Abstract
Objective
To report our experience using mycophenolate mofetil as first-line treatment for dermatomyositis-associated interstitial lung disease.
Methods
We examined the medical records of all 16 dermatomyositis patients with interstitial lung disease seen in our outpatient university hospital dermatology clinic between May 26, 2006 and May 25, 2009. In this retrospective case series, we describe the clinical course of the four patients with definitive evidence of interstitial lung disease on radiologic imaging who were treated with mycophenolate mofetil and had pulmonary data available to document their outcome. All patients also received prednisone.
Results
All three patients with at least one year of follow-up on mycophenolate mofetil experienced complete normalization of pulmonary function tests (including diffusing capacity for carbon monoxide) and resolution of dyspnea. They were also able to reduce their prednisone doses. The only patient with pre- and post-treatment chest computed tomography imaging had total resolution of her interstitial opacities. The patient with only five months of post-treatment follow-up experienced an improvement in diffusing capacity for carbon monoxide from 44 to 77% predicted but no change in dyspnea.
Conclusion
These promising data indicate that mycophenolate mofetil may be a useful therapy for interstitial lung disease in dermatomyositis patients, but larger studies are needed to more definitively evaluate this medication’s role in therapy.
Interstitial lung disease (ILD) is commonly observed in patients with dermatomyositis (1), but few studies address treatment of ILD in this population. Prior reports document treatment of polymyositis and/or dermatomyositis-associated ILD with various immunosuppressants, including cyclosporine, tacrolimus, and cyclophosphamide (2). However, these therapies are all aggressive and are associated with a variety of serious side effects.
Several recent small retrospective and uncontrolled prospective studies of scleroderma patients and other miscellaneous connective tissue disease (CTD) patients describe treatment of ILD with mycophenolate mofetil (MMF), an immunosuppressive agent with a relatively favorable safety profile. We add to the current literature with this case series of four dermatomyositis patients (one with skin disease and symptomatic muscle disease, one with skin disease and subclinical muscle disease, and two with skin disease and no muscle disease) who were successfully treated with MMF for ILD. To our knowledge, this is the first report documenting use of MMF for ILD specifically in dermatomyositis patients.
Patients and Methods
Between May 26, 2006 and May 25, 2009, 16 dermatomyositis patients seen in our outpatient dermatology clinic were diagnosed with possible or definite ILD. Of these patients, four had definitive ILD (per radiologic imaging), were treated with MMF, and had available data to document their pulmonary outcome. These patients are included in this report.
The other 12 patients are not included in the report for the following reasons: not treated with MMF (eight), the radiologic diagnosis of ILD was equivocal (two), no radiologic pulmonary images were available (one), and no pulmonary data (radiologic or PFTs) were available to document the outcome of MMF treatment (one).
All diagnoses of ILD were confirmed by chest high resolution computed tomography (HRCT). HRCT images were reviewed by our pulmonologist author (MEK). The diagnosis of dermatomyositis was made based on clinical skin findings by our dermatologist author (VPW). Dermatomyositis patients with proximal muscle weakness and objective evidence of myositis were termed classic dermatomyositis. Although previous studies have used the terms amyopathic and hypomyopathic dermatomyositis to describe patients with minimal or no muscle weakness, these terms were not used in this study (3). Systemic immunosuppression for two months or greater in the first six months after skin disease onset is an exclusion criterion for amyopathic and hypomyopathic dermatomyositis (due to the theoretical possibility that such treatment prevented development of muscle disease), and all patients in this report received early immunosuppression for their lung disease and/or other symptoms. Patients with minimal or no muscle symptoms were thus classified as early-treated hypomyopathic dermatomyositis if there was objective evidence of myositis and early-treated amyopathic dermatomyositis if there was no such evidence.
All patients in this case series were part of a previous retrospective cohort study assessing the prevalence of ILD in dermatomyositis patients. The institutional review board at the University of Pennsylvania granted exempt approval for this study.
