Abstract
PURPOSE
To compare the effects of topical cyclosporine A 0.05% (Restasis) with those of prednisolone acetate 1.00% (Pred Forte) on corneal haze after photorefractive keratectomy (PRK).
SETTING
Gavin Herbert Eye Institute, University of California, Irvine–Orange, California, USA.
DESIGN
Experimental study.
METHODS
After −9.00 diopter PRK, 15 rabbits were divided into 3 groups and treated for 4 weeks with prednisolone acetate 1.00% or cyclosporine A 0.05% or neither (control). Corneal haze was measured by in vivo confocal microscopy preoperatively and 2, 4, 6, 8, and 12 weeks postoperatively. At 12 weeks, the corneas were evaluated for collagen organization by ex vivo 2-photon second-harmonic generation and stromal cell density.
RESULTS
Corneal haze was significantly less in the prednisolone acetate group than in the cyclosporine and control groups during the first 6 weeks postoperatively (P < .02). At 8 weeks, there was no significant difference between the 3 groups. There was no significant difference in haze between the cyclosporine group and control group at any time. The stroma was also significantly thinner in the prednisolone acetate group than in the other groups for the first 4 weeks postoperatively (P < .02). Second-harmonic generation scar thickness measurements at 12 weeks were not significantly different between the groups, although the prednisolone acetate group tended to have lower stromal cell density.
CONCLUSION
Cyclosporine A 0.05% had no effect on wound healing after PRK, while prednisolone acetate 1.00% significantly reduced peak corneal haze but had no effect on long-term corneal haze after discontinuation of the drug.
Photorefractive keratectomy (PRK) and laser in situ keratomileusis are commonly used procedures to correct refractive errors. However, opacification of the anterior stroma, better known as corneal haze, is a potential postsurgical complication that causes reduced contrast sensitivity, glare, and, in severe cases, reduced visual acuity.1,2 Clinically significant haze occurs in 0.5% to 5.0% of cases.3,4 Studies5,6 have shown that corneal haze is associated with the activation, migration, and differentiation of stromal keratocytes to corneal myofibroblasts that markedly scatter light and are seen as brightly reflecting cells by in vivo confocal microscopy. Myofibroblast differentiation is also known to be caused by the release of transforming growth factor-β (TGF-β), which leads to down regulation of keratocyte crystallin protein expression of transketolase and aldehyde dehydrogenase 3A1/1A1, which is thought to regulate stromal cell light scattering.7 Furthermore, blocking TGF-β–induced myofibroblast differentiation using neutralizing antibodies significantly reduces corneal haze after PRK.8
Infiltrating leukocytes and monocytes are a major source of TGF-β during wound healing and are generally thought to be involved in keratocyte activation and myofibroblast differentiation. To reduce the inflammatory response, postoperative topical steroidal agents, such as prednisolone acetate 1.00% (Pred Forte), are the current standard of care in the United States. These agents delay corneal wound healing and the appearance of myofibroblasts. However, the efficacy of steroid treatment remains questionable. In addition to its use after photorefractive surgery, prednisolone acetate 1.00% is used broadly for prophylaxis of inflammation after ocular surgery and for steroid-responsive inflammation of the conjunctiva, cornea, and anterior segment. Corticosteroidal agents, used cautiously, are some of the most potent and effective modalities of treatment available for ocular inflammation.9 However, cataract and elevated intraocular pressure are reported as adverse effects secondary to the use of steroidal agents.10–21 As alternatives to steroid therapy, studies have evaluated the effects of nonsteroidal antiinflammatory agents,22–25 tranilast,26 cysteine,27 and antioxidants such as vitamin E, among others.28
Cyclosporine A is an immunosuppressant with widespread use to control rejection of organ transplants and to treat autoimmune and inflammatory conditions. The mechanism of action includes inhibition of activation of T-lymphocytes and inhibition of apoptosis in a variety of cells.29 Ophthalmic use of cyclosporine formulated by a pharmacy with doses between 0.05% to 2.00% has been useful for several ocular inflammatory and immune diseases such as Sjögren syndrome, vernal keratoconjunctivitis, atopic keratoconjunctivitis, Theodore superior limbic keratoconjunctivitis, childhood ocular rosacea, Thygeson keratitis, Herpes simplex virus, Mooren ulcer, and corneal allograft rejection among others.30 In particular, cyclosporine has proven efficacious against inflammation associated with dry-eye disease.30–32 The purpose of this study was to evaluate whether a topical cyclosporine formulation (cyclosporine A 0.05% ophthalmic emulsion [Restasis]) that has been commercially available as a U.S. Food and Drug Administration–approved treatment for dry-eye syndrome is effective in reducing corneal haze compared with the standard topical steroid treatment (prednisolone acetate ophthalmic suspension 1.00%) after PRK in rabbits.
MATERIALS AND METHODS
Rabbits
Fifteen rabbits were used in this study. All procedures were approved by the University of California–Irvine Institutional Animal Care and Use Committee and conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Rabbits were anesthetized with intramuscular injections of xylazine (5 mg/kg) (Anased) and ketamine (22 mg/kg). Tetracaine hydrochloride 0.5% eyedrops were instilled into each right eye, and a speculum was used to open the eyelids. An 8.0 mm trephine (Bausch & Lomb) was used to mark the central cornea, after which the epithelium was removed with a Bard Parker #11 surgical blade (Becton Dickinson AcuteCare). Next, 6.00 mm diameter, −9.00 diopter PRK was performed using a LadarVision excimer laser (Alcon Laboratories, Inc.). After surgery, rabbits were randomly assigned to 1 of 3 groups of 5 rabbits each to receive prednisolone acetate 1.00%, cyclosporine A 0.05%, or neither (control). Prednisolone acetate 1.00% was administered 4 times a day during the first week and tapered over the next 3 weeks. Cyclosporine A 0.05% was administered twice a day for 4 weeks. In addition, eyes received topical gentamicin sulfate 3 times a day for 3 days and intramuscular buprenorphine (Buprenex, 0.1 mg/kg) twice a day as needed for relief of pain. After 12 weeks, the rabbits were humanely killed by an intravenous injection of sodium pentobarbital and phenytoin sodium (130 mg/kg and 17 mg/Kg, respectively).
