 0 (0) |
| Skin peeling | 1 (0.5) | 0 (0) |
| Systemic | ||
| Eosinophilia | 114 (81) | 2 (1) |
| Flu-like symptoms/aches | 31 (18) | 0 (0) |
| Fever | 21 (12) | 0 (0) |
| Chills | 12 (7) | 0 (0) |
| Fatigue | 11 (6) | 0 (0) |
| Pruritus | 9 (5) | 0 (0) |
| Rash | 9 (5) | 0 (0) |
| Nausea | 7 (4) | 0 (0) |
| Headache | 5 (3) | 0 (0) |
| Leukocytosis | 4 (2) | 0 (0) |
| Pruritus (not at vaccine site) | 4 (2) | 0 (0) |
| Lymphadenopathy | 3 (2) | 0 (0) |
| Neutrophilia | 2 (1) | 0 (0) |
| Urticaria | 2 (1) | 0 (0) |
| Ecchymosis | 1 (0.5) | 0 (0) |
| Hyperglycemia | 1 (0.5) | 0 (0) |
| Night sweats | 1 (0.5) | 0 (0) |
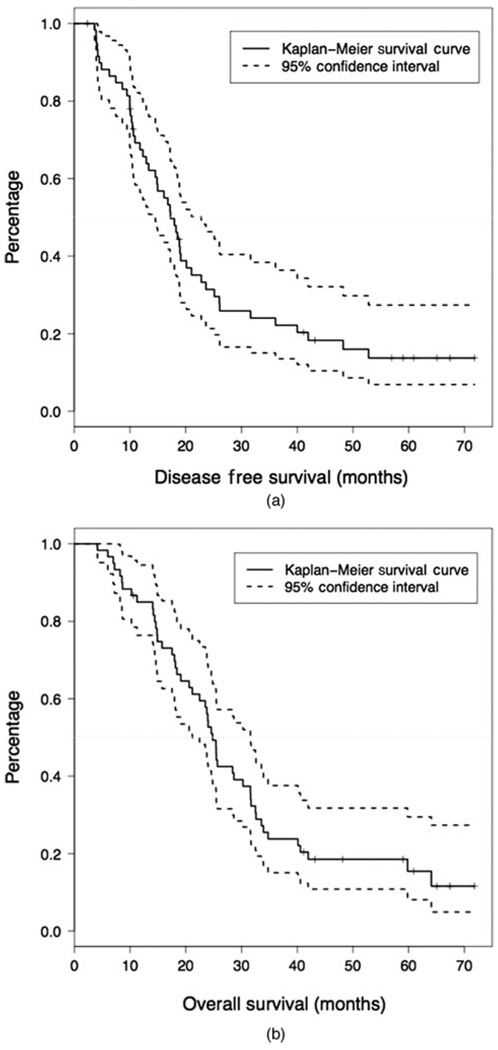
Figure 2 shows disease free survival (Figure 2A) and overall survival (Figure 2B). The median disease-free survival for all evaluable patients is 17.3 months (95% CI, 14.6–22.8) and a median overall survival of 24.8 months (95% CI, 21.2–31.6). The percentage of individuals who are disease-free after 1 year is 67.4% (95% CI, 56.4–80.6%) and who are alive after 1 year is 85.0% (95% CI, 76.3–94.5%). In univariate models, positive lymph nodes and margins were significantly associated with a decrease in overall survival (HR: 3.43, 95% CI, 1.22–9.62, P = 0.01 and HR: 1.85, 95% CI, 1.00–3.42, P = 0.05). A multivariable model was not used due to the strong relationship between margin and lymph node status.
FIGURE 2.
A, Disease-free survival after treatment with allogeneic, irradiated, GM-CSF secreting pancreatic tumor cells as immunotherapy. The survival function is plotted with 95% confidence intervals. The median is denoted with a solid line. B, Overall survival after treatment with allogeneic, irradiated, GM-CSF secreting pancreatic tumor cells as immunotherapy. The survival function is plotted with 95% confidence intervals. The median is denoted with a solid line.
