Abstract
Conditional responses in rodents such as locomotion have been reported for drugs of abuse and similar to the placebo response in humans, may be associated with the expectation of reward. We examined several conditional opioid-like responses and the influence of drug expectation on conditioned place preference and concomitant conditional locomotion. Male C57BL/6J mice were conditioned with the selective mu opioid receptor agonist fentanyl (0.2 mg/kg, i.p.) in a novel context and subsequently given a vehicle injection. In separate experiments, locomotor activity, Straub tail, hot plate sensitivity, and conditioned place preference (CPP) were measured. Mice exhibited multiple conditional opioid-like responses including conditional hyperlocomotion, a conditional pattern of opioid-like locomotion, Straub tail, analgesia, and place preference. Modulating drug expectation via administration of fentanyl to “demonstrator” mice in the home cage did not affect the expression of conditioned place preference or the concomitant locomotor activity in “observer” mice. In summary, Pavlovian conditioning of an opioid in a novel context induced multiple conditional opioid-like behaviors and provides a model for studying the neurobiological mechanisms of the placebo response in mice.
Keywords: Associative learning, Context-dependent, Opiate, Expectancy, Pain, Open field, Cocaine
1. Introduction
Conditional stimuli associated with drugs of abuse can induce drug-like physiological responses and contribute to craving in addicts (Childress et al., 1986; O’Brien et al., 1992). A common feature of rewarding drugs in rodents is the induction of hyperlocomotion (Wise and Bozarth, 1987) which can become conditioned to a novel context and can be expressed in the absence of drug (Hayashi et al., 1980; Stewart, 1983). With respect to amphetamines, it has been shown that both the amount and pattern of conditional locomotion can mimic the acute drug response (Gold et al., 1989). As such, contexts and cues associated with the drug may induce drug-like motivational states (Stewart, 1983) with the expectation of receiving it and thus, conditional locomotion may be part of a more general placebo response.
In humans, the placebo response is mostly studied experimentally in the context of analgesia (Beecher, 1955). Placebo analgesia can depend on the release of endogenous opioids as first inferred from opioid antagonist studies (Levine et al., 1978) and later demonstrated using advanced neuroimaging techniques (Wager et al., 2007; Zubieta et al., 2005). The hypothesis that dopamine and the expectation of reward (e.g., symptomatic relief) can contribute to the placebo response via dopamine release was first supported by the observation of more striatal dopamine release in Parkinson’s patients who are placebo responders (de la Fuente-Fernandez et al., 2001). It has subsequently been shown that the expectation of reward in humans (money) can induce dopamine-mediated placebo analgesia which correlates with the degree of dopamine transmission in the nucleus accumbens (Scott et al., 2007). Furthermore, in rodents, the expectation of a rewarding stimulus in rats (candy) can induce naloxone-reversible analgesia (Dum and Herz, 1984). Thus, the placebo response can depend on both opioidergic and dopaminergic mechanisms, as recently demonstrated under the same experimental conditions in humans (Scott et al., 2008).
With respect to opioids, in addition to hyperlocomotion, acute opioid administration also induces other overt classical responses in rodents such as analgesia and Straub tail. Pavlovian conditioning of locomotor activity and analgesia have been observed in rodents (Bardo and Valone, 1994; Hayashi et al., 1980; Levine et al., 1984; Stewart, 1983; Valone et al., 1998). Conditional opioid-opposite responses have also been reported following repeated administration in rodents and in opiate addicts (Childress et al., 1986; Siegel et al., 2000; Wikler, 1973) and may be more associated with extensive drug exposure.
The demonstration of multiple opioid-like responses would further support the hypothesis that conditional locomotion is part of a general placebo response and provides a rationale for testing a hypothesis related to this interpretation. Namely, does the expectation of reward contribute to drug-like conditional responses in rodents as it does with placebo responses in humans (de la Fuente-Fernandez et al., 2001; Scott et al., 2007)? The conditioned place preference assay provides an opportunity for examining how drug reward and its expectation might relate to conditional responses such as locomotor activity. Here, a rewarding drug is paired with a distinct context. After training, animals will demonstrate an increase in time spent on the drug-paired side, providing an index of reward (Tzschentke, 2007), that is accompanied by a conditional increase in locomotor activity, e.g., following opioid exposure (Vezina and Stewart, 1987).
In the present study, we first wished to test the hypothesis that mice conditioned with an opioid in a novel context would consequently demonstrate multiple conditional opioid-like placebo responses. We examined two previously reported conditional behaviors, hyperlocomotion (Stewart, 1983) and analgesia (Bardo and Valone, 1994; Miller et al., 1990; Valone et al., 1998) and two novel conditional behaviors, namely a pattern of opioid-like locomotion and Straub tail. The results indicate that mice exhibit multiple conditional opioid-like behavioral responses, which is consistent with a placebo response. We next tested the hypothesis that reward expectation influences drug preference and the conditional locomotor activity that accompanies it. In order to modulate the expectation of drug, we used the drug-induced behavior of the cage mates (“demonstrators”) as a cue for predicting either drug or no drug in “observer” mice. The results indicate that mice exhibited conditioned place preference indicating drug reward; however, there was no effect of demonstrator cue-induced drug expectation on the expression conditioned place preference or the concomitant locomotor activity under these conditions.
2. Materials and methods
Because several experiments were conducted in this study, Table 1 provides a list with the experiments, experimental groups and the N size, the behavioral measurement, the contexts in which they were measured, and the figures to which they correspond. Fig. 1 provides a schematic of the time lines of the conditioning protocols for each behavioral assay.
Table 1.
Sequence of behavioral testing. Listed are the experiments that were performed. Separate mice were used for each row. Details of the experiments, groups, measurements, and conditioning contexts are described in Section 2
| Experiments | Groups and N | Measurement | Conditioning context | Figure |
|---|---|---|---|---|
| Fentanyl-induced and conditional locomotion |
Control: N = 9 Conditioned: N = 10 Unconditioned: N = 10 |
Distance (cm) Percent distance in outer zone |
Open field | 1a, 2a and b and 3 |
| Cocaine-induced and conditional locomotion |
Control: N = 10 Conditioned: N = 11 Unconditioned: N = 10 |
Distance (cm) Percent distance in outer zone |
Open field | 1a and 2c and d |
| Acute and conditional Straub tail to fentanyl | Control: N = 8 Conditioned: N = 8 Unconditioned: N = 8 |
Percent time that the tape is above the line |
Unheated hot plate | 1b and 4a and b |
| No acute or conditional Straub tail to cocaine |
Control: N = 4 Conditioned: N = 4 Unconditioned: N = 3 |
Percent time that the tape is above the line |
Unheated hot plate | 1b and 4c and d |
| Acute fentanyl analgesia | Vehicle: N = 11 Fentanyl: N = 9 |
Hot plate latency (s) | 52.5 °C hot plate | 5a |
| Conditional analgesia | Control: N = 10 Conditioned: N = 19 Unconditioned: N = 14 |
Hot plate latency (s) | Train: unheated hot plate Test: 52.5 °C hot plate |
1b and 5b |
| Effect of drug expectation on conditional place preference and locomotion |
Daily: N = 21 Drug: N = 19 No drug: N = 21 |
Time on fentanyl side (s); distance (cm) |
Train: drug- and vehicle-paired chambers Test: access to all 3 chambers |
1c and 6a and b |
| Effect of vehicle injections on change in preference and locomotor activity |
N = 15 | Time spent on all three sides (s); distance (cm) |
Train: vehicle in both chambers. Test: access to all 3 chambers |
6c and d |
Fig. 1.
