Abstract
ATP-binding cassette (ABC) transporters are multispanning membrane proteins that utilize ATP to move a broad range of substrates across cellular membranes. ABC transporters are involved in a number of human disorders and diseases [1]. Overexpression of a subset of the transporters has been closely linked to multidrug resistance in both bacteria and viruses and in cancer. A poorly understood and important aspect of ABC transporter biology is the role of phosphorylation as a mechanism to regulate transporter function. In this review, we summarize the current literature addressing the role of phosphorylation in regulating ABC transporter function. A comprehensive list of all the phosphorylation sites that have been identified for the human ABC transporters is presented, and we discuss the role of individual kinases in regulating transporter function. We address the potential pitfalls and difficulties associated with identifying phosphorylation sites and the corresponding kinase(s), and we discuss novel techniques that may circumvent these problems. We conclude by providing a brief perspective on studying ABC transporter phosphorylation.
Keywords: ABC transporter, CK2, kinase, LC/MS, PKA, PKC, phosphorylation, regulation
1. INTRODUCTION
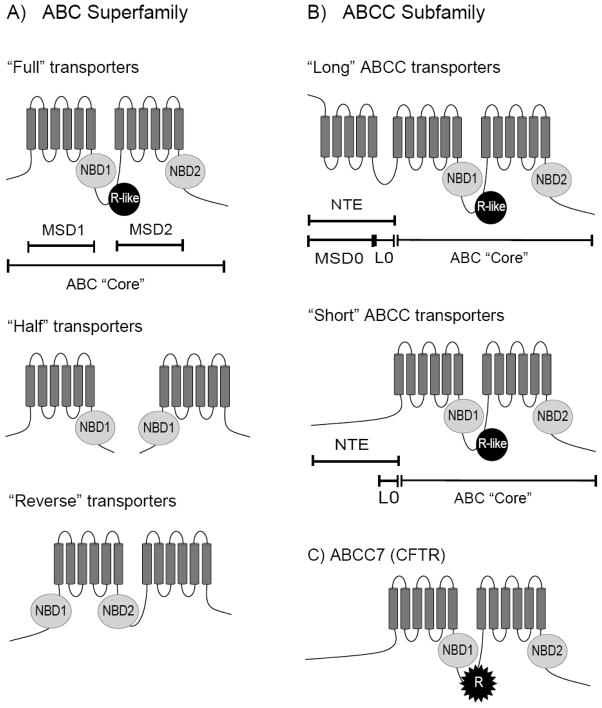
ATP-Binding Cassette (ABC) transporters are found in species ranging from bacteria to man [2]. ABC transporters are involved in a variety of human health related problems and diseases (Table 1). The transporters are found in a number of cellular organelles, including the mitochondria, vacuole/lysosome, and plasma membrane, and are responsible for the transport of chemical compounds across cellular membranes in a nucleotide dependent manner [1–4]. The ABC transporters have been divided into 5 subfamilies according to sequence similarity of their nucleotide binding domains (NBDs): ABCA-ABCD and ABCG (ABCE and ABCF are not transporters, have no membrane spans, and are localized to the cytosol) [1,3]. In general, the ABC transporters structurally consist of an “ABC Core” which contains two membrane spanning domains (MSDs or TMDs) and two NBDs that are connected by intracellular loops (Fig. 1) [1,2]. However, transporters can exist as heterodimers of “Half” transporters or “Long” ABC transporters, which contain an additional N-terminal extension (NTE) (Fig. 1) [1,4–6].
Table 1.
ABC Transporter-Associated Diseases
| Disease | Transporter(s) | Reference |
|---|---|---|
| Tangier disease | ABCA1 | [132] |
| Stargardt disease | ABCA4 | [133] |
| Immune deficiency | ABCB2 and ABCB3 | [134, 135] |
| Progressive familial intrahepatic cholestasis (PFIC) | ABCB4 and ABCB11 | [136] |
| Sideroblastic anemia and ataxia | ABCB7 | [137] |
| Dubin Johnson Syndrome (DJS) | ABCC2 | [138] |
| Pseudoxanthoma Elasticum (PXE) | ABCC6 | [139] |
| Cystic fibrosis | ABCC7 | [140] |
| Persistent hypoglycemia of infancy | ABCC8 and ABCC9 | [141] |
| Adrenoleukodystrophy | ABCD1 | [142] |
| Sitosterolemia | ABCG5 and ABCG8 | [143] |
| Cancer | ABCB1, ABCC1, and ABCG2 | [1, 55, 144–146] |
Fig. 1. Predicted topology of ATP-Binding Cassette (ABC) transporters.
(A) In general, ABC transporters are structurally characterized as having an ABC “Core” containing two membrane spanning domains (MSDs), also called transmembrane spanning domains (TMDs), and two intracellular nucleotide binding domains (NBDs). A number of ABC transporters exist as “Half” transporters which form homo- and/or heterodimers. (B) The ABCC subfamily has N-terminal extension (NTE) in addition to the ABC “Core”, The NTE of “Long” ABCC proteins consists of an extra membrane spanning domain (MSD0, TMD0) and an extra linker domain (L0) (also called cytoplasmic loop 3 (CL3)). In “Short” ABCC proteins, the MSD0 is absent. (C) The linker region between NBD1 and MSD2 of ABCC7 (CFTR) is extensively phosphorylated and is thus referred to as the regulatory domain (R domain). Similar domains are found in other ABC transporters and are referred to here as the “R-like” domain (annotated in Fig. 1A).
Although much is known about the function of the ABC transporters, post-translational regulation of transporter function has remained relatively uncharacterized until recently. A significant problem associated with identifying proteins that regulate ABC transporter function is the detection of interacting proteins. For example, identifying an interacting kinase(s) and the corresponding post-translational modification(s) is difficult [4,7]. The difficulty arises from the fact that ABC transporters are tethered to the membrane but site-specific or truncation mutants often misfold or un- fold and as a result traffic incorrectly [4]. The development and improvement of a number of techniques including the membrane yeast-two hybrid (MYTH) assay and proteomic methods, such as co-immunoprecipitation-affinity chromatography followed by liquid chromatography-mass spectrometry (LC/MS) proteomic analysis, over the last decade has greatly reduced this barrier [4]. Many phosphorylation sites within ABC transporters have been identified as a result of these improvements. In many cases, the corresponding kinase(s) has also been identified [4,7]. This review will focus on regulation of ABC transporter function via phosphorylation, the kinases that are involved, and the current and future technologies that are and will be available to identify new kinases that phosphorylate the ABC transporters as well as their corresponding phosphorylation sites.
2. PHOSPHORYLATION AS A MECHANISM OF ABC TRANSPORTER REGULATION
Phosphorylation is one of the most common mechanisms of post-translational protein regulation in the cell [8]. A broad range of cellular proteins are regulated via kinase-mediated phosphorylation. Phosphorylation occurs under greatly varying conditions in response to multiple stimuli [9,10]. Phosphorylation is critically important for the regulation of a number of cellular processes including transcription, ubiquitination, protein degradation, protein subcellular localization, and, most notably, protein function [10]. The availability of ATP as a donor and the ease of reversibility of phosphorylation are likely the reasons why the cell has so extensively adopted phosphorylation as a mechanism to regulate so many cellular processes [8]. Therefore, it is not surprising that abnormal phosphorylation is associated with many human diseases and conditions (misregulation of a number of kinases is strongly associated with multidrug resistance (MDR) in cancer) [9,10]. Many diseases occur as the result of mutated phosphorylation sites [8]. It is important to note that many toxins and pathogens exert their effects by altering cellular phosphorylation [8]. One such example of a phosphorylation-altering toxin is microcystin, which is effluxed by the green algae that produces it via the mycH ABC transporter. This toxin inhibits Type 1 and 2A protein phosphatases. Importantly, microcystin is a substrate for P-gp (ABCB1) in fish and is believed to regulate the expression and possibly the function of fish P-gp via inhibition of protein phosphatases [11–13].
A critical gap in our understanding of ABC transporter biology is the mechanism by which the transporters are regulated at the post-translational level [4]. A number of studies have identified phosphorylation as a mechanism of transporter regulation. Phosphorylation sites have been identified in members of almost every ABC transporter subfamily from yeast to man. It is reasonable to believe that all the transporters are regulated by phosphorylation and a variety of other post-translational modifications to some extent. Regulation of ABC transporter function by phosphorylation provides cells with a simple, low energy, fast, and efficient way to change transporter function. Other modifications that may regulate ABC transporter function or expression, such as sumoylation or ubiquitination, require more energy and are less efficient. A comprehensive list of phosphorylation sites identified by LC/MS for the human ABC transporters is provided in Table 2. Below we will discuss the role of phosphorylation as a mechanism to regulate the function of each ABC subfamily. We will conclude this review with a prospectus for how modern proteomics will aid in identifying the post-translational regulators and post-translational modifications of the ABC transporter family.
Table 2.