In Vivo Confocal Microscopy
Under anesthesia, animals received a baseline scan and were then followed postoperatively at 2, 4, 6, 8, and 12 weeks using in vivo confocal microscopy to measure corneal thickness and haze as described previously.33 In vivo confocal microscopy examinations were performed using a tandem-scanning confocal microscope (Tandem Scanning Corp.) with a ×24 surface-contact objective (numerical aperture, 0.6; working distance, 1.5 mm). One drop of hydroxypropyl methylcellulose 2.5% (Gonak) was placed on the tip of the objective as a coupling gel. All camera settings were kept constant throughout the experiment. For each examination, repeated through-focus data sets were obtained from the central corneal region to identify the thinnest stromal region or center of the photoablated stromal surface. Three to 5 through-focus data sets were collected around this region for measurement of epithelial thickness, stromal thickness, and haze using purpose-designed software described in an earlier publication.34
Ex Vivo Confocal Microscopy
After the animals were humanely killed, the corneas were fixed by anterior chamber perfusion with paraformaldehyde 2% in phosphate-buffered saline (PBS) (pH 7.4). After perfusion, the corneas were dissected and left in 2% paraformaldehyde–PBS overnight. Corneal blocks (2.0 mm × 2.0 mm) from the central region were then scanned with a 510 Meta laser scanning confocal microscope (Zeiss) equipped with a mode-locked titanium–sapphire infrared femtosecond laser (Chameleon, Coherent, Inc.) for generation of nonlinear optical signals. Initially, corneal fibrosis was evaluated by assessing collagen organization using nonlinear optical second-harmonic generation. This nonlinear signal is generated from non-centrosymmetric materials, such as collagen, and has been used to measure corneal fibrosis and assess collagen organization in the cornea.35 Specifically, second-harmonic generation signals were generated using 800 nm infrared femtosecond laser pulses and the second harmonic-generation emission signal was collected at 400 nm. Second-harmonic generation forward-scattered signals passing through the tissue were collected using a 0.8 NA condenser lens with a narrow bandpass filter (400/50) placed in front of the transmission light detector. The backward-scattered second-harmonic generation signals were detected using a Meta detector (Zeiss) set to collect 390 to 410 nm bandwidth. After second-harmonic generation imaging, corneal tissue was stained en bloc with nucleic acid stain (DAPI, 300 nM, Molecular Probes, Invitrogen) in 50% TD buffer (dimethyl sulfoxide 0.5%, Triton X 0.5%, dextran 40 2.5% in PBS, pH 7.4) to quantify cell density. Nuclear staining was then detected by 2-photon excited fluorescence using the femtosecond laser tuned to 740 nm.
Cell Density and Corneal Fibrosis Measurements
Cell density was measured by counting the stromal cell nuclei in the anterior stroma immediately underlying the corneal epithelium within a volume measuring 225 µm × 225 µm × 50 µm deep into the stroma as described previously.36 Corneal fibrosis was assessed using second-harmonic generation by measuring the thickness of the irregular/fibrotic collagen layer underlying the corneal epithelium as described previously.35 Briefly, nonlinear optical imaging of second-harmonic generated signals was used to measure the thickness of the irregular collagen deposited along the anterior corneal surface 12 weeks after PRK. The thickness of the fibrotic layer was measured by determining the distance from the anterior stroma to the interface between fibrotic and normal collagen fibers. A mean value was then calculated for each image stack and the mean of 3 independent image stacks was taken to determine the thickness of the fibrotic layer for an individual eye.
Statistical Analysis
All results are reported as the mean ± standard deviation. Differences between treatment groups were assessed by 2-way repeated-measures analysis of variance and Bonferroni multiple comparisons (Sigma Stat version 3.11, Systat Software Inc.).
RESULTS
Epithelial and Stromal Thickness
After PRK, 14 eyes had an uneventful follow-up. One rabbit in the prednisolone acetate group developed infectious conjunctivitis that resolved with topical ofloxacin treatment for 7 days. This eye was removed from the study.
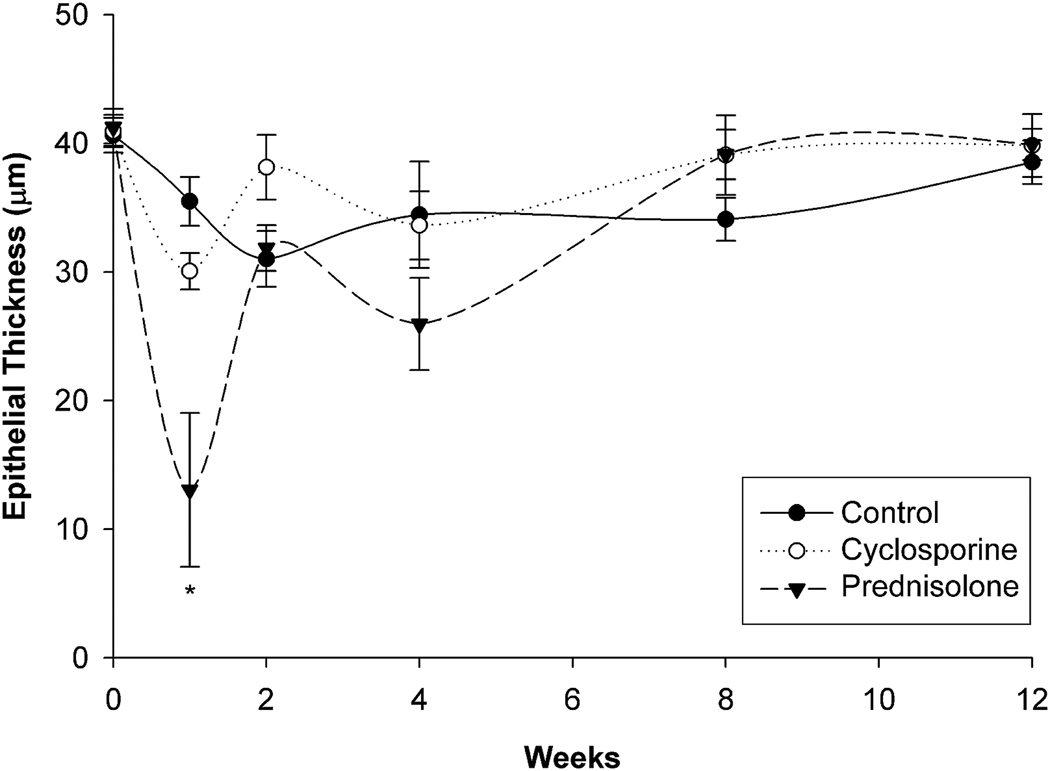
Figure 1 and Table 1 show the changes in epithelial thickness. In the control group, epithelial thickness was not significantly different at any time point after surgery. In the cyclosporine group, the epithelial thickness was significantly reduced 1 week after surgery compared with baseline (P = .002); there were no other significant differences between baseline and any other time point. In the prednisolone acetate group, there was also significant epithelial thinning 1 week after surgery (P < .005); the epithelium in this group was significantly thinner than in the other 2 groups (P < .02). The epithelium was also significantly thinner at the end of prednisolone acetate treatment (4 weeks) compared with baseline, although there was no significant difference with the thickness in the other 2 groups at that time point. No significant difference was detected between the 3 groups 12 weeks after PRK.