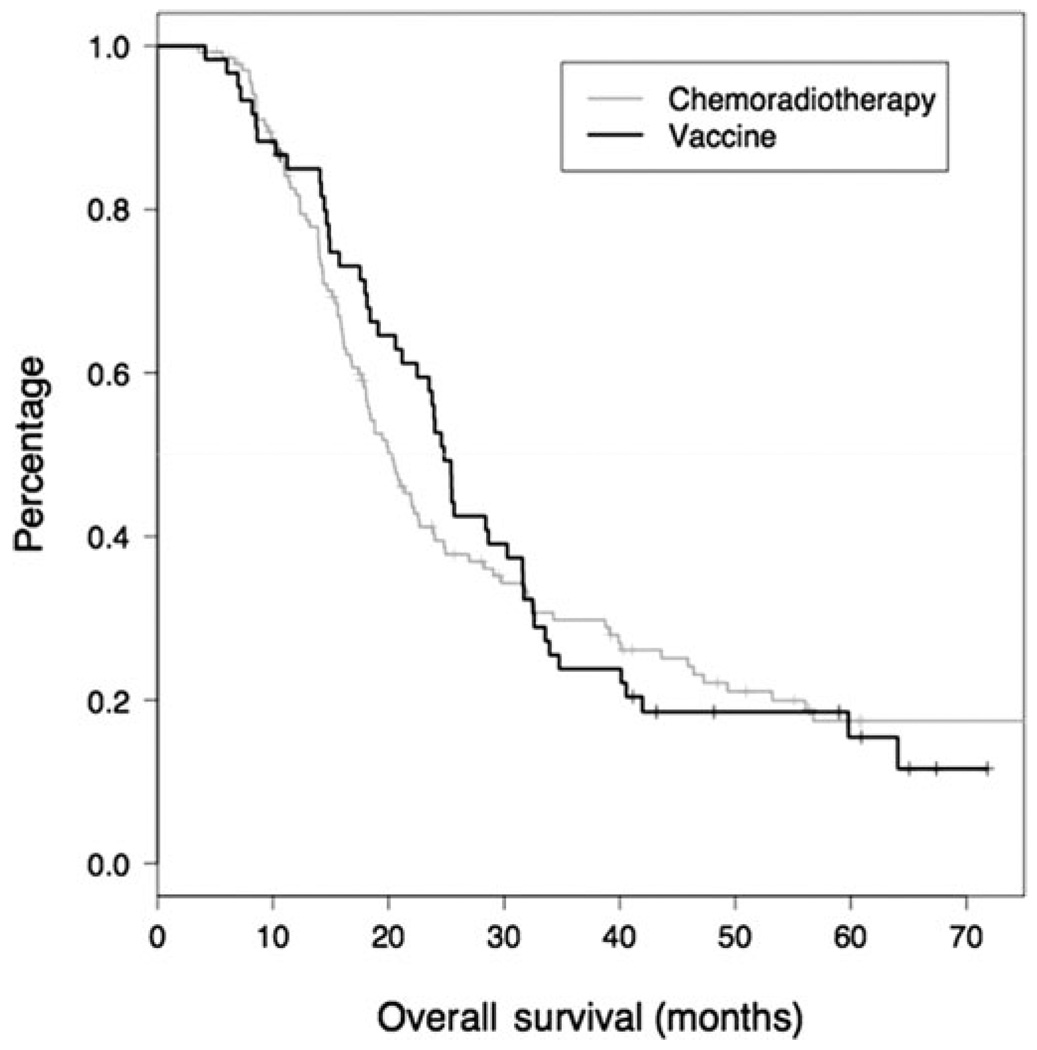
This study also compares patients treated with immunotherapy integrated with adjuvant chemoradiation and a historical cohort treated at the Johns Hopkins Hospital with surgery followed by adjuvant chemoradiation alone. The median and 1 year overall survival of patients included in the historical cohort were 20.3 months (95% CI, 18.0–23.9) and 81.8% (95% CI, 75.4–88.6%), respectively. The Kaplan-Meier curves suggest that there might be a benefit from vaccine over chemoradiation in the first 2 years after surgery (Figure 3). However, there was no significant difference in the median overall survival (HR: 0.96, 95% CI, 0.68–1.35, P = 0.8). A comparison with a subset of Johns Hopkins Hospital patients matched on tumor size, nodal status, and margin status produced nearly identical results (data not shown), and did not favor the immunotherapy group.