Phosphorylation Sites of the ABC Transporters Identified by MS Analysis of Tryptic Derived Peptides
| Transporter | Amino acid | Site | Reference |
|---|---|---|---|
| ABCA1 | SERINE | 1042 | [14,15] |
| THREONINE | 1242 | ||
| THREONINE | 1243 | ||
| SERINE | 1255 | ||
| SERINE | 2054 | ||
| ABCA2 | SERINE | 1327 | [147] |
| SERINE | 1331 | ||
| TYROSINE | 1694 | [148] | |
| TYROSINE | 2178 | [149] | |
| TYROSINE | 2186 | [148] | |
| THREONINE | 2412 | [149] | |
| ABCA3 | TYROSINE | 1265 | [148] |
| TYROSINE | 1268 | ||
| TYROSINE | 1349 | ||
| ABCA4 | TYROSINE | 400 | [148] |
| ABCA5 | TYROSINE | 22 | [148] |
| TYROSINE | 58 | ||
| TYROSINE | 1299 | [150] | |
| ABCA6 | NONE IDENTIFIED | ||
| ABCA7 | NONE IDENTIFIED | ||
| ABCA8 | NONE IDENTIFIED | ||
| ABCA9 | NONE IDENTIFIED | ||
| ABCA10 | TYROSINE | 307 | [151] |
| SERINE | 568 | [152] | |
| ABCA11 | NONE IDENTIFIED | ||
| ABCA12 | TYROSINE | 811 | [150] |
| TYROSINE | 814 | ||
| ABCA13 | TYROSINE | 1773 | [148] |
| ABCB1 | SERINE | 644 | [149] |
| SERINE | 658 | ||
| SERINE | 660 | ||
| SERINE | 661 | [23,24] | |
| SERINE | 667 | ||
| SERINE | 671 | ||
| SERINE | 683 | [149] | |
| SERINE | 686 | [23,24] | |
| ABCB2 | NONE IDENTIFIED | ||
| ABCB3 | THREONINE | 458 | [149] |
| TYROSINE | 597 | [150] | |
| TYROSINE | 693 | ||
| ABCB4 | NONE IDENTIFIED | ||
| ABCB5 | NONE IDENTIFIED | ||
| ABCB6 | NONE IDENTIFIED | ||
| ABCB7 | TYROSINE | 345 | [148] |
| TYROSINE | 356 | [148] | |
| ABCB8 | NONE IDENTIFIED | ||
| ABCB9 | SERINE | 530 | [153] |
| ABCB10 | NONE IDENTIFIED | ||
| ABCB11 | NONE IDENTIFIED | ||
| ABCC1 | TYROSINE | 490 | [148] |
| SERINE | 915 | [154,155] | |
| SERINE | 917 | [156] | |
| SERINE | 918 | [150] | |
| SERINE | 919 | [157,158] | |
| TYROSINE | 920 | [148,159,160] | |
| SERINE | 930 | [161,162] | |
| TYROSINE | 1508 | [160] | |
| ABCC2 | SERINE | 283 | [149,154] |
| TYROSINE | 616 | [157,158] | |
| THREONINE | 873 | [149] | |
| SERINE | 878 | ||
| TYROSINE | 885 | ||
| SERINE | 900 | ||
| ABCC3 | SERINE | 908 | [149,163] |
| SERINE | 911 | ||
| ABCC4 | SERINE | 313 | [152] |
| SERINE | 314 | ||
| SERINE | 601 | [164] | |
| THREONINE | 646 | [147,149,155] | |
| TYROSINE | 1259 | [150] | |
| ABCC5 | TYROSINE | 10 | [160] |
| SERINE | 505 | [149] | |
| SERINE | 509 | ||
| THREONINE | 513 | ||
| ABCC6 | SERINE | 681 | [156] |
| SERINE | 902 | [165] | |
| SERINE | 1310 | [156] | |
| ABCC7 | SERINE | 422 | [166] |
| SERINE | 489 | ||
| SERINE | 511 | [86] | |
| TYROSINE | 515 | [167] | |
| SERINE | 519 | [71] | |
| SERINE | 557 | ||
| THREONINE | 582 | [77] | |
| THREONINE | 604 | ||
| SERINE | 641 | [71] | |
| SERINE | 660 | [63,166,168] | |
| SERINE | 670 | [71] | |
| THREONINE | 682 | [77] | |
| SERINE | 686 | [63] | |
| THREONINE | 690 | [71] | |
| SERINE | 700 | [63,166,168] | |
| SERINE | 707 | [77] | |
| SERINE | 712 | [166,168] | |
| SERINE | 728 | [71] | |
| SERINE | 737 | [63,166,168] | |
| SERINE | 742 | [71] | |
| SERINE | 753 | [168] | |
| SERINE | 756 | [71] | |
| THREONINE | 757 | ||
| THREONINE | 760 | ||
| SERINE | 768 | [63,166,168] | |
| THREONINE | 787 | [71] | |
| THREONINE | 788 | ||
| SERINE | 790 | [63] | |
| SERINE | 795 | [63,166,168] | |
| SERINE | 813 | ||
| THREONINE | 1176 | [156] | |
| ABCC8 | TYROSINE | 798 | [150] |
| ABCC9 | NONE IDENTIFIED | ||
| ABCC10 | THREONINE | 463 | [169] |
| SERINE | 467 | ||
| ABCC11 | TYROSINE | 993 | [157,158] |
| ABCC12 | NONE IDENTIFIED | ||
| ABCD1 | SERINE | 733 | [149,154,170] |
| ABCD2 | NONE IDENTIFIED | ||
| ABCD3 | TYROSINE | 143 | [152] |
| ABCD4 | NONE IDENTIFIED | ||
| ABCG1 | NONE IDENTIFIED | ||
| ABCG2 | TYROSINE | 362 | [105] |
| ABCG4 | NONE IDENTIFIED | ||
| ABCG5 | NONE IDENTIFIED | ||
| ABCG8 | NONE IDENTIFIED | ||
3. ABC TRANSPORTER SUBFAMILIES; CONSEQUENCES OF THEIR PHOSPHORYLATION
3.1. ABCA
The ABCA subfamilies of proteins are involved in a variety of cellular functions and are associated with a number of human diseases [1,2]. Most notable is the role of ABCA1 in cholesterol efflux and Tangier disease. ABCA4 is critically important for the efflux of the retinol-AC conjugate from retinal pigment epithelium cells. Mutations in ABCA4 result in Stargardt disease, which is similar to macular degeneration [1,2]. Of these two transporters, ABCA1 has been the most extensively studied. For many years protein kinase A (PKA) was known to play an important role in regulating cholesterol efflux; however, the mechanism for this regulation was unknown. In 2002 PKA-regulated cholesterol efflux was finally shown to be the result of direct phosphorylation of ABCA1[14]. The study identified two PKA phosphorylation sites at serine (Ser)1042 within NBD1 and the homologous site, Ser2054, within NBD2 (it is thought that the NBDs share significant homology due to gene duplication) [14]. Although both sites are PKA phosphorylation sites in vitro, mutation analysis in vivo strongly suggests that Ser2054 is the dominant phosphorylation site [14]. Phosphorylation of Ser2054 is constitutive and required for full transporter activity. Mutation of Ser2054 to alanine (Ala) results in a 50% decrease in ABCA1-mediated cholesterol efflux in vitro [14]. In 2004, Roosbeek et. al. published biochemical evidence for phosphorylation of the CK2 consensus sites at threonine (Thr)1242, Thr1243, and Ser1255 within the “R-like” domain (Fig. 1), a shorter version of the ABCC7 R domain found between NBD1 to MSD2 [15]. Mutation of these sites individually to alanine (Ala) results in a partial loss of protein function [15]. Further, treatment of cells with a CK2 inhibitor resulted in decreased phosphorylation of recombinant NBD1-R1 (from ABCA1), which supports the initial finding that Thr1242, Thr1243, and Ser1255 are phosphorylated directly by CK2 [15].
Importantly, phosphorylation of ABCA1 plays a role in protein stability [16,17]. Martinez et. al. showed that phosphorylation of ABCA1 at Thr1286 and Thr1305, within the “R-like” domain, promotes calpain-mediated ABCA1 degradation [16]. Mutation of both sites to Ala resulted in a 3.1-fold increase in cell surface expression and a 2.3-fold increase in cholesterol efflux as compared to wild type (WT) [16]. Further, Yamauchi et al. provided evidence suggesting that ABCA1 is stabilized through a protein kinase Cα(PKCα)-dependent phosphorylation mechanism [17]. Together, these studies suggest a role for phosphorylation in the regulation of ABCA1 protein activity and stabilization/degradation.
3.2. ABCB
The ABCB subfamily of ABC transporters is a structurally and functionally diverse group of proteins that is conserved in all mammals (reviewed elsewhere) [1,2,18]. Members of the ABCB subfamily have been directly linked to multiple diseases including cholestasis, immune deficiency, and sideroblastic anemia, and MDR in cancer [19]. The most recognized of the ABCB subfamily is probably ABCB1, which is more commonly referred to as MDR1 or p-glycoprotein, followed by ABCB2 and ABCB3, the transporter-associated with antigen presenting proteins (TAPs) [1,3,19].
3.2.1. ABCB1
Overexpression of ABCB1 is strongly associated with the multidrug resistance (MDR) phenotype in a broad range of cancers [1,20]. Although the role of ABCB1 in cancer has been extensively studied, very little is known about the role of ABCB1 in normal cellular metabolism and cell protection from environmental stress. In addition, post-translational regulation of ABCB1 function is poorly understood. Interestingly, a large number of studies have suggested that ABCB1 is phosphorylated in vivo; however, it is still unclear what role phosphorylation plays in regulating ABCB1 function [8,9,20–43]. In 1987, Mellado and Horwitz published the first evidence suggesting that ABCB1 was phosphorylated [38]. Their work showed that phosphorylation of ABCB1 increased when cells were treated with cAMP. Treatment of partially purified ABCB1 with recombinant PKA (i.e., the catalytic subunit) results in increased phosphorylation of ABCB1 in vitro [38]. It is now apparent that ABCB1 is likely phosphorylated by a number of kinases, including PKC and PKA. A number of conflicting studies have been published as to the role of phosphorylation in regulating ABCB1 function and yet no clear consensus has been reached [21–25,29,31–32,36,41,42].
Interestingly, it is important to note that a large number of studies have shown that many of the known inhibitors of PKA, PKC, and many other kinases are both substrates and/or inhibitors of ABCB1 function [44–48]. There is increasing evidence that the same is true for some of the ABCC and ABCG subfamilies of transporters. The role of the kinase inhibitors as substrates and inhibitors of the ABC transporters is extremely important and critical to evaluating the effectiveness of kinase inhibitor use in the clinic [44–48]. Although of extreme importance, the role of kinase inhibitors as substrates and inhibitors of the ABC transporters is not covered in this review. Excellent reviews on this subject can be found elsewhere [44–48].