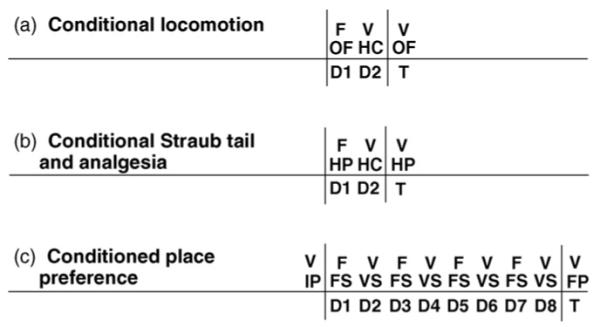
Timelines for Pavlovian conditioning. The drug treatments, training contexts, and training days for conditioning are listed between the two vertical lines. The treatment and context on Test Day are listed to the right of the second vertical line. F = fentanyl; V = vehicle; D = day of training; T = Test Day; OF = open field; HC = home cage; HP = hot plate (unheated during training and Test Day for Straub tail; heated on Test Day for analgesia); IP = Initial Preference; FS = fentanyl-paired side of the place preference chambers; VS = vehicle-paired side of the place preference chambers; FP = Final Preference. For conditional locomotion (a) and fentanyl-induced conditional Straub tail (b), counterbalancing of whether drug or vehicle was administered as the first injection was employed.
2.1. Drugs
The mu opioid receptor agonist fentanyl citrate (NIDA, Bethesda, MD) was chosen because of its selectivity for the mu opioid receptor, its rapid onset of biological activity, and its short duration of action. These properties were considered likely to facilitate the association between the environmental context, cues and the internal drug cues. As a comparison, cocaine hydrochloride (Sigma, St. Louis) was administered in some experiments because of its similar rate of onset and duration of action. Fentanyl and cocaine conditioning were conducted as separate experiments with their own respective control groups (Table 1). Drugs were dissolved in sterile, double deionized water and were administered intraperitoneally (i.p.) in a volume of 10 ml/kg.
2.2. Mice
Naïve, male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) of 10–12 weeks old were used for each experiment (Table 1). Mice were housed four per cage, provided unlimited access to food and water, and were maintained on a 12:12 light/dark cycle (lights off at 18:00 h). Experiments were conducted during the light phase of the cycle from 9:00 to 14:00 h. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Institutional Animal Care and Use Committee.
2.3. Locomotion
The open field (MedAssociates©, St. Albans, VT) was located in a separate, dimly lit room adjacent to the vivarium. The size of the open field was 27.9 cm × 27.9 cm × 20.3 cm height. Locomotor activity was tracked by 16 photobeams spaced evenly apart on both x and y axes. The outer zone was 3.95 cm wide on each side and the central zone was 20 cm × 20 cm. The chambers were washed thoroughly with 25% Simple Green© before the start of the first session and following each 30 min session, just prior to placement of the subsequent mice into the chambers. The patterns of locomotion during training and Test Day were determined for each mouse by calculating the distance traveled in the outer zone (over 5 or 30 min), dividing this value by the total distance traveled (over 5 or 30 min), and multiplying this value by 100. Outer zone data are presented as the percent distance traveled in the outer zone.
Conditional locomotion was induced by administering one injection of either fentanyl (0.2 mg/kg, i.p.) or cocaine (15 mg/kg, i.p.) in the open field and one injection of vehicle (i.p.) in the home cage (“Conditioned”). In order to facilitate Pavlovian conditioning and minimize latent inhibitory learning, we did not habituate mice to the open field prior to training. Following the drug injection, Conditioned mice were immediately placed in the open field and confined for 30 min. Following the vehicle injection, Conditioned mice were injected and placed immediately back into their home cage. “Control” mice received vehicle (i.p.) in both contexts using the same injection procedure. “Unconditioned” mice received fentanyl or cocaine in the home cage and vehicle in the open field using the same injection procedure. On Test Day, mice of all 3 groups received a vehicle injection (i.p.) and were immediately placed in the open field and recorded for activity over 30 min. The order of training was counterbalanced for Days 1 and 2 such that approximately half the mice received the first injection in the open field and the other half received the first injection in the home cage (Fig. 1a).
2.4. Straub tail
Straub tail is an erection of the tail that is caused by contraction of the sacrococcygeal dorsalis muscles (Bilbey and Grossman, 1960). It is one hallmark response to opioid treatment in rodents that is mediated by the mu opioid receptor (Nath et al., 1994). We developed an automated procedure for measuring fentanyl-induced Straub tail and validated this procedure by demonstrating that locomotor activity did not confound our measurement by checking the response in cocaine-treated mice. We then examined Pavlovian conditioning of the Straub tail response with either fentanyl (0.2 mg/kg, i.p.) or cocaine (15 mg/kg, i.p.) in the same mice.
In measuring Straub tail, a piece of white label tape (1 cm2) was applied 1 cm from the tip of the mouse tail just prior to injection (i.p.) and placement on the unheated hot plate which had the same dimensions as the open field. We used the unheated hot plate instead of the open field because it contained a transparent plexiglass wall (20 cm in height) surrounding it through which the mouse could be visualized and recorded. A thin piece of white label tape (1.5 mm in width) was placed horizontally across the plexiglass, 3.5 cm above the floor. Mice were recorded for 30 min post-injection with a digital camera along a horizontal plane at a height of 3.5 cm above the floor (the approximate height of a 25 g male C57BL/6J mouse walking on all four paws). The amount of time that the piece of tape on the mouse tail was above the white line was calculated using EthoVision® video tracking software (Noldus Information Technology; Wageningen, The Netherlands) and is represented as a percentage of the total time (30 min for training; 1 min for test). During training, Control and Unconditioned mice that received vehicle on the unheated hot plate often chewed off the white tape (starting at 10–15 min post-vehicle), whereas drug-treated Conditioned mice appeared unbothered by it. Importantly, during the 1 min test for conditional Straub tail, none of the mice from any group attempted to remove the tape.