Post-immunotherapy induction of mesothelin-specific CD8+ T cells correlates with DFS in HLA-0101 and HLA-0201 patients.
FIGURE 3.
Comparison of overall survival for a historical patient cohort resected at JHH who received chemoradiation therapy alone versus patients who received chemoradiation and immunotherapy on study.
Two HLA-A0101 and 2 HLA-A0201 binding mesothelin epitopes have already been identified by a previously described method.16 Six new HLA-A0101 and 4 new HLA-A0201 binding mesothelin epitopes were identified using immunized lymphocytes from 2 immunotherapy treated patients to screen overlapping peptides spanning the entire mesothelin protein. We used an ELISPOT assay16 to evaluate the number of cytokine expressing CD8+ T cells specific for each of the mesothelin peptides before and after the first and final vaccinations (Table 4). Because screening was only performed to identify new HLA-A0101 and HLA-A0201 binding epitopes, we limited our ELISPOT analysis to the 25 HLA-A0101+ and 23 HLA-A0201+ patients, and to 1 HLA-A0207+ patient for whom pre- and postimmunotherapy lymphocytes were available covering 43 of the 60 treated patients (6 patients expressed bothHLA-A1 and HLA-A2).
TABLE 4.
Identified HLA-A0201 and HLA-A0101 Binding Mesothelin Epitopes
| Peptide Number |
Amino Acid Position |
Peptide Sequence | HLA-restriction |
|---|---|---|---|
| 31D | 153–161 | RGAPERQRL | HLA-A0101 |
| 32D | 158–166 | RQRLLPAAL | HLA-A0101 |
| 36A | 175–183 | LLSEADVRA | HLA-A0101 |
| 37C | 182–190 | RALGGLACD | HLA-A0101 |
| 44C | 217–225 | DQQEAARAA | HLA-A0101 |
| 45E | 224–232 | AALQGGGPP | HLA-A0101 |
| 16F | 80–88 | RVRELAVAL | HLA-A0201 |
| 32E | 159–167 | QRLLPAALA | HLA-A0201 |
| 44F | 220–228 | EAARAALQG | HLA-A0201 |
| 45D | 223–231 | RAALQGGGP | HLA-A0201 |
Peripheral blood leukocytes from 2 vaccinated participants demonstrating prolonged disease-free survival were used to screen an overlapping mesothelin peptide library. Shown are the amino acid positions and sequences of the 6 newly identified HLA-A0101 binding (top) and 4 newly identified HLA-A0201 binding (bottom) mesothelin-derived 9mer epitopes.
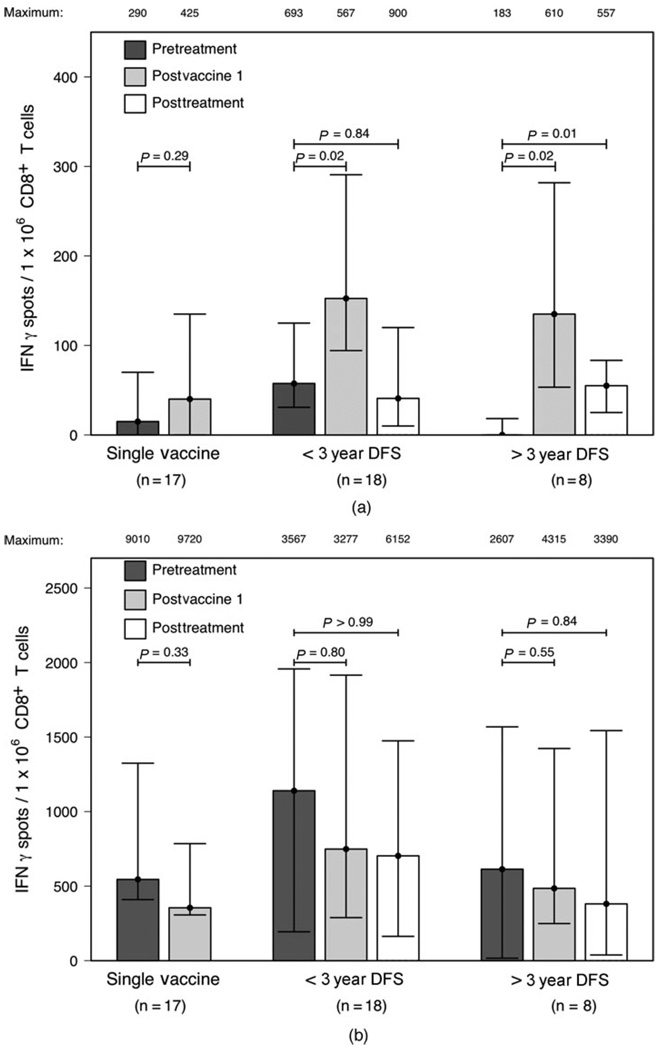
CD8+ T cells specific for at least one mesothelin peptide were detected after the first immunotherapy treatment in the majority (38 of 43) of participants analyzed. Four of the 5 patients who did not demonstrate a T cell response to any of the studied mesothelin epitopes after the first treatment recurred before the second scheduled immunotherapy treatment. We pooled the ELISPOT data for patient subgroups focusing on the mesothelin peptide to which each individual subject demonstrated a maximum frequency of responding T cells (Figure 4A). By this analysis, when compared to pretreatment