Abstract
In order to develop improved radioligands for imaging brain CB1 receptors with positron emission tomography (PET) based on rimonabant (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide; 1), we synthesized compounds 9a–s in which the N-piperidinyl ring was replaced with a 4-(4-cyano-tetrahydro-2H-pyranyl) or 1-cyano-cyclohexyl ring. Such changes were expected to be almost isosteric with 1, confer greater metabolic resistance and, in the case of the 4-(4-cyano-tetrahydro-2H-pyranyl) compounds, substantially reduce lipophilicity. One derivative, 1-(2-bromophenyl)-N-(1-cyanocyclohexyl)-5-(4-methoxyphenyl)-4-methyl-pyrazole-3-carboxamide (9n), showed high affinity (Ki = 15.7 nM) and selectivity for binding to CB1 receptors. The corresponding 4-(4-cyano-tetrahydro-2H-pyranyl) derivative (9m) also showed quite high affinity for CB1 receptors (Ki = 62 nM), but was found to have even higher affinity (Ki = 29 nM) for the structurally unrelated 18 kDa translocator protein (TSPO). Some other minor structural changes among 9a–s were also found to switch binding selectivity from CB1 receptors to TSPO or vice versa. These unexpected findings and their implications for the development of selective ligands or PET radioligands for CB1 receptors or TSPO are discussed in relation to current pharmacophore models of CB1 receptor and TSPO binding sites.
Keywords: CB1 receptor, CB2 receptor, TSPO receptor, pharmacophore
Introduction
Marijuana (Cannabis sativa) is one of the oldest known plant-derived substances used medicinally or as a drug of abuse.1 Marijuana’s principle psychoactive phytocannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC)a,2 binds with high affinity to two known receptor subtypes, cannabinoid type-1 (CB1)3 and cannabinoid type-2 (CB2)4. Both the CB1 and CB2 receptor sub-types are G-protein coupled receptors (GPCRs) with 7-transmebrane domains. They have 48% protein sequence homology.5 CB1 receptors have high density in brain and are also located in some peripheral tissues, whereas CB2 receptors are mostly located in peripheral tissues.5 Brain CB1 receptors are believed to play a role in the regulation of neurotransmitter (e.g., glutamate and γ-aminobutyric acid (GABA)) release through retrograde endocannabinoid-mediated depolarization-induced suppression of inhibition.6 Alterations in brain CB1 receptor densities and function have been implicated in several psychiatric disorders, such as drug dependence,7 obesity,8 depression,9 and schizophrenia10. Therefore, there is keen interest to study brain CB1 receptors in relation to such disorders in living human subjects with molecular imaging techniques, such as positron emission tomography (PET), and suitably selective radioligands.11
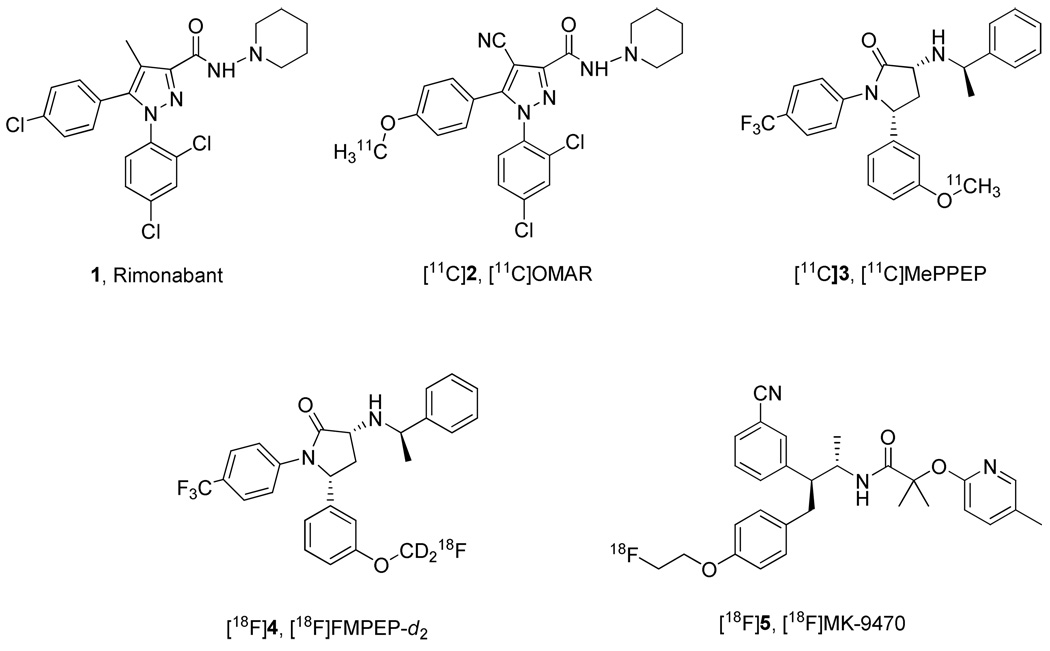
In 1994 Sanofi Recherche, presently Sanofi-Aventis, introduced the first high-affinity and selective CB1 receptor ‘antagonist’, rimonabant (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide; SR141716A 1, Chart 1).12 This ligand is now considered to be an inverse-agonist. The key 1,5-diarylpyrazole pharmacophore of 1 has been modified extensively to evaluate substituent-effects on receptor affinity, selectivity and physiochemical properties.13 Additionally, 1 has been modified to allow labeling with a short-lived imaging radionuclide (e.g., carbon-11 or fluorine-18) in the development of PET radioligands.11 Compound 1 is highly lipophilic with a computed cLogD value of 6.32 (Table 1), and hence many candidate PET radioligands derived from 1 retain high lipophilicity.11 This is usually detrimental to the success of a PET radioligand14–18 because high lipophilicity generally promotes low and poorly measureable free radioligand fraction in plasma, low radioligand penetration from blood to brain, and high non-specific binding to brain tissue. Therefore, success in the development of PET radioligands from 1 has been quite limited. PET CB1 receptor radioligands developed from other structural platforms also tend to be quite lipophilic, although some are gaining application in human subjects.11 Examples are [11C]OMAR19 (2), [11C] MEPPEP20,21 (3), [18F]FMPEP-d222 (4), and [18F]MK-947023 (5) (Chart 1).
Chart 1.
Structures of 1 and some PET CB1 receptor radioligands in human use.