In examining acute fentanyl-induced Straub tail, following placement of the white tape on the tail, Conditioned mice were injected with fentanyl and immediately placed on the unheated hot plate and recorded for 30 min. The next day, Conditioned mice received vehicle (i.p.) in the home cage. “Unconditioned” mice received one injection of vehicle on the unheated hot plate for 30 min and one injection of fentanyl in the home cage. Control mice received vehicle (i.p.) in both environments. For fentanyl conditioning, the order of training was counterbalanced such that the injection on Day 1 was either on the unheated hot plate (“fentanyl first”) or in the home cage (“vehicle first”). The Unconditioned counterparts received fentanyl in the home cage on the same days. On Test Day, mice of all three groups received a vehicle injection and were placed on the unheated hot plate (Fig. 1b). Because we only observed conditional Straub tail when fentanyl was given as the first injection on the unheated hot plate during training (fentanyl first), in order to facilitate the observation of conditional responses, we eliminated counterbalancing for any of the subsequent experiments such that fentanyl was always the first treatment in experimental groups receiving fentanyl. As such, in examining conditional Straub tail with cocaine, Conditioned mice received cocaine as the first injection on the unheated hot plate during training.
2.5. Analgesia
In assessing acute fentanyl analgesia using the hot plate assay, mice were injected in the home cage with either vehicle (i.p.) or fentanyl (0.2 mg/kg, i.p.) and 10 min later, were tested for analgesia on the 52.5 °C hot plate. The latency to lick the hindpaw was recorded with a stopwatch to the nearest 0.1 s. We have found “licking” to be the most reliable response in the C57BL/6J strain because it is an unambiguous, uniform response unlike “flicking” (which can assume many forms such as intermittent fluttering) and because the few mice that flick first will always lick within 1–2 s.
The unheated hot plate served as the drug training context because it was the same size as the open field and thus, could serve as both the training context and the nociceptive stimulus on Test Day. Conditioned mice received fentanyl (0.2 mg/kg, i.p.) on Day 1 and were immediately placed on the unheated hot plate and confined for 30 min. On Day 2, Conditioned mice received a vehicle injection (i.p.) in the home cage. Unconditioned mice received a fentanyl injection in the home cage on Day 1 and vehicle in the open field on Day 2. “Control” mice received a vehicle injection on the unheated hot plate on Day 1 and vehicle in the home cage on Day 2. On Test Day, immediately following a vehicle injection, each mouse was placed on the 52.5 °C hot plate and the latency to lick the hindpaw was recorded by an experimenter blind to treatment (Fig. 1b).
2.6. Conditioned place preference
Conditioned place preference was conducted using an unbiased, counterbalanced, three-chamber design as previously described (Skoubis et al., 2001). Standard fluorescent lighting from the room ceiling supplied a low level of illumination in the chambers (38.5 cm in height). Each box (TruScan, Coulbourn Instruments, Allentown, PA) was divided into 2 conditioning chambers on the left and right sides and a neutral compartment in the middle. The middle, neutral compartment was gray and triangular (40.5 cm × 28.5 cm by 28.5 cm; 406 cm2) and contained guillotine doors flanking the two sides of it which allowed access to the two conditioning chambers. The two conditioning chambers were trapezoid in shape (20.25 cm × 41 cm by 28.5 cm; 613 cm2) and were not directly accessible to each other; i.e., the mice had to exit one conditioning chamber via the neutral chamber to gain access to the second chamber. The conditioning chamber on the left side of the neutral compartment contained a cow-print pattern with lemon-scented filter paper. The conditioning chamber on the right side of the neutral compartment contained a checkered pattern with almond-scented filter paper. The scented filter paper (2 cm × 10 cm) was attached to a paper clip that hung approximately 25 cm above the floor of the chambers.
In assessing Initial Preference, mice received a vehicle injection (i.p.), were immediately placed into the neutral compartment, and were allowed free access to the conditioning chambers (the guillotine doors were removed). The time spent in each chamber and the total distance traveled were recorded over 15 min. The next day, drug conditioning commenced whereby mice received four trials of fentanyl on odd days of training (0.2 mg/kg, i.p.; Days 1, 3, 5, and 7) and four trials of vehicle on even days of training (i.p.; Days 2, 4, 6, and 8) during which time they were confined to the assigned chamber (via inserting the guillotine door) for 30 min (Fig. 1c). The drug-paired chamber was randomly assigned to either the left or right side with approximately equal N’s for each side. During Final Preference, the guillotine doors were removed and mice received a vehicle injection (i.p.), were immediately placed into the neutral compartment, and were allowed free access to all three chambers (Fig. 1c). The time spent in each chamber and the total distance traveled were recorded over 15 min. Change in preference was indicated by comparing the time spent on the drug-paired side during Initial Preference and Final Preference.
2.7. Drug administration to demonstrator mice to modulate drug expectation in observer mice
In determining the effect of drug expectation on the expression of conditioned place preference and the concomitant locomotor activity (Table 2), a between-subjects design was employed whereby fentanyl administration to the first 2 demonstrators in the home cage predicted fentanyl in the fentanyl-paired chamber (the “Drug” group), or predicted a vehicle injection in the vehicle-paired chamber (the “No Drug” group) for the last 2 observers of the cage. On alternating days, vehicle administration to the demonstrators predicted either vehicle (the “Drug” group) or fentanyl (the “No Drug” group) to the observers in the opposite chamber. For the “Daily” observer group, the demonstrators were given fentanyl daily during training. The inter-injection interval for demonstrators in the home cage and observers in the conditioning chambers was 10 min, which is the peak time for the locomotor stimulating effect of fentanyl. For Initial Preference, observers were given a vehicle injection (i.p.), immediately placed in the neutral compartment, and tested for initial preference for 15 min. No treatment was given to the demonstrators. During training, following appropriate treatment of the demonstrators in the home cage, observers of all three groups were given fentanyl (Days 1, 3, 5, and 7) and vehicle (Days 2, 4, 6, 8) in the assigned chambers 10 min following injection of the demonstrator mice. On Test Day, fentanyl was administered to the demonstrators of all three groups in the home cage. Ten minutes later, the observers were given a vehicle injection (i.p.) and immediately placed in the neutral compartment and tested for Final Preference for 15 min. Preference was not measured in demonstrator mice. Due to computer hardware/software failure during the 15 min run on Test Day, data from 5 mice could not be retrieved (N = 1 for Daily, N = 3 for Drug, N = 1 for No Drug).