levels, significantly enhanced mesothelin peptide-specific responses were observed after the first (P = 0.02) and final immunotherapy (P = 0.01) in the group of participants remaining disease-free for greater than 3 years. However, postimmunotherapy mesothelin-specific responses were only significantly enhanced after the first (P = 0.02) but not the final (P = 0.84) treatment in the group of participants who had recurred within 3 years. Furthermore, postimmunotherapy mesothelin-specific responses were not significantly enhanced in the group of patients who recurred early, after receiving only the first immunotherapy (P = 0.29). Nearly identical results were obtained when T cell frequencies to the entire pool of mesothelin peptides were compared (data not shown). In addition to measuring CD8+ T cell responses to mesothelin peptides, we also measured responses to the positive control CMV/EBV/Influenza A (CEF) peptide pool in each participant at every time point. Although CEF pool responses were detected in all participants analyzed, postimmunotherapy changes in CEF pool-specific responses were not detected in any of the groups (Figure 4B). It is important to note that the 3 year DFS cut off was used for these analyses to ensure that we were capturing late recurring patients in the recurrence group.
FIGURE 4.
Postimmunotherapy enhancement of mesothelin-specific CD8+ T cell responses in HLA-A0101+ and HLA-A201+ patients correlates with disease-free survival. IFN gamma ELISPOT assays were performed to measure the frequencies of CD8+ T cells specific for (A) mesothelin epitopes and (B) the CEF pool in peripheral blood leukocytes isolated from HLA-A1+ and HLA-A2+ patients for which pre- and posttreatment lymphocytes were available. Patients were divided into 3 groups: those receiving only one treatment (Single Vaccine, n = 17), those receiving multiple treatments who recurred within 3 years (DFS < 3 year, n = 18), and those receiving multiple treatments who remained disease-free for greater than 3 years (DFS > 3 years, n = 8). Mesothelin responses are reported for the epitope to which each individual patient showed a maximum response. Shown for each group is the median number of IFN gamma-secreting CD8+ T cells per 1 × 106 CD8+ T cells above background measured against irrelevant tumor antigens with the interquartile range. The maximum value for each group is listed above each column. Responses were measured before treatment (pretreatment), 14 days after the first immunotherapy treatment (postvaccine 1) and if given multiple treatments, 28 days after the final immunotherapy treatment (posttreatment). Postimmunotherapy responses were compared to pretreatment responses using 2-tailed Wilcoxon sign-rank tests and the calculated P-values are shown.
