Abstract
Autophagy is a bulk degradation process in which cytosolic proteins and organelles are degraded through lysosomes. To evaluate autophagic flux in cardiac myocytes, we generated adenovirus and cardiac-specific transgenic mice harboring tandem fluorescent mRFP-GFP-LC3. Starvation significantly increased the number of mRFP-GFP-LC3 dots representing both autophagosomes and autolysosomes per cell, suggesting that autophagic flux is increased in cardiac myocytes. H2O2 significantly increased autophagic flux, which was attenuated in the presence of N-2-mercaptopropionyl glycine (MPG), an antioxidant, suggesting that oxidative stress stimulates autophagy in cardiac myocytes. Myocardial ischemia/reperfusion (I/R) increased both autophagosomes and autolysosomes, thereby increasing autophagic flux. Treatment with MPG attenuated I/R-induced increases in oxidative stress, autophagic flux, and Beclin-1 expression, accompanied by a decrease in the size of myocardial infarction (MI)/area at risk (AAR), suggesting that oxidative stress plays an important role in mediating autophagy and myocardial injury during I/R. MI/AAR after I/R was significantly reduced in beclin1+/− mice, whereas beclin1+/− mice treated with MPG exhibited no additional reduction in the size of MI/AAR after I/R. These results suggest that oxidative stress plays an important role in mediating autophagy during I/R, and that activation of autophagy through oxidative stress mediates myocardial injury in response to I/R in the mouse heart. Antioxid. Redox Signal. 14, 2179–2190.
Introduction
Autophagy is a bulk degradation process in which cytosolic proteins and organelles are sequestrated by double-membrane structures of unknown origin called autophagosomes, transferred to lysosomes, and degraded by proteases therein (14). Autophagy is generally an adaptive mechanism by which amino acids and fatty acids are recycled for ATP production, and damaged organelles, protein aggregates, and exogenous pathogens, all of which could lead to cellular malfunction and cell death, are removed (15). However, excessive activation of autophagy can be toxic and may even induce cell death, termed type II programmed cell death (15).
Increasing lines of evidence suggest that autophagy is activated during various pathologic conditions in the heart (25). For example, both chronic and acute myocardial ischemia activate autophagy in the myocardium (18, 33), and autophagy also is activated during cardiac hypertrophy, atrophy, and heart failure (25). However, the function of autophagy in the heart appears to be stimulus dependent. For example, we showed previously that although autophagy can be protective during acute myocardial ischemia and chronic hibernation, it can be detrimental during myocardial reperfusion after a short period of ischemia [ischemia/reperfusion (I/R)] (18, 32). Because autophagy plays an important role in mediating survival and death of cardiac myocytes in a stimulus-dependent manner, it is important to determine whether autophagy is stimulated during various pathophysiologic conditions in vivo and to evaluate its functional significance.
Importantly, evaluating whether autophagy is stimulated is not an easy task, especially at the organ level in vivo. Autophagy is a dynamic process involving nucleation, autophagosome formation, fusion of autophagosomes to lysosomes, and degradation in lysosomes, each of which is governed by multiple signaling mechanisms (22). Although the assessment of autophagosome formation at a single time point may be used for evaluating the extent of autophagy, the results could be misleading because the extent of autophagosome formation often dissociates from the level of autophagic flux (14). Conversely, tandem fluorescent mRFP-GFP-LC3 (tf-LC3), recently developed by Kimura et al. (13), allows one to evaluate the extent of autophagosome and autolysosome formation simultaneously, because LC3 puncta labeled with both GFP and mRFP represent autophagosomes, whereas those labeled with mRFP alone represent autolysosomes. As GFP-LC3 mice developed by Mizushima and colleagues (23) have been a very useful tool for assessing the level of autophagosome formation in mice in vivo, we reasoned that transgenic mice harboring tf-LC3 could also be a useful tool for conveniently evaluating the extent of autophagic flux in organs in vivo.
Here we generated transgenic mice with cardiac-specific expression of tf-LC3 (Tg-tf-LC3) and evaluated the effect of I/R on autophagic flux in the heart. Accumulating lines of evidence suggest that oxidative stress plays an important role in mediating autophagy in various cell types and organs (12). Because oxidative stress is an important cause of myocardial injury at the time of reperfusion (17), and because our previous results suggest that activation of autophagy during the reperfusion phase is detrimental (18), we hypothesized that oxidative stress activates autophagy during I/R and that activation of autophagy by oxidative stress plays an important role in mediating I/R injury in the heart. We tested (a) whether tf-LC3 can be used to evaluate autophagic flux in cardiac myocytes, (b) whether Tg-tf-LC3 can be used to evaluate the extent of autophagy during I/R in the mouse heart in vivo, and (c) whether oxidative stress plays an essential role in mediating autophagy and myocardial injury during I/R in the mouse heart in vivo.
Materials and Methods
Antibodies
Antibodies used in the study include those against LC3 (MBL Intl. Corp.; PD014), p62 (ARP Inc.; 03-GP62-C), Beclin1 (BD Biosciences; 612112), and α-Tubulin (Sigma-Aldrich; T6199).
Primary culture of cardiac myocytes
Primary cultures of left ventricular cardiac myocytes were prepared from 1-day-old Crl: (WI) BR-Wistar rats (Harlan Laboratories), as described previously (18). A cardiac myocytes–enriched fraction (>95%) was obtained by centrifugation through a discontinuous Percoll gradient (18). After 24 h of culture in gelatin-coated culture dishes, the media were changed to serum-free Dulbecco's modified Eagle's medium (DMEM)/F-12 (Mediatech Inc.). For amino acid deprivation (AAD), myocytes were washed 3 times with phosphate-buffered saline (PBS) and incubated with amino acid–free, serum-free Hanks Balanced Salt Solution (HBSS; Mediatech Inc.) for 2 h, as described previously (18).