A number of studies support a role for phosphorylation in the regulation of ABCB1 function [21,22,25,30,31,34,36,39–42]. Overwhelming evidence suggests that PKC is a major player in ABCB1 phosphorylation and regulation [22–25,30–32,37,39,40,42,43]. Ex vivo purification of ABCB1 followed by tryptic digestion and peptide sequencing via Edman degradation identified that human ABCB1 is phosphorylated at putative PKC phosphorylation sites: serine 661, 667, and 671 [24,39]. Supporting these findings, in vitro kinase assays performed on small peptides derived from the “R-like” domain of ABCB1 identified serine 661, 667, and 671 as potential in vivo PKC phosphorylation sites [23,27,28]. PKC-dependent phosphorylation of ABCB1 is stimulated by the PKC activator 12-O-tetradecanoylphorbol-13-acetate (TPA) and okadaic acid [24] and inhibited by sphingosine stereoisomers [40]. Work by Idriss et. al. examining ABCB1 function in purified vesicles from sf9 cells suggests that phosphorylation within the “R-like” domain is specific for PKCα and not PKCε [31]. Further phosphorylation within the “R-like” domain regulates ABCB1 ATPase activity [31,42]. PKCα-mediated phosphorylation appears to regulate ABCB1-dependent efflux of anions, which suggests a role for ABCB1 in Cl− channel regulation [30–32,42–43]. Interestingly, mutation of all the possible PKC phosphorylation sites within the “R-like” domain of ABCB1 does not alter ABCB1 function [29]. However, the results of this study must be taken with caution as the function of the mutants was assessed in yeast and not in mammalian cells [29]. It is not uncommon to find that studies performed in yeast with mammalian homologues differ in their results as compared with the homologous studies carried out in human/mammalian cells [49–51].
In addition to PKC, evidence suggests that PKA and CK2 phosphorylate ABCB1 and regulate ABCB1 function [24,27–28,39]. PKA phosphorylates serine 667, 671, and 683 in human ABCB1 in vitro and in vivo [24,28,39]. These studies suggest that serine 667 and 671 are substrates for both PKC and PKA. CK2 phosphorylates up to five sites of ABCB1 within a small tryptic fragment (631–658) within the “R-like” domain similar to PKC and PKA [27]. Interestingly, GTP can stimulate ABCB1 phosphorylation and the intracellular ATP/GTP ratio has a clear effect on ABCB1 phosphorylation status [33,35]. In conclusion, ABCB1 function is regulated by phosphorylation and this regulation is quite dynamic due to the involvement of multiple kinases and multiple phosphorylation sites [34].
3.2.2. ABCB2 and ABCB3 (TAP1 and TAP2)
ABCB2 and ABCB3 are commonly referred to as the ATP-binding cassette transporters associated with antigen processing or TAPs [1,2]. ABCB2 and ABCB3 are required for the transport of antigenic peptides from the cytosol into the lumen of the ER for the assembly of histocompatibility antigen complex class I [1,2]. Heterodimerization is required for ABCB2 and ABCB3 to carry-out their cellular function [1,2]. Li et al. showed that a 43-kDa kinase can be found associated with the ABCB2/3 complex [52]. ABCB2/3 transport activity is inhibited by phosphorylation [52]. However, with such limited number of studies analyzing the role of phosphorylation on ABCB2/3 function, no significant conclusions can be made.
3.3. ABCC
The ABCC transporters are distinct from other ABC transporters by two unique features: 1) many transport glutathione conjugates (GS-X) or cotransport glutathione (GSH) and a substrate; and 2) the subfamily has an additional N-terminal extension beyond the “ABC Core” (Fig. 1) [2,53–55]. The N-terminal extension comes in two forms, a long form (comprising 5 additional membrane spans and a cytosolic loop) and a short form (comprising a very large N-terminal cytosolic domain of about 300 amino acids) (Fig. 1) [2,53,54]. The ABCC subfamily is one of the largest ABC transporter subfamilies. The ABCCs are involved in a variety of human diseases including pseudoxanthoma elasticum, cystic fibrosis, and MDR. ABCC7, more commonly referred to as the cystic fibrosis conductance regulator (CFTR) [2], is the most characterized of all the ABC transporters with respect to phosphorylation. Phosphorylation of the ABCC subfamily is discussed below.
3.3.1. ABCC2
ABCC2 is the gene that is responsible for Dubin-Johnson Syndrome (DJS) [1,2]. One of the major roles of ABCC2 in humans is thought to be the transport of conjugated bilirubin across the plasma membrane and into the canacular space where it can be removed from the body via hepatobiliary excretion 1,2]. Mutations that render ABCC2 non-functional prohibit the efflux of conjugated bilirubin from the liver. The loss of ABCC2 function or a reduction in transporter function results in the intracellular accumulation of both conjugated bilirubin (glucuronide-bilirubin) and unconjugated bilirubin in the liver [56,57]. Therefore, one mechanism of potential treatment of DJS patients would be to upregulate ABCC2 function, and hence, a critical understanding of ABCC2 regulation is required. To date, only three papers have reported on the posttranslational modification of ABCC2 [58–60]. Of these three reports, only one provides significant evidence to suggest a role for phosphorylation in ABCC2 regulation [60]. Although further investigation is needed, the initial studies described here suggest that patients with Dubin Johnsons Syndrome may benefit from treatment with kinase inhibitors.
3.3.2. ABCC7
ABCC7, or CFTR as it is more commonly known, is the Cl-channel that is responsible for cystic fibrosis when mutated [18,61]. ABCC7 is a short ABCC similar to ABCC4 and 5 (Fig. 1). Mutations in ABCC7, most notably a deletion of phenylalanine (Phe)508, result in misfolding of the protein and retention in the endoplasmic reticulum (ER) [18,61]. Of all the members of the ABC transporter superfamily, ABCC7 regulation via kinase-mediated phosphorylation is the most extensively characterized (see the list of identified phosphorylation sites in Table 2). The first evidence of ABCC7 regulation via phosphorylation was by Tabcharani et. al. and Picciotto et. al. in 1991 [62–64]. Their studies suggested that ABCC7 is regulated by PKA and PKC in response to cyclic AMP (cAMP) [62–64]. It has now been almost 20 years since this initial discovery and only very recent work has begun to shed light on the mechanism by which phosphorylation regulates ABCC7 function. ABCC7 phosphorylation and regulation have been extensively reviewed elsewhere [40,65–71], and thus we will briefly summarize the findings and discuss them in the context of the larger ABC transporter superfamily.
A review of the literature suggests that ABCC7 is extensively phosphorylated throughout the R domain, a cytosolic region of the protein that links the NBD1 to MSD2 see Table 2 and Fig. (1). ABCC7 function appears to be regulated by phosphorylation within NBD1 by PKC [72–74], and by extensive PKA- and PKC-mediated phosphorylation within the R domain [64,72,75–82]. In addition, more recent work suggests that ABCC7 function is regulated to a lesser extent by AMPK- and CK2-mediated phosphorylation within the R domain, and dephosphorylation by protein phosphatases [62,83–87].
The R domain of ABCC7 is rich in putative PKA phosphorylation sites [62–64,66,67,71]. In general, individual or combined mutation of PKA sites results in decreased transporter function [62–64,66,67,69,71,72,76,77,80,88–90]. The stimulatory effect of PKA on ABCC7 via phosphorylation is additive with a maximal induction of ABCC7 transporter function around 3-fold over that of the unphosphorylated protein [66,67,71]. Importantly, detailed analysis of the phosphorylation sites relative to each other suggests that phosphorylation at any one site is directly and indirectly affected by phosphorylation at another phosphorylation site(s) within ABCC7 [66,67,71]. PKA has been shown to require AKAP (Ezrin) to physically interact with ABCC7 and inhibition of this interaction blocks PKA phosphorylation of the R domain [82]. This finding suggests that phosphorylation of ABCC7 by PKA is regulated by AKAP.
Similar to PKA, ABCC7 has multiple PKC consensus sites within the R domain [62–64,66,67,71]. Regulation of ABCC7 function via PKC-mediated phosphorylation within the R domain is influenced by PKA- and PKC-mediated phosphorylation at other, non-R domain localized phosphorylation sites [66,67,71]. Therefore, PKA- and PKC-mediated ABCC7 regulation is extremely complex and involves multiple phosphorylation sites [66,67,71]. In the future, it will be very important to analyze the phosphorylation status of ABCC7 under various cellular conditions.
Recent studies shed new light on the mechanism by which phosphorylation regulates ABCC7 function through the R domain [70,78,91,92]. A combination of NMR, computational, and three dimensional cryomicroscopy studies indicate that phosphorylation of the R domain results in conformational changes that alter the function of ABCC7 [68,70,78,92]. The computational studies suggest that the change afforded by phosphorylation pushes the R domain away from the core and NBDs which results in an increase in the apparent size/radius of the protein [68]. This computational model is further supported by three dimensional cryomicroscopy studies which identified that in the absence of phosphorylation of the R domain, the NBDs and the core are more compact in shape and structure [68,70,92]. The culmination of these findings was recently published by Kanelis et al. In this study, the authors demonstrate by NMR that the unphosphorylated R domain of ABCC7 folds into the NBDs and the core. Phosphorylation of the R domain inhibits these interaction [92]. Together these studies suggest a model in which phosphorylation of the R domain prevents “compacting” of the ABCC7 protein and opens the core to accept ions for transport [92]. These findings provide important insight into the overall role of phosphorylation in the regulation of all ABC transporters. Although the extensive cytosolic loop in ABCC7 that connects NBD1 to MSD0 is not strictly conserved in other ABC transporters, a similar structure is found in every member of the ABC transporter superfamily [2,19].
3.3.3. ABCC8/9
ABCC8 and ABCC9, more commonly called sulfonyl urea receptors 1 and 2 (SUR1 and SUR2), are “Long” ABCC transporters similar to ABCC1 and ABCC2 [18,19,93]. Unlike other members of the ABCC subfamily, ABCC8 and ABCC9 are part of a multiprotein complex containing multiple subunits of the potassium inward rectifier protein, Kir6. 2. This large protein complex forms classical KATP channels [94]. Mutations in ABCC8, ABCC9, and Kir6.2 result in familial persistent hyperinsulinemic hypoglycemia (PHIP) [18,19,93,94] and appear to play an important role in cardiomyocyte function [95]. Similar to many other cellular ion channels, the KATP channels are highly regulated at multiple levels including post-translationally by phosphorylation [96–102].