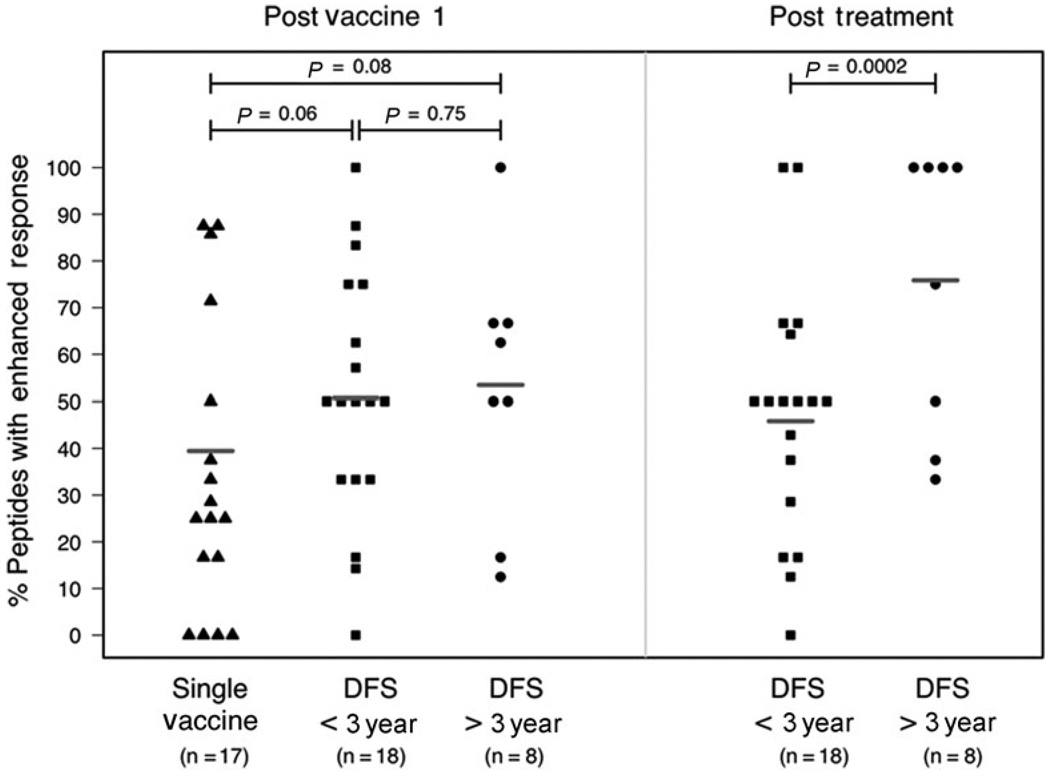
Interestingly, significant differences in the repertoires of mesothelin peptides for which enhanced responses were measured were not observed among groups after the first immunotherapy. Enhanced T cell responses were observed to over 50% of the mesothelin peptides after the first immunotherapy in participants given multiple treatments and 38.7% of the peptides in participants given a single treatment. However, an expansion in the repertoire of mesothelin-derived epitopes targeted after the final immunotherapy was observed in the group of patients who demonstrated longer disease-free survival (increased from 53.4% to 75.9%). In contrast, a reduction in the number of targeted mesothelin epitopes was observed in the group of patients who had recurred within 3 years (decrease from 50.7% to 45.8%). Consequently, after multiple treatments, a larger mesothelin-specific T cell repertoir was observed in participants demonstrating longer disease-free survival (P = 0.0002) (Figure 5).
FIGURE 5.
Longer disease-free survival is associated with an expansion in the repertoire of CD8+ T cells targeted at mesothelin epitopes in HLA-A0101+ and HLA-0201+ patients. IFN gamma ELISPOT assays were performed to measure the frequencies of CD8+ T cells specific for mesothelin epitopes as described in Figure 4. Patients were divided into 3 groups: those receiving only 1 treatment (single vaccine, n = 17), those receiving multiple treatments who recurred within 3 years (DFS < 3 year, n = 18), and those receiving multiple treatments who remained disease-free for greater than 3 years (DFS > 3years, n = 8). Shown are the percentage of mesothelin peptides per patient for which a postimmunotherapy enhancement, defined as a 2-fold or greater increase in the number of IFN gamma-producing peptide-specific T cells was measured after the first (postvaccine 1) and the final immunotherapy treatments (posttreatment, only for patients receiving multiple treatments). Bars represent the overall percentage of peptides for which an enhanced response was measured for each group. Group repertoires were compared using logistic regression and the calculated P values are shown.