Figure 1.
Quantification of epithelial thickness after PRK (* = P < .02 compared with control and cyclosporine).
Table 1.
Epithelial thickness over time.
| Mean Epithelial Thickness (µm) ± SD | |||
|---|---|---|---|
| Week | Control | Cyclosporine | Prednisolone Acetate |
| Baseline | 40 ± 3 | 40 ± 2 | 41 ± 2 |
| 1 | 35 ± 4 | 30 ± 3* | 13 ± 11*† |
| 2 | 31 ± 4 | 38 ± 5 | 31 ± 3 |
| 4 | 34 ± 9 | 33 ± 5 | 25 ± 7* |
| 6 | 34 ± 5 | 33 ± 5 | 36 ± 6 |
| 8 | 34 ± 3 | 39 ± 6 | 39 ± 3 |
| 12 | 38 ± 3 | 39 ± 5 | 39 ± 2 |
Note: All statistical comparisons by 2-way repeated-measures analysis of variance (1-factor repetition)
P < .005 compared with baseline in each group
P < .02 compared with control and ![]() groups
groups
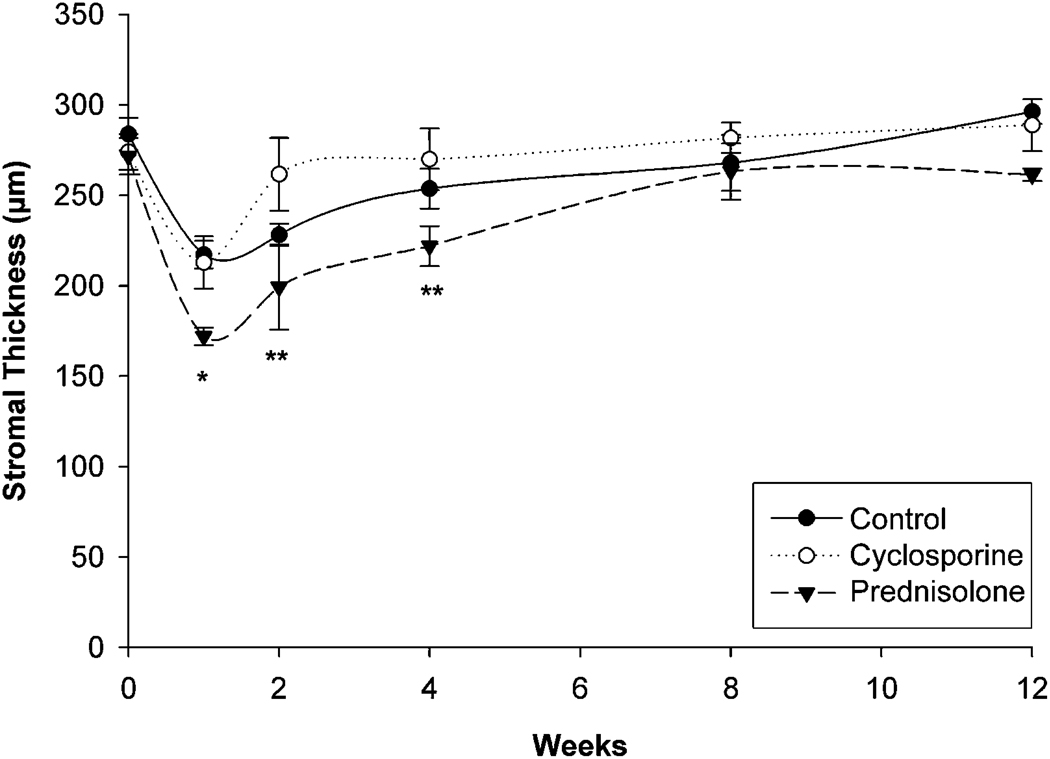
Figure 2 and Table 2 show the changes in stromal thickness. All groups had significant stromal thinning 1 week after PRK (P < .005), with a gradual return of stromal thickness over time. The stroma in the prednisolone acetate group, however, showed significant thinning up to 4 weeks after PRK (P < .005) and was significantly thinner than in the other 2 groups up to 6 weeks after surgery (P < .02). These findings suggest that prednisolone acetate significantly delayed the normal wound-healing response compared with that in the control and cyclosporine groups.
Figure 2.
Quantification of stromal thickness after PRK (* = P < .02 compared with control and cyclosporine; ** = P < .02 compared with cyclosporine).
Table 2.
Stromal thickness over time.