Table 2.
Effect of drug expectation on conditional place preference and locomotor activity. In examining the influence of drug expectation on conditional place preference and locomotor activity, fentanyl administration to demonstrator mice predicted one of three situations for observer mice: fentanyl on the fentanyl-paired side (“Drug”), vehicle on the vehicle-paired side (“No Drug”), or simply an injection whereby fentanyl was given daily to the demonstrator mice (“Daily”). On Test Day, fentanyl was administered to demonstrators of all three observer groups and 10 min later, observers received a vehicle injection in the neutral compartment and were allowed free access to all three chambers
| Observer groups |
Assay | Train: fentanyl to demonstrators in the home cage |
Train: vehicle to demonstrators in the home cage |
Test |
|---|---|---|---|---|
| Daily | Conditional place preference and concomitant locomotion |
Predicts fentanyl and vehicle injections on both sides |
N.A.: demonstrators receive fentanyl again in home cage |
Fentanyl to demonstrators: observers expect an injection |
| Drug | Conditional place preference and concomitant locomotion |
Predicts fentanyl on the fentanyl-paired side |
Predicts vehicle on the vehicle-paired side |
Fentanyl to demonstrators: observers expect fentanyl on the fentanyl-paired side |
| No Drug | Conditional place preference and concomitant locomotion |
Predicts vehicle on the vehicle-paired side |
Predicts fentanyl on the fentanyl-paired side |
Fentanyl to demonstrators: observers expect vehicle on the vehicle-paired side |
We did not measure the social impact of fentanyl administration to demonstrator cage mates upon the observer cage mates in the place preference experiment.
2.8. Analysis
ANOVA followed by Tukey’s post hoc comparison or Student’s t-test was used for analyses with p < 0.05 considered significant. Spearman’s correlation coefficients are reported with p-values.
3. Results
3.1. Acute and conditional locomotion to fentanyl and cocaine
In Fig. 2a, in examining the effect of acute fentanyl on locomotion, repeated measures ANOVA indicated a main effect of drug (F2,26 = 37.76; p < 0.0001), time (F5,130 = 17.30; p < 0.0001), and an interaction of drug with time (F10,130 = 10.08; p < 0.0001). Subsequent one-way ANOVAs of the six 5-min time bins (effect of drug: F2,26 = 78.22, 67.74, 46.24, 26.78, 13.58, 10.48; p < 0.05) followed by Tukey’s post hoc comparison indicated that mice receiving acute fentanyl (Conditioned) exhibited significantly greater locomotor activity than Control and Unconditioned mice receiving vehicle at all six time bins (p < 0.05) (Fig. 2a).
Fig. 2.
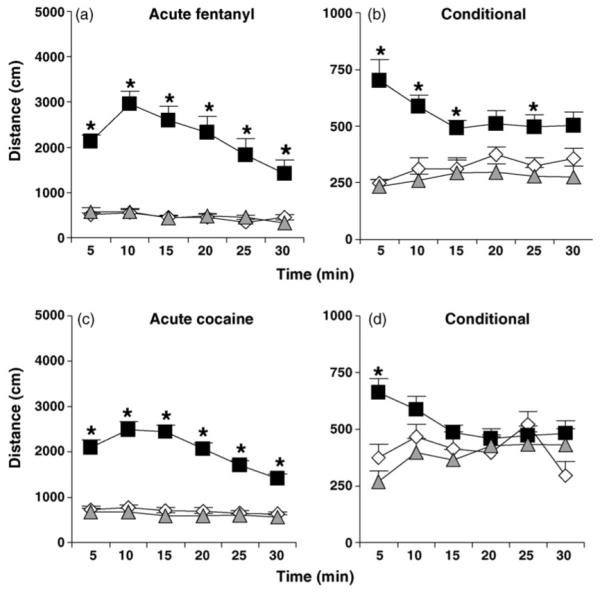
Acute and conditional locomotion. (a) and (b) Acute and conditional locomotion to fentanyl (0.2 mg/kg, i.p.). (c) and (d) Acute and conditional locomotion to cocaine (15 mg/kg, i.p.). Open diamonds = Control mice receiving vehicle injections in the open field and home cage during training. Black squares = Conditioned mice receiving drug in the open field and vehicle in the home cage during training. Gray triangles = Unconditioned mice receiving drug in the home cage and vehicle in the open field and during training. “*” = Significantly different from Control and Unconditioned mice (p < 0.05). Data are presented as the mean ± S.E.M.
Fig. 2 billustrates conditional locomotion to fentanyl in response to a subsequent vehicle injection. Two-way repeated measures ANOVA indicated a main effect of conditioning (F2,26 = 15.31; p < 0.05), no effect of time (F5,130 < 1), and an interaction of conditioning with time (F10,130 = 3.80; p < 0.05). Subsequent one-way ANOVAs of the six 5-min time bins (effect of conditioning: F2,26 = 19.92, 17.05, 4.87, 6.51, 6.92, 4.88; p < 0.05) followed by Tukey’s post hoc comparison indicated that Conditioned mice differed from Control and Unconditioned mice at 5, 10, 15, and 25 min (p < 0.05) whereas at 20 and 30 min, Conditioned and Control mice did not differ significantly from each other (Fig. 2b).
In considering the possibility that conditional locomotion was due to a lack of habituation during training (Carey et al., 2008; Tirelli and Terry, 1998), we compared the first 5 min of activity of Conditioned mice on Test Day with the first 5 min of activity of Control mice on Day 1 in the open field. Repeated measures ANOVA indicated no effect of conditioning (F1,17 = 3.05; p > 0.05), an effect of time (F4,68 = 7.72; p < 0.05), and an interaction of conditioning with time (F4,68 = 2.88; p < 0.05). Unpaired t-test indicated that Conditioned mice showed marginally greater activity than Control mice during the first minute (t17 = 2.08; p = 0.053) that was significant during the third minute (t17 = 2.69; p = 0.016) (data not shown). Thus, this indicates that conditional locomotion is in part explained by drug conditioning and also suggests that a lack of habituation of Conditioned mice to the open field during training could also contribute.
Fig. 2c and d illustrate acute and conditional locomotion in response to cocaine (15 mg/kg, i.p.). In examining acute cocaine-induced locomotion, two-way repeated measures ANOVA indicated a main effect of drug (F2,28 = 91.96; p < 0.05), time (F5,140 = 21.41; p < 0.05) and an interaction of drug with time (F10,140 = 13.03; p < 0.05). One-way ANOVAs of the six 5-min time bins (effect of drug: F2,28 = 47.61, 68.72, 94.72, 72.30, 66.74, 49.38; p <0.05) followed by Tukey’s post hoc comparison indicated that mice receiving acute cocaine (Conditioned) traveled a greater distance than mice receiving Control and Unconditioned mice receiving vehicle at all six time bins (p < 0.05) (Fig. 2c).