DISCUSSION
This single arm, single institution phase II study of allogeneic GM-CSF secreting cell lines as immunotherapy given in sequence with chemoradiation after pancreaticoduodenectomy supports the following 3 conclusions. First, the administration of up to 5 repetitive treatments with gene modified pancreas tumor cells is well tolerated in resected pancreatic cancer patients when sequenced with adjuvant chemoradiation. Second, the survival of patients receiving this immunotherapy in sequence with chemoradiation compares favorably with published data for resected pancreas cancer that report overall survivals ranging from 15 to 20 months.2–11 Third, the induction and maintenance of enhanced postimmunotherapy mesothelin-specific T cell responses is associated with prolonged disease-free survival in the HLA-A1+ and HLA-A2+ patients who were analyzed.
These data confirm our phase I study findings that postimmunotherapy induction of mesothelin-specific CD8+ T cells correlates with improved DFS.12,16 In this phase II study, we extended our analyses to multiple HLA-A0101 and HLA-A0201 epitopes that derive from mesothelin. We observed that patients who remained disease-free demonstrated an increase in the number of epitopes against which their lymphocytes responded after the final immunotherapy treatment. One possible explanation for this finding is that each patient has a different T cell repertoire with different response capabilities. A second possibility is that each patient’s tumor, or antigen-presenting cells, processes different HLA epitopes for presentation to their T cells. These data suggest that the expansion of the T cell repertoire recognizing a more complete panel of antigen epitopes rather than the quantitation of one epitope specific T cell population, may be a better predictor of long-term DFS associated with this immunotherapy. Although we have demonstrated a correlation of the posttreatment induction of mesothelin-specific T cell responses with improved overall response, we have not directly demonstrated that mesothelin is the target antigen expressed by pancreatic tumors in this study. However, we have previously reported that mesothelin-specific T cells can lyse pancreas tumor cells that naturally expressed mesothelin.16
It is interesting that mesothelin-specific T cell frequencies were higher at baseline in the patients who demonstrated shorter DFS. Although it is possible that elevated baseline frequencies may predict poorer prognosis, the significance of this finding is currently unclear. In light of these data, baseline levels will be closely monitored in future studies to further investigate the relevance of the baseline response.
Recent studies have implicated a negative role for Tregs in the clinical outcome of patients with cancer. Specifically, these studies have demonstrated that increased numbers of tumor-infiltrating Tregs are associated with poorer prognosis.21,22 In concordance with previous studies, immunohistochemical analysis of resected pancreatic cancer tissue revealed the presence of tumor-infiltrating Tregs. However, unlike other studies, we found that these Tregs were present in substantial numbers in all patient specimens tested (data not shown). Consequently, we were unable to determine the impact of Tregs on survival or on the induction of mesothelin-specific T cell responses. However, the common presence of Tregs in pancreas cancers highlights the importance of targeting the suppressive function of these cells in future studies even in patients with earlier stage disease.
In conclusion, these findings provide the rationale for conducting a multicenter phase II adjuvant study, which will be led by The Cancer and Leukemia Group B (CALGB) in 2010. These data also provide the rationale for additional boosting beyond 18 months after pancreaticoduodenectomy. Finally, these data support the further evaluation of mesothelin-specific T cells as a surrogate marker of disease-free survival.
ACKNOWLEDGMENTS
Dr. Laheru and Dr. Jaffee had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Laheru, Jaffee, Yeo, Abrams, Hruban, and Piantadosi made the study concept and design. Acquisition of data was undertaken by Lutz, Laheru, Onners, Solt, Tartakovsky, Choi, Biedrzycki, Kobrin, and Sharma. Herman, Lutz, Laheru, Jaffee, Piantadosi, Sugar, and Illei undertook analysis and interpretation of data. Drafting of the manuscript was done by Laheru, Lutz, Jaffee, Herman, and Sugar. Critical revision of the manuscript for important intellectual input was done by Hruban, Yeo, Lillemoe, Cameron, Le, Donehower, and Jaffee. Statistical analysis was undertaken by Piantadosi, Sugar, and Lutz. Jaffee and Laheru obtained funding. Administrative, technical or material support was provided by Solt. Study supervision was done by Jaffee and Biedrzycki.