| Mean Stromal Thickness (µm) ± SD | |||
|---|---|---|---|
| Week | Control | Cyclosporine | Prednisolone Acetate |
| Baseline | 283 ± 19 | 273 ± 23 | 271 ± 19 |
| 1 | 217 ± 15* | 212 ± 30* | 171 ± 11*† |
| 2 | 228 ± 12* | 261 ± 34 | 199 ± 39*‡ |
| 4 | 253 ± 19 | 269 ± 35 | 221 ± 17*‡ |
| 6 | 278 ± 23 | 284 ± 33 | 237 ± 26† |
| 8 | 267 ± 34 | 281 ± 18 | 263 ± 31 |
| 12 | 296 ± 15 | 288 ± 32 | 261 ± 6 |
Note: All statistical comparisons by 2-way repeated-measures analysis of variance (1-factor repetition
P < .005 compared with baseline in each group
P < .02 compared with control and ![]() groups
groups
P < .02 compared with ![]() alone
alone
Haze Formation
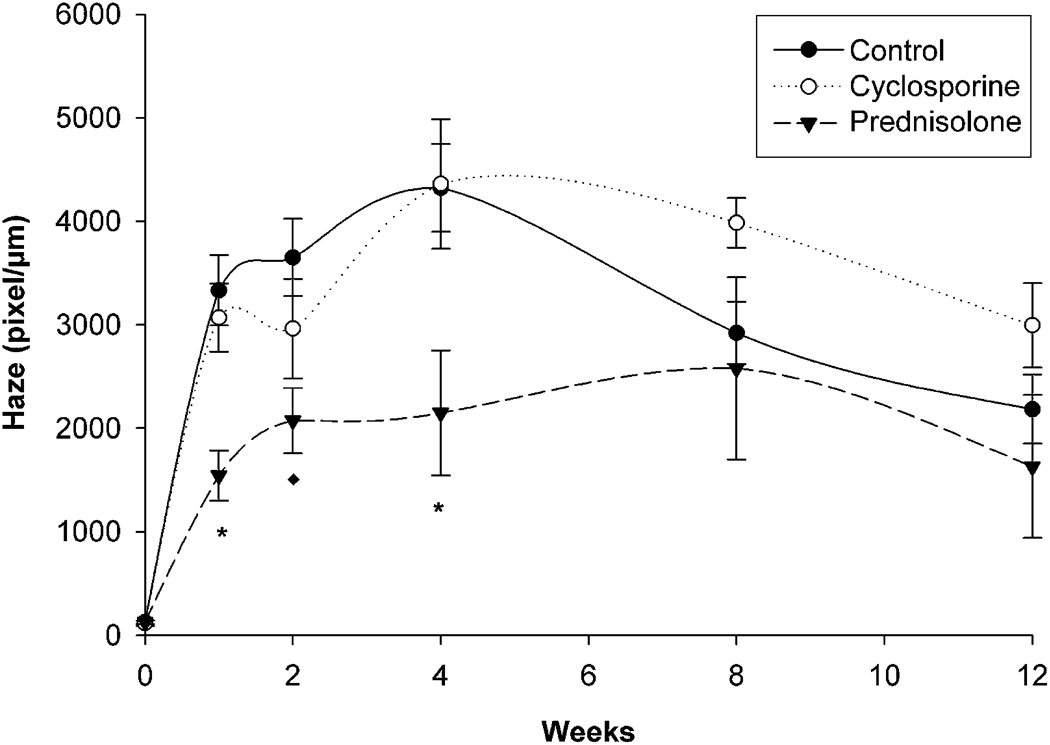
Figure 3 and Table 3 show the changes in corneal haze. In the control group, corneal haze increased up to 4 weeks after PRK, peaked, and then gradually declined up to 12 weeks, although it remained significantly elevated above baseline (P < .005). A similar pattern was observed in the cyclosporine group, with no significant differences between that group and the control group. Although the pattern was similar in the prednisolone acetate group, the level of haze was significantly less than in the control and cyclosporine groups from 1 week to 6 weeks after surgery (P < .02). Furthermore, the level of haze at 12 weeks was not significantly different than baseline levels in the prednisolone acetate group.
Figure 3.
Quantification of corneal haze after PRK (*= P < .02 compared with control and cyclosporine; ** P < .02 compared with control).
Table 3.
Corneal haze over time.
| Mean Corneal Haze (Pixels/µm) ± SD | |||
|---|---|---|---|
| Week | Control | Cyclosporine | Prednisolone Acetate |
| Baseline | 126 ± 37 | 115 ± 62 | 124 ± 38 |
| 1 | 3332 ± 766* | 3069 ± 737* | 1542 ± 486*† |
| 2 | 3650 ± 832* | 2962 ± 1075* | 2072 ± 631*‡ |
| 4 | 4323 ± 949* | 4360 ± 1398* | 2148 ± 1206*† |
| 6 | 3656 ± 1253* | 3620 ± 585* | 2050 ± 1094*† |
| 8 | 2920 ± 672* | 3984 ± 534* | 2577 ± 1761* |
| 12 | 2184 ± 748* | 2993 ± 910* | 1629 ± 1381 |
Note: All statistical comparisons by 2-way repeated-measures analysis of variance (1-factor repetition)
P < .005 compared with baseline in each group
P < .02 compared with control and ![]() groups
groups
P < .02 compared with control group alone
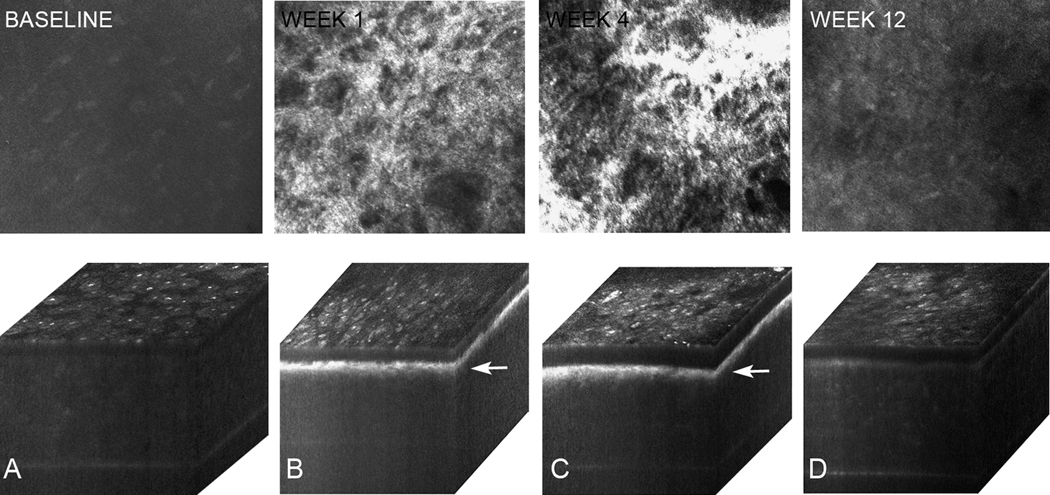
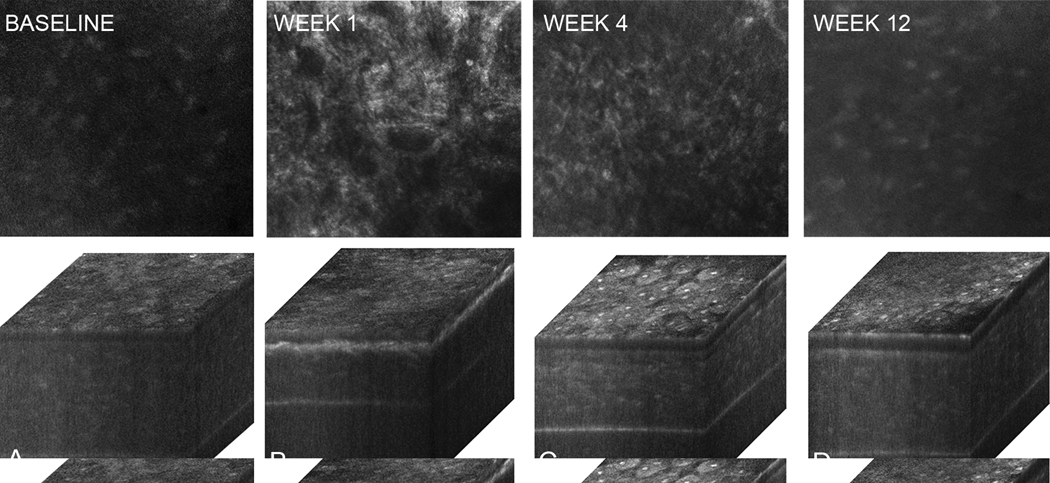
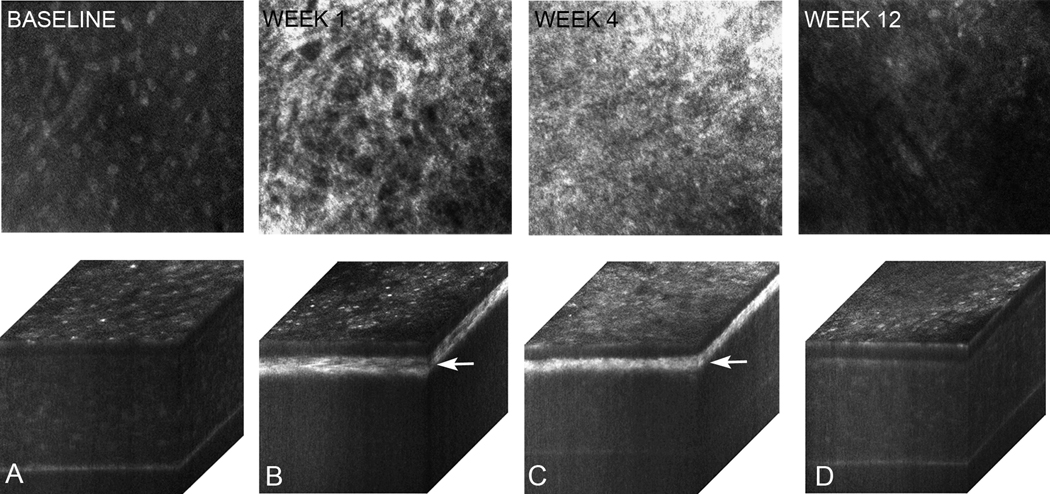