In examining conditional locomotion to cocaine, two-way repeated measures ANOVA indicated no effect of conditioning (F2,28 = 3.05; p > 0.05) or time (F5,140 = 2.01; p > 0.05), but an interaction of conditioning with time (F10,140 = 4.61; p < 0.05). One-way ANOVA of the first 5-min bin, but not the other time bins, indicated a main effect of conditioning (F2,28 = 13.80; p < 0.05). Tukey’s post hoc comparison indicated that Conditioned mice showed greater activity than Control and Unconditioned mice (p < 0.05) (Fig. 2d).
In considering the possibility that conditional locomotion was due to a lack of habituation during training, we compared the first 5 min of activity of cocaine-Conditioned mice on Test Day with the activity of Control mice receiving vehicle on Day 1 in the open field. Repeated measures ANOVA indicated no main effect of conditioning (F1,19 < 1), an effect of time (F4,76 = 10.87; p < 0.05), and an interaction of conditioning with time (F4,76 = 3.69; p < 0.05). Although there was a significant interaction, unpaired t-test indicated that none of the five time points were significantly different from each other (p > 0.05; data not shown). Thus, cocaine-induced conditional locomotion could in part be due to a lack of habituation of drug-treated mice during conditioning.
3.2. Acute and conditional pattern of opioid-like locomotion
Fig. 3 shows the pattern of acute (3a; totaled over 30 min) and conditional locomotor activity (3b; totaled over 5 min) following fentanyl administration (same mice from Fig. 2a and b). Acutely, there was a main effect of drug over 30 min (F2,26 = 141.65; p < 0.05) that was explained by Conditioned mice demonstrating greater outer zone activity than Control and Unconditioned mice (p < 0.05; Tukey’s) (Fig. 3a). Following conditioning, one-way ANOVA indicated a main effect of conditioning (F2,26 = 8.49; p < 0.05) that was explained by Conditioned mice traveling a greater percentage of the distance in the outer zone during the first 5 min relative to both Control and Unconditioned mice (p < 0.05) (Fig. 3b). The illustrations at the bottom of Fig. 3 show the locomotor activity of a single representative mouse from each group following the first minute on Test Day. Individual mice were chosen that were similar to the group average percent conditional outer zone activity over 5 min.
Fig. 3.
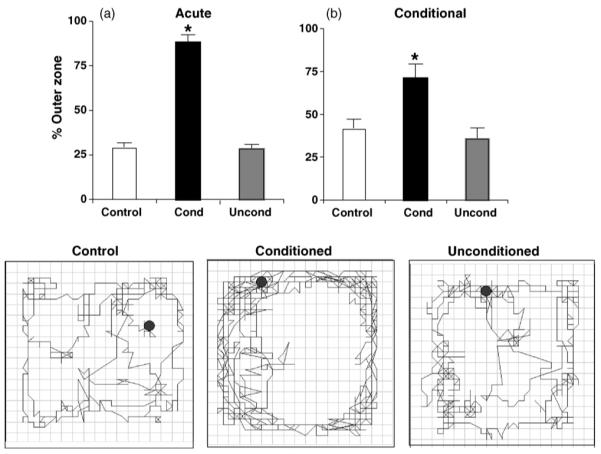
Acute and conditional pattern of opioid locomotion. (a) Percent fentanyl-induced outer zone activity over 30 min. (b) Percent conditional outer zone activity during the first 5 min. “*” = Conditioned mice (Cond) differed significantly different from Control and Unconditioned mice (Uncond; p < 0.05). Data are presented as the mean ± S.E.M. In the bottom illustrations, the first minute of locomotor activity is shown for a single mouse from each group that is representative of the average 5-min time bin (individual ± Control mouse = 43.8% versus the group average of 42.3%; individual Conditioned mouse = 78.3% versus the group average of 72.0%; individual Unconditioned mouse = 43.7% versus the group average of 36.5%).
In examining the effect of acute cocaine on outer zone activity, there was no effect of drug (F2,28 = 3.13; p > 0.05) (data not shown). In examining possible conditional outer zone activity, there was no effect of conditioning (F2,28 < 1) (data not shown).
3.3. Acute and conditional Straub tail
Fig. 4a and b illustrate acute and conditional Straub tail following fentanyl. Acutely, there was a main effect of drug (F2,21 = 58.86; p < 0.05). Conditioned mice receiving fentanyl exhibited significant Straub tail relative to Control and Unconditioned mice (p < 0.05, Tukey’s; Fig. 4a).
Fig. 4.
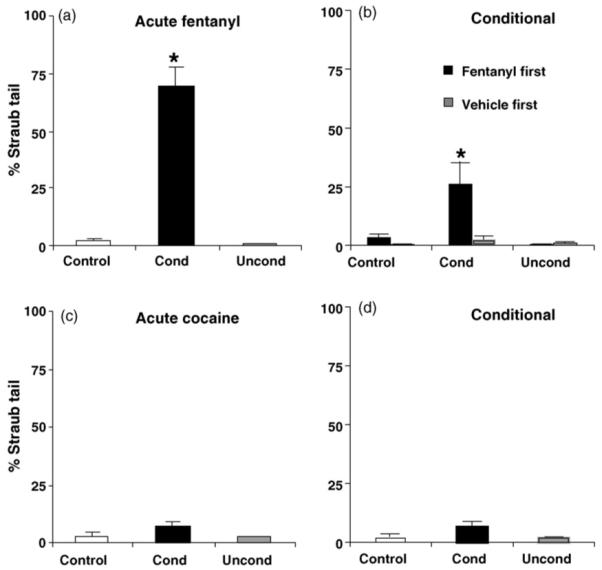
Acute and conditional Straub tail. (a) Acute fentanyl-induced Straub tail (0.2 mg/kg, i.p.). (b) Conditional Straub tail for the first minute following a vehicle injection. Conditional Straub tail depended on whether the first training injection was fentanyl (“fentanyl first”) or vehicle (“vehicle first”). “*” = Conditioned mice (Cond) significantly different from Control and Unconditioned mice (Uncond) following “fentanyl first” conditioning (p < 0.05). (c) Lack of cocaine response over 30 min (15 mg/kg, i.p.; given as the first injection in Conditioned mice; p > 0.05). (d) Lack of cocaine conditional response for the first minute following a vehicle injection (p > 0.05). Data are presented as the mean ± S.E.M.