Results
All four patients experienced the onset of ILD within one year of the onset of their dermatomyositis symptoms. Two patients had early-treated amyopathic dermatomyositis, one patient had early-treated hypomyopathic dermatomyositis, and one patient had classic dermatomyositis. HRCT scans of the chest in all patients showed bibasilar-predominant ground glass and reticular opacities with mild or no honeycombing. The patients were all obese, with body mass indices of 30.7–52.1. See Table 1 for more detailed patient characteristics.
Table 1.
Patient characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age, gender, race | 65, F, Caucasian | 65, M, Caucasian | 37, F, Caucasian | 62, F, Caucasian |
| BMI | 36.0 | 30.7 | 52.1 | 38.6 |
| Smoking history | 40 packs years, quit when she was diagnosed with ILD | 17 pack years, quit approximately 30 years before dermatomyositis onset | 36 pack years, quit 3 years before dermatomyositis onset | Never smoked |
| Relevant PMH | Obstructive sleep apnea | Diabetes mellitus type 2 | Obstructive sleep apnea | None |
| Dermatomyositis type | Early-treated amyopathic | Classic | Early-treated amyopathic | Early-treated hypomyopathic |
| Years since dermatomyositis onset | 4 | 3 | 1.5 | 2 |
| Skin findings | Gottron’s papules, V-neck and back of the neck erythema, cuticular dystrophy, mechanic hands, periorbital edema | Gottron's sign, periungual erythema, mechanic hands, periorbital edema | Gottron’s sign, periungual erythema, dystrophic cuticles, erythematous scaly plaques on elbows | Gottron’s papules, V-neck and back of neck erythema, mechanic hands, heliotrope rash, periorbital edema |
| Muscle findings | Minimal/no muscle symptoms, normal CK and aldolase, normal MRI (bilateral thighs) | Proximal muscle weakness, CK 639, aldolase 18.6, EMG abnormal (inflammatory myopathy) | Minimal/no muscle symptoms; normal CK and aldolase | Minimal/no muscle symptoms, normal CK and aldolase, EMG abnormal (possible chronic myopathy) |
| Antibody status | ||||
| ANA | Negative | Negative | Negative | Negative |
| Anti-Jo-1 | Negative | Negative | Negative | Negative |
|
Years since ILD onset |
4 | 2.5 | 0.75 | 2 |
| Duration of MMF treatment | 3 years, 2 months | 1 year, 2 months | 5 months | 1 year, 1 month |
| Outcome of respiratory symptoms | Improved (no dyspnea) | Improved (no dyspnea) | No change | Improved (no dyspnea) |
| Outcome of PFTs (%predicted): MMF start, final, [lowest] | DLCO: 70, 88, [46] | DLCO: 37, 82, [37] | DLCO: 44, 77, [44] | DLCO: 66, 82, [66] |
| FVC: 99, 121, [77] | FVC: 76, 87, [76] | FVC: 65, 69, [65] | FVC: 73, 83, [64] | |
| TLC: 99, 89, [70] | TLC: 77, 85, [77] | TLC: 73, 71, [71] | TLC: 56, 86, [56] | |
| Outcome of HRCT changes | Improved (interstitial opacities completely resolved) | NA (no HRCT before treatment) | NA (no HRCT after treatment) | NA (no HRCT before treatment) |
| Prednisone dose at MMF start | 60 mg daily | 60 mg daily | 40 mg daily | 15 mg daily |
| Prednisone dose at latest date of follow-up | 0 mg daily | 4 mg daily | 40 mg daily | 4 mg daily |
ANA=antinuclear antibodies, BMI=body mass index, CK=creatine kinase, DLCO=diffusing capacity for carbon monoxide, EMG=electromyography, FVC=forced vital capacity, F=female, HRCT=high resolution computed tomography (of the chest), ILD=interstitial lung disease, MMF=mycophenolate mofetil, M=male, NA=not applicable, PFTs=pulmonary function tests, PMH=past medical history, TLC=total lung capacity
Upon being diagnosed with ILD, all patient were treated with 3000 mg daily of MMF in divided doses (titrated up from a starting dose of 1000–2000 mg daily) as first-line therapy. Three of four patients were also treated with high-dose prednisone (maximum dose 40–60 mg daily). The fourth patient had already been on prednisone for 8 months (maximum dose 60 mg daily) prior to her diagnosis with dermatomyositis and ILD, and her prednisone was tapered when MMF was added. All three patients with at least one year follow-up on MMF experienced complete normalization of PFTs and resolution of dyspnea. These patients were also able to substantially decrease their prednisone doses while on MMF (starting dose 15–60 mg daily, final dose 0–4 mg daily). The remaining patient had been treated with MMF for only 5 months at the latest date of follow-up and did not report improvement in her dyspnea but did have a large increase in diffusing capacity for carbon monoxide (DLCO; from 44% to 77% predicted) while taking MMF. See Table 1 for a summary of the outcome of each patient.