Figures 4 to 6 show in vivo confocal microscopy single images taken within the anterior stroma (46 µm depth from the superficial epithelium) and 3-dimensional (3-D) orthogonal cabinet projections of rabbit corneas 1, 4, and 12 weeks after PRK in the control group (Figure 4), cyclosporine group (Figure 5), and prednisolone acetate group (Figure 6). The single images show anterior stroma containing highly reflective myofibroblasts in the control group and cyclosporine group eyes. Images from the prednisolone acetate group show a noticeable lack of myofibroblasts 1 week and 4 weeks after PRK. Marked light scattering in the anterior stroma in the orthogonal cabinet projections was also detected in the control group and cyclosporine group 1 weeks and 4 weeks after PRK; the scattering was markedly reduced by 12 weeks. In the prednisolone acetate group, increased light scattering was noticed at 1 week, although it was significantly less than in the control and cyclosporine groups. The scattering in the prednisolone acetate group declined progressively at 4 weeks and 12 weeks. Overall, these findings suggest that prednisolone acetate 1.00% treatment reduced corneal haze by delaying wound healing and the appearance of myofibroblasts in the wound and confirm the role of cellular light scattering in the development of corneal haze.
Figure 4.
In vivo confocal microscopy through-focusing single images of anterior stroma obtained at 46 µm depth from the surface epithelium and 3-D orthogonal projections of the same eye at baseline (A) and postoperatively at 1 week (B), 4 weeks (C), and 12 weeks (D) in the control group. Note the increased reflectivity (arrows) at 1 and 4 weeks after PRK that gradually returned toward normal at 12 weeks.
Figure 6.
In vivo confocal microscopy through focusing single images of anterior stroma obtained at 46 µm depth from the surface epithelium and 3-D orthogonal projections of the same eye at baseline (A) and postoperatively at 1 week (B), 4 weeks (C), and 12 weeks (D) in the prednisolone acetate group. Note that that these eyes show much less haze at 1 week (A) with fewer myofibroblasts in the anterior stroma.
Figure 5.
In vivo confocal microscopy through focusing single images of anterior stroma obtained at 46 µm depth from the surface epithelium and 3-D orthogonal projections of the same eye at baseline (A) and postoperatively at 1 week (B), 4 weeks (C), and 12 weeks (D) in the cyclosporine group. Similar to the control eyes, there was an increased reflectivity (arrows) at 1 week and 4 weeks that gradually returned toward normal at 12 weeks.
Corneal Fibrosis and Stromal Cell Density
Based on measurements using nonlinear optical imaging of second-harmonic generation signals, the scar thickness at 12 weeks was not significantly different between the 3 groups. The mean thickness was 57 ± 11 µm in the control group, 55 ± 14 µm in the cyclosporine group, and 54 ± 9 µm in the prednisolone acetate group. These results indicate that although prednisolone acetate 1.00% reduced light scattering and delayed wound healing and myofibroblast differentiation, the delay did not affect the long-term wound-healing response and the deposition of fibrotic tissue after termination of treatment.