Table 1.
cLogD values and Ki values at CB1 and CB2 receptors and at TSPO for known CB1 receptor ligands 1, 3, 5, new ligands 9a–s, and known TSPO ligands 10–12.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligand | R1 | R2 | R3 | R4 | R5 | cLogDa | CB1Ki (nM)b | CB2Ki (nM)b | TSPO Ki (nM)b |
| 1 | 6.32 | 1.4 ± 0.2 | 927 ± 66 | N.A. | |||||
| 3 | 4.76 | 0.472c | > 1,980c | N.A. | |||||
| 5 | 4.98 | 0.70d | 44d | N.A. | |||||
| 9a | 2-Br | H | H | O | H | 3.61 | 297 ± 31 | > 10,000 | 3,110 ± 572 |
| 9b | 2-F | H | Me | O | H | 3.55 | > 10,000 | 2,180 ± 224 | 394 ± 62.4 |
| 9c | 2-Cl | H | Me | O | H | 3.80 | > 10,000 | > 10,000 | 436 ±40.5 |
| 9d | 2-Cl | H | Me | O | Me | 3.77 | > 10,000 | > 10,000 | 880 ± 36.2 |
| 9e | 2-Br | H | Me | O | H | 3.81 | > 10,000 | > 10,000 | 576 ± 27.3 |
| 9f | 2-Br | H | Me | CH2 | H | 5.85 | > 10,000 | > 10,000 | 75.2 ± 2.51 |
| 9g | 2-Br | H | CN | O | H | 3.22 | 437 ± 44 | > 10,000 | 3,690 ± 519 |
| 9h | 3-Br | H | Me | O | H | 3.81 | > 10,000 | 226 ± 9.5 | 115 ± 4.9 |
| 9i | 4-Br | H | Me | O | H | 3.81 | 259 ± 26 | 655 ± 36.5 | 420 ± 20.5 |
| 9j | 2-I | H | Me | O | H | 3.92 | > 10,000 | > 10,000 | 762 ± 51.0 |
| 9k | 2-Ph | H | Me | O | H | 4.63 | 500 ± 58 | > 10,000 | 2,800 ± 116 |
| 9l | 2-F | 4-MeO | Me | O | H | 3.66 | 115 ± 11 | 2,460 ± 214 | 132 ± 4.91 |
| 9m | 2-Br | 4-MeO | Me | O | H | 3.94 | 62.2 ± 12.8 | 1,890 ± 350 | 29.0 ± 3.0 |
| 9n | 2-Br | 4-MeO | Me | CH2 | H | 5.87 | 15.7 ± 2.3 | 2,750 ± 183 | 279 ± 52.3 |
| 9o | 3-Br | 4-MeO | Me | O | H | 3.94 | 680 ± 108 | 234 ± 13.8 | 61.8 ± 2.2 |
| 9p | 2-I | 4-MeO | CN | O | H | 3.86 | > 10,000 | 2,370 ± 168 | 1,920 ± 116 |
| 9q | 3-Br | 4-MeO | Br | O | H | 4.00 | 356 ± 40 | 175 ± 10.4 | 155 ± 6.7 |
| 9r | 2-Br | 4-F | Me | O | H | 4.08 | 55 ± 6 | > 10,000 | 492 ± 28.2 |
| 9s | 2-Br | 3-F | Me | O | H | 4.08 | > 10,000 | > 10,000 | 693 ± 28.1 |
| 10 | 4.89 | N.A. | N.A. | 1.0± 0.0 | |||||
| 11 | 4.29 | N.A. | N.A. | 5.70e | |||||
The possible efficacious roles of the nitrile group in pharmacophore development have recently been highlighted,24 and include its ability to act as a carbonyl or halogen isostere, to act as a hydroxyl or carboxyl surrogate, and to reduce lipophilicity without adverse steric effect. In our search for leads to improved PET radioligands for CB1 receptors, we considered that the replacement of the N-piperidinyl ring in 1 with a 1-cyanocyclohexyl or 4-(4-cyano-tetrahydro-2H-pyranyl) ring (Scheme 1) would be a minor steric perturbation and would confer lower lipophilicity. Moreover, these rings, because of their nitrile substitution, may show greater resistance to metabolism than the replaced piperidinyl ring, which has been shown to be the exclusive region of metabolism of 1 in vitro25 and in vivo25,26. We therefore set out to synthesize a set of compounds with replaced N-piperidinyl rings and to evaluate their structure-activity relationships at CB1 receptors. Selective high-affinity CB1 ligands were discovered and also very unexpectedly selective high-affinity ligands for a structurally and functionally distinct site, the 18 kDa translocator protein, previously known as the peripheral benzodiazepine receptor (PBR) and now known as TSPO.27 These findings and their implications for ligand and PET radioligand development are discussed and rationalized in the context of an identified level of congruence between previously proposed pharmacophore models of the CB1 receptor and TSPO.
Scheme 1.
The main structural changes made to 1 in the newly synthesized set of ligands.
Results and Discussion
Chemistry
N-(4-Cyano-tetrahydro-2H-pyran-4-yl) and N-(1-cyanocyclohexyl) derivatives of 1,5-diarylpyrazole 3-carboxamides constituted the two core scaffolds of all newly synthesized compounds. Substituents in the 2-, 3- and 4-positions of the pyrazole-N1 and -C5 aryl rings, and at the pyrazole 3-carboxamide and 4-positions of the core scaffolds were also varied. Because of our strong interest in developing radioligands for imaging with PET, nearly all of the new ligands were designed to be amenable to labeling with cyclotron-produced, carbon-11 (t1/2 = 20.4 min) by commonly used procedures such as C-11C-methylation (9b–f, 9h–o, 9r and 9s), O-11C-methylation (9l–q), N-11C-methylation (9d), or 11C-cyanation (9g, and 9p).28
Compounds were synthesized as shown in Scheme 2. Substituted anilines were first converted into diazonium salts and then treated with ethyl 2-chloroacetoacetate under basic conditions to give 6a–g in good yields. A series of aryl ketones (7a–f) were converted into enamines with morpholine, TiCl4 and DIPEA (diisopropylethylamine) in toluene solution using a modified general procedure.29 Compounds 6a–g were then treated with the morpholine enamines under basic conditions to give the 1,5-diarylpyrazoles 8a–k, 8m and 8n in low but useful yields by a regioselective 1,3-dipolar cycloaddition reaction.30 Treatment of the acyl acetonitrile, 7h with 6c gave the diarylpyrazole 8p in low yield. Diarylpyrazole 8l was prepared in high yield by bromination of 8k with NBS (N-bromosuccinimide) using acetonitrile as a polar solvent to favor the formation of bromine. Each of the diarylpyrazoles 8a–q were hydrolyzed to the acids, converted into acyl chlorides and treated with either 4-amino-tetrahydro-2H-pyran-4-carbonitrile or 1-amino-cyclohexanecarbonitrile HCl to give the desired carboxamides (9a–c and 9e–s). Treatment of 9c with NaH and MeI gave the tertiary amide 9d in high yield.