Although one patient had an episode of tinea pedis and onychomycosis while immunosuppressed with MMF and prednisone, the four patients reported no other adverse effects attributed to the MMF.
Patient 1 was referred to our dermatology clinic six months after beginning prednisone for newly diagnosed ILD (confirmed by HRCT and a DLCO of 46% predicted). Although her pulmonary disease and skin symptoms had responded well to high-dose prednisone (60 mg daily for two weeks, then 40 mg daily), tapering her prednisone below 40 mg daily had resulted in worsening skin and respiratory symptoms. Upon presentation to our dermatology clinic, the patient was diagnosed with early-treated amyopathic dermatomyositis. For treatment of her skin and lung disease, she was started on MMF, and her prednisone was increased to 60 mg daily.
After beginning MMF, the patient’s pulmonary symptoms slowly improved, and her prednisone was carefully tapered over a time span of greater than 2 years. Thirteen months after starting MMF (on prednisone 10.5 mg daily), the patient denied dyspnea, her PFTs were essentially within normal limits (DLCO was borderline low at 79% predicted), and HRCT chest imaging showed complete resolution of her previous parenchymal opacities (see Figure 1). Her skin symptoms also improved on the MMF and remained stable as her prednisone was tapered. At her latest follow-up, approximately 3 years after starting MMF, the patient’s PFTs were normal, she was asymptomatic from a respiratory standpoint, and her skin disease was stable.
Figure 1.
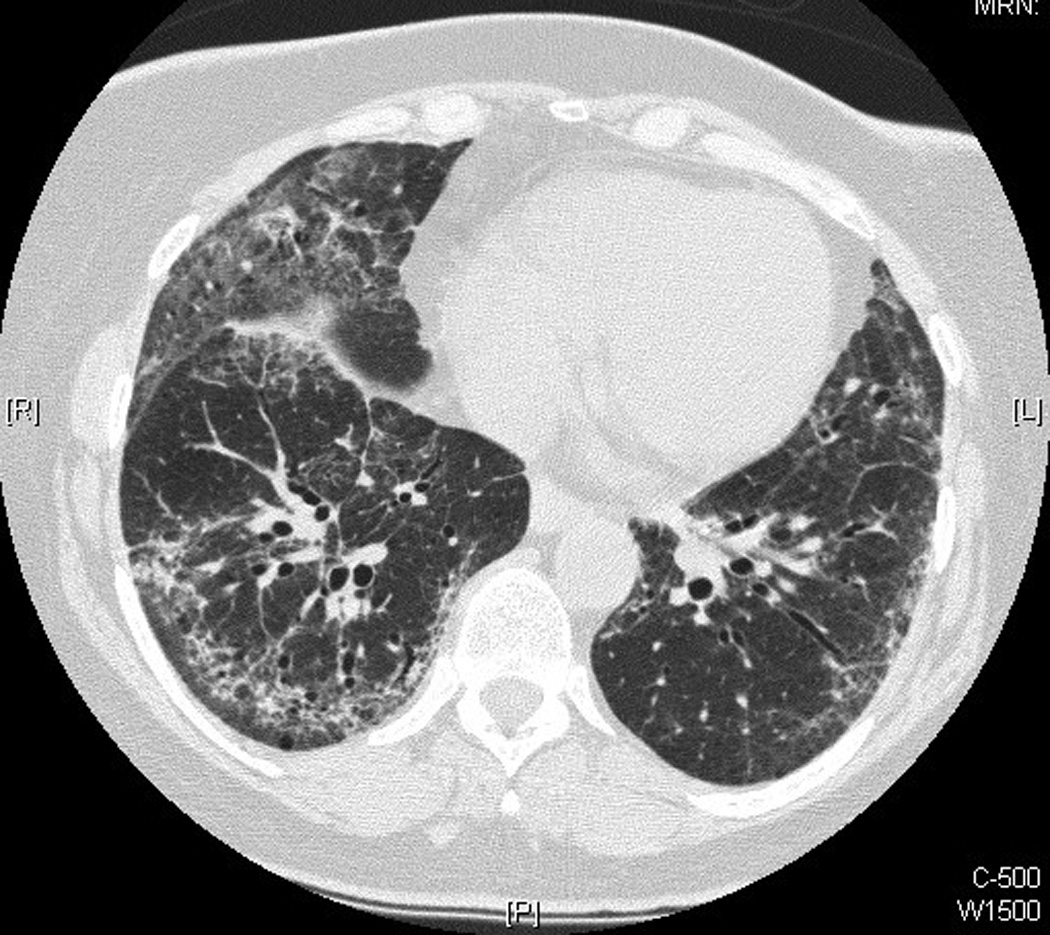
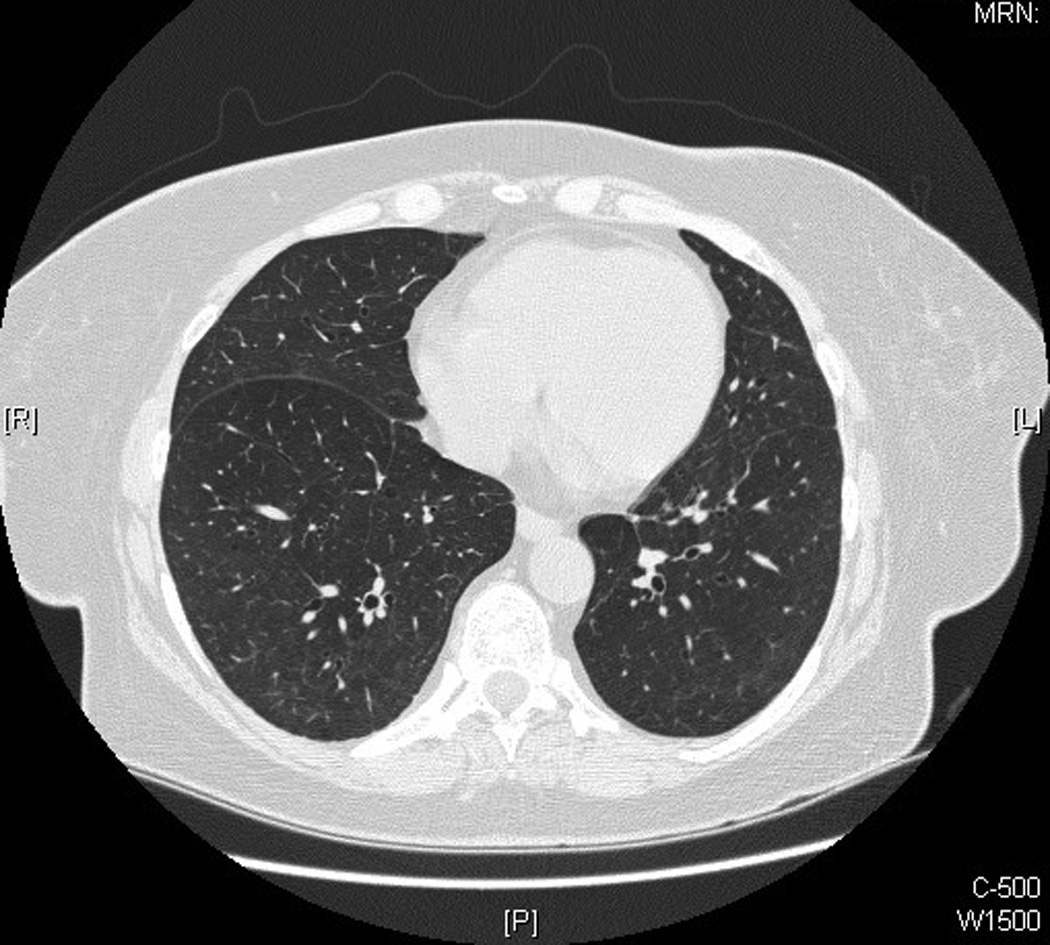
See Table 2 for details of the patient’s serial PFTs and her associated respiratory symptoms and prednisone and MMF doses.