Multiple studies suggest that ABCC8 is regulated by phosphorylation. One such study reports that ABCC8 (SUR1) is phosphorylated at Ser1571 in a PKA-dependent manner [96]. Phosphorylation of ABCC8 by PKA regulates the basal state and function of the KATP channels, including burst duration and interburst intervals[96]. Interestingly, the same study identified a PKA phosphorylation site at Ser372 in Kir6.2 and demonstrated that this site induces KATP channel activity in vivo [96]. This work is supported by Lin et. al. which identified a second phosphorylation site within Kir6.2 at Thr224 [98]. In addition to PKA regulation, ABCC8 may be regulated by PKC [100]. Work by Ribalet et. al. suggests that phosphorylation of both Kir6.2 and ABCC8 is required for proper function of the KATP channel [100]. To this end, cells expressing functional KATP channels were inhibited by specific PKC inhibitors [100].
Similar to ABCC8, ABCC9 (SUR2) is phosphorylated by PKA [99,101–103] at Thr633, Ser1387, and Ser1465 [99,103]. Phosphorylation of ABCC9 by PKA induces activation of the KATP channel [99,103]. This work, together with the studies described above for ABCC8, suggests a role for multisite phosphorylation as a complex mechanism for regulating KATP channel activity [99]. A challenge to studying the mechanism by which phosphorylation regulates ABCC8 and ABCC9 is determining how their interaction with Kir6.2 is affected. Therefore, it will be interesting to see if recent advances in phosphoproteomics and structural biology will lead to new insights into the role of phosphorylation in regulating KATP channel activity.
3.4. ABCD
The ABCD subfamilies of ABC transporters are located within the membrane of the peroxisome [1,2]. There are four members of this subfamily, ABCD1–4 [1,2]. Mutations within ABCD1 are responsible for the human disease, adrenoleukodystrophy [1,2]. To date, only one study has reported that members of the ABCD subfamily are regulated by phosphorylation [104]. Tanaka et. al. reported that ABCD1 and ABCD3 are phosphorylated in vivo and this phosphorylation appears to alter transporter function [104]. Unlike many of the other ABC transporters, which are phosphorylated by Ser/Thr-specific kinases, ABCD1 and ABCD3 appear to be phosphorylated by a tyrosine kinase [104]. In conclusion, it is reasonable to believe that ABCD1 and ABCD3 are regulated via phosphorylation; however, a considerable amount of work remains before any substantial conclusions can be drawn.
3.5. ABCG
The ABCG subfamily of transporters is an extremely diverse subfamily of transporters comprised of five half transporters (ABCG 1,2,4,5, and 8) [2]. The ABCG subfamily includes ABCG2 (BCRP), which is has been strongly associated with drug resistance in cancer, and ABCG5 and ABCG8, which have been shown to be responsible for sitosterolemia [2]. Similar to the ABCD subfamily, only one report to date has identified phosphorylation as a potential regulatory mechanism in this subfamily [105]. Xie et. al. reported that phosphorylation of ABCG2 is required for proper function [105]. Their work suggests that the kinase Pim-1 phosphorylates ABCG2 at Thr362 and that this phosphorylation modulates dimerization of the ABCG2 molecules, which is a requirement for proper function [105]. Mutation of Thr362 to alanine results in cytoplasmic compartmentalization of ABCG2 thereby inhibiting proper localization at the plasma membrane and protein dimerization [105]. Further work is necessary to determine the overall extent to which ABCG2 is phosphorylated.
4. REGULATION OF NON-MAMMALIAN ABC TRANSPORTERS: A BRIEF OVERVIEW
ABC transporters are found in all organisms from bacteria to man [2,18]. The role of ABC transporters in microorganisms has been associated with the efflux of a number of compounds and ions including salt ions, sugars, lipids, and toxins [106–114]. In bacteria and yeast, ABC transporters are associated with multidrug resistance and increased organism virulence [106–114]. Therefore, a better understanding of ABC transporter biology and biochemistry is of critical importance in order to improve treatment of microorganism-associated diseases and infections. In addition, microorganisms, such as the yeast Saccharomyces cerevisiae, are excellent models for the study of ABC transporter function and regulation [4,115,116]. In recent years, high throughput genomic and protein interaction studies involving yeast ABC transporters have proven extremely useful in identifying new regulatory pathways including those involving phosphorylation [4,115,116]. Recent advances in phosphoproteomics of the whole yeast proteome have been extremely useful in identifying a number of phosphorylation sites within the ABC transporters [110,117–120]. Together, these approaches have provens the utility and importance of yeast and other microorganisms as model systems to characterize ABC transporter function.
To date a number of studies in yeast and bacteria have identified mechanisms by which ABC transporter function is regulated by phosphorylation [121–129]. In yeast, the ABC transporters that have been suggested to be regulated by phosphorylation include Ste6p, Ycf1p, Pdr5p, and Cdr1p [122–127,129]. Phosphorylation of Ste6p, a protein required for yeast mating, appears to play a critical role in regulating protein localization and recycling [122,123]. The activity of Pdr5p is regulated by Sit4p-mediated phosphorylation [126]. Similarly, Ycf1p function is regulated by the yeast casein kinase 2 alpha protein, Cka1p [124]. Phosphorylation has been suggested to play a dual role for Cdr1p [129] by altering the activity and stability of the transporter [129]. Similar studies can be found for bacterial ABC transporters [121,128]. Mycobacterium tuberculosis pathogenicity in mice requires the ABC transporter, Rv1747 [121,128]. Here pathogenicity associated with Rv1747 is regulated, in part, via a Ser/Thr kinase [121,128]. Further insight into the mechanisms regulating ABC transporter phosphorylation in microorganisms may allow for the development of novel kinase inhibitor-based therapeutics for the treatment of microorganismal-associated diseases and infections.
5. PHOSPHOPROTEOMICS: PROSPECTUS WITH RESPECT TO ABC’S
With the increasing development of new phosphopeptide enrichment techniques and the increasing sensitivity of mass spectrometers, the identification of phosphorylation sites on proteins has become quite routine [130]. With the ever increasing number of high quality proteomic cores being established, liquid chromatography-mass spectroscopy (LC/MS) has quickly become the technique of choice [130]. Unlike many of the traditional techniques used to identify phosphorylation of a protein, such as two dimensional gel electrophoresis (2D SDS-PAGE) analysis of [32P]-labeled protein lysates, LC/MS does not require radioactivity, it is more sensitive, and has the ability to identify multiple phosphorylation sites in multiple samples during a single run. This allows for a direct comparison of the phosphorylation status of a single protein from two separate samples. LC/MS in this manner utilizes a technique called stable isotope labeling of amino acids in tissue culture or SILAC [130].
One of the most important developments in recent years may be the use of immobilized metal affinity chromatography (IMAC) to greatly enrich phosphopeptides from whole-cell trypsinized lysate protein preparations [131]. Currently, ultra high performance liquid chromatography (UHPLC) and nano-HPLC MS/MS analysis in combination with various dissociative MS techniques (ECD and ETD) have increased detection sensitivity well beyond what was capable even a year ago [131]. Improvements in all these areas will increase our ability to detect phosphorylation sites within the ABC transporters, a development that will be essential for understanding the complex regulation of ABC transporter function via multisite phosphorylation.
Although the development of highly sensitive phosphoproteomic detection techniques has vastly improved our ability to identify sites of phosphorylation, it remains extremely difficult to identify phosphorylation sites within membrane bound proteins [7]. As with the study of membrane proteins in general, a great limitation to their biochemical analysis is our ability to enrich them [7]. Therefore, it has become critically important to consider one or multiple enrichment steps [7]. To use LC/MS phosphoproteomics successfully to identify ABC transporter phosphorylation sites, multiple techniques have been used. These techniques include both classical and modern methodologies such as density gradient centrifugation, co-immunoprecipitation (possibly by biotinylation), and purification from cell debris with various detergents [7]. Other techniques that will continue to play an important role in the identification of kinases and kinase-transporter interactions are cross-linking technologies and the modified yeast two hybrid assay (iMYTH). “Cell-shaving” may provide unique insights into the status of the externally oriented loops of the ABC transporters [4,7]. These techniques are extensively reviewed by Cordwell et. al. [7].
In conclusion, advanced tools are available to further address how phosphorylation regulates ABC transporter function. More detailed analysis of this type will provide useful insight into not only the cellular function of ABC transporters but also into how these proteins are regulated. Ultimately, the identification of kinases that regulate transporter function may aid in the development of novel kinase inhibitor-based therapies that will more effectively treat ABC transporter-related diseases.
Acknowledgments
CMP is supported by NIH P20RR020171. The authors thank Dr. Mary Vore for her support and her effort in the review of this manuscript. The authors would also like to thank Dr. Zhe-Sheng Chen for the opportunity to submit this manuscript into this review issue of Current Pharmaceutical Biotechnology.
LIST OF ABBREVIATIONS
- ABC
ATP-binding Cassette
- BCRP
Breast Cancer Related Protein
- CFTR
Cystic Fibrosis Transductance Regulator
- IMAC
Ion Metal Affinity Chromatography
- LC/MS
Liquid Chromatography/Mass Spectrometry
- MRP
Multidrug Resistance-associated Protein
- P-gp
P-Glycoprotein
- TAP
Tandem Affinity Purification
- UHPLC
Ultra High Performance Liquid Chromatography
- ECD
Electron Capture Dissociation
- ETD
Electron Transfer Dissociation
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest for the authors of this paper.