Scheme 2.
Syntheses of 9a–s. Reagents, conditions and yields: a) HCl-aq, NaNO2, AcONa, ethyl 2-chloroacetoacetate, 52–83%; b) for 7a–f, morpholine, DIPEA, TiCl4, toluene, MgSO4; c) DIPEA, MeCN or EtOH, 18–26%; d) ref. 35; e) DIPEA, t-BuOH or MeCN, reflux, 16 h, 6–8%, ref. 36; f) NBS, MeCN, 91%; g) 1: LiOH, aq.-THF; 2: oxalyl chloride, DMF (cat.), DCM; 3: 4-amino-tetrahydro-2H-pyran-4-carbonitrile or 1-aminocyclohexanecarbonitrile HCl, DIPEA, DCM, 34–71% (for 9a–e, 9g–n and 9p–s), 36–68% for 9f and 9o; h) NaH (60% dispersion in oil), DMF, MeI, 64%
Computed ligand lipophilicities
The most common structural change made to the scaffold of 1, namely replacement of the N-(piperidin-1-yl)-4H-pyrazole-3-carboxamide moiety with an N-(4-cyano-tetrahydro-2H-pyran-4-yl)-4H-pyrazole-3-carboxamide moiety was computed to reduce lipophilicity by about 0.65 cLogD units. Consequently, the majority of the prepared ligands have much lower cLogD values than 1 (Table 1). These cLogD values approach values in the range that is generally preferred for PET radioligands (~ 1.5– 3.5).14–18 Replacement of the 4-(4-cyano-tetrahydro-2H-pyranyl) group with a 1-cyano-cyclohexyl group increases the computed lipophilicity substantially (Table 1). Nevertheless, the prepared examples still have substantially lower lipophilicity than 1.
Structure–affinity relationships
The sequence homologies and structures of TSPO, and CB1 and CB2 receptors are each highly conserved between species.5,27 Therefore, the binding affinities of ligands determined for each of these sites in tissue from one species in vitro are expected to be quite representative of those in other animal species. Prompted by the serendipitous and unexpected discovery of the high affinity of 9m for TSPO in a broad receptor screen of this compound (see later), each new ligand was assayed at TSPO, CB1, CB2 and GABAA-Bz sites (Table 1). All compounds exhibited low affinity (Ki > 10 µM, n = 4) for the GABAA-Bz site. Across the synthesized series of compounds, the Ki values for CB1 and CB2 receptors and TSPO ranged widely, namely from 15.7 to > 10,000 nM, 175 to > 10,000 nM and 29.0 to > 10,000 nM, respectively.
The substituent in the N 1aryl ring (R1, Table 1) was mainly varied with respect to halogen (F, Cl, Br and I) and position. In compounds having a pyrazole-C5 phenyl substituent (R2, Table 1) (9b–f, 9h–k), such variations failed to generate compounds with affinity for CB1 or CB2 receptors in the Ki < 100 nanomolar range. All these compounds had measurable affinity for TSPO, with the majority of 4-(4-cyano-tetrahydro-2H-pyranyl) compounds showing less than micromolar affinity. One compound, the relatively lipophilic 1-cyanocyclohexyl compound 9f, showed relatively higher TSPO affinity (Ki = 75 nM). Remarkably, this compound was wholly selective for TSPO versus CB1 or CB2 receptors. Generally, high-affinity TSPO ligands are tertiary amides and their secondary amide counterparts have lower affinity.31,32 However, methylation of the amido nitrogen in the ligand 9c led to compound 9d with a marked two-fold reduction in TSPO receptor affinity (to Ki = 880 nM), but with retained selectivity versus CB1 or CB2 receptors. Introduction of the large phenyl substituent into the 2-position of the N1-aryl ring in place of halogen (Cl, Br or I), as in 9k, switched the selectivity from TSPO to CB1 receptors. A 4-bromo substituent, as in 9i, also induced selectivity for CB1 receptors. In this group of compounds, replacement of the 4-methyl group (R3, Table 1) on the pyrazole ring with hydrogen (9a) or nitrile (9g) conferred CB1 selectivity.
Introduction of a substituent into the C5-aryl ring (R2, Table 1) had a major influence on receptor affinity and selectivity. Compounds with a 4-methoxy substituent in the C5-aryl ring plus a 2-fluoro or 2-bromo substituent on the N1-aryl ring (9l–n) showed quite high affinity for both CB1 receptors and TSPO, with very low affinity for CB2 receptors. Remarkably, the 4-(4-cyano-tetrahydro-2H-pyranyl) compound (9m) with a 2-bromo substituent in the C5-aryl ring showed high affinity (Ki = 29 nM) for binding to TSPO with two-fold preference over binding to CB1 receptors. Even more remarkably, replacement of the tetrahydro-2H-pyranyl ring oxygen atom with a methylene group, as in the 1-cyano-cyclohexyl analog (9n), caused selectivity to be reversed and affinity for CB1 receptors to be enhanced (Ki = 15.7 nM). In the whole group of pyrazole-C5 4-methoxyphenyl compounds (9l–q), binding site affinity and selectivity were sensitive to position and type of substituent in the pyrazole-C5 aryl ring. Thus, a compound with a 3-bromo substituent (9o) showed quite a high affinity (Ki = 62 nM) and selectivity for binding to TSPO versus CB1 receptors. This compound also showed very low affinity for CB2 receptors, as did the other N1-3-bromophenyl compounds (9h, 9q). A 2-iodo substituent in the N1-aryl ring and a 4-nitrile group in the pyrazole ring (9p) led to very low affinity at CB1 and CB2 receptors and at TSPO. Introduction of a 4-fluoro substituent into the C5-aryl ring, as in 9r, in place of a 4-methoxy substituent enhanced selectivity of binding to CB1 receptors versus TSPO. However, shift of this substituent to the 3-position, as in 9s, abolished high-affinity CB1 receptor binding.
Receptor and binding site screening of 9m and 9n
Ligands 9m and 9n at 10 µM concentration showed < 50% inhibition (n = 4) for radioligand binding to the following receptors and binding sites: 5-HT1A,E, 5-HT1D (CycMEP), 5-HT2A–C, 5-HT3, 5-HT5A, 5-HT6, 5-HT7, α1A,B, α2A–C, β1,3, D1–4, DAT, DOR, H1–4, M1–5, MOR, NET, SERT, σ1,2, and V1A,1B,2. The Ki values (n = 3) of 9m at 5-HT1A, 5-HT1D, D5 and KOR were 6,280 ± 1,100, > 10,000, > 10,000 and 798 ± 84 nM, respectively. The corresponding Ki values for 9n were 1,130 ± 96, > 10,000, > 10,000 and 597 ± 67 nM. Thus, 9m is highly selective for TSPO and CB1 receptors versus other tested binding sites and 9n is highly selective for CB1 receptors versus all other tested binding sites and receptors.