Table 2.
Longitudinal pulmonary details
| Patient No. |
Months since MMF start |
MMF dose (mg daily) |
Pred- nisone dose (mg daily) |
DLCO | FVC | FEV1/ FVC (%) |
TLC | SpO2 (on room air) |
Wt (lbs) |
Medication changes after PFT results |
Dyspnea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −6 | 0 | 0 | 46 | 77 | 81 | 70 | 84% (walk) 93% (rest) |
217 | Began prednisone 60 mg daily | DOE for 2 months |
| 1 | −3 | 0 | 40 | 80 | 125 | 79 | 119 | 94% (walk) 97% (rest) |
217 | Began prednisone taper | DOE improved |
| 1 | −1 | 0 | 30 | 70 | 99 | 82 | 99 | NA | 217 | 1 month later: increased prednisone to 60 mg daily (taper after 2 months), MMF added | DOE increased |
| 1 | 13 | 3000 | 10.5 | 79 | 106 | 78 | 99 | 96% (rest) | 200 | Continued prednisone taper | No DOE |
| 1 | 24 | 3000 | 3 | 83 | 104 | 79 | 97 | 96% (rest) | 217 | Finished prednisone taper over next 4 months | No DOE |
| 1 | 37 | 2000 | 0 | 88 | 121 | 78 | 89 | 95% (rest) | 230 | Continued MMF taper (began taper 4 months ago) | No DOE |
| 2 | 0 | 0 | 0 | 37 | 76 | 83 | 77 | NA | 200 | Began prednisone 60 mg daily and MMF | Noted DOE in retrospect |
| 2 | 7 | 3000 | 25 | 76 | 82 | 77 | 83 | 97% (rest) | 216 | Continued prednisone taper (began taper 2 months ago) | No DOE |
| 2 | 14 | 2000 | 4 | 82 | 87 | 78 | 85 | 98% (rest) | 214 | Continued prednisone taper | No DOE |
| 3 | −1 | 0 | 7.5 | 44 | 65 | 87 | 73 | NA | 305 | 1 month later: added MMF, increased prednisone to 40 mg daily, stopped methotrexate (12.5 mg weekly) | Noted DOE in retrospect |
| 3 | 5 | 3000 | 40 | 77 | 69 | 86 | 71 | 100% (rest) | 363 | Started prednisone taper | No change in DOE |
| 4 | −1 | 0 | 15 | 66 | 73 | 69 | 56 | NA | 210 | 1 month later: MMF added, prednisone taper started | Mild DOE |
| 4 | 6 | 3000 | 10 | 69 | 64 | 78 | 77 | 99% (rest) | 207 | Continued prednisone taper | DOE improved |
| 4 | 12 | 3000 | 4 | 82 | 83 | 83 | 86 | 100% (rest) | 211 | Continued prednisone taper | No DOE |
All PFT values (DLCO, FVC, FEV1, TLC) except FEV1/FVC are percent of predicted value. DLCO=diffusing capacity for carbon monoxide, DOE=dyspnea on exertion, FEV1=forced expiratory volume in one second, FVC=forced vital capacity, MMF=mycophenolate mofetil, PFT=pulmonary function test, SpO2=saturation of peripheral oxygen, TLC=total lung capacity
Patient 2 denied dyspnea when he was diagnosed with classic dermatomyositis, but screening pulmonary function tests performed at the time of his diagnosis revealed a DLCO of 37% predicted. He was started on MMF and 60 mg daily prednisone for his muscle disease, skin symptoms, and suspected ILD. His ILD was later confirmed with HRCT imaging. The patient experienced a dramatic improvement in his energy level and skin and muscle symptoms during the three months after he started prednisone and MMF. He also reported that his breathing was improved (noting in retrospect that he had been short of breath for a year). Five months after initiating MMF he began a prednisone taper. At his latest date of follow-up (14 months after beginning MMF), he was on 4 mg daily of prednisone, he denied muscle or respiratory symptoms, his skin disease was stable, and his PFTs had completely normalized. HRCT imaging at this time showed stable ILD in comparison to imaging from 8 months prior, but no pre-MMF HRCT scans were available.