Adenoviruses
The tf-LC3 plasmid construct (13) (courtesy of Dr Tamotsu Yoshimori, Osaka University, Osaka, Japan) was used to generate an adenovirus (Ad-tf-LC3) by using shuttle vector pDC316 and the Admax system (Microbix). Ad-LacZ was used as the control. Cardiac myocytes were transduced with 15 multiplicities of infection (MOIs) of adenovirus for 24 h.
Preparation of cell lysates and tissue homogenates
Protein lysates were prepared from myocytes cultured in 6-cm culture dishes by using boiled (95°C for 2 min) 2 × SDS sample buffer containing 4% SDS, 20% glycerol, 120 mM Tris-HCl (pH 6.8), 0.01% bromophenol blue, and 5% β-mercaptoethanol. The protein samples were immediately boiled again at 95°C for 3 min.
Heart-tissue homogenates were prepared by using RIPA buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% Igepal CA-630, and 0.5% sodium deoxycholate with protease inhibitors (Sigma; P8340) at a 1:400 dilution. After determining the protein concentration with BCA Assay (Thermo Scientific), equal amounts of proteins were loaded on SDS-PAGE gels with 6 × sample buffer containing 0.35 M Tris-HCl (pH 6.8), 10.28% SDS, 36% glycerol, 0.01% bromophenol blue, and 5% β-mercaptoethanol.
Immunoblot analysis
In vivo samples containing 35 μg of proteins were subjected to SDS-PAGE, as described previously (18). Densitometric analyses of the blots were carried out by using the public domain ImageJ program (NIH, Baltimore, MD).
Evaluation of fluorescent LC3 puncta
Cardiac myocytes cultured on coverslips were transduced with Ad-tf-LC3 at 15 MOI. Twenty-four hours after adenovirus transduction, the cells were washed with PBS, fixed with 4% paraformaldehyde, mounted with a reagent containing 4′,6-diamidino-2-phenylindole (DAPI) (Vectashield; Vector Laboratories, Inc.), and viewed under a fluorescence microscope (Nikon Eclipse E800). The number of GFP and mRFP dots was determined by manual counting of fluorescent puncta in five fields from three different myocyte preparations with a 60 × objective. The nuclear number was evaluated by counting the number of DAPI-stained nuclei in the same field. The number of dots per cell was obtained by dividing the total number of dots by the number of nuclei in each microscopic field.
For in vivo determination of the number of fluorescent LC3 dots, fresh heart slices were embedded with Tissue-Tek OCT compound (Sakura Finetechnical Co., Ltd.) and frozen at −80°C. Sections 10 μm thick were obtained from the frozen tissue samples by using a cryostat (CM3050 S; Leica), air-dried for 30 min, fixed by washing in 95% ethanol for 10 min, mounted by using a reagent containing DAPI, and viewed under a fluorescence microscope.
Dihydroethidium staining
The method used to identify the presence of oxidative stress with dihydroethidium (DHE) fluorescence was described previously (1).
Transgenic mice
Transgenic mice with cardiac-specific expression of tf-LC3 (Tg-tf-LC3) were generated on an FVB background with the murine α-Myosin Heavy Chain promoter, kindly provided by Dr. J. Robbins (Children's Hospital, Cincinnati, OH). Non-transgenic (NTg) mice were used as controls. Beclin-1–heterozygous knockout (beclin1+/−) mice (courtesy of Dr. Beth Levine, University of Texas Southwestern, Dallas, TX) were described previously (18). For starvation studies, Tg-tf-LC3 mice were starved for 24 h, following which the mice were sacrificed. All protocols concerning the use of animals were approved by the Institutional Animal Care and Use Committee at the University of Medicine and Dentistry of New Jersey.
Oxidative stress and antioxidant treatment in vitro
Cardiac myocytes were treated with 200 μM hydrogen peroxide (H2O2) for 1 h and 1 mM N-2-mercaptopropionyl glycine (MPG) for 4 h to induce oxidative stress and antioxidant treatment, respectively.
I/R surgery and antioxidant treatment in vivo
The method of inducing I/R in mice was described previously (18). Mice generated on an FVB background were subjected to ischemia for 45 min and to reperfusion for either 2 or 24 h. Mice generated on a C57BL/6 background were subjected to 30-min ischemia followed by reperfusion for 2 or 24 h. MPG, 100 mg/kg, was injected intraperitoneally into mice 24 h and 1 h before occlusion of the artery.
Assessment of area at risk and infarct size
The method used to determine the area at risk (AAR) and infarct size after I/R by using triphenyltetrazolium chloride (TTC) staining was described previously (18).
Statistics
Data are expressed as mean ± SEM. Statistical analyses between groups of two were conducted with the unpaired Student t test. Groups of three or more were analyzed with one-way ANOVA, followed by the Newman–Keuls multiple comparison test. A value of p < 0.05 was considered statistically significant.
Results
tf-LC3 is a useful tool for evaluating autophagic flux in cardiac myocytes
Autophagic flux being a dynamic process, it is insufficient to evaluate only autophagosome formation to determine the extent of autophagy. It is imperative to distinguish between increased autophagosome formation and decreased autophagosome clearance (14). tf-LC3 staining is a very useful tool for evaluating the extent of autophagic flux, as it helps to differentiate between autophagosomes and autolysosomes (13). GFP fluorescence is quenched in the acidic pH of the lysosomal compartment, thereby limiting the use of GFP-LC3 to the identification of autophagosomes. However, mRFP continues to fluoresce, and mRFP-LC3 can be used to identify both autophagosomes and autolysosomes. By using tf-LC3 and determining the number of red dots that overlay green dots and appear yellow in merged images, the number of autophagosomes can be evaluated. The red dots that do not overlay green dots and appear red in merged images indicate autolysosome formation (13).