Relationship of ligand structures to previously proposed CB1 receptor and TSPO pharmacophore models
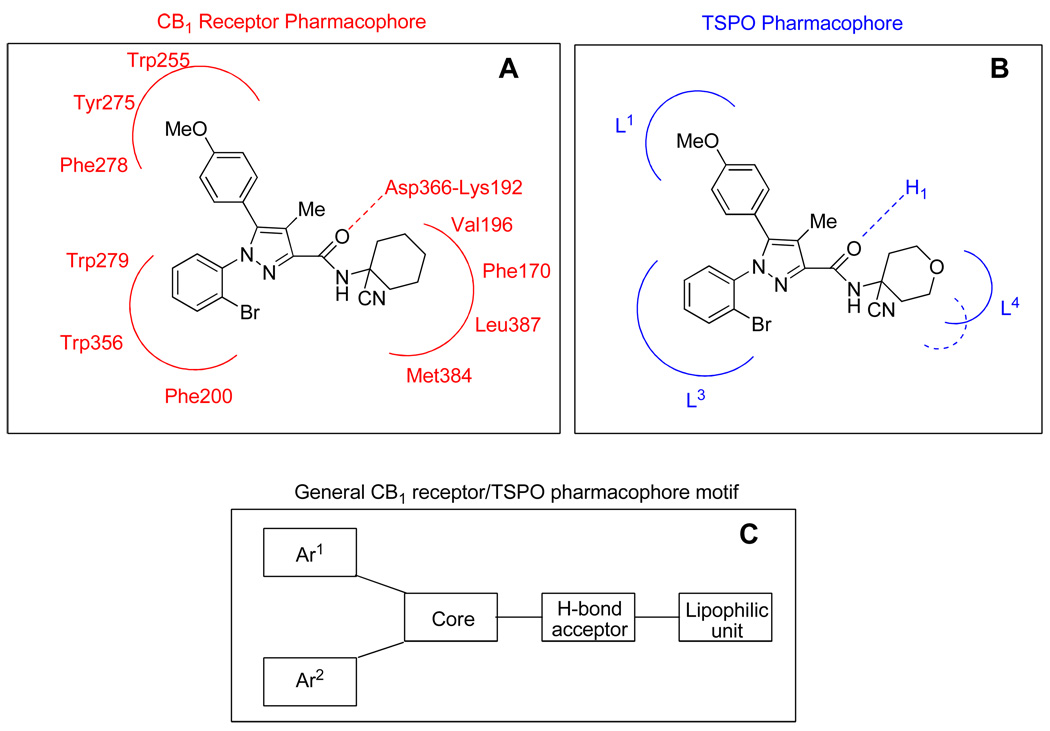
CB1 receptors and TSPO are structurally distinct proteins. The CB1 receptor belongs to the family of GPCRs having 7–transmembrane domains and considerable information has been obtained on the likely topography and make-up of the binding site for ligands structurally resembling 1. This information has been summarized and a pharmacophore model proposed.13 The model proposes i) a hydrogen bond interaction between the amide oxygen of the ligand and the Asp366-Lys192 salt bridge in the receptor, ii) a pocket formed by Val 196, Phe 170, Leu 387 and Met 384 that can accommodate a lipophilic alicyclic group, and iii) two distal pockets, one formed by Trp255, Tyr 275 and Phe 278 and the other by Trp279, Trp356 and Phe 200 which can each accept a substituted phenyl ring, as depicted in Panel A, Scheme 3. It may be hypothesized that the highest affinity and most CB1-selective ligand found in this study, 9n, fits into the proposed pharmacophore in the same manner as the prototypical selective CB1 receptor ligand 1 (Panel A, Scheme 3). TSPO protein is not a GPCR, but a tryptophan-rich 18 kDa protein that is a component of a trimeric complex with the 32 kDa voltage–dependent anion channel and the 30 kDa adenine nucleotide translocase to constitute the mitochodrial permeability transition pore.27 Relatively less is known about the amino acids composing the binding site for high-affinity TSPO ligands from a plethora of structural classes. Nevertheless, a pharmacophore model has been proposed which comprises three lipophilic pockets (dubbed L1, L3 and L4) and an H-bond donor group (Panel B, Scheme 3).33–35 This pharmacophore model accommodates classical TSPO ligands such as the isoquinoline 3-carboxamide 10 (see Table 1 for structure) and TSPO ligands from some other structural classes, including the recently described N1-methyl-2-phenylindol-3-ylglyoxylamides, of which 11 (Table 1) is a recent example.36 The model also accommodates the highest affinity TSPO ligand found in this study, namely 9m (Panel B, Scheme 3).
Scheme 3.
Compatibility of some new ligands with previously proposed pharmacophore models. A: Compatibility of 9n with a proposed13 CB1 receptor pharmacophore model. B: Compatibility of 9m with a proposed31–33 TSPO receptor pharmacophore model. C: Common general features of proposed CB1 receptor and TSPO pharmacophore models. Known CB1-selective ligands such as 1–5 (Chart 1) and known TSPO-selective ligands 10 and 11 (Table 1) are examples of compounds from different structural classes that are compatible with the pharmacophore motif shown in panel C.
We observe that the previously described pharmacophore models for CB1 receptors and TSPO are strikingly similar in the arrangement of key domains of binding site-ligand interaction; that is the pharmacophores appear quite congruent at this macro level of description. Ligands with the general structural motif shown in Panel C (Scheme 3) therefore have potential to bind to either or both pharmacophores. This provides a rational basis for our unexpected findings on binding selectivity. Clearly, our CB1 and TSPO ligand binding data also reveal marked sensitivities to subtle differences in ligand structure, as exemplified by 9m and 9n (Table 1). At a deeper level of description the pharmacophores would be expected to be noticeably different and this would account for the severe alterations or even reversals of ligand selectivity with minor structural changes.
Implications for PET radioligand development
As for CB1 receptors, the development of PET radioligands for imaging brain and peripheral TSPO currently attracts immense effort.32 This effort on TSPO radioligands is driven by their potential to become important biomarkers of inflammation in a variety of neuropsychiatric and peripheral conditions. The majority of radioligands being developed for CB1 receptors or TSPO share the general structural motif depicted in Panel C (Scheme 3). PET radioligands are required to bind selectively to their target proteins. Therefore, a message from this study is to screen candidate PET radioligands for CB1 receptors for high affinity binding at TSPO and vice versa in order to confirm adequate target selectivity. Screens against CB2 receptors are also of course advised.