See Table 2 for details of the patient’s serial PFTs and his associated respiratory symptoms and prednisone and MMF doses.
Patient 3 had no pulmonary complaints when she was diagnosed with early-treated amyopathic dermatomyositis, but screening pulmonary function tests revealed a DLCO of 44% predicted, and a subsequent HRCT confirmed ILD. After her diagnosis of ILD, she noted in retrospect that she had experienced exertional dyspnea for the past few months. The patient was already on methotrexate and prednisone (7.5 mg daily) for treatment of inflammatory arthritis. Following her diagnosis of ILD, her prednisone was increased to 40 mg daily, and she was started on MMF. Her methotrexate was also discontinued at this time because ILD is rare but well-known toxicity of methotrexate. Five months after she started MMF (the latest date of follow-up), the patient’s skin symptoms had improved. Her dyspnea was unchanged, but her DLCO had increased from 44 to 77% predicted. Given the impressive improvement in DLCO and the likely multifactorial nature of her dyspnea (morbid obesity, obstructive sleep apnea), a prednisone taper was started. Notably, this patient’s history of methotrexate use prevents us from definitively determining the etiology of her ILD (methotrexate versus dermatomyositis) and the cause of her improved DLCO (addition of mycophenolate mofetil and prednisone dosage increase versus methotrexate discontinuation). However, dermatomyositis was felt to be a much more likely source of the patient’s ILD than methotrexate due to the time course of her ILD (chronic symptom onset and no rapid improvement following methotrexate withdrawal) and the rarity of methotrexate-induced interstitial lung disease (particularly chronic ILD) (4, 5).
See Table 2 for details of the patient’s serial PFTs and her associated respiratory symptoms and prednisone and MMF doses.
Patient 4 had already completed 8 months of prednisone (maximum dose 60 mg daily) therapy for her rash and cough when she was referred to our dermatology clinic and diagnosed with early-treated hypomyopathic dermatomyositis and ILD. At the time of her diagnosis, she was started on MMF and began a gradual taper of her prednisone (from 15 mg daily). The patient experienced an improvement in her respiratory symptoms over the subsequent months. One year after starting MMF (her latest date of follow-up), she was down to 4 mg prednisone daily, her pulmonary function tests were within normal limits, and she denied dyspnea. However, she still had active skin disease at this time.
See Table 2 for details of the patient’s serial PFTs and her associated respiratory symptoms and prednisone and MMF doses.
Discussion
In this small case series, all three patients with dermatomyositis-associated ILD who were treated for at least one year with MMF and prednisone experienced complete normalization of pulmonary function tests (including DLCO) and resolution of dyspnea. These patients were also able to reduce their daily prednisone doses. Although MMF has been disappointing in patients with idiopathic pulmonary fibrosis (6), this medication has recently begun to emerge as a potential treatment for ILD in CTD patients.