References
- 1.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 2.Dean M, Allikmets R. Evolution of ATP-binding cassette transporter genes. Curr Opin Genet Dev. 1995;5:779–785. doi: 10.1016/0959-437x(95)80011-s. [DOI] [PubMed] [Google Scholar]
- 3.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 4.Paumi CM, Chuk M, Snider J, Stagljar I, Michaelis S. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol Mol Biol Rev. 2009;73:577–593. doi: 10.1128/MMBR.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haimeur A, Conseil G, Deeley RG, Cole SP. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. doi: 10.2174/1389200043489199. [DOI] [PubMed] [Google Scholar]
- 6.Taglicht D, Michaelis S. Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol. 1998;292:130–162. doi: 10.1016/s0076-6879(98)92012-2. [DOI] [PubMed] [Google Scholar]
- 7.Cordwell SJ, Thingholm TE. Technologies for plasma membrane proteomics. Proteomics. 2009;10(4):611–627. doi: 10.1002/pmic.200900521. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur J Biochem. 2001;268:5001–5010. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson LN. The regulation of protein phosphorylation. Biochem Soc Trans. 2009;37:627–641. doi: 10.1042/BST0370627. [DOI] [PubMed] [Google Scholar]
- 10.Salazar C, Hofer T. Multisite protein phosphorylation--from molecular mechanisms to kinetic models. FEBS J. 2009;276:3177–3198. doi: 10.1111/j.1742-4658.2009.07027.x. [DOI] [PubMed] [Google Scholar]
- 11.Ame MV, Baroni MV, Galanti LN, Bocco JL, Wunderlin DA. Effects of microcystin-LR on the expression of P-glycoprotein in Jenynsia multidentata. Chemosphere. 2009;74:1179–1186. doi: 10.1016/j.chemosphere.2008.11.068. [DOI] [PubMed] [Google Scholar]
- 12.Contardo-Jara V, Pflugmacher S, Wiegand C. Multi-xenobiotic-resistance a possible explanation for the insensitivity of bivalves towards cyanobacterial toxins. Toxicon. 2008;52:936–943. doi: 10.1016/j.toxicon.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Pearson LA, Hisbergues M, Borner T, Dittmann E, Neilan BA. Inactivation of an ABC transporter gene, mcyH, results in loss of microcystin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Appl Environ Microbiol. 2004;70:6370–6378. doi: 10.1128/AEM.70.11.6370-6378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See RH, Caday-Malcolm RA, Singaraja RR, Zhou S, Silverston A, Huber MT, Moran J, James ER, Janoo R, Savill JM, Rigot V, Zhang LH, Wang M, Chimini G, Wellington CL, Tafuri SR, Hayden MR. Protein kinase A site-specific phosphorylation regulates ATP-binding cassette A1 (ABCA1)-mediated phospholipid efflux. J Biol Chem. 2002;277:41835–41842. doi: 10.1074/jbc.M204923200. [DOI] [PubMed] [Google Scholar]
- 15.Roosbeek S, Peelman F, Verhee A, Labeur C, Caster H, Lensink MF, Cirulli C, Grooten J, Cochet C, Vandekerckhove J, Amoresano A, Chimini G, Tavernier J, Rosseneu M. Phosphorylation by protein kinase CK2 modulates the activity of the ATP binding cassette A1 transporter. J Biol Chem. 2004;279:37779–37788. doi: 10.1074/jbc.M401821200. [DOI] [PubMed] [Google Scholar]
- 16.Martinez LO, Agerholm-Larsen B, Wang N, Chen W, Tall AR. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J Biol Chem. 2003;278:37368–37374. doi: 10.1074/jbc.M307161200. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi Y, Hayashi M, Abe-Dohmae S, Yokoyama S. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J Biol Chem. 2003;278:47890–47897. doi: 10.1074/jbc.M306258200. [DOI] [PubMed] [Google Scholar]
- 18.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 19.Dean M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005;400:409–429. doi: 10.1016/S0076-6879(05)00024-8. [DOI] [PubMed] [Google Scholar]
- 20.Ueda K, Cornwell MM, Gottesman MM, Pastan I, Roninson IB, Ling V, Riordan JR. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys Res Commun. 1986;141:956–962. doi: 10.1016/s0006-291x(86)80136-x. [DOI] [PubMed] [Google Scholar]
- 21.Chambers TC. Identification of phosphorylation sites in human MDR1 P-glycoprotein. Methods Enzymol. 1998;292:328–342. doi: 10.1016/s0076-6879(98)92026-2. [DOI] [PubMed] [Google Scholar]
- 22.Chambers TC, McAvoy EM, Jacobs JW, Eilon G. Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J Biol Chem. 1990;265:7679–7686. [PubMed] [Google Scholar]
- 23.Chambers TC, Pohl J, Glass DB, Kuo JF. Phosphorylation by protein kinase C and cyclic AMP-dependent protein kinase of synthetic peptides derived from the linker region of human P-glycoprotein. Biochem J. 1994;299(Pt 1):309–315. doi: 10.1042/bj2990309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers TC, Pohl J, Raynor RL, Kuo JF. Identification of specific sites in human P-glycoprotein phosphorylated by protein kinase C. J Biol Chem. 1993;268:4592–4595. [PubMed] [Google Scholar]
- 25.Chambers TC, Zheng B, Kuo JF. Regulation by phorbol ester and protein kinase C inhibitors, and by a protein phosphatase inhibitor (okadaic acid), of P-glycoprotein phosphorylation and relationship to drug accumulation in multidrug-resistant human KB cells. Mol Pharmacol. 1992;41:1008–1015. [PubMed] [Google Scholar]
- 26.Cohen P. The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 27.Glavy JS, Horwitz SB, Orr GA. Identification of the in vivo phosphorylation sites for acidic-directed kinases in murine mdr1b P-glycoprotein. J Biol Chem. 1997;272:5909–5914. doi: 10.1074/jbc.272.9.5909. [DOI] [PubMed] [Google Scholar]
- 28.Glavy JS, Wolfson M, Nieves E, Han EK, Yang CP, Horwitz SB, Orr GA. Identification of in vivo phosphorylation sites for basic-directed kinases in murine mdr1b P-glycoprotein by combination of mass spectrometry and site-directed mutagenesis. Methods Enzymol. 1998;292:342–358. doi: 10.1016/s0076-6879(98)92027-4. [DOI] [PubMed] [Google Scholar]
- 29.Goodfellow HR, Sardini A, Ruetz S, Callaghan R, Gros P, McNaughton PA, Higgins CF. Protein kinase C-mediated phosphorylation does not regulate drug transport by the human multidrug resistance P-glycoprotein. J Biol Chem. 1996;271:13668–13674. doi: 10.1074/jbc.271.23.13668. [DOI] [PubMed] [Google Scholar]
- 30.Hardy SP, Goodfellow HR, Valverde MA, Gill DR, Sepulveda V, Higgins CF. Protein kinase C-mediated phosphorylation of the human multidrug resistance P-glycoprotein regulates cell volume-activated chloride channels. EMBO J. 1995;14:68–75. doi: 10.1002/j.1460-2075.1995.tb06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idriss H, Urquidi V, Basavappa S. Selective modulation of P-glycoprotein’s ATPase and anion efflux regulation activities with PKC alpha and PKC epsilon in Sf9 cells. Cancer Chemother Pharmacol. 2000;46:287–292. doi: 10.1007/s002800000172. [DOI] [PubMed] [Google Scholar]
- 32.Idriss HT, Hannun YA, Boulpaep E, Basavappa S. Regulation of volume-activated chloride channels by P-glycoprotein: phosphorylation has the final say. J Physiol. 2000;524(Pt 3):629–636. doi: 10.1111/j.1469-7793.2000.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lelong IH, Cardarelli CO, Gottesman MM, Pastan I. GTP-stimulated phosphorylation of P-glycoprotein in transporting vesicles from KB-V1 multidrug resistant cells. Biochemistry. 1994;33:8921–8929. doi: 10.1021/bi00196a009. [DOI] [PubMed] [Google Scholar]
- 34.Lelong-Rebel IH, Cardarelli CO. Differential phosphorylation patterns of P-glycoprotein reconstituted into a proteoliposome system: insight into additional unconventional phosphorylation sites. Anticancer Res. 2005;25:3925–3935. [PubMed] [Google Scholar]
- 35.Lelong-Rebel IH, Rebel G, Cardarelli CO, Pastan I, Gottesman MM. Modulation by the ATP/GTP ratio of the phosphorylation level of P-glycoprotein and of various plasma membrane proteins of KB-V1 multidrug resistant cells. Anticancer Res. 2003;23:2363–2375. [PubMed] [Google Scholar]
- 36.Ma LD, Marquardt D, Takemoto L, Center MS. Analysis of P-glycoprotein phosphorylation in HL60 cells isolated for resistance to vincristine. J Biol Chem. 1991;266:5593–5599. [PubMed] [Google Scholar]
- 37.Masanek U, Stammler G, Volm M. Modulation of multidrug resistance in human ovarian cancer cell lines by inhibition of P-glycoprotein 170 and PKC isoenzymes with antisense oligonucleotides. J Exp Ther Oncol. 2002;2:37–41. doi: 10.1046/j.1359-4117.2002.01004.x. [DOI] [PubMed] [Google Scholar]
- 38.Mellado W, Horwitz SB. Phosphorylation of the multidrug resistance associated glycoprotein. Biochemistry. 1987;26:6900–6904. doi: 10.1021/bi00396a005. [DOI] [PubMed] [Google Scholar]
- 39.Orr GA, Han EK, Browne PC, Nieves E, O’Connor BM, Yang CP, Horwitz SB. Identification of the major phosphorylation domain of murine mdr1b P-glycoprotein. Analysis of the protein kinase A and protein kinase C phosphorylation sites. J Biol Chem. 1993;268:25054–25064. [PubMed] [Google Scholar]
- 40.Sachs CW, Ballas LM, Mascarella SW, Safa AR, Lewin AH, Loomis C, Carroll FI, Bell RM, Fine RL. Effects of sphingosine stereoisomers on P-glycoprotein phosphorylation and vinblastine accumulation in multidrug-resistant MCF-7 cells. Biochem Pharmacol. 1996;52:603–612. doi: 10.1016/0006-2952(96)00312-7. [DOI] [PubMed] [Google Scholar]
- 41.Staats J, Marquardt D, Center MS. Characterization of a membrane-associated protein kinase of multidrug-resistant HL60 cells which phosphorylates P-glycoprotein. J Biol Chem. 1990;265:4084. [PubMed] [Google Scholar]