The ligand 9m possesses too low affinity and selectivity to be developed as a PET radioligand for TSPO, but could perhaps be a lead to better candidates. [11C]2 (Chart 1) has been developed as a promising PET radioligand for brain CB1 receptors and is now in use in human subjects. This radioligand is selective with moderately high affinity (Kb = 30 nM) and a cLogD value of 5.3. By comparison, ligand 9n also shows excellent selectivity for CB1 receptors, high affinity (Ki = 16 nM) and only somewhat higher cLogD value (5.87; Table 1). The labeling of 9n with carbon-11, which is feasible in its 4-methoxy group, for evaluation in vivo may therefore be warranted.
Conclusion
Modification of the prototypical CB1 receptor ligand 1, primarily by replacing the N-piperidinyl ring with a 4-(4-cyano-tetrahydro-2H-pyranyl) or 1-cyano-cyclohexyl ring, led respectively to one high-affinity CB1 selective ligand (9n), and also surprisingly to a ligand with a preference to bind with high affinity to TSPO (9m). Many of the prepared ligands showed quite high affinity for both CB1 and TSPO. These unexpected findings may be rationalized on the basis of the observed congruence between previously proposed phamacophore models for CB1 receptors and TSPO.
Experimental
Materials
All reagents and solvents (ACS or HPLC grade) were purchased from commercial sources and used as supplied. 1-(2-Chlorophenyl)-N-methyl-N-sec-butyl-isoquinoline-3-carboxamide (PK 11195, 10) was purchased from Tocris Biosciences. Chloro[(phenyl)hydrazono]ethyl acetates 6a–d were synthesized according to a previously described procedure.30,37 The 1,5-diarylpyrazoles 8o and 8q were prepared as previously described.38,39
General methods
1H (400 MHz) and 13C (100 MHz) NMR spectra were recorded at r.t. on a Varian-400 spectrometer (Varian, Walnut Creek, CA) or Avance-400 spectrometer (Brucker; Billerica, MA). Chemical shifts are reported in δ units (ppm) downfield relative to the chemical shift of tetramethylsilane. Signals are quoted as s (singlet), d (doublet), dd (double doublet), dt (double triplet), t (triplet), q (quartet) or m (multiplet). High-resolution mass spectra (HRMS) were determined at the University of Illinois at Urbana (Champaign, IL, USA) or Notre Dame Mass Spectrometry Facility (Notre Dame, Indiana). Melting points were determined using Mel-temp II (Laboratory Devices, Holliston, MA, USA) or Mel-temp apparatus (Electrothermal, Fisher Scientific, USA) and are uncorrected. Flash column chromatography was performed on silica gel (230–400 mesh, 60 Å) (Sigma-Aldrich). Elemental analyses were acquired from Midwest Microlab, LLC (Indianapolis, IN, USA).
Chemistry
Chloro[(2-phenylphenyl)hydrazono]ethyl acetate (6e)
A solution of 2-aminobiphenyl (7.6 g, 45 mmol) in concentrated HCl (12 M, 5.4 mL) plus H2O (200 mL) was stirred in a 1-L round-bottomed flask for 30 min. The reaction mixture was cooled to about 5 °C in an ice-water bath. A solution of sodium nitrite (3.2 g, 46 mmol) in H2O (10 mL) was slowly added to the reaction mixture so that the reaction temperature remained below 15 °C. The reaction mixture was then stirred for another 30 min. A separate solution of sodium acetate (3.5 g, 43 mmol) and ethyl 2-chloroacetoacetate (6.2 mL, 45 mmol) in EtOH-H2O (400 mL, 4:1 v/v) was added dropwise. The reaction mixture was allowed to warm to r.t. and stirred overnight (16 h). The precipitate was filtered off, washed with water and dried to give 6e (11.3 g, 83 %) as a light yellow solid. Mp 82–84 °C; 1H NMR δ 8.60 (s, 1H), 7.69 (d, J = 8.4 Hz, 1H), 7.51–7.36 (m, 6H), 7.26–7.22 (m, 1H), 7.12 (t, J = 6.8 Hz, 1H), 4.40 (q, J = 7.2 Hz, 2H), 1.41 (t, J = 6.8 Hz, 3H).
Chloro[(3-bromophenyl)hydrazono]ethyl acetate (6f)
The procedure described for the synthesis of 5e was applied to 3-bromo-aniline to give 6f (52%) as a light brown solid. Mp 84–86 °C; 1H NMR δ 8.60 (s, 1H), 7.69 (d, J = 8.4 Hz, 1H), 7.53 (t, J = 6.8, 2H), 7.45–7.36 (m, 4H), 7.26 (t, J = 6.8 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 4.40 (q, J = 7.2 Hz, 2H), 1.41 (t, J = 6.8 Hz, 3H).
Chloro[(4-bromophenyl)hydrazono]ethyl acetate (6g)
The procedure described for the synthesis of 5e was applied to 4-bromo-aniline to give 6g (78%) as a pale white solid. Mp 84–86 °C; 1H NMR δ 8.30 (s, 1H), 7.44 (t, J = 1.6, 1H), 7.21–7.11 (m, 3H), 4.43 (q, J = 7.2 Hz, 2H), 1.43 (t, J = 6.8 Hz, 3H).
Ethyl 1-(2-fluorophenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxylate (8a)
Morpholine (33 mL, 358 mmol) was added to a suspension of MgSO4 (4 g) in toluene 60 mL) and was stirred at r.t. TiCl4 (47 mL, 1 M in toluene) was slowly added to give a dark green suspension. Propiophenone (7a, 8.0 g, 60 mmol) and DIPEA (52 mL, 298 mmol) were dissolved separately in toluene (20 mL). This mixture was slowly added to the solution containing TiCl4 and morpholine. The reaction was heated to 60 °C for 16 h, cooled to r.t. and then filtered. The cake was washed with toluene, which was then added to the filtrate. The combined filtrate was concentrated in vacuo to give crude the morpholine enamine (8.2 g, 68%) as a pale yellow oil.
DIPEA (7.2 mL, 41.4 mmol) was added to a stirred solution of 6a (3.3 g, 13.7 mmol) and the morpholine enamine (2.8 g, 13.7 mmol) in EtOH (100 mL). The mixture was stirred at r.t. for 16 h and then concentrated in vacuo. The resulting crude product was purified by column chromatography on silica gel (20% EtOAc in hexanes) to give 8a (1.1 g, 25%) as a white solid. Mp 66–68 °C; 1H NMR δ 7.47 (td, J = 1.6 and 7.6 Hz, 1H), 7.32–7.29 (m, 4H), 7.18–7.15 (m, 3H), 7.02 (td, J = 1.6 and 7.6 Hz), 4.49 (q, J = 7.2 Hz, 2H), 2.35 (s, 3H), 1.43 (t, J = 6.8 Hz, 3H).