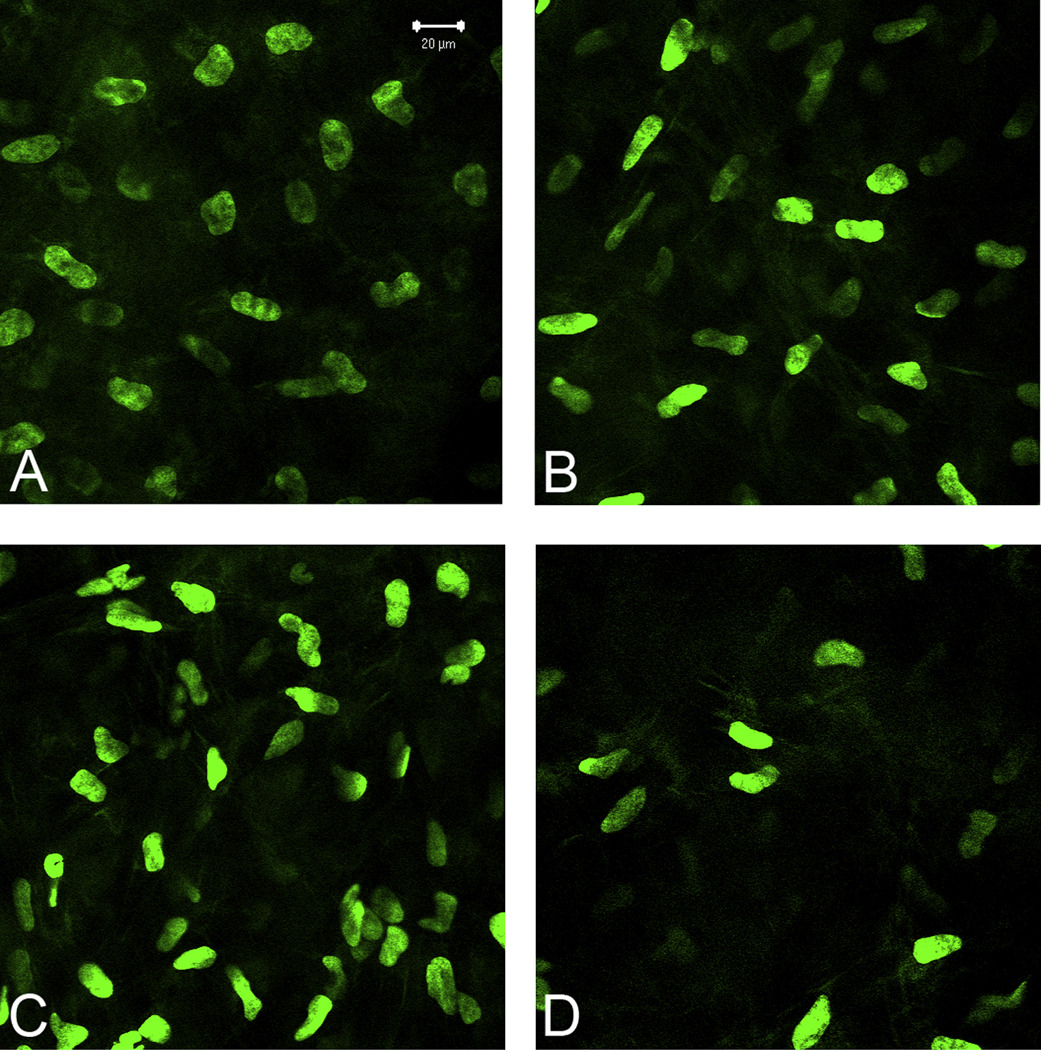
Quantification of cell density in the anterior stroma found that eyes in the prednisolone acetate group had a lower cell density than eyes in the other 2 groups (Figure 7). The mean measured density just below the epithelium was 43 723 ± 20 490 cells/mm3 in the prednisolone acetate group, 67 080 ± 29 516 cells/mm3 in the cyclosporine group, and 63 077 ± 18 887cells/mm3 in the control group. However, there was no significant difference between any of the groups (P = .1), perhaps because the power of the test with α = 0.050 was 0.242, well below the desired power of 0.800. Nevertheless, the findings suggest that delayed wound healing by prednisolone acetate 1.00% may suppress the normal cellular infiltration into the wound but does not affect long-term fibrosis.
Figure 7.
Single plane images showing nuclear density (green) of corneas at (A) baseline and in the (B) control group, (C) cyclosporine group, and (D) prednisolone acetate group. Images were obtained within the anterior stroma (44 µm from the surface epithelium) in the central area of ablation. Note the reduced cell density in the prednisolone acetate eye.
DISCUSSION
In this study, we compared the effects of prednisolone acetate 1.00% (Pred Forte) eyedrops with those of cyclosporine 0.05% (Restasis) on corneal haze, stromal cell density, and fibrosis. Although steroid treatment significantly delayed corneal wound healing and the appearance of postsurgical corneal haze during treatment, there was no significant long-term effect on the deposition of fibrotic tissue or corneal haze. In contrast, cyclosporine 0.05% had no effect on the development of corneal haze or fibrosis.
Previous studies6,8,37 found that repopulation of the anterior stroma with activated keratocytes and myofibroblasts after PRK increased cellular light scattering and corneal haze and was dependent in part on the release of the inflammatory cytokine TGF-β. Modulation of the inflammatory response in the first 72 hours after PRK has also been shown to reduce corneal haze formation and affect visual outcomes.19,28 In this context, steroids, well known as antiinflammatory agents that inhibit immune response, collagen synthesis, and neovascularization, have been used widely to prevent corneal haze. Our data support this contention and indicate that steroidal agents inhibit the formation of haze during the first month after PRK. This reduction in haze appears to be due in part to a delay in the wound-healing response, as evidenced by the significantly thinner corneal stroma up to 4 weeks after surgery. In addition, steroidal agents appear to block the appearance of myofibroblasts, as seen by the reduction in cellular light scattering in the anterior cornea, and they significantly reduced the overall level of corneal haze. However, suspension of steroid therapy 1 month after surgery was followed by the reappearance of haze at 8 weeks. Taken together, our study is in agreement with others that report a transient effect of steroidal agents in reducing haze21 and question the long-term effectiveness of these agents in preventing corneal haze and loss of visual performance.14–17
Cyclosporine A is thought to inhibit inflammation by reducing proinflammatory cytokines, markers of apoptosis and activated T-lymphocytes.29,31,38,39 In our study, topical cyclosporine A, used at 0.05% twice a day, had no effect on corneal wound healing. Recently, Shii et at.40 showed that cyclosporin A eyedrops can inhibit fibrosis and inflammatory cell infiltration in murine type 1 allergic conjunctivitis by affecting the expression of interleukin (IL)-4, IL-5, and Thy2 cytokine production without suppressing the expression of TGF-β. This response was different from that of steroidal agents, which suppressed TGF-β in addition to IL-4 and IL-5. Because TGF-β is a key inflammatory cytokine that regulates keratocyte activation and myofibroblast differentiation in the cornea, the lack of effect of cyclosporine 0.05% on corneal haze may be related to its inability to suppress TGF-β synthesis, TGF-β release, or both. Additional studies are needed to evaluate whether higher doses of cyclosporine or combined therapy would affect long-term haze formation after photorefractive surgery.