Several small retrospective studies (10–28 patients each) of CTD-ILD patients, including two scleroderma-only studies (7, 8) and two studies of miscellaneous CTD patients (9, 10), document stabilization of PFTs in the majority of patients after treatment with MMF (some patients also received glucocorticoids). Some of these studies also report improvement of respiratory symptoms (10) and decreased prednisone doses following MMF therapy (9, 10). Two small (<10 patients each), uncontrolled prospective studies focusing on scleroderma patients with recent-onset ILD who were treated with MMF and glucocorticoids as first-line therapy show improved PFTs (including DLCO), HRCT imaging, and symptoms in most patients (11, 12). Notably, in some of these studies (and in our report), many patients were treated with glucocorticoids and MMF, which limits our ability to determine the contribution of each individual drug to the clinical outcome.
The clinical outcome of our patients was excellent in comparison to the existing literature. Due to the small number of patients in our report, it is difficult to make any inferences from our data. However, given that our report is limited to dermatomyositis patients (including two patients with early-treated amyopathic dermatomyositis and one with early-treated hypomyopathic dermatomyositis) with newly diagnosed ILD, our experience suggests that this patient population may be particularly responsive to first-line therapy with MMF.
To our knowledge, this is the first report specifically documenting the use of MMF for treatment of dermatomyositis-associated ILD and the first report of CTD-ILD patients treated with MMF to include dermatomyositis patients with minimal or no muscle symptoms. One of the case series of CTD-ILD patients treated with MMF includes two polymyositis patients but no dermatomyositis patients (10). The other case series includes five polymyositis/dermatomyositis patients and one dermatomyosits/Sjogren’s syndrome patient, but the authors group all CTD types together when reporting their results and do not detail the clinical courses of individual patients or CTD groups (9).
Although our retrospective data collection may have underestimated the incidence of MMF-related side effects experienced by our patients, no patients were forced to reduce their MMF dosages or to discontinue MMF due to side effects. The low rate of side effects observed in this study is consistent with what has been reported in other CTD-ILD studies and with the favorable safety profile of MMF. Common side effects of MMF include gastrointestinal and urinary symptoms (usually resolve with continued use) and hematologic abnormalities (usually reversible with dose reduction or discontinuation) (13). MMF is also associated with an increased risk of infection, but opportunistic infections appear to be rare in the dermatology population (13). The risk of malignancy conferred by MMF treatment is uncertain; malignancies have been reported in psoriasis patients treated with MMF , but a cohort study of 85 psoriasis patients demonstrated no increased risk in MMF-treated patients relative to the general population (13).
Of note, all four patients in this report were obese. Although this finding may merely be coincidental, it is interesting since there is no known association between obesity and ILD or obesity and dermatomyositis.
Mycophenolic acid, the active form of mycophenolate mofetil, inhibits inosine monophosphate dehydrogenase, a rate-limiting enzyme for de novo synthesis of guanosine nucleotides (14). The end result is decreased T and B lymphocyte proliferation. MMF has also been shown to inhibit fibrosis via direct suppression of fibroblast function (14). The combination of immunosuppressive and antifibrotic properties may be especially helpful for treatment of autoimmune-associated fibrotic disease, including ILD.
Our own clinical experience and a recent retrospective review of 12 dermatomyositis patients demonstrate that MMF can be an effective steroid-sparing agent for recalcitrant skin and muscle manifestations of dermatomyositis (15). Although the small number of patients in this report limits our ability to make generalizations, our pulmonary data indicate that MMF may also be useful for ILD in dermatomyositis patients. Hopefully our promising results will encourage further exploration of this medication for treatment of dermatomyositis-associated ILD.