Ethyl 1-(2-chlorophenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxylate (8b)
The procedure described for the synthesis of 8a was applied to 6b and 7a to yield 8b (21%) as a solid. Mp 116–118 °C; 1H NMR δ 7.42 (dd, J = 1.6 and 7.2 Hz, 1H), 7.36–7.26 (m, 6H), 7.17–7.14 (m, 2H), 4.49 (q, J = 7.2 Hz, 2H), 2.36 (s, 3H), 1.45 (t, 6.8 Hz, 3H).
Ethyl 1-(2-bromophenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxylate (8c)
The procedure described for the synthesis of 8a was applied to 6c and 7a to yield 8c (23%) as a solid. Mp 116–118 °C; 1H NMR δ 7.54 (dd, J = 1.6 and 7.2 Hz, 1H), 7.39 (dd, J = 1.6 and 7.2 Hz, 1H), 7.33–7.16 (m, 7H), 4.49 (q, J = 7.2 Hz, 2H), 2.37 (s, 3H), 1.45 (t, J = 6.8, 3H).
Ethyl 1-(3-bromophenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxylate (8d)
The procedure described for the synthesis of 8a was applied to 6f and 7a to yield 8d (15%) as a semi-solid; 1H NMR δ 7.56 (t, J = 2.0 Hz, 1H), 7.45–7.39 (m, 4H), 7.17–7.00 (m, 4H), 4.49 (q, J = 7.2 Hz, 2H), 2.39 (s, 3H), 1.43 (t, J = 6.8, 3H).
Ethyl 1-(4-bromophenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxylate (8e)
The procedure described for the synthesis of 8a was applied to 6g and 7a to yield 8e (25%) as a solid. Mp 76–78 °C; 1H NMR δ 7.42–7.37 (m, 5H), 7.16–7.17 (m, 4H), 4.49 (q, J = 7.2 Hz, 2H), 2.39 (s, 3H), 1.43 (t, J = 6.8, 3H).
Ethyl 1-(2-iodophenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxylate (8f)
The procedure described for the synthesis of 8a was applied to 6d and 7a to yield 8f (22%) as a solid. Mp 122–124 °C; 1H NMR δ 7.80 (d, J = 8.0 Hz, 1H), 7.34–7.27 (m, 5H), 7.20–7.18 (m, 2H), 7.08–7.04 (m, 1H), 4.49 (q, J = 7.2 Hz, 2H), 2.37 (s, 3H), 1.45 (t, J = 6.8 Hz, 3H).
Ethyl 1-(2-phenylphenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxylate (8g)
The procedure described for the synthesis of 8a was applied to 6e and 7a to yield 8g (17%) as a semi-solid. 1H NMR δ 7.73 (d, J = 7.2 Hz, 1H), 7.21 (dd, J = 1.6 and 8.0 Hz, 1H), 7.17–7.12 (m, 2H), 7.08 (p, J = 8.4 Hz, 4H), 6.51 (d, J = 7.6 Hz, 2H), 6.42 (d, J = 7.6 Hz, 2H), 4.45 (q, J = 7.2 Hz, 2H), 2.17 (s, 3H), 1.48 (t, J = 7.2 Hz).
Ethyl 1-(2-bromophenyl)-5-phenyl-1H-pyrazole-3-carboxylate (8h)
The procedure described for the synthesis of 8a was applied to 6c and 7b to yield 8h (24%) as a solid. Mp 90–92 °C; 1H NMR δ 7.62 (d, J = 8.4 Hz, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 7.6 Hz, 1H), 7.34–7.20 (m, 6H), 7.10 (s, 1H), 4.49 (q, J = 7.2 Hz, 2Hz), 1.45 (t, J = 6.8 Hz).
Ethyl 1-(2-fluorophenyl)-5-(4-methoxyphenyl)-4-methyl-1H-pyrazole-3-carboxylate (8i)
The procedure described for the synthesis of 8a was applied to 6a and 7c to yield 8i (19%) as a white solid. Mp 86–88 °C; 1H NMR δ 7.46 (td, J = 1.6 and 7.6 Hz, 1H), 7.35–7.29 (m, 1H), 7.18 (t, J = 7.6 Hz, 1H), 7.09 (d, J = 11.2 Hz, 2H), 7.04 (t, J = 9.2 Hz), 6.85 (d, J = 11.2 Hz, 2H), 4.48 (q, J = 7.2 Hz, 2H), 3.79 (s, 3H), 2.33 (s, 3H), 1.45 (t, J = 6.8 Hz, 3H).
Ethyl 1-(3-bromophenyl)-5-(4-methoxyphenyl)-4-methyl-pyrazole-3-carboxylate (8j)
The procedure described for the synthesis of 8a was applied to 6f and 7g to yield 8j (12%) as a semi-solid. 1H NMR δ 7.57 (t, J = 1.9 Hz, 1H) 7.46–7.44 (m, 1H), 7.22–7.11 (m, 3H), 7.06–7.04 (m, 2H), 6.93 (d, J = 8.8), 4.47 (q, J = 7.2 Hz, 2H), 3.84 (s, 3H), 2.29 (s, 3H), 1.45 (t, J = 6.8 Hz, 3H).
Ethyl 1-(3-bromophenyl)-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxylate (8k)
The procedure described for the synthesis of 8a was applied to 6f and 7c to yield 8k (26%) as a solid. Mp 64–68 °C; 1H NMR δ 7.61 (d, J = 8.4 Hz, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.33 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.03 (s, 1H), 6.80 (d, J = 7.2 Hz, 2H), 4.49 (q, J = 7.2 Hz, 2H), 3.78 (s, 3H), 1.45 (t, J = 6.8 Hz, 3H).
Ethyl 4-bromo-1-(3-bromophenyl)-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxylate (8l)
NBS (334 mg, 1.9 mmol) was added to a stirred solution of 8k (500 mg, 1.2 mmol) in MeCN (25 mL). The reaction mixture was heated to reflux for 8 h and then concentrated in vacuo. The crude was purified with flash chromatography (hexanes–EtOAc, 8:2 v/v) to give 8l (543 mg, 91%). Mp 118–120 °C; 1H NMR δ 7.61 (t, J = 2.0 Hz, 1H), 7.46 (dd, J = 6.8 Hz, 1H), 7.21 (d, J = 9.3 Hz, 2H), 7.16 (t, J = 8.4 Hz, 1H), 7.06 (d, J = 7.6 Hz, 1H), 6.93 (d, J = 8.4 Hz, 2H), 4.50 (q, J = 7.2 Hz, 2H), 3.84 (s, 3H), 1.47 (t, J = 6.8 Hz, 3H).