In examining conditional Straub tail, one-way ANOVA indicated a main effect of conditioning (F2,21 = 4.10; p < 0.05). Tukey’s post hoc comparison indicated that Conditioned mice demonstrated significant Straub tail relative to the Unconditioned mice (p < 0.05) but not Control mice (p > 0.05). Upon further examination of conditional Straub tail, when the order of treatment during conditioning was considered (fentanyl as the first or second injection), two-way ANOVA indicated a main effect of conditioning (F2,18 = 6.84; p < 0.05), order (F1,18 = 6.79; p < 0.05), and an interaction of conditioning with order (F2,18 = 5.14; p < 0.05) (N = 4 per treatment per order). When fentanyl was administered first, there was a main effect of conditioning (F2,9 = 6.08; p < 0.05) that was explained by Conditioned mice demonstrating significant Straub tail relative to Control and Unconditioned mice (p < 0.05; Tukey’s; Fig. 4b). In mice receiving vehicle as the first injection, there was no main effect of conditioning (F2,9 = 1.70; p > 0.05) (Fig. 4b; “vehicle first”). Thus, with the intent of maximizing conditional opioid responses, experiments for the remainder of this study were carried out such that fentanyl was always administered first to the experimental groups receiving fentanyl.
Fig. 4c and d illustrates the lack of acute and conditional Straub tail response to cocaine. Acutely, there was no effect of drug (F2,8 = 3.2; p > 0.05). In examining possible conditioning effects, one-way ANOVA indicated no main effect of conditioning (F2,8 < 1) (Fig. 4d). Thus, although cocaine induced acute and conditional locomotion, it did not produce acute or conditional Straub tail, confirming that our measure of Straub tail is not confounded by locomotor activity.
3.4. Acute and conditional analgesia
Fig. 5 illustrates acute and conditional analgesia to fentanyl. Acutely, fentanyl (0.2 mg/kg, i.p.) produced a significant increase in hot plate latency relative to vehicle-treated mice at 10 min post-injection (t18 = 3.88; p < 0.05) (Fig. 5a). In examining conditional analgesia, separate mice were trained using the unheated hot plate as the drug context and on Test Day, the 52.5 °C hot plate was used to assess conditional analgesia. One-way ANOVA indicated a main effect of conditioning (F2,40 = 9.95; p < 0.05). Tukey’s post hoc comparison indicated that Conditioned mice showed significantly greater hot plate latencies in response to a vehicle injection than Control and Unconditioned mice (p < 0.05) (Fig. 5b).
Fig. 5.
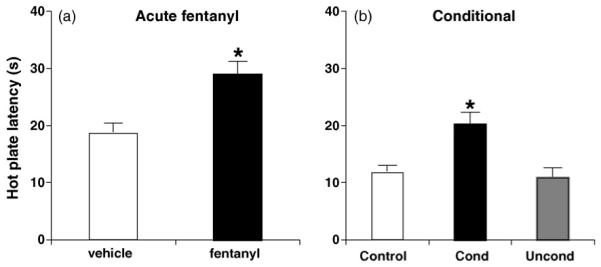
Acute and conditional analgesia. (a) Acute fentanyl-induced analgesia on the 52.5 °C hot plate (0.2 mg/kg, i.p.). “*” = Significantly different from vehicle (p < 0.05). (b) Conditional analgesia on the 52.5 °C hot plate. “*” = Conditioned mice (Cond) significantly different from Control and Unconditioned mice (Uncond; p < 0.05). Data are presented as the mean ± S.E.M.
In examining the possibility that locomotor activity interfered with the hot plate response, distance was estimated by the number of traverses across the hot plate (27 cm) by an experimenter blind to treatments. Spearman’s correlations indicated no significant correlation between average velocity (total distance traveled before hot plate response/hot plate latency) and hot plate latency in any of the groups (Control: rs = −0.33, p = 0.35; Conditioned: rs = −0.059, p = 0.81; Unconditioned: rs = −0.19, p = 0.53), providing evidence that locomotor activity did not compete with the hot plate response.
3.5. Effect of drug expectation on conditioned place preference and locomotor activity
Table 2 represents the procedure used to modulate drug expectation in observer mice via drug administration to demonstrator mice.
Fig. 6a and b illustrate the lack of effect of demonstrators on the expression of conditioned place preference and the concomitant locomotion in observer mice of all three groups (Daily, Drug, and No Drug). With respect to conditioned place preference, repeated measures ANOVA indicated a main effect of day (F1,58 = 38.26; p < 0.05), but no effect of demonstrator (F2,58 < 1) nor an interaction (F2,58 < 1). The Daily, Drug, and No Drug groups all exhibited a significant increase in time spent in the drug-paired side (paired t-test: t20 = 4.33, t18 = 2.85, t20 = 3.86; p < 0.05, respectively), indicating drug reward (Fig. 6a). In considering the first 5 min, there was no effect of day (F1,58 = 2.24; p > 0.05) or demonstrator (F2,58 < 1), indicating no change in preference for the drug-paired side.
Fig. 6.
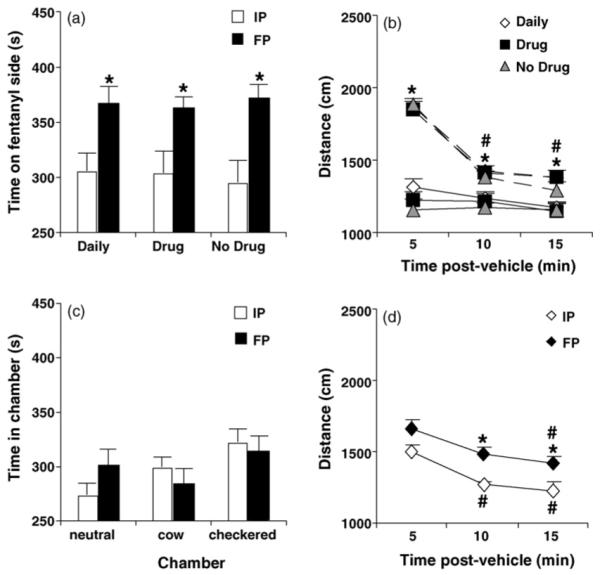
Effect of drug expectation on conditioned place preference and locomotion. (a) Fentanyl administration to demonstrator mice during training served as a cue for predicting simply an injection (“Daily”), predicting drug on the drug-paired side (“Drug”) or for predicting vehicle on the vehicle-paired side (“No Drug”) in observer mice (see Table 2). On Test Day, 10 min following fentanyl administration to demonstrators, observers of all three groups were tested for Final Preference. IP = Initial Preference over 15 min. FP = Final Preference over 15 min. There was no effect of demonstrator cues on the expression of conditioned place preference (p > 0.05). All three groups of observer mice showed significant preference for fentanyl (* = different from IP; p < 0.05). (b). There was no effect of demonstrator cues on locomotor activity during FP (p > 0.05). Mice exhibited greater activity during FP (dashed lines) compared to IP (solid lines; *p < 0.05; collapsed across observer groups) and greater activity during the first 5 min of FP compared to 10 min or 15 min (#p < 0.05; collapsed across observer groups). (c) No change in time spent in the three chambers from IP to FP in separate, naive mice given vehicle injections in both environments during training (p > 0.05). Neutral = neutral chamber (gray). Cow = cow-print chamber. Checkered = checkered chamber. (d) There was a significant increase in locomotor activity during FP at the 10-min and 15-min time bins (*p < 0.05). # = Significantly different from the 5-min bin (p < 0.05). Data are presented as the mean ± S.E.M.