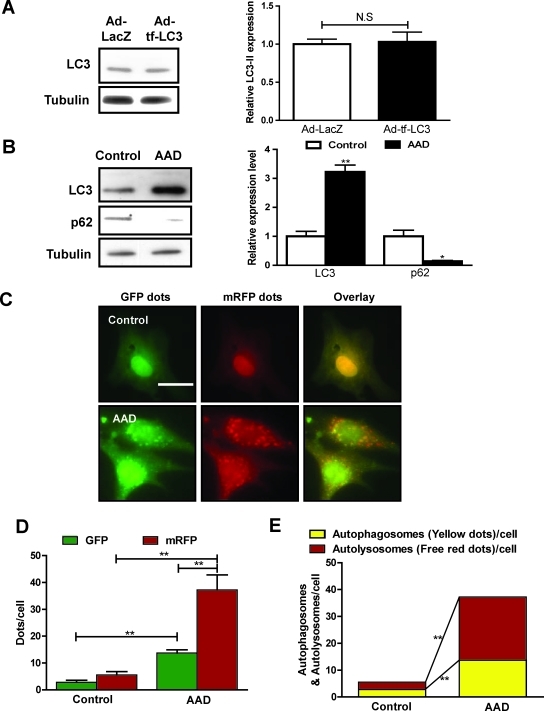
To evaluate autophagic flux in cultured cardiac myocytes, we generated an adenovirus harboring tf-LC3 (Ad-tf-LC3). Cardiac myocytes were transduced with Ad-tf-LC3 for 24 h. Expression of Ad-tf-LC3 does not affect the level of endogenous LC3 (Fig. 1A). We used amino acid deprivation (AAD) as a model to study the regulation of autophagic flux in cultured cardiac myocytes. Expression of LC3-II was significantly increased after 2 h of AAD (Fig. 1B). Expression of p62, a polyubiquitin-binding protein known to be sequestered and degraded during autophagy (2, 26), was significantly decreased after AAD, indicating enhanced autophagy (Fig. 1B). We then evaluated the extent of autophagosome and autolysosome formation by using fluorescent LC3 puncta. The numbers of GFP and mRFP dots per cell were both significantly increased after AAD (Fig. 1C and D). In the merged images, more free red dots than yellow dots were seen, indicating significantly increased autolysosome formation compared with autophagosomes (Fig. 1E), and suggesting that AAD increases autophagic flux. Thus, the results of the analysis using Ad-tf-LC3 well corroborate those obtained with conventional methods.
FIG. 1.
tf-LC3 can be used to evaluate autophagic flux in cultured cardiac myocytes. (A) Neonatal rat ventricular myocytes were transduced with adenovirus harboring tf-LC3 (Ad-tf-LC3) or control Ad-LacZ for 24 h. Immunoblot and densitometric analyses indicating expression of LC3-II are shown. Tubulin was used as an internal control. (B) Cardiac myocytes were subjected to amino acid deprivation (AAD) by treatment with serum-free, amino acid–free Hanks balanced salt solution for 2 h. Immunoblot and densitometric analyses indicating expression of LC3-II, p62, and tubulin are shown. (C) Myocytes were transduced with Ad-tf-LC3 for 24 h and were subjected to AAD for 2 h. Representative images of fluorescent LC3 puncta are shown. (D) Mean number of GFP and mRFP dots per cell. (E) Mean number of autophagosomes (dots with both red and green color; i.e., dots with yellow color in merged images) and autolysosomes (dots with only red but not green color; i.e., dots with red color in merged images) per cell. Adenovirus was transduced at 15 MOI. Results represent the means from at least three independent experiments. *p < 0.05; **p < 0.01; N.S., not significant. Scale bar represents 50 μm. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Generation of transgenic mice with cardiac-specific expression of tf-LC3 (Tg-tf-LC3)
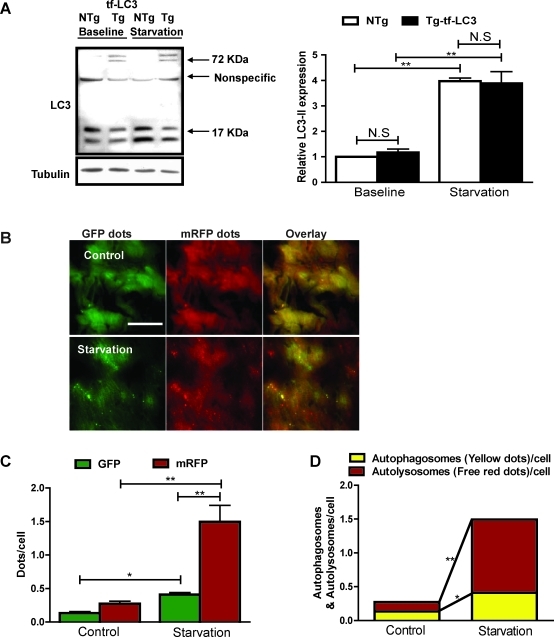
To evaluate the level of autophagic flux in the heart in vivo with tf-LC3, we generated transgenic mice with cardiac-specific expression of tf-LC3 (Tg-tf-LC3). Expression of tf-LC3 does not affect the level of endogenous LC3 at baseline or in response to starvation for 24 h in vivo (Fig. 2A). Starvation increased the level of endogenous LC3-II in both Tg-tf-LC3 and non-transgenic (NTg) control mice (Fig. 2A). Starvation also increased both green and red dots in Tg-tf-LC3 mice, and the merged images showed that starvation significantly increased both yellow dots representing autophagosome formation and red dots representing autolysosome formation in the mouse heart (Fig. 2B–D). These results suggest that Tg-tf-LC3 mice are useful for evaluating autophagic flux in the heart in vivo.