Ethyl 1-(2-bromophenyl)-5-(4-fluorophenyl)-4-methyl-1H-pyrazole-3-carboxylate (8m)
The procedure described for the synthesis of 8a was applied to 6c and 7d to yield 8m (20%) as a white solid. Mp 109–110 °C; 1H NMR δ 7.56 (d, J = 7.6 Hz, 1H), 7.39–7.32 (m, 2H), 7.25–7.23 (m, 1H), 7.17 (dd, J = 5.6 and 3.2 Hz, 2H), 7.01 (t, J = 8.4 Hz, 2H), 4.49 (q, J = 7.2 Hz, 2H), 2.34 (s, 3H), 1.45 (t, J = 6.8 Hz, 3H).
Ethyl 1-(2-bromophenyl)-5-(3-fluorophenyl)-4-methyl-1H-pyrazole-3-carboxylate (8n)
The procedure described for the synthesis of 8a was applied to 6c and 7e to yield 8n (19%) as a white solid. Mp 106–108 °C; 1H NMR δ 7.57 (d, J = 76. Hz, 1H), 7.40–7.32 (m, 2H), 7.29–7.24 (m, 2H), 7.02 (t, J = 6.4 Hz, 1H), 6.96 (d, J = 8.0 Hz, 1H), 6.90 (d, J = 9.6 Hz, 1H), 4.49 (q, J = 7.2 Hz, 2H), 2.34 (s, 3H), 1.45 (t, J = 6.8 Hz, 3H).
Ethyl 1-(2-bromophenyl)-4-cyano-5-phenyl-1H-pyrazole-3-carboxylate (8p)
A solution of 6c (10.5 g, 34.4 mmol), benzoylacetonitrile (7h, 5.0 g, 34.4 mmol) and DIPEA (13.3 g, 103.2 mmol) was refluxed in MeCN (100 mL) for 16 h. The reaction mixture was then concentrated in vacuo and the crude product extracted with ether (3 × 100 mL). The ethereal extracts were combined and concentrated in vacuo. The crude was purified with flash chromatography (hexanes–EtOAc, 7:3 v/v) to give 8p (1.1 g, 8%) as a pale white solid. Mp 106–108 °C; 1H NMR δ 7.63 (d, J = 8.0 Hz, 1H), 7.46–7.35 (m, 8H), 4.56 (q, J = 7.2 Hz, 2H), 1.49 (t, J = 6.8 Hz, 3H).
1-(2-Bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-5-phenyl-1H-pyrazole-3-carboxamide (9a)
An aqueous solution (6 mL) of LiOH (819 mg, 34.2 mmol) was added to a stirred solution of 8c (500 mg, 966 µmol) in THF (60 mL) and heated to reflux for 6 h. The reaction was cooled to r.t. and volatile compounds were removed in vacuo. The residue was dissolved in water (50 mL) and neutralized with aq. HCl. The resulting precipitate was filtered-off and dried in vacuo yielding crude acid (242 mg, 52%). DMF (1 drop) and oxalyl chloride (66 µL, 777 µmol) were added to a stirred solution of the acid (200 mg, 518 µmol) in dry dichloromethane (DCM; 10 mL). After bubbling had ceased, the reaction was concentrated in vacuo. A solution of 4-amino-tetrahydro-2H-pyran-4-carbonitrile (101 mg, 673 µmol) and DIPEA (90 µL, 777 µmol) in DCM (10 mL) was added to the acid chloride and stirred at r.t. for 2 h. The volatile compounds were removed in vacuo and the crude residue purified with silica gel chromatography (hexanes–EtOAc, 60:40 v/v) to yield 9a (58% from acid) as a white solid. Mp 226–228 °C; 1H NMR δ 7.68 (d, J = 8.0 Hz, 1H), 7.44–7.39 (m, 2H), 7.39–7.35 (m, 1H), 7.35–7.25 (m, 3H), 7.12 (s, 1H), 7.06 (s, 1H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.88 (td, J = 2.0 and 12.4 Hz, 2H), 2.57 (d, J = 12.8 Hz, 2H), 2.11 (td, J = 4.4 and 10.0 Hz); HRMS (tof): [M+Na]+, calc’d for C22H1979BrN4NaO2, 473.0584; found, 473.0562, error 4.5 ppm; Anal. calc’d for C22H19BrN4O2: C 58.5, H 4.2, N 12.4; found C 58.4, H 4.3, N 12.3.
N-(4-Cyano-tetrahydro-2H-pyran-4-yl)-1-(2-fluorophenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxamide (9b)
The procedure described for the synthesis of 9a was applied to 8a and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9b (68% from acid) as a white solid. Mp 212–214 °C; 1H NMR δ 7.40–7.32 (m, 5H), 7.21–7.05 (m, 5H), 4.00 (dt, J = 3.2 and 12.8 Hz, 2H), 3.87 (td, J = 2.0 and 12.4 Hz, 2H), 2.56 (d, J = 12.8 Hz, 2H), 2.40 (s, 3H), 2.07–2.04 (m, 2H); HRMS (tof): [M+H]+, calc’d for C23H22FN4O2, 405.1721; found, 405.1711, error 2.5 ppm; Anal. calc’d for C23H21FN4O2 ˙0.3 H2O: C 67.4, H 5.3, N 13.7; found C 67.4, H 5.3, N 13.7.
1-(2-Chlorophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxamide (9c)
The procedure described for the synthesis of 9a was applied to 8b and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9c (56% from acid) as a white solid. Mp 208–210 °C; 1H NMR δ 7.43–7.28 (m, 7H), 7.15–7.12 (m, 2H), 7.10 (s, 1H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.86 (td, J = 2.0 and 12.4 Hz, 2H), 2.55 (d, J = 12.8 Hz, 2H), 2.40 (s, 3H), 2.06–2.04 (m, 2H); HRMS (tof): [M+Na]+, calc’d for C23H21ClN4NaO2, 443.1245; found, 443.1231, error 3.2 ppm; Anal. calc’d for C23H21ClN4O2: C 65.6, H 5.0, N 13.3; found C 65.7, H 5.0, N 13.0.