In Fig. 6b, in examining locomotor activity during preference and the influence of demonstrator-induced drug expectation, two-factor repeated measures ANOVA indicated no effect of demonstrator (F2,58 < 1), an effect of day (F1,58 = 177.74; p < 0.05), an effect of time (F2,116 = 106.70; p < 0.05), and an interaction of day with time (F2,116 = 80.01; p < 0.05). Because there was no effect of demonstrator on day or time and because there was no interaction of demonstrator with day or time, data were collapsed across the Daily, Drug, and No Drug groups. The effect of Day was explained by locomotor activity during Final Preference being greater than that exhibited during Initial Preference for the 5-min bin (t60 = 14.93; p < 0.05), the 10-min bin (t60 = 7.49; p < 0.05), and the 15-min bin (t60 = 5.84; p < 0.05). The interaction of day with time was explained by mice showing greater activity for the 5-min bin compared to the 10 and 15-min bins during Final Preference (p < 0.05, Tukey’s) whereas none of the time bins differed significantly from each other during Initial Preference (p > 0.05).
In Fig. 6c and d, in order to examine if there was any non-specific change in preference for the three chambers or locomotion that was not due to drug treatment, Initial Preference was assessed in naive mice for 15-min following a vehicle injection (i.p.) in the neutral compartment. On training days 1–8, mice received four vehicle injections in the cow-pattern chamber and on alternating days, received four vehicle injections in the checkered-pattern chamber (counterbalanced for which chamber was experienced first). For Final Preference, mice were administered a vehicle injection in the neutral compartment and allowed free access to all three chambers for 15 min. One mouse jumped out of the chamber during Test Day and thus, could not be analyzed. As such, a total of 15 mice were tested (Table 1). Two-factor repeated measures ANOVA indicated no main effect of day (F1,14 < 1), no effect of chamber (F2,28 = 1.50; p > 0.05), and no interaction of day with chamber (F2,28 = 1.61; p > 0.05; Fig. 6c).
Regarding locomotor activity, repeated measures ANOVA indicated a main effect of day (F1,14 = 13.80; p < 0.05) and time (F2,28 = 31.99; p < 0.05) but no interaction of day with time (F2,28 < 1). The effect of day was explained by a significant increase in activity during Final Preference at the 10-min bin (t14 = 4.54; p < 0.05) and 15-min bin (t14 = 3.07; p < 0.05; Fig. 6d). Thus, the increase in locomotor activity observed during Final Preference for fentanyl is clearly most specific to Pavlovian conditioning during the first 5-min bin (Fig. 6b) whereas at the 10 and 15-min bins, the increase could be due to a non-specific increase in activity (Fig. 6d). The effect of time in Fig. 6d was explained by activity being significantly greater during the 5-min bin compared to the 10-min bin and 15-min bin during Initial Preference and compared to the 15 min bin during Final Preference (p < 0.05; Tukey’s).
4. Discussion
Opioid administration to mice in a novel context elicited several conditional opioid-like placebo responses, including the novel observations of conditional Straub tail and a pattern of opioid-like locomotor activity. Because the placebo response is hypothesized to be mediated by the expectation of reward (de la Fuente-Fernandez et al., 2001), we modulated drug expectation of observer mice via drug administration to demonstrator mice in the home cage and examined its effect on the expression of reward and conditional locomotion. Under these conditions, we did not find an effect of the demonstrator cues on reward or conditional locomotion in observer mice.
Fentanyl produced acute and conditional locomotion (Fig. 2a and b) as well as acute and conditional outer zone activity in the open field (Fig. 3). While this is the first report of a learned pattern of opioid-like locomotor activity, thigmotaxis, or “wall hugging,” has been observed with acute morphine and the selective mu opioid receptor agonist DAMGO in C57BL/6J mice (Hodgson et al., 2009; Mickley et al., 1990). Furthermore, a previous study demonstrated that the amount and pattern of amphetamine hyperlocomotion can become conditioned with several pairings (Gold et al., 1989). Cocaine at a systemic dose of 15 mg/kg produced acute hyperlocomotion (Fig. 2c) that was comparable to the amount observed with 0.2 mg/kg fentanyl (Fig. 2a). However, cocaine did not produce significant thigmotaxis or conditional outer zone activity (data not shown). This indicates that conditional outer zone activity is not a product of conditional locomotion per se, but is specific to a particular drug.
This is the first report to demonstrate conditional Straub tail following opioid administration (Fig. 4b). While cocaine produced acute and conditional locomotion (Fig. 2c and d), it did not produce acute or conditional Straub tail (Fig. 4c and d). This demonstrates that our measure of Straub tail is not confounded by a general increase in locomotor activity. Interestingly, conditional Straub tail was only observed when fentanyl was administered as the first injection during training. This suggests that there may be a latent inhibitory learning process following exposure to the vehicle injection procedure that limits association with some of the drug effects.
The conditional analgesia following one-trial opioid conditioning (Fig. 5b), although consistent with human studies of Pavlovian conditioning (Amanzio and Benedetti, 1999) and some rodent studies (Bardo and Valone, 1994; Miller et al., 1990; Valone et al., 1998), is in contrast to other studies demonstrating a conditional response that is opposite the acute effect, such as conditional hyperalgesia (Siegel, 1975). Most previous studies involved multiple trials and thus, the conditional behaviors will likely differ. Following repeated trials, analgesic tolerance develops and is accompanied by opposing responses including spontaneous and opioid-induced hyperalgesia (Bryant et al., 2005). This hyperalgesia is in part mediated by associative learning processes (Siegel, 1977, 1978) and can sensitize with multiple exposures (Celerier et al., 2001) and thus, might become more readily associated with the context and cues. Thus, simultaneous association of both drug-like and opponent responses could prevent the detection of conditional analgesia in some instances.