FIG. 2.
Transgenic mice harboring tf-LC3 can be used to study autophagy in the heart. Transgenic mice harboring cardiac-specific expressed tf-LC3 (Tg-tf-LC3) were generated. Age-matched non-transgenic mice (NTg) were used as controls. The mice were starved for 24 h, after which they were sacrificed, and the hearts were removed for biochemical analyses. (A) Immunoblot analyses indicating expression of endogenous LC3 and mRFP-GFP-LC3, both at baseline and after 24-h starvation in the Tg-tf-LC3 and NTg mice. Bands of endogenous LC3-I and LC3-II were detected at ∼17 kDa, and those of mRFP-GFP-LC3 were detected at ∼72 kDa. A nonspecific band was observed at ∼55 kDa. Quantitative analyses showed that starvation significantly increases the level of endogenous LC3-II in both NTg and Tg-tf-LC3. (B) Frozen sections were made from hearts of Tg-tf-LC3 mice at baseline and after starvation. Representative images of fluorescent LC3 puncta, GFP dots, mRFP dots, and their merged images are shown. (C) Mean numbers of green and red dots per cell. (D) Mean numbers of autophagosomes represented by yellow dots in merged images and autolysosomes represented by red dots in merged images per cell. Results represent the means from at least three mice. *p < 0.05; **p < 0.01; N.S., not significant. Scale bar represents 50 μm. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Antioxidant MPG inhibits oxidative stress–induced autophagy in cardiac myocytes in vitro
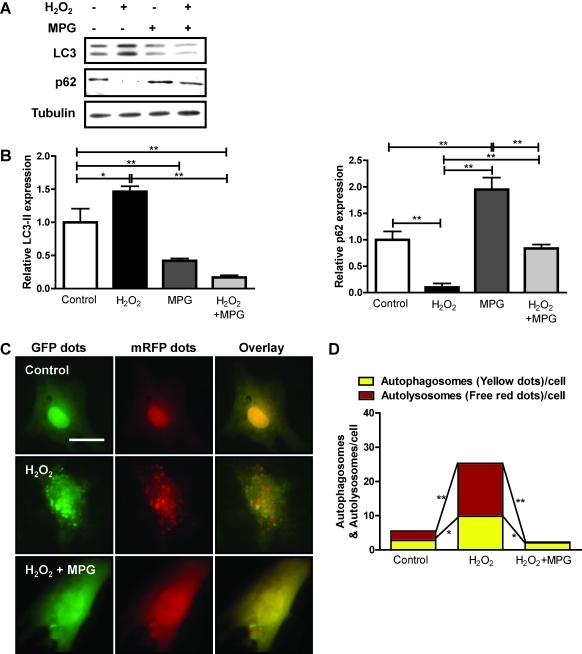
Oxidative stress plays an important role in mediating myocardial cell death in response to stress, such as I/R (6). Oxidative stress also stimulates autophagy in some cell types (6, 12). To evaluate the effect of oxidative stress on autophagy, we treated cardiac myocytes with 200 μM H2O2 for 1 h. H2O2 treatment significantly increased LC3-II and decreased p62, suggesting that oxidative stress stimulates autophagy in cardiac myocytes (Fig. 3A, B). In cardiac myocytes transduced with Ad-tf-LC3, H2O2 increased the number of green and red dots, and the merged images showed that H2O2 increased both yellow dots representing autophagosomes and red dots representing autolysosomes (Fig. 3C, D). These results suggest that oxidative stress enhances autophagic flux in cultured myocytes.
FIG. 3.
N-2-Mercaptopropionyl glycine (MPG) inhibits hydrogen peroxide (H2O2)-induced autophagy in vitro. Cardiac myocytes were treated with 1 mM antioxidant, MPG, for 4 h, followed by 200 μM H2O2 for 1 h. (A) Immunoblots indicating expression of LC3, p62, and tubulin. (B) Densitometric analyses. (C) Cardiac myocytes were transduced with 15 MOI of Ad-tf-LC3 for 24 h, after which they were treated with H2O2 in the presence or absence of MPG at the indicated doses and times. Representative images of fluorescent LC3 puncta are shown. Scale bar represents 50 μm. (D) Mean number of autophagosomes represented by yellow dots in merged images and autolysosomes represented by red dots in merged images per cell. Results represent the means from at least three independent experiments. *p < 0.05; **p < 0.01. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
To understand further the role of oxidative stress in mediating autophagy in cardiac myocytes, we used the antioxidant MPG. MPG is an amino acid derivative that contains a sulfhydryl or thiol group, and is known to act as a free radical scavenger (21). Treatment of cardiac myocytes with 1 mM MPG for 4 h significantly attenuated H2O2-induced increases in LC3-II and decreases in p62 accumulation (Fig. 3A, B), and significantly reduced both autophagosome and autolyososome formation, as evaluated by Ad-tf-LC3 (Fig. 3C, D). Taken together, the antioxidant, MPG, inhibits oxidative stress–induced autophagy in cardiac myocytes.