In conclusion, we suggest that cyclosporine 0.05% has no effect on corneal wound healing after PRK while prednisolone acetate 1.00% significantly reduces peak corneal haze but has no effect on long-term corneal fibrosis after its discontinuation.
Acknowledgments
Supported by the National Eye Institute, Bethesda, Maryland (grants EY07348, EY016663), Discovery Eye Foundation, the Skirball Program in Molecular Ophthalmology, and by a grant from Research to Prevent Blindness, Inc., New York, New York, USA.
Footnotes
Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
REFERENCES
- 1.Alió JL, Artola A, Claramonte PJ, Ayala MJ, Sánchez SP. Complications of photorefractive keratectomy for myopia: two year follow-up of 3000 cases. J Cataract Refract Surg. 1998;24:619–626. doi: 10.1016/s0886-3350(98)80256-3. [DOI] [PubMed] [Google Scholar]
- 2.Loewenstein A, Lipshitz I, Varssano D, Lazar M. Complications of excimer laser photorefractive keratectomy for myopia. J Cataract Refract Surg. 1997;23:1174–1176. doi: 10.1016/s0886-3350(97)80311-2. [DOI] [PubMed] [Google Scholar]
- 3.Meyer JC, Stulting RD, Thompson KP, Durrie DS. Late onset of corneal scar after excimer laser photorefractive keratectomy. Am J Ophthalmol. 1996;121:529–539. doi: 10.1016/s0002-9394(14)75427-3. [DOI] [PubMed] [Google Scholar]
- 4.Seiler T, Wollensak J. Results of a prospective evaluation of photorefractive keratectomy at 1 year after surgery. Ger J Ophthalmol. 1993;2:135–142. [PubMed] [Google Scholar]
- 5.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 6.Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 7.Jester JV. Corneal crystallins and the development of cellular transparency. [Accessed December 23, 2010];Semin Cell Dev Biol. 2008 19:82–93. doi: 10.1016/j.semcdb.2007.09.015. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2275913/pdf/nihms-42563.pdf. [DOI] [PMC free article] [PubMed]
- 8.Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGFβ modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr Eye Res. 1998;17:736–747. [PubMed] [Google Scholar]
- 9.McGhee CNJ, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids; benefits and risks. Drug Saf. 2002;25:33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Aras C, Özdamar A, Aktunç R, Erçikan C. The effects of topical steroids on refractive outcome and corneal haze, thickness, and curvature after photorefractive keratectomy with a 6.0-mm ablation diameter. Ophthalmic Surg Lasers. 1998;29:621–627. [PubMed] [Google Scholar]
- 11.Baek S-H, Chang J-H, Choi S-Y, Kim W-J, Lee J-H. The effect of topical corticosteroids on refractive outcome and corneal haze after photorefractive keratectomy. J Refract Surg. 1997;13:644–652. doi: 10.3928/1081-597X-19971101-11. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee A, Shah S, Galway G. Effects of topical corticosteroids after photorefractive keratectomy. J Refract Surg. 1997;13:S454–S455. doi: 10.3928/1081-597X-19970801-18. [DOI] [PubMed] [Google Scholar]
- 13.Choi SY, Kim HY, Kim JY, Wee WR, Lee JH. Two-year follow-up of eyes without topical corticosteroid treatment after photorefractive keratectomy (PRK) [Accessed December 23, 2010];Korean J Ophthalmol. 1998 12:25–29. doi: 10.3341/kjo.1998.12.1.25. Available at: http://ekjo.org/Synapse/Data/PDFData/0065KJO/kjo-12-25.pdf. [DOI] [PubMed]
- 14.Corbett MC, O’Brart DPS, Marshall J. Do topical corticosteroids have a role following excimer laser photorefractive keratectomy? J Refract Surg. 1995;11:380–387. doi: 10.3928/1081-597X-19950901-15. [DOI] [PubMed] [Google Scholar]
- 15.Gartry DS, Kerr Muir MG, Lohmann CP, Marshall J. The effect of topical corticosteroids on refractive outcome and corneal haze after photorefractive keratectomy; a prospective, randomized, double-blind trial. Arch Ophthalmol. 1992;110:944–952. doi: 10.1001/archopht.1992.01080190050028. [DOI] [PubMed] [Google Scholar]
- 16.Machat JJ. Double-blind corticosteroid trial in identical twins following photorefractive keratectomy. Refract Corneal Surg. 1993;9 suppl:S105–S107. [PubMed] [Google Scholar]
- 17.O’Brart DPS, Lohmann CP, Klonos G, Corbett MC, Pollock WST, Kerr-Muir MG, Marshall J. The effects of topical corticosteroids and plasmin inhibitors on refractive outcome, haze, and visual performance after photorefractive keratectomy; a prospective, randomized, observer-masked study. Ophthalmology. 1994;101:1565–1574. doi: 10.1016/s0161-6420(94)38032-8. [DOI] [PubMed] [Google Scholar]
- 18.Tengroth B, Fagerholm P, Soderberg P, Hamberg-Nystrom H, Epstein D. Effect of corticosteroids in postoperative care following photorefractive keratectomies. Refract Corneal Surg. 1993;9 suppl:S61–S64. [PubMed] [Google Scholar]
- 19.Vetrugno M, Maino A, Quaranta GM, Cardia L. The effect of early steroid treatment after PRK on clinical and refractive outcomes. [Accessed December 23, 2010];Acta Ophthalmol Scand. 2001 79:23–27. doi: 10.1034/j.1600-0420.2001.079001023.x. Available at: http://onlinelibrary.wiley.com/doi/10.1034/j.1600-0420.2001.079001023.x/pdf. [DOI] [PubMed]