- 42.Szabo K, Bakos E, Welker E, Muller M, Goodfellow HR, Higgins CF, Varadi A, Sarkadi B. Phosphorylation site mutations in the human multidrug transporter modulate its drug-stimulated ATPase activity. J Biol Chem. 1997;272:23165–23171. doi: 10.1074/jbc.272.37.23165. [DOI] [PubMed] [Google Scholar]
- 43.Vanoye CG, Castro AF, Pourcher T, Reuss L, Altenberg GA. Phosphorylation of P-glycoprotein by PKA and PKC modulates swelling-activated Cl- currents. Am J Physiol. 1999;276:C370. doi: 10.1152/ajpcell.1999.276.2.C370. [DOI] [PubMed] [Google Scholar]
- 44.Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A, Boudriot U, Neubauer A. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267–1275. doi: 10.1038/sj.leu.2404638. [DOI] [PubMed] [Google Scholar]
- 45.Ozvegy-Laczka C, Cserepes J, Elkind NB, Sarkadi B. Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resist Updat. 2005;8:15–26. doi: 10.1016/j.drup.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, Bates SE, Shen T, Ashby CR, Jr, Fu LW, Ambudkar SV, Chen ZS. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 47.Shi Z, Tiwari AK, Shukla S, Robey RW, Kim IW, Parmar S, Bates SE, Si QS, Goldblatt CS, Abraham I, Fu LW, Ambudkar SV, Chen ZS. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem Pharmacol. 2009;77:781–793. doi: 10.1016/j.bcp.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009;37:359–365. doi: 10.1124/dmd.108.024612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richter S, Geldner N, Schrader J, Wolters H, Stierhof YD, Rios G, Koncz C, Robinson DG, Jurgens G. Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature. 2007;448:488–492. doi: 10.1038/nature05967. [DOI] [PubMed] [Google Scholar]
- 50.Bowring CE, Llewellyn DH. Differences in HAC1 mRNA processing and translation between yeast and mammalian cells indicate divergence of the eukaryotic ER stress response. Biochem Biophys Res Commun. 2001;287:789–800. doi: 10.1006/bbrc.2001.5633. [DOI] [PubMed] [Google Scholar]
- 51.Morreale G, Conforti L, Coadwell J, Wilbrey AL, Coleman MP. Evolutionary divergence of valosin-containing protein/cell division cycle protein 48 binding interactions among endoplasmic reticulum-associated degradation proteins. FEBS J. 2009;276:1208–1220. doi: 10.1111/j.1742-4658.2008.06858.x. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Salter-Cid L, Vitiello A, Preckel T, Lee JD, Angulo A, Cai Z, Peterson PA, Yang Y. Regulation of transporter associated with antigen processing by phosphorylation. J Biol Chem. 2000;275:24130–24135. doi: 10.1074/jbc.M003617200. [DOI] [PubMed] [Google Scholar]
- 53.Hipfner DR, Gauldie SD, Deeley RG, Cole SP. Detection of the M(r) 190,000 multidrug resistance protein, MRP, with monoclonal antibodies. Cancer Res. 1994;54:5788. [PubMed] [Google Scholar]
- 54.Slovak ML, Ho JP, Bhardwaj G, Kurz EU, Deeley RG, Cole SP. Localization of a novel multidrug resistance-associated gene in the HT1080/DR4 and H69AR human tumor cell lines. Cancer Res. 1993;53:3221–3225. [PubMed] [Google Scholar]
- 55.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 56.Keitel V, Nies AT, Brom M, Hummel-Eisenbeiss J, Spring H, Keppler D. A common Dubin-Johnson syndrome mutation impairs protein maturation and transport activity of MRP2 (ABCC2) Am J Physiol Gastrointest Liver Physiol. 2003;284:G165–174. doi: 10.1152/ajpgi.00362.2002. [DOI] [PubMed] [Google Scholar]
- 57.Keppler D, Konig J. Hepatic secretion of conjugated drugs and endogenous substances. Semin Liver Dis. 2000;20:265–272. doi: 10.1055/s-2000-9391. [DOI] [PubMed] [Google Scholar]
- 58.Ito K, Wakabayashi T, Horie T. Mrp2/Abcc2 transport activity is stimulated by protein kinase Calpha in a baculo virus co-expression. system. Life Sci. 2005;77:539–550. doi: 10.1016/j.lfs.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 59.Minami S, Ito K, Honma M, Ikebuchi Y, Anzai N, Kanai Y, Nishida T, Tsukita S, Sekine S, Horie T, Suzuki H. Posttranslational regulation of Abcc2 expression by SUMOylation system. Am J Physiol Gastrointest Liver Physiol. 2009;296:G406–413. doi: 10.1152/ajpgi.90309.2008. [DOI] [PubMed] [Google Scholar]
- 60.Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- 61.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007. [PubMed] [Google Scholar]
- 62.Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- 63.Picciotto MR, Cohn JA, Bertuzzi G, Greengard P, Nairn AC. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1992;267:12742–12752. [PubMed] [Google Scholar]
- 64.Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991;66:1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- 65.Aleksandrov AA, Aleksandrov LA, Riordan JR. CFTR (ABCC7) is a hydrolyzable-ligand-gated channel. Pflugers Arch. 2007;453:693–702. doi: 10.1007/s00424-006-0140-z. [DOI] [PubMed] [Google Scholar]
- 66.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hegedus T, Aleksandrov A, Mengos A, Cui L, Jensen TJ, Riordan JR. Role of individual R domain phosphorylation sites in CFTR regulation by protein kinase A. Biochim Biophys Acta. 2009;1788:1341–1349. doi: 10.1016/j.bbamem.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Hegedus T, Serohijos AW, Dokholyan NV, He L, Riordan JR. Computational studies reveal phosphorylation-dependent changes in the unstructured R domain of CFTR. J Mol Biol. 2008;378:1052–1063. doi: 10.1016/j.jmb.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seibert FS, Chang XB, Aleksandrov AA, Clarke DM, Hanrahan JW, Riordan JR. Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum. Biochim Biophys Acta. 1999;1461:275–283. doi: 10.1016/s0005-2736(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Aleksandrov LA, Zhao Z, Birtley JR, Riordan JR, Ford RC. Architecture of the cystic fibrosis transmembrane conductance regulator protein and structural changes associated with phosphorylation and nucleotide binding. J Struct Biol. 2009;167:242–251. doi: 10.1016/j.jsb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev. 1999;79:S77. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- 72.Chappe V, Hinkson DA, Zhu T, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation of protein kinase C sites in NBD1 and the R domain control CFTR channel activation by PKA. J Physiol. 2003;548:39–52. doi: 10.1113/jphysiol.2002.035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Csanady L, Chan KW, Nairn AC, Gadsby DC. Functional roles of nonconserved structural segments in CFTR’s NH2-terminal nucleotide binding domain. J Gen Physiol. 2005;125:43–55. doi: 10.1085/jgp.200409174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino WB, Hendrickson WA, Hunt JF, Kearins MC, Lorimer D, Maloney PC, Post KW, Rajashankar KR, Rutter ME, Sauder JM, Shriver S, Thibodeau PH, Thomas PJ, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–292. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Becq F, Jensen TJ, Chang XB, Savoia A, Rommens JM, Tsui LC, Buchwald M, Riordan JR, Hanrahan JW. Phosphatase inhibitors activate normal and defective CFTR chloride channels. Proc Natl Acad Sci USA. 1994;91(19):9160–9164. doi: 10.1073/pnas.91.19.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang XB, Tabcharani JA, Hou YX, Jensen TJ, Kartner N, Alon N, Hanrahan JW, Riordan JR. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- 77.Chappe V, Hinkson DA, Howell LD, Evagelidis A, Liao J, Chang XB, Riordan JR, Hanrahan JW. Stimulatory and inhibitory protein kinase C consensus sequences regulate the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 2004;101:390–395. doi: 10.1073/pnas.0303411101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dulhanty AM, Riordan JR. Phosphorylation by cAMP-dependent protein kinase causes a conformational change in the R domain of the cystic fibrosis transmembrane conductance regulator. Biochemistry. 1994;33:4072. doi: 10.1021/bi00179a036. [DOI] [PubMed] [Google Scholar]
- 79.Mathews CJ, Tabcharani JA, Chang XB, Jensen TJ, Riordan JR, Hanrahan JW. Dibasic protein kinase A sites regulate bursting rate and nucleotide sensitivity of the cystic fibrosis transmembrane conductance regulator chloride channel. J Physiol. 1998;508(Pt 2):365–377. doi: 10.1111/j.1469-7793.1998.365bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seibert FS, Tabcharani JA, Chang XB, Dulhanty AM, Mathews C, Hanrahan JW, Riordan JR. cAMP-dependent protein kinase-mediated phosphorylation of cystic fibrosis transmembrane conductance regulator residue Ser-753 and its role in channel activation. J Biol Chem. 1995;270:2158–2162. doi: 10.1074/jbc.270.5.2158. [DOI] [PubMed] [Google Scholar]
- 81.Broughman JR, Sun L, Umar S, Sellin JH, Morris AP. Chronic PKC-beta2 activation in HT-29 Cl.19a colonocytes prevents cAMP-mediated ion secretion by inhibiting apical membrane CFTR targeting. Am J Physiol Gastrointest Liver Physiol. 2006;291:G331–334. doi: 10.1152/ajpgi.00356.2005. [DOI] [PubMed] [Google Scholar]
- 82.Sun F, Hug MJ, Bradbury NA, Frizzell RA. Protein kinase A associates with cystic fibrosis transmembrane conductance regulator via an interaction with ezrin. J Biol Chem. 2000;275:14360–14366. doi: 10.1074/jbc.275.19.14360. [DOI] [PubMed] [Google Scholar]
- 83.Thelin WR, Kesimer M, Tarran R, Kreda SM, Grubb BR, Sheehan JK, Stutts MJ, Milgram SL. The cystic fibrosis transmembrane conductance regulator is regulated by a direct interaction with the protein phosphatase 2A. J Biol Chem. 2005;280:41512–41520. doi: 10.1074/jbc.M507308200. [DOI] [PubMed] [Google Scholar]