MPG protects against myocardial reperfusion injury by partial inhibition of autophagy
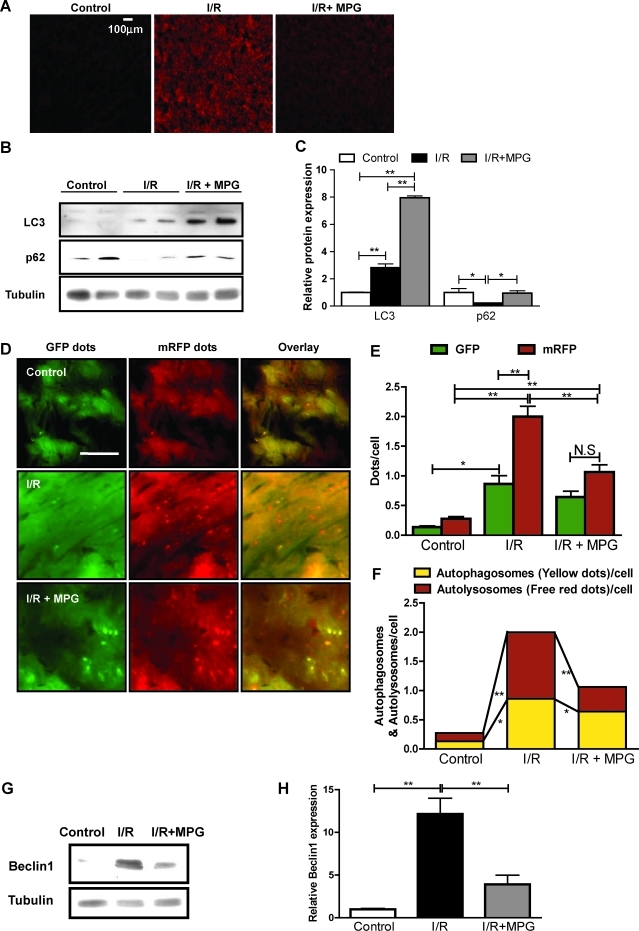
Reperfusion of the ischemic myocardium increases production of reactive oxygen species (ROS), which play an important role in mediating reperfusion injuries (19). In mouse hearts subjected to 45 min of ischemia followed by 2 h of reperfusion, DHE fluorescence, an indicator of superoxide, was significantly increased (Fig. 4A). However, when mice were injected intraperitoneally with 100 mg/kg of the antioxidant, MPG, 24 h and 1 h before ischemia followed by reperfusion, the amount of oxidative stress was significantly decreased (Fig. 4A).
FIG. 4.
MPG partially inhibits autophagy after ischemia/reperfusion (I/R). The 3- to 4-month-old wild-type FVB strain mice were subjected to 45-min myocardial ischemia followed by 2-h reperfusion. Antioxidant MPG (100 mg/kg) was injected intraperitoneally 24 h and 1 h before ischemia. (A) Frozen sections were prepared from the mouse hearts and stained with DHE to evaluate the presence of oxidative stress. Scale bar indicates 100 μm. (B) Immunoblots indicating expression of LC3, p62, and tubulin in mouse hearts subjected to I/R in the presence or absence of MPG. (C) Densitometric analyses. (D) Tg-tf-LC3 mice were subjected to I/R in the presence or absence of MPG. Representative images of fluorescent LC3 puncta are shown. Scale bar indicates 50 μm. (E) Mean numbers of green and red dots per cell. (F) Mean numbers of autophagosomes represented by yellow dots in merged images and autolysosomes represented by red dots in merged images per cell. Results represent the means from at least three independent experiments. (G) Immunoblots indicating expression of Beclin-1 and tubulin after I/R in the presence or absence of MPG. (H) Densitometric analyses. Results represent the means from at least three mice each. *p < 0.05; **p < 0.01; N.S., not significant. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
We showed previously that autophagy is robustly increased during I/R and has a detrimental effect (18). To evaluate the role of oxidative stress in mediating autophagy during I/R, mice were subjected to I/R in the presence or absence of MPG. As previously reported, I/R significantly increased LC3-II and decreased p62 accumulation, indicative of increased autophagy (Fig. 4B, C). Although treatment with MPG increased the level of LC3-II, it also increased accumulation of p62, indicating that autophagic flux is inhibited (Fig. 4B, C). To evaluate further the formation of autophagosomes and autolysosomes in cardiac myocytes during I/R, Tg-tf-LC3 mice were subjected to I/R in the presence and absence of MPG. I/R significantly increased red and green puncta, with more autolysosome formation, as evidenced by red puncta, than autophagosome formation, indicated by yellow puncta in merged images (Fig. 4D–F). Treatment with MPG partially decreased both green and red dots after I/R (Fig. 4D, E). Overlaid images showed that MPG significantly decreased I/R-induced increases in yellow dots and red dots, with a more prominent effect on red dots, indicating that MPG significantly decreased autophagic flux during I/R (Fig. 4F). These results suggest that an MPG-sensitive mechanism, most likely oxidative stress, plays an important role in mediating autophagy in response to I/R in the heart.
We showed previously that Beclin-1, a key molecule mediating autophagy, is significantly upregulated during I/R, and that autophagy is induced in a Beclin-1–dependent manner (18). Although MPG did not affect expression of Beclin-1 at baseline (data not shown), MPG significantly attenuated I/R-induced upregulation of Beclin-1 (Fig. 4G, H). Beclin-1 forms a complex with class III phosphatidylinositol 3-kinase and other autophagy-related proteins, and is localized to the pre-autophagosomal structure mediating vesicle nucleation (18, 28). However, Beclin-1 does not affect the conjugation of LC3-I with phosphatidylethanolamine, leading to LC3-II formation (18, 29). This may explain why treatment with MPG did not affect LC3-II accumulation, while significantly inhibiting autophagosome formation.