Acknowledgments
Funding/support: This material is based upon work supported by the National Institutes of Health, including NIH K24-AR 02207 (Werth) AND by a Merit Review Grant from the Department of Veterans Affairs Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
References
- 1.Fathi M, Lundberg IE. Interstitial lung disease in polymyositis and dermatomyositis. Curr Opin Rheumatol. 2005 Nov;17(6):701–706. doi: 10.1097/01.bor.0000179949.65895.53. [DOI] [PubMed] [Google Scholar]
- 2.Kameda H, Takeuchi T. Recent advances in the treatment of interstitial lung disease in patients with polymyositis/dermatomyositis. Endocr Metab Immune Disord Drug Targets. 2006 Dec;6(4):409–415. doi: 10.2174/187153006779025775. [DOI] [PubMed] [Google Scholar]
- 3.Gerami P, Schope JM, McDonald L, Walling HW, Sontheimer RD. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis sine myositis): A missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006 Apr;54(4):597–613. doi: 10.1016/j.jaad.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Dawson JK, Graham DR, Desmond J, Fewins HE, Lynch MP. Investigation of the chronic pulmonary effects of low-dose oral methotrexate in patients with rheumatoid arthritis: A prospective study incorporating HRCT scanning and pulmonary function tests. Rheumatology (Oxford) 2002 Mar;41(3):262–267. doi: 10.1093/rheumatology/41.3.262. [DOI] [PubMed] [Google Scholar]
- 5.Salaffi F, Manganelli P, Carotti M, Subiaco S, Lamanna G, Cervini C. Methotrexate-induced pneumonitis in patients with rheumatoid arthritis and psoriatic arthritis: Report of five cases and review of the literature. Clin Rheumatol. 1997 May;16(3):296–304. doi: 10.1007/BF02238967. [DOI] [PubMed] [Google Scholar]
- 6.Walter N, Collard HR, King TE., Jr Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006 Jun;3(4):330–338. doi: 10.1513/pats.200602-016TK. [DOI] [PubMed] [Google Scholar]
- 7.Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008 Feb;133(2):455–460. doi: 10.1378/chest.06-2861. [DOI] [PubMed] [Google Scholar]
- 8.Zamora AC, Wolters PJ, Collard HR, Connolly MK, Elicker BM, Webb WR, et al. Use of mycophenolate mofetil to treat scleroderma-associated interstitial lung disease. Respir Med. 2008 Jan;102(1):150–155. doi: 10.1016/j.rmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Swigris JJ, Olson AL, Fischer A, Lynch DA, Cosgrove GP, Frankel SK, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006 Jul;130(1):30–36. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 10.Saketkoo LA, Espinoza LR. Experience of mycophenolate mofetil in 10 patients with autoimmune-related interstitial lung disease demonstrates promising effects. Am J Med Sci. 2009 May;337(5):329–335. doi: 10.1097/MAJ.0b013e31818d094b. [DOI] [PubMed] [Google Scholar]
- 11.Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology (Oxford) 2006 Aug;45(8):1005–1008. doi: 10.1093/rheumatology/kei211. [DOI] [PubMed] [Google Scholar]
- 12.Vanthuyne M, Blockmans D, Westhovens R, Roufosse F, Cogan E, Coche E, et al. A pilot study of mycophenolate mofetil combined to intravenous methylprednisolone pulses and oral low-dose glucocorticoids in severe early systemic sclerosis. Clin Exp Rheumatol. 2007 Mar–Apr;25(2):287–292. [PubMed] [Google Scholar]
- 13.Zwerner J, Fiorentino D. Mycophenolate mofetil. Dermatol Ther. 2007 Jul–Aug;20(4):229–238. doi: 10.1111/j.1529-8019.2007.00136.x. [DOI] [PubMed] [Google Scholar]
- 14.Roos N, Poulalhon N, Farge D, Madelaine I, Mauviel A, Verrecchia F. In vitro evidence for a direct antifibrotic role of the immunosuppressive drug mycophenolate mofetil. J Pharmacol Exp Ther. 2007 May;321(2):583–589. doi: 10.1124/jpet.106.117051. [DOI] [PubMed] [Google Scholar]
- 15.Edge JC, Outland JD, Dempsey JR, Callen JP. Mycophenolate mofetil as an effective corticosteroid-sparing therapy for recalcitrant dermatomyositis. Arch Dermatol. 2006 Jan;142(1):65–69. doi: 10.1001/archderm.142.1.65. [DOI] [PubMed] [Google Scholar]