- 84.Kongsuphol P, Cassidy D, Hieke B, Treharne KJ, Schreiber R, Mehta A, Kunzelmann K. Mechanistic insight into control of CFTR by AMPK. J Biol Chem. 2009;284:5645–5653. doi: 10.1074/jbc.M806780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pagano MA, Arrigoni G, Marin O, Sarno S, Meggio F, Treharne KJ, Mehta A, Pinna LA. Modulation of protein kinase CK2 activity by fragments of CFTR encompassing F508 may reflect functional links with cystic fibrosis pathogenesis. Biochemistry. 2008;47:7925–7936. doi: 10.1021/bi800316z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Treharne KJ, Crawford RM, Xu Z, Chen JH, Best OG, Schulte EA, Gruenert DC, Wilson SM, Sheppard DN, Kunzelmann K, Mehta A. Protein kinase CK2, cystic fibrosis transmembrane conductance regulator, and the deltaF508 mutation: F508 deletion disrupts a kinase-binding site. J Biol Chem. 2007;282:10804–10813. doi: 10.1074/jbc.M610956200. [DOI] [PubMed] [Google Scholar]
- 87.King JD, Jr, Fitch AC, Lee JK, McCane JE, Mak DO, Foskett JK, Hallows KR. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am J Physiol Cell Physiol. 2009;297:C94–101. doi: 10.1152/ajpcell.00677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Csanady L, Seto-Young D, Chan KW, Cenciarelli C, Angel BB, Qin J, McLachlin DT, Krutchinsky AN, Chait BT, Nairn AC, Gadsby DC. Preferential phosphorylation of R-domain Serine 768 dampens activation of CFTR channels by PKA. J Gen Physiol. 2005;125:171–186. doi: 10.1085/jgp.200409076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dahan D, Evagelidis A, Hanrahan JW, Hinkson DA, Jia Y, Luo J, Zhu T. Regulation of the CFTR channel by phosphorylation. Pflugers Arch. 2001;443(Suppl 1):S92. doi: 10.1007/s004240100652. [DOI] [PubMed] [Google Scholar]
- 90.Ostedgaard LS, Baldursson O, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by its R domain. J Biol Chem. 2001;276:7689–7692. doi: 10.1074/jbc.R100001200. [DOI] [PubMed] [Google Scholar]
- 91.Baker JM, Hudson RP, Kanelis V, Choy WY, Thibodeau PH, Thomas PJ, Forman-Kay JD. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat Struct Mol Biol. 2007;14:738. doi: 10.1038/nsmb1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanelis V, Hudson RP, Thibodeau PH, Thomas PJ, Forman-Kay JD. NMR evidence for differential phosphorylation-dependent interactions in WT and DeltaF508 CFTR. EMBO J. 2010;29:263–277. doi: 10.1038/emboj.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 94.Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 95.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 96.Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human K(ATP) channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J. 1999;18:4722. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin YF, Chai Y. Functional modulation of the ATP-sensitive potassium channel by extracellular signal-regulated kinase-mediated phosphorylation. Neuroscience. 2008;152:371–380. doi: 10.1016/j.neuroscience.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J. 2000;19:942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 100.Ribalet B, John SA, Weiss JN. Regulation of cloned ATP-sensitive K channels by phosphorylation, MgADP, and phosphatidylinositol bisphosphate (PIP(2)): a study of channel rundown and reactivation. J Gen Physiol. 2000;116:391–410. doi: 10.1085/jgp.116.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi Y, Chen X, Wu Z, Shi W, Yang Y, Cui N, Jiang C, Harrison RW. cAMP-dependent protein kinase phosphorylation produces interdomain movement in SUR2B leading to activation of the vascular KATP channel. J Biol Chem. 2008;283:7523–7530. doi: 10.1074/jbc.M709941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, Zhu D, Jiang C. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta. 2008;1778:88–96. doi: 10.1016/j.bbamem.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol. 2007;293:R1205–1214. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanaka AR, Tanabe K, Morita M, Kurisu M, Kasiwayama Y, Matsuo M, Kioka N, Amachi T, Imanaka T, Ueda K. ATP binding/hydrolysis by and phosphorylation of peroxisomal ATP-binding cassette proteins PMP70 (ABCD3) and adrenoleukodystrophy protein (ABCD1) J Biol Chem. 2002;277:40142–40147. doi: 10.1074/jbc.M205079200. [DOI] [PubMed] [Google Scholar]
- 105.Xie Y, Xu K, Linn DE, Yang X, Guo Z, Shimelis H, Nakanishi T, Ross DD, Chen H, Fazli L, Gleave ME, Qiu Y. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J Biol Chem. 2008;283:3349–3356. doi: 10.1074/jbc.M707773200. [DOI] [PubMed] [Google Scholar]
- 106.Kiran MD, Akiyoshi DE, Giacometti A, Cirioni O, Scalise G, Balaban N. OpuC--an ABC transporter that is associated with Staphylococcus aureus pathogenesis. Int J Artif Organs. 2009;32:600–610. doi: 10.1177/039139880903200909. [DOI] [PubMed] [Google Scholar]
- 107.Colicchio R, Ricci S, Lamberti F, Pagliarulo C, Pagliuca C, Braione V, Braccini T, Tala A, Montanaro D, Tripodi S, Cintorino M, Troncone G, Bucci C, Pozzi G, Bruni CB, Alifano P, Salvatore P. The meningococcal ABC-Type L-glutamate transporter GltT is necessary for the development of experimental meningitis in mice. Infect Immun. 2009;77:3578–3587. doi: 10.1128/IAI.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lamarche MG, Wanner BL, Crepin S, Harel J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev. 2008;32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- 109.Mishra NN, Prasad T, Sharma N, Payasi A, Prasad R, Gupta DK, Singh R. Pathogenicity and drug resistance in Candida albicans and other yeast species. A review. Acta Microbiol Immunol Hung. 2007;54:201–235. doi: 10.1556/AMicr.54.2007.3.1. [DOI] [PubMed] [Google Scholar]
- 110.Niimi M, Tanabe K, Wada S, Yamazaki A, Uehara Y, Niimi K, Lamping E, Holmes AR, Monk BC, Cannon RD. ABC transporters of pathogenic fungi: recent advances in functional analyses. Nippon Ishinkin Gakkai Zasshi. 2005;46:249–260. doi: 10.3314/jjmm.46.249. [DOI] [PubMed] [Google Scholar]
- 111.Upcroft P. Multiple drug resistance in the pathogenic protozoa. Acta Trop. 1994;56:195–212. doi: 10.1016/0001-706x(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 112.Del Sorbo G, Schoonbeek H, De Waard MA. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet Biol. 2000;30:1–15. doi: 10.1006/fgbi.2000.1206. [DOI] [PubMed] [Google Scholar]
- 113.Davidson AL, Chen J. ATP-binding cassette transporters in bacteria. Annu Rev Biochem. 2004;73:241–261. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 114.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cai J, Gros P. Overexpression, purification, and functional characterization of ATP-binding cassette transporters in the yeast, Pichia pastoris. Biochim Biophys Acta. 2003;1610:63–76. doi: 10.1016/s0005-2736(02)00718-6. [DOI] [PubMed] [Google Scholar]
- 116.Sipos G, Kuchler K. Fungal ATP-binding cassette (ABC) transporters in drug resistance & detoxification. Curr Drug Targets. 2006;7:471–481. doi: 10.2174/138945006776359403. [DOI] [PubMed] [Google Scholar]
- 117.Albuquerque CP, Smolka MB, Payne SH, Bafna V, Eng J, Zhou H. A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol Cell Proteomics. 2008;7:1389–1396. doi: 10.1074/mcp.M700468-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gruhler A, Olsen JV, Mohammed S, Mortensen P, Faergeman NJ, Mann M, Jensen ON. Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol Cell Proteomics. 2005;4:310–327. doi: 10.1074/mcp.M400219-MCP200. [DOI] [PubMed] [Google Scholar]
- 119.Li X, Gerber SA, Rudner AD, Beausoleil SA, Haas W, Villen J, Elias JE, Gygi SP. Large-scale phosphorylation analysis of alpha-factor-arrested Saccharomyces cerevisiae. J Proteome Res. 2007;6:1190–1197. doi: 10.1021/pr060559j. [DOI] [PubMed] [Google Scholar]
- 120.Smolka MB, Albuquerque CP, Chen SH, Zhou H. Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc Natl Acad Sci USA. 2007;104:10364–10369. doi: 10.1073/pnas.0701622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Curry JM, Whalan R, Hunt DM, Gohil K, Strom M, Rickman L, Colston MJ, Smerdon SJ, Buxton RS. An ABC transporter containing a forkhead-associated domain interacts with a serine-threonine protein kinase and is required for growth of Mycobacterium tuberculosis in mice. Infect Immun. 2005;73:4471–4477. doi: 10.1128/IAI.73.8.4471-4477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelm KB, Huyer G, Huang JC, Michaelis S. The internalization of yeast Ste6p follows an ordered series of events involving phosphorylation, ubiquitination, recognition and endocytosis. Traffic. 2004;5:165–180. doi: 10.1111/j.1600-0854.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 123.Kolling R. Mutations affecting phosphorylation, ubiquitination and turnover of the ABC-transporter Ste6. FEBS Lett. 2002;531:548–552. doi: 10.1016/s0014-5793(02)03621-9. [DOI] [PubMed] [Google Scholar]
- 124.Paumi CM, Chuk M, Chevelev I, Stagljar I, Michaelis S. Negative regulation of the yeast ABC transporter Ycf1p by phosphorylation within its N-terminal extension. J Biol Chem. 2008;283:27079–27088. doi: 10.1074/jbc.M802569200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Subba Rao G, Bachhawat AK, Gupta CM. Two-hybrid-based analysis of protein-protein interactions of the yeast multidrug resistance protein, Pdr5p. Funct Integr Genomics. 2002;1:357–366. doi: 10.1007/s10142-001-0040-4. [DOI] [PubMed] [Google Scholar]