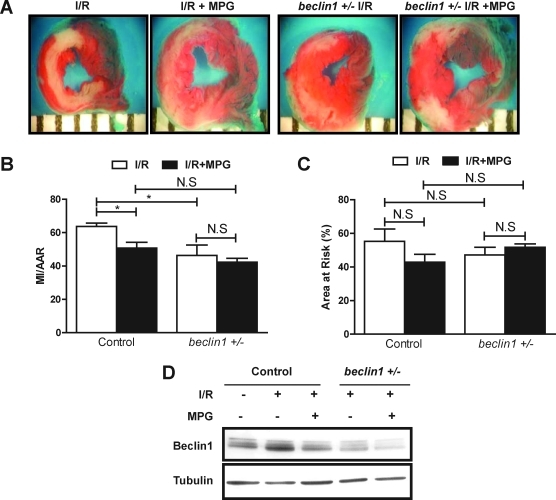
MPG plays a cytoprotective role during I/R in dog and mouse hearts (11, 20, 21). In mouse hearts subjected to 45-min ischemia and 24-h reperfusion, although no significant difference was found in the area at risk (AAR) (Fig. 5A, C), the size of myocardial infarction (MI)/AAR, as evaluated by triphenyltetrazolium chloride staining, was significantly decreased in the presence of MPG (Fig. 5A, B), suggesting that oxidative stress plays an important role in mediating I/R injury in our model, consistent with previous results (18).
FIG. 5.
MPG decreases infarction size after I/R through inhibition of autophagy. (A–C) The 3- to 4-month-old wild-type mice and beclin1 heterozygous knockout mice (beclin1+/-) bred on a C57 background were each subjected to 30 min of myocardial ischemia followed by 24 h of reperfusion. Antioxidant MPG (100 mg/kg) was injected intraperitoneally 24 h and 1 h before ischemia. Mice were sacrificed, and the hearts were removed. Myocardial infarction size was determined after staining with triphenyltetrazolium chloride (TTC). (A) Representative images of LV tissue sections from wild-type and beclin1+/- mice subjected to IR in the presence or absence of MPG. (B) The myocardial infarction (MI) size/area at risk (AAR) expressed as a percentage. (C) The percentage of area at risk. (D) Mice were subjected to 30 min of ischemia followed by 2 h of reperfusion. Immunoblots indicating the expression of Beclin-1 and tubulin are shown. Results represent the means from at least three mice each. *p < 0.05; **p < 0.01; N.S., not significant. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Because MPG treatment inhibited both autophagic flux and myocardial injury, and because stimulation of autophagy during I/R could be detrimental (18), the MPG-induced reduction in infarct size may be mediated through its inhibition of autophagy. To test this hypothesis, we used beclin1 heterozygous knockout mice (beclin1+/−), in which autophagy during I/R is significantly attenuated (18). As shown previously, beclin1+/− mice exhibited a significantly smaller size of MI/AAR after I/R (Fig. 5A, B), suggesting that Beclin-1–induced autophagy after I/R is detrimental (18). Although MPG significantly reduced the size of MI/AAR in control mice, it did not further reduce the size of MI/AAR in beclin1+/− mice (Fig. 5A, B). Neither MPG nor downregulation of Beclin-1 significantly affected the size of AAR (Fig. 5C). These results are consistent with the notion that MPG inhibits I/R injury through suppression of autophagy. We confirmed that I/R-induced upregulation of Beclin-1 is suppressed both by MPG and in beclin1+/− mice, either alone or in combination (Fig. 5D).
Discussion
In this study, we showed that tf-LC3 can be used to evaluate the level of autophagic flux in cardiac myocytes both in vitro and in vivo. By using Tg-tf-LC3, we demonstrated that I/R increases autophagic flux in cardiac myocytes through an oxidative stress–dependent mechanism in vivo.
tf-LC3 is a useful tool for evaluating the formation of both autophagosomes and autolysosomes simultaneously in cardiac myocytes. The results of our experiments with tf-LC3 showed that AAD increases both autophagosomes and autolysosomes, an observation also supported by conventional indexes of autophagy, including increases in LC3-II and decreases in p62 accumulation in cardiac myocytes. Although autophagosomes accumulate when fusion between autophagosomes and lysosomes is inhibited, a concurrent increase in both autophagosomes and autolysosomes indicates that autophagic flux is stimulated (14). Although autophagic flux can be evaluated by comparing the extent of autophagosome accumulation in the presence or absence of inhibitors of lysosomal fusion or degradation or both, such interventions are usually toxic, which would confound analyses of the role of autophagy in mediating biologic functions in vivo (14, 24). Tg-tf-LC3 mice are useful not only in that the extent of autophagic flux can be monitored in vivo, but also in that one can investigate the effect of various interventions on formation of autophagosomes and autolysosomes independently.
Terada and colleagues (23, 31) previously reported double-transgenic mice generated by cross-breeding cardiac-specific mCherry-LC3 mice with systemic GFP-LC3 mice. Compared with those mice, our transgenic mice with cardiac-specific expression of a single transgene, tf-LC3, have several advantages. First, because either green or red color can be emitted from the same LC3 molecule in Tg-tf-LC3, more-accurate quantification of autophagosomes and autolysosomes can be made than in the cross between cardiac-specific mCherry-LC3 and systemic GFP-LC3 mice, in which the number of mCherry-LC3 and GFP-LC3 molecules are not identical and GFP-LC3 is not cardiac myocyte specific. Second, the mouse model reported by Terada requires cross-breeding between mCherry-LC3 mice and GFP-LC3 mice, whereas our model does not, because both GFP and mRFP are expressed in a single transgene. Finally, because tf-LC3 is expressed in a cardiac myocyte–specific manner in Tg-tf-LC3, our model allows one to quantitate the extent of autophagic flux specifically in cardiac myocytes.