Conditioned place preference developed to fentanyl as previously reported (Mucha and Herz, 1985). In the place conditioning chambers, we found no effect of drug expectation on the amount of conditioned place preference (Fig. 6a) or locomotion (Fig. 6b). With regard to conditional locomotion, all three groups had access to and entered their drug conditioning chambers and thus, the added effect of drug expectation on conditional responses might not be as easily observed as it would be in an unconditioned context where it could be isolated from the drug environment. Future efforts will attempt to isolate the effects of reward expectation on multiple conditional responses by presenting drug-predictive cues in an unconditioned context.
We have provided converging evidence for a placebo response in mice following Pavlovian conditioning of an opioid in a novel environment. Although the use of drug administration in demonstrator mice as a cue for modulating drug expectation was ineffective in changing conditional responses in observer mice in the place conditioning chambers, it is possible that these cues may be effective under different experimental conditions. Furthermore, it will be important to determine the influence of demonstrator drug cues on other complex behaviors relevant to addiction in observer mice such as drug withdrawal and reinstatement of drug seeking. Last, mouse models may be developed for other conditions that respond well to placebo treatments (and possibly reward expectation) such as Parkinson’s disease and depression. For example, a recent human study in Parkinson’s patients used Pavlovian conditioning of the anti-Parkinson’s drug apomorphine to generate a robust reduction in muscle rigidity (Benedetti et al., 2004). Thus, mouse models of Pavlovian conditioning could lead to mechanistic insight into the neurobiology of the placebo response across multiple clinical conditions.
Acknowledgment
We thank Dr. Matthew J. Sanders for technical assistance with open field.
Role of funding source
Funding for this study was provided by DA05010, MH015795, and the Shirley and Stefan Hatos Center for Neuropharmacology. NIDA and NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J. Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Valone JM. Morphine-conditioned analgesia using a taste cue: dissociation of taste aversion and analgesia. Psychopharmacology (Berl) 1994;114:269–274. doi: 10.1007/BF02244848. [DOI] [PubMed] [Google Scholar]
- Beecher HK. The powerful placebo. J. Am. Med. Assoc. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat. Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- Bilbey DJL, Grossman MH. The anatomical basis of the Straub tail phenomenon. Br. J. Pharmacol. 1960;15:540–543. doi: 10.1111/j.1476-5381.1960.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Zaki PA, Carroll FI, Evans CJ. Opioids and addiction: emerging pharmaceutical strategies for reducing reward and opponent processes. Clin. Neurosci. Res. 2005;5:103–115. [Google Scholar]
- Carey RJ, Damianopoulos EN, Shanahan AB. Cocaine conditioned behavior: a cocaine memory trace or an anti-habituation effect. Pharmacol. Biochem. Behav. 2008 doi: 10.1016/j.pbb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J. Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Role of conditioning factors in the development of drug dependence. Psychiatr. Clin. North Am. 1986;9:413–425. [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Dum J, Herz A. Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacol. Biochem. Behav. 1984;21:259–266. doi: 10.1016/0091-3057(84)90224-7. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Spatial pattern analysis reveals similarities between amphetamine conditioned and unconditioned locomotion. Behav. Pharmacol. 1989;1:209–220. [PubMed] [Google Scholar]
- Hayashi T, Ohashi K, Tadokoro S. Conditioned drug effects to d-amphetamine- and morphine-induced motor acceleration in mice: experimental approach for placebo effect. Jpn. J. Pharmacol. 1980;30:93–100. doi: 10.1254/jjp.30.93. [DOI] [PubMed] [Google Scholar]
- Hodgson S, Hofford R, Buckman S, Wellman P, Eitan S. Morphine-induced stereotyped thigmotaxis could appear as enhanced fear and anxiety in some behavioural tests. J. Psychopharmacol. 2009 doi: 10.1177/0269881108100797. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- Levine JD, Feldmesser M, Tecott L, Lane S, Gordon NC. The role of stimulus intensity and stress in opioid-mediated analgesia. Brain Res. 1984;304:265–269. doi: 10.1016/0006-8993(84)90329-9. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Mulvihill MA, Postler MA. Brain mu and delta opioid receptors mediate different locomotor hyperactivity responses of the C57BL/6J mouse. Psychopharmacology (Berl) 1990;101:332–337. doi: 10.1007/BF02244050. [DOI] [PubMed] [Google Scholar]
- Miller JS, Kelly KS, Neisewander JL, McCoy DF, Bardo MT. Conditioning of morphine-induced taste aversion and analgesia. Psychopharmacology (Berl) 1990;101:472–480. doi: 10.1007/BF02244224. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Nath C, Gupta MB, Patnaik GK, Dhawan KN. Morphine-induced straub tail response: mediated by central mu2-opioid receptor. Eur. J. Pharmacol. 1994;263:203–205. doi: 10.1016/0014-2999(94)90543-6. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann. N.Y. Acad. Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Siegel S. Evidence from rats that morphine tolerance is a learned response. J. Comp. Physiol. Psychol. 1975;89:498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- Siegel S. Morphine tolerance acquisition as an associative process. J. Exp. Psychol. Anim. Behav. Process. 1977;3:1–13. doi: 10.1037//0097-7403.3.1.1. [DOI] [PubMed] [Google Scholar]
- Siegel S. Pavlovian conditioning analysis of morphine tolerance. NIDA Res. Monogr. 1978:27–53. [PubMed] [Google Scholar]
- Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp. Clin. Psychopharmacol. 2000;8:276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Matthes HW, Walwyn WM, Kieffer BL, Maidment NT. Naloxone fails to produce conditioned place aversion in mu-opioid receptor knock-out mice. Neuroscience. 2001;106:757–763. doi: 10.1016/s0306-4522(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Stewart J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug self-adminstration. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1983;7:591–597. doi: 10.1016/0278-5846(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Terry P. Amphetamine-induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes. Behav. Pharmacol. 1998;9:409–419. doi: 10.1097/00008877-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valone JM, Randall CK, Kraemer PJ, Bardo MT. Olfactory cues and morphine-induced conditioned analgesia in rats. Pharmacol. Biochem. Behav. 1998;60:115–118. doi: 10.1016/s0091-3057(97)00554-6. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioned locomotion and place preference elicited by tactile cues paired exclusively with morphine in an open field. Psychopharmacology (Berl) 1987;91:375–380. doi: 10.1007/BF00518195. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence, implications of a conditioning theory for research and treatment. Arch. Gen. Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