- 126.Chen XJ. Activity of the Kluyveromyces lactis Pdr5 multidrug transporter is modulated by the Sit4 protein phosphatase. J Bacteriol. 2001;183:3939–3948. doi: 10.1128/JB.183.13.3939-3948.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eraso P, Martinez-Burgos M, Falcon-Perez JM, Portillo F, Mazon MJ. Ycf1-dependent cadmium detoxification by yeast requires phosphorylation of residues Ser908 and Thr911. FEBS Lett. 2004;577:322–326. doi: 10.1016/j.febslet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 128.Molle V, Soulat D, Jault JM, Grangeasse C, Cozzone AJ, Prost JF. Two FHA domains on an ABC transporter, Rv1747, mediate its phosphorylation by PknF, a Ser/Thr protein kinase from Mycobacterium tuberculosis. FEMS Microbiol Lett. 2004;234:215–223. doi: 10.1016/j.femsle.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 129.Wada S, Tanabe K, Yamazaki A, Niimi M, Uehara Y, Niimi K, Lamping E, Cannon RD, Monk BC. Phosphorylation of candida glabrata ATP-binding cassette transporter Cdr1p regulates drug efflux activity and ATPase stability. J Biol Chem. 2005;280:94–103. doi: 10.1074/jbc.M408252200. [DOI] [PubMed] [Google Scholar]
- 130.Rogers LD, Foster LJ. Phosphoproteomics--finally fulfilling the promise? Mol Biosyst. 2009;5:1122. doi: 10.1039/b905580k. [DOI] [PubMed] [Google Scholar]
- 131.Chen G, Pramanik BN. Application of LC/MS to proteomics studies: current status and future prospects. Drug Discov Today. 2009;14:465–471. doi: 10.1016/j.drudis.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 132.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 133.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 134.Powis SJ, Howard JC, Butcher GW. The major histocompatibility complex class II-linked cim locus controls the kinetics of intracellular transport of a classical class I molecule. J Exp Med. 1991;173:913. doi: 10.1084/jem.173.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Momburg F, Roelse J, Neefjes J, Hammerling GJ. Peptide transporters and antigen processing. Behring Inst Mitt. 1994;94:26–36. [PubMed] [Google Scholar]
- 136.Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, Tanner MS, Kagalwalla AF, Nemeth A, Pawlowska J, Baker A, Mieli-Vergani G, Freimer NB, Gardiner RM, Thompson RJ. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 137.Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, Koeller DM. Mutation of a putative mitochondrial iron transporter gene (ABC7) in X-linked sideroblastic anemia and ataxia (XLSA/A) Hum Mol Genet. 1999;8:743. doi: 10.1093/hmg/8.5.743. [DOI] [PubMed] [Google Scholar]
- 138.Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25:1539–1542. doi: 10.1002/hep.510250635. [DOI] [PubMed] [Google Scholar]
- 139.Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci USA. 2000;97:6001–6006. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 141.Nestorowicz A, Wilson BA, Schoor KP, Inoue H, Glaser B, Landau H, Stanley CA, Thornton PS, Clement JPt, Bryan J, Aguilar-Bryan L, Permutt MA. Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet. 1996;5:1813–1822. doi: 10.1093/hmg/5.11.1813. [DOI] [PubMed] [Google Scholar]
- 142.Mosser J, Lutz Y, Stoeckel ME, Sarde CO, Kretz C, Douar AM, Lopez J, Aubourg P, Mandel JL. The gene responsible for adrenoleukodystrophy encodes a peroxisomal membrane protein. Hum Mol Genet. 1994;3:265–271. doi: 10.1093/hmg/3.2.265. [DOI] [PubMed] [Google Scholar]
- 143.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 144.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Roninson IB. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 146.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 147.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 148.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1230. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 149.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hornbeck PV, Chabra I, Kornhauser JM, Skrzypek E, Zhang B. PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics. 2004;4(6):1551–1561. doi: 10.1002/pmic.200300772. [DOI] [PubMed] [Google Scholar]
- 151.Tao WA, Wollscheid B, O’Brien R, Eng JK, Li XJ, Bodenmiller B, Watts JD, Hood L, Aebersold R. Quantitative phosphoproteome analysis using a dendrimer conjugation chemistry and tandem mass spectrometry. Nat Methods. 2005;2:591–598. doi: 10.1038/nmeth776. [DOI] [PubMed] [Google Scholar]
- 152.Imami K, Sugiyama N, Kyono Y, Tomita M, Ishihama Y. Automated phosphoproteome analysis for cultured cancer cells by two-dimensional nanoLC-MS using a calcined titania/C18 biphasic column. Anal Sci. 2008;24:161–166. doi: 10.2116/analsci.24.161. [DOI] [PubMed] [Google Scholar]
- 153.Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, Li J, Cohn MA, Cantley LC, Gygi SP. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci USA. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Korner R, Greff Z, Keri G, Stemmann O, Mann M. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell. 2008;31:438–448. doi: 10.1016/j.molcel.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 155.Mayya V, Lundgren DH, Hwang SI, Rezaul K, Wu L, Eng JK, Rodionov V, Han DK. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal. 2009;2:ra46. doi: 10.1126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]
- 156.Gauci S, Helbig AO, Slijper M, Krijgsveld J, Heck AJ, Mohammed S. Lys-N and trypsin cover complementary parts of the phosphoproteome in a refined SCX-based approach. Anal Chem. 2009;81:4493–4501. doi: 10.1021/ac9004309. [DOI] [PubMed] [Google Scholar]
- 157.Brill LM, Motamedchaboki K, Wu S, Wolf DA. Comprehensive proteomic analysis of Schizosaccharomyces pombe by two-dimensional HPLC-tandem mass spectrometry. Methods. 2009;48:311–319. doi: 10.1016/j.ymeth.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brill LM, Xiong W, Lee KB, Ficarro SB, Crain A, Xu Y, Terskikh A, Snyder EY, Ding S. Phosphoproteomic analysis of human embryonic stem cells. Cell Stem Cell. 2009;5:204–213. doi: 10.1016/j.stem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jorgensen C, Sherman A, Chen GI, Pasculescu A, Poliakov A, Hsiung M, Larsen B, Wilkinson DG, Linding R, Pawson T. Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science. 2009;326:1502–1509. doi: 10.1126/science.1176615. [DOI] [PubMed] [Google Scholar]
- 160.Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, Possemato A, Nardone J, Innocenti G, Wetzel R, Wang Y, MacNeill J, Mitchell J, Gygi SP, Rush J, Polakiewicz RD, Comb MJ. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci USA. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oppermann FS, Gnad F, Olsen JV, Hornberger R, Greff Z, Keri G, Mann M, Daub H. Large-scale proteomics analysis of the human kinome. Mol Cell Proteomics. 2009;8:1751–1764. doi: 10.1074/mcp.M800588-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thingholm TE, Larsen MR, Ingrell CR, Kassem M, Jensen ON. TiO(2)-based phosphoproteomic analysis of the plasma membrane and the effects of phosphatase inhibitor treatment. J Proteome Res. 2008;7:3304–3313. doi: 10.1021/pr800099y. [DOI] [PubMed] [Google Scholar]
- 163.Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci USA. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sui S, Wang J, Yang B, Song L, Zhang J, Chen M, Liu J, Lu Z, Cai Y, Chen S, Bi W, Zhu Y, He F, Qian X. Phosphoproteome analysis of the human Chang liver cells using SCX and a complementary mass spectrometric strategy. Proteomics. 2008;8:2024–2434. doi: 10.1002/pmic.200700896. [DOI] [PubMed] [Google Scholar]
- 165.Yu LR, Zhu Z, Chan KC, Issaq HJ, Dimitrov DS, Veenstra TD. Improved titanium dioxide enrichment of phosphopeptides from HeLa cells and high confident phosphopeptide identification by cross-validation of MS/MS and MS/MS/MS spectra. J Proteome Res. 2007;6:4150–4162. doi: 10.1021/pr070152u. [DOI] [PubMed] [Google Scholar]
- 166.Townsend RR, Lipniunas PH, Tulk BM, Verkman AS. Identification of protein kinase A phosphorylation sites on NBD1 and R domains of CFTR using electrospray mass spectrometry with selective phosphate ion monitoring. Protein Sci. 1996;5:1865–1873. doi: 10.1002/pro.5560050912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang Y, Du D, Fang L, Yang G, Zhang C, Zeng R, Ullrich A, Lottspeich F, Chen Z. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signaling. EMBO J. 2006;25:5058–5070. doi: 10.1038/sj.emboj.7601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Neville DC, Rozanas CR, Price EM, Gruis DB, Verkman AS, Townsend RR. Evidence for phosphorylation of serine 753 in CFTR using a novel metal-ion affinity resin and matrix-assisted laser desorption mass spectrometry. Protein Sci. 1997;6:2436–2445. doi: 10.1002/pro.5560061117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cantin GT, Yi W, Lu B, Park SK, Xu T, Lee JD, Yates JR., 3rd Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J Proteome Res. 2008;7:1346–1351. doi: 10.1021/pr0705441. [DOI] [PubMed] [Google Scholar]
- 170.Wang B, Malik R, Nigg EA, Korner R. Evaluation of the low-specificity protease elastase for large-scale phosphoproteome analysis. Anal Chem. 2008;80:9526–9533. doi: 10.1021/ac801708p. [DOI] [PubMed] [Google Scholar]