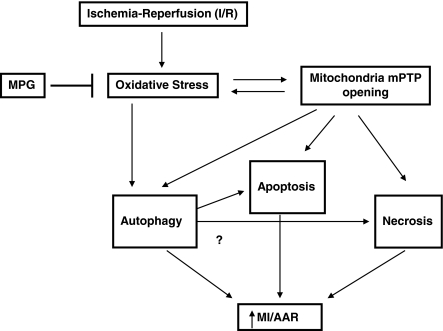
With our unique mouse model, we here provide evidence that oxidative stress plays an important role in mediating autophagy during reperfusion (Fig. 6). Reperfusion causes various cellular responses relevant to autophagy, including oxidative stress, mitochondrial permeability transition pore (mPTP) opening, endoplasmic reticulum stress, Ca2+ overloading, and mitochondrial damage (7, 17, 30). However, to our knowledge, the role of oxidative stress in mediating increases in autophagic flux during I/R has not been clearly demonstrated. Our results suggest that increases in oxidative stress are both necessary and sufficient for inducing autophagy during I/R in cardiac myocytes.
FIG. 6.
Schematic representation of the role of oxidative stress in mediating autophagy and myocardial injury in response to I/R. Myocardial I/R stimulates autophagy through increases in oxidative stress. Stimulation of autophagy plays an important role in mediating myocardial injury.
Oxidative stress affects autophagy through multiple mechanisms. During starvation, H2O2 directly oxidizes HsAtg4 and inhibits its cysteine protease activity, thereby causing increased LC3 lipidation and autophagy in cancer cell lines (27). Oxidative stress affects lysosomal membrane permeability through disulfide bond formation in the lysosomal membrane proteins (6, 12). Autophagy is also activated as a compensatory response to damages in intracellular organelles (12). For example, increases in oxidative stress in mitochondria induce opening of the mPTP, leading to depolarization of the mitochondrial membrane potential, swelling, and damage to mitochondrial proteins (3, 5, 9). Cyclophilin D, a component of the mPTP, is involved in starvation-induced mitophagy in cardiac myocytes (4). BH3-only proteins, such as Bnip3, are upregulated during mPTP opening and mediate autophagy during I/R (10, 30). Oxidative stress also triggers ER stress, which, in turn, induces autophagy (17, 30).
Although the molecular mechanism by which oxidative stress induces autophagy during myocardial reperfusion remains to be elucidated, we showed previously that Beclin-1 is dramatically upregulated during the reperfusion phase in the mouse heart, which, in turn, plays an essential role in mediating increases in autophagy (18). Because MPG treatment significantly inhibited upregulation of Beclin-1 during the reperfusion phase, it is possible that oxidative stress induces autophagy through upregulation of Beclin-1. Whether the dramatic upregulation of Beclin-1 during the reperfusion phase is mediated through a transcriptional mechanism, and, if so, which transcription factor is involved in this process, remains to be elucidated.
Our results showed that MPG treatment inhibited formation of both autophagosomes and autolysosomes during reperfusion. Interestingly, we noted that the formation of the latter is more strongly affected. Although the precise mechanism by which this regulation takes place is yet to be identified, it is possible that mechanisms mediating autolysosome formation, such as the fusion of autophagosomes and lysosomes, may be sensitive to oxidative stress. Interestingly, a study by Li et al. (16) showed that MPG neutralizes 3-aminopropanol, a neurotoxin formed during cerebral ischemia that damages the lysosome.
Although oxidative stress activates various mechanisms leading to an increase in death of cardiac myocytes (19), we propose that oxidative stress induces reperfusion injury in part through stimulation of autophagy. Several lines of evidence support our hypothesis. First, suppression of oxidative stress inhibits autophagy during the reperfusion phase, and a similar extent of autophagy suppression by Beclin-1 downregulation was sufficient to reduce the level of reperfusion injury. Second, although MPG significantly reduced the size of MI/AAR in control mice, it did not further reduce the size of MI/AAR when autophagy is suppressed in beclin1+/− mice, consistent with the notion that oxidative stress regulates cell survival/death through regulation of autophagy. Whether stimulation of autophagy can be detrimental during reperfusion is controversial (8, 18). Although our results indicating that MPG suppresses both autophagy and myocardial injury support the notion that autophagy during reperfusion is detrimental, further studies are needed to elucidate the functional significance of autophagy during I/R.
At present, the contribution of autophagic cell death to overall myocardial injury is unknown. Because I/R-induced increases in apoptosis are attenuated in beclin1+/− mice, it is possible that autophagy and apoptosis may be linked. Although mPTP opening may initially induce both autophagy and apoptosis, severe depletion of ATP caused by mPTP opening may lead to necrosis as well. Whether strong activation of lysosomal degradation by autophagy contributes to necrotic cell death during I/R remains to be elucidated.
In summary, our results suggest that tf-LC3 is a useful tool for investigating the level of autophagic flux in the heart and the cardiac myocytes therein. With Tg-tf-LC3 mice, we showed that oxidative stress plays an important role in stimulating autophagic flux, which contributes to myocardial injury during I/R in vivo. We propose that suppression of oxidative stress during the reperfusion phase prevents myocardial injury in part through suppression of excessive autophagy.
Abbreviations Used
- AAD
amino acid deprivation
- AAR
area at risk
- Ad
adenovirus
- DAPI
4′,6-diamidino-2-phenylindole
- DHE
dihydroethidium
- GFP
green fluorescent protein
- H2O2
hydrogen peroxide
- I/R
ischemia/reperfusion
- MI
myocardial infarction size
- MOI
multiplicities of infection
- MPG
N-2-mercaptopropionyl glycine
- mPTP
mitochondrial permeability transition pore
- mRFP
monomeric red fluorescent protein
- NTg
non-transgenic
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- tf-LC3
tandem fluorescent mRFP-GFP-LC3
- Tg
transgenic
- TTC
triphenyltetrazolium chloride
Acknowledgments
We thank Drs. Tamotsu Yoshimori and Beth Levine for tf-LC3 and beclin1+/− mice, respectively. We thank Daniela Zablocki for critical reading of the manuscript.