Supported by: NCI K23 Award # CA093566–01A1, NCI RO1CA88058–01, NCI RAID Program, NCI SPORE Program in Gastrointestinal Cancers, the Lustgarten Foundation, the Viragh Family Foundation, and the Skip Viragh Charities.
Also supported in part by Cell Genesys, Inc and Cerus Corp.
Footnotes
Under a licensing agreement between Cell Genesys and the Johns Hopkins University, the University is entitled to milestone payments and royalties on sales of the vaccine product described in this manuscript. Funding for some of the studies described was provided by Cerus (now Anza) Corporation. Under a licensing agreement involving mesothelin, between Anza and the Johns Hopkins University, Drs. Jaffee and Hruban are entitled to a share of royalties received by the University. The terms of these arrangements are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Trial Registration Clinicaltrials.gov identifier: NCT00084383.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Yeo CJ, Pluth-Yeo T, Hruban R, et al. Cancer of the Pancreas. In: DeVita VT, editor. Principles and Practice of Oncology. 7th ed. Philadelphia: J.B. Lippincott Co; 2005. pp. 945–986. [Google Scholar]
- 3.Stockten DD, Bückler MW, Dervenis C, et al. Meta-analysis of randomized adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92(8):1372–1381. doi: 10.1038/sj.bjc.6602513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 5.Bakkevold KE, Arnesjo B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and ampulla of vater-results of a controlled, prospective, randomized multicentre study. Eur J Cancer. 1993;29(5):698–6703. doi: 10.1016/s0959-8049(05)80349-1. [DOI] [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocrcinoma of the pancreas- 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 7.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-Fluorouracil after curative resection of cancer of the pancreas and periampullary region. Ann Surg. 1999;230(6):776–784. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 9.Regine WF, Winter KW, Abrams R, et al. Fluorouracil versus gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection for of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 10.Picozzi VJ, Kozarek RE, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185(5):476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 11.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative intent resection of pancreatic cancer. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 12.Jaffee EM, Hruban R, Biedrzycki B, et al. A novel allogeneic GM-CSF secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19(1):145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 13.Cao D, Maitra A, Saavedra JA, et al. Expression of novel markers of pancreatic ductal adenocarcinoma in pancreatic nonductal neoplasms: additional evidence of different genetic pathways. Mod Pathol. 2005;18(6):752–761. doi: 10.1038/modpathol.3800363. [DOI] [PubMed] [Google Scholar]
- 14.Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124(6):838–845. [PubMed] [Google Scholar]
- 15.Li M, Bharadwaj U, Zhang R, et al. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7(2):286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross priming by antigen presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200(3):297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffee EM, Schutte M, Gossett J, et al. Development and characterization of a cytokine secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci Am. 1998;4(3):194–203. [PubMed] [Google Scholar]
- 18.Jaffee EM, Abrams RA, Cameron JL, et al. A phase I trial of lethally irradiated allogeneic pancreatic tumor cells transfected with the GM-CSF gene for the treatment of pancreatic adenocarcinoma. Hum Gene Ther. 1998;9(13):1951–1971. doi: 10.1089/hum.1998.9.13-1951. [DOI] [PubMed] [Google Scholar]
- 19.Lawless J. Statistical Models and Methods for Lifetime Data. John Wiley & Sons; 1982. [Google Scholar]
- 20.R Development Core Team; R. R Foundation for Statistical Computing. Vienna, Austria: 2007. [Accessed October 15, 2010]. A language and environment for statistical computing. ISBN3–900051-07–0 http://www.R-project.org. [Google Scholar]
- 21.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 22.Hiraoka N, Onozato K, Kosuge T, et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]