- 20.Yamaguchi T, Murat D, Kimura I, Negishi K, Yuki K, Tsubota K, Ohtake Y. Diagnosis of steroid-induced glaucoma after photorefractive keratectomy. J Refract Surg. 2008;24:413–415. doi: 10.3928/1081597X-20080401-17. [DOI] [PubMed] [Google Scholar]
- 21.You X, Bergmanson JP, Zheng XM, MacKenzie IC, Boltz RL, Aquavella JV. Effect of corticosteroids on rabbits corneal keratocytes after photorefractive keratectomy. J Refract Surg. 1995;11:460–467. doi: 10.3928/1081-597X-19951101-12. [DOI] [PubMed] [Google Scholar]
- 22.Arshinoff SA, Mills MD, Haber S. Pharmacotherapy of photorefractive keratectomy. J Cataract Refract Surg. 1996;22:1037–1044. doi: 10.1016/s0886-3350(96)80116-7. [DOI] [PubMed] [Google Scholar]
- 23.Baek S-H, Choi S-Y, Chang J-H, Wee W-R, Lee J-H. Short-term effects of flurbiprofen and diclofenac on refractive outcome and corneal haze after photorefractive keratectomy. J Cataract Refract Surg. 1997;23:1317–1323. doi: 10.1016/s0886-3350(97)80109-5. [DOI] [PubMed] [Google Scholar]
- 24.Durrie DS, Kennard MG, Boghossian AJ. Effects of nonsteroidal ophthalmic drops on epithelial healing and pain in patients undergoing bilateral photorefractive keratectomy (PRK) Adv Ther. 2007;24:1278–1285. doi: 10.1007/BF02877774. [DOI] [PubMed] [Google Scholar]
- 25.Kaji Y, Amano S, Oshika T, Obata H, Ohashi T, Sakai H, Shirasawa E, Tsuru T, Yamashita H. Effect of anti-inflammatory agents on corneal wound-healing process after surface excimer laser keratectomy. J Cataract Refract Surg. 2000;26:426–431. doi: 10.1016/s0886-3350(99)00358-2. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto S, Sakai T, Iwaki Y, Tobari I, Hamano S. Effects of tranilast on cultured rabbit corneal keratocytes and corneal haze after photorefractive keratectomy. Jpn J Ophthalmol. 1999;43:355–362. doi: 10.1016/s0021-5155(99)00103-3. [DOI] [PubMed] [Google Scholar]
- 27.Meduri A, Grenga PL, Scorolli L, Ceruti P, Ferreri G. Role of cysteine in corneal wound healing after photorefractive keratectomy. Ophthalmic Res. 2009;41:76–82. doi: 10.1159/000187623. [DOI] [PubMed] [Google Scholar]
- 28.Bilgihan A, Bilgihan K, Yis Ö, Sezer C, Akyol G, Hasanreisoglu B. Effects of topical vitamin E on corneal superoxide dismutase, glutathione peroxidase activities and polymorphonuclear leucocyte infiltration after photorefractive keratectomy. [Accessed December 23, 2010];Acta Ophthalmol Scand. 2003 81:177–180. doi: 10.1034/j.1600-0420.2003.00042.x. Available at: http://onlinelibrary.wiley.com/doi/10.1034/j.1600-0420.2003.00042.x/pdf. [DOI] [PubMed]
- 29.Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Jabs DA, Levy-Clarke GA, Foster CS. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117:576–584. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54:321–338. doi: 10.1016/j.survophthal.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Perry HD, Donnenfeld ED. Topical 0.05% cyclosporin in the treatment of dry eye. Expert Opin Pharmacother. 2004;5:2099–2107. doi: 10.1517/14656566.5.10.2099. [DOI] [PubMed] [Google Scholar]
- 32.Pflugfelder SC. Integrating Restasis into the management of dry eye. Int Ophthalmol Clin. 2006;46(4):101–103. doi: 10.1097/01.iio.0000212137.85298.98. [DOI] [PubMed] [Google Scholar]
- 33.Jester JV, Petroll WM, Cavanagh HD. Measurement of tissue thickness using confocal microscopy. Methods Enzymol. 1999;307:230–245. doi: 10.1016/s0076-6879(99)07016-0. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Jester JV, Cavanagh HD, Black TD, Petroll WM. On-line 3-dimensional confocal imaging in vivo. [Accessed December 23, 2010];Invest Ophthalmol Vis Sci. 2000 41:2945–2953. Available at: http://www.iovs.org/content/41/10/2945.full.pdf. [PubMed]
- 35.Farid M, Morishige N, Lam L, Wahlert A, Steinert RF, Jester JV. Detection of corneal fibrosis by imaging second harmonic-generated signals in rabbit corneas treated with mitomycin C after excimer laser surface ablation. [Accessed December 23, 2010];Invest Ophthalmol Vis Sci. 2008 49:4377–4383. doi: 10.1167/iovs.08-1983. Available at: http://www.iovs.org/content/49/10/4377.full.pdf. [DOI] [PMC free article] [PubMed]
- 36.Petroll WM, Cavanagh HD, Jester JV. Three-dimensional imaging of corneal cells using in vivo confocal microscopy. J Microsc. 1993;170:213–219. doi: 10.1111/j.1365-2818.1993.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 37.Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Fruman DA, Klee CB, Bierer BE, Burakoff SJ. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. [Accessed December 23, 2010];Proc Natl Acad Sci USA. 1992 89:3686–3690. doi: 10.1073/pnas.89.9.3686. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC525555/pdf/pnas01083-0026.pdf. [DOI] [PMC free article] [PubMed]
- 39.Halloran PF, Kung L, Noujaim J. Calcineurin and the biological effect of cyclosporine and tacrolimus. Transplant Proc. 1998;30:2167–2170. doi: 10.1016/s0041-1345(98)00577-6. [DOI] [PubMed] [Google Scholar]
- 40.Shii D, Nakagawa S, Shinomiya K, Yoshimi M, Katsuta O, Oda T, Nakamura M. Cyclosporin A eye drops inhibit fibrosis and inflammatory cell infiltration in murine type I allergic conjunctivitis without affecting the early-phase reaction. Curr Eye Res. 2009;34:426–437. doi: 10.1080/02713680902866980. [DOI] [PubMed] [Google Scholar]