This work was supported in part by U.S. Public Health Service grants HL 59139, HL67724, HL69020, HL91469, AG27211, and HL102738, and by Foundation of Leducq Transatlantic Network of Excellence.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ago T. Kuroda J. Pain J. Fu C. Li H. Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2004;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorkoy G. Lamark T. Brech A. Outzen H. Perander M. Overvatn A. Stenmark H. Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bopassa JC. Michel P. Gateau-Roesch O. Ovize M. Ferrera R. Low-pressure reperfusion alters mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2005;288:H2750–H2755. doi: 10.1152/ajpheart.01081.2004. [DOI] [PubMed] [Google Scholar]
- 4.Carreira RS. Lee Y. Ghochani M. Gustafsson AB. Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;6 doi: 10.4161/auto.6.4.11553. [epub ahead of print.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Dorado D. Agullo L. Sartorio CL. Ruiz-Meana M. Myocardial protection against reperfusion injury: the cGMP pathway. Thromb Haemost. 2009;101:635–642. [PubMed] [Google Scholar]
- 6.Gurusamy N. Das DK. Autophagy, redox signaling, and ventricular remodeling. Antioxid Redox Signal. 2009;11:1975–1988. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafsson AB. Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustafsson AB. Gottlieb RA. Eat your heart out: role of autophagy in myocardial ischemia/reperfusion. Autophagy. 2008;4:416–421. doi: 10.4161/auto.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 10.Hamacher-Brady A. Brady NR. Logue SE. Sayen MR. Jinno M. Kirshenbaum LA. Gottlieb RA. Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz LD. Fennessey PV. Shikes RH. Kong Y. Marked reduction in myocardial infarct size due to prolonged infusion of an antioxidant during reperfusion. Circulation. 1994;89:1792–1801. doi: 10.1161/01.cir.89.4.1792. [DOI] [PubMed] [Google Scholar]
- 12.Kiffin R. Bandyopadhyay U. Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 13.Kimura S. Noda T. Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 14.Klionsky DJ. Abeliovich H. Agostinis P. Agrawal DK. Aliev G. Askew DS. Baba M. Baehrecke EH. Bahr BA. Ballabio A. Bamber BA. Bassham DC. Bergamini E. Bi X. Biard-Piechaczyk M. Blum JS. Bredesen DE. Brodsky JL. Brumell JH. Brunk UT. Bursch W. Camougrand N. Cebollero E. Cecconi F. Chen Y. Chin LS. Choi A. Chu CT. Chung J, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine B. Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W. Yuan XM. Ivanova S. Tracey KJ. Eaton JW. Brunk UT. 3-Aminopropanol, formed during cerebral ischaemia, is a potent lysosomotropic neurotoxin. Biochem J. 2003;371:429–436. doi: 10.1042/BJ20021520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui Y. Kyoi S. Takagi H. Hsu CP. Hariharan N. Ago T. Vatner SF. Sadoshima J. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui Y. Takagi H. Qu X. Abdellatif M. Sakoda H. Asano T. Levine B. Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 19.Misra MK. Sarwat M. Bhakuni P. Tuteja R. Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- 20.Mitsos SE. Askew TE. Fantone JC. Kunkel SL. Abrams GD. Schork A. Lucchesi BR. Protective effects of N-2-mercaptopropionyl glycine against myocardial reperfusion injury after neutrophil depletion in the dog: evidence for the role of intracellular-derived free radicals. Circulation. 1986;73:1077–1086. doi: 10.1161/01.cir.73.5.1077. [DOI] [PubMed] [Google Scholar]
- 21.Mitsos SE. Fantone JC. Gallagher KP. Walden KM. Simpson PJ. Abrams GD. Schork MA. Lucchesi BR. Canine myocardial reperfusion injury: protection by a free radical scavenger, N-2-mercaptopropionyl glycine. J Cardiovasc Pharmacol. 1986;8:978–988. [PubMed] [Google Scholar]
- 22.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 23.Mizushima N. Yamamoto A. Matsui M. Yoshimori T. Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizushima N. Yoshimori T. Levine B. Methods in mammalian autophagy research. Cell. 2006;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishida K. Kyoi S. Yamaguchi O. Sadoshima J. Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 26.Pankiv S. Clausen TH. Lamark T. Brech A. Bruun JA. Outzen H. Overvatn A. Bjorkoy G. Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 27.Scherz-Shouval R. Shvets E. Fass E. Shorer H. Gil L. Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha S. Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27(suppl 1):S137–S148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki K. Kirisako T. Kamada Y. Mizushima N. Noda T. Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takagi H. Matsui Y. Sadoshima J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal. 2007;9:1373–1381. doi: 10.1089/ars.2007.1689. [DOI] [PubMed] [Google Scholar]
- 31.Terada M. Nobori K. Munehisa Y. Kakizaki M. Ohba T. Takahashi Y. Koyama T. Terata Y. Ishida M. Iino K. Kosaka T. Watanabe H. Hasegawa H. Ito H. Double transgenic mice crossed GFP-LC3 transgenic mice with alphaMyHC-mCherry-LC3 transgenic mice are a new and useful tool to examine the role of autophagy in the heart. Circ J. 2008;74:203–206. doi: 10.1253/circj.cj-09-0589. [DOI] [PubMed] [Google Scholar]
- 32.Yan L. Sadoshima J. Vatner DE. Vatner SF. Autophagy in ischemic preconditioning and hibernating myocardium. Autophagy. 2009;5:709–712. doi: 10.4161/auto.5.5.8510. [DOI] [PubMed] [Google Scholar]
- 33.Yan L. Vatner DE. Kim SJ. Ge H. Masurekar M. Massover WH. Yang G. Matsui Y. Sadoshima J. Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]