1-(2-Chlorophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-N,4-dimethyl-5-phenyl-1H-pyrazole-3-carboxamide (9d)
NaH (60% in dispersion) (17 mg, 428 µmol) was added to a stirred solution of 9c (90 mg, 223 µmol) and methyl iodide (61 mg, 428 µmol) in DMF (5 mL). The reaction was stirred for 1 h and then concentrated in vacuo. The crude was purified with flash chromatography (hexanes–EtOAc, 8:2 v/v) to give 9d (62 mg, 64%) as a pale white solid. Mp 182–184 °C; 1H NMR δ 7.45 (d, J = 9.2 Hz, 1H), 7.35–7.20 (m, 6H), 7.18–7.14 (m, 2H), 4.09 (dd, J = 2.8 and 12.8 Hz, 2H), 3.45 (s, 3H), 2.58 (d, J = 2.8 Hz, 2H), 2.28 (s, 3H), 2.17 (td, J = 4.0 and 12.0 Hz, 2H); HRMS (tof): [M+Na]+, calc’d for C24H23ClN4NaO2, 457.1402; found, 457.1386, error 3.4 ppm; Anal. calc’d for C24H23ClN4O2: C 66.3, H 5.3, N 12.9; found C 65.9, H 5.3, N 12.6.
1-(2-Bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxamide (9e)
The procedure described for the synthesis of 9a was applied to 8c and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9e (58% from acid) as a white solid. Mp 188–190 °C; 1H NMR δ 7.62 (d, J = 8.0, 1H), 7.36–7.28 (m, 6H), 7.18–7.14 (m, 2H), 7.10 (s, 1H), 4.01 (td, J = 2.0 and 12.4 Hz, 2H), 3.87 (td, J = 2.0 and 12.4 Hz, 2H), 2.56 (d, J = 12.8 Hz, 2H), 2.41 (s, 3H), 2.07–2.04 (m, 2H); HRMS (tof): [M+H]+, calc’d for C23H2279BrN4O2, 465.0921; found, 465.0908, error 2.8 ppm; Anal. calc’d for C23H21BrN4O2: C 59.4, H 4.5, N 12.0; found C 59.5, H 4.6, N 11.7.
1-(2-Bromophenyl)-N-(1-cyanocyclohexyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxamide (9f)
The procedure described for the synthesis of 9a was applied to 8c and 1-aminocyclohexanecarbonitrile HCl to yield 9f (62% from acid) as a white solid. Mp 212–214 °C; 1H NMR δ 7.61 (d, J = 8.0 Hz, 7.37–7.25 (m, 6H), 7.16–7.14 (m, 2H), 7.04 (s, 1H), 2.51 (bs, 2H), 2.41 (s, 3H), 1.78–1.69 (m, 7H), 1.32 (bs, 1H). HRMS (tof): [M+H]+, calc’d for C24H2379BrN4NaO, 485.0953; found, 485.0947, error 3.8 ppm; Anal. calc’d for C24H23BrN4O: C 62.2, H 5.0, N 12.1; found C 62.1, H 5.2, N 12.3.
1-(2-Bromophenyl)-4-cyano-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-5-phenyl-1H-pyrazole-3-carboxamide (9g)
The procedure described for the synthesis of 9a was applied to 8p and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9g (71% from acid) as a solid. Mp 224–226 °C; 1H NMR δ 7.69 (d, J = 8.4 Hz, 1H), 7.49–7.32 (m, 8H), 6.95 (s, 1H), 4.02 (dt, J = 3.2 and 12.8 Hz, 2H), 3.86 (td, J = 2.0 and 12.4 Hz, 2H), 2.58 (d, J = 12.8 Hz, 2H), 2.09–2.04 (m, 2H); HRMS (tof): [M+H]+, calc’d for C23H1979BrN5O2, 476.0717; found, 476.0700, error 3.6 ppm; Anal. for C23H18BrN5O2: C 58.0, H 3.8, N 14.7; found C 57.6, H 3.9, N 14.5.
1-(3-Bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxamide (9h)
The procedure described for the synthesis of 9a was applied to 8d and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9h (53% from acid) as a white solid. Mp 138–140 °C; 1H NMR δ 7.56 (t, J = 2 Hz, 1H), 7.45 (m, 4H), 7.17–7.12 (m, 3H), 7.10 (s, 1H), 7.00 (d, J = 7.2 Hz, 1H), 4.02 (dt, J = 3.2 and 12.8 Hz, 2H), 3.88 (td, J = 2.0 and 12.4 Hz, 2H), 2.59 (d, J = 12.8 Hz, 2H), 2.36 (s, 3H), 2.10–2.04 (m, 2H); HRMS (tof): [M+Na]+, calc’d for C23H2179BrN4NaO2, 487.0740; found, 487.0726, error 2.8 ppm; Anal. calc’d for C23H21BrN4O2:C 59.4, H 4.5, N, 12.0; found C 59.3, H 4.5, N 12.0.
1-(4-Bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxamide (9i)
The procedure described for the synthesis of 9a was applied to 8e and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9i (34% from acid) as a solid. Mp 176–178 °C; 1H NMR δ 7.46 (dt, J = 2.4 and 9.2 Hz, 2H), 7.41–7.38 (m, 3H), 7.16–7.13 (m,3H), 7.11 (dt, J = 2.4 and 9.2 Hz, 2H), 4.02 (dt, J = 3.2 and 12.8 Hz, 2H), 3.88 (td, J = 2.0 and 12.4 Hz, 2H), 2.57 (d, J = 12.8 Hz, 2H), 2.36 (s, 3H), 2.08–2.04 (m, 2H); HRMS (tof): [M+Na]+, calc’d for C23H2179BrN4NaO2, 487.0740; found, 487.0721, error 3.9 ppm; Anal. calc’d for C23H21BrN4O2: C 59.4, H 4.5, N 12.0; found C 59.2, H 4.6, N 11.9.
N-(4-Cyano-tetrahydro-2H-pyran-4-yl)-1-(2-iodophenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxamide (9j)
The procedure described for the synthesis of 9a was applied to 8f and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9j (61% from acid) as a solid. Mp 200–202 °C; 1H NMR δ 7.88 (dd, J = 1.2 and 7.6 Hz, 1H), 7.39 (td, J = 1.2 and 7.6 Hz, 1H), 7.31–7.29 (m, 3H), 7.27 (dd, J = 1.6 and 7.6, 1H), 7.18–7.16 (m, 2H), 7.14–7.09 (m, 2H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.87 (td, J = 2.0 and 12.4 Hz, 2H), 2.56 (d, J = 12.8 Hz, 2H), 2.41 (s, 3H), 2.08–2.04 (m, 2H); HRMS (tof): [M+H]+, calc’d for C23H22IN4O2, 513.0728; found, 513.0780, error 0.5 ppm; Anal. calc’d for C23H21IN4O2: C 53.9, H 4.1, N 10.9: found C 54.0, H 4.1, N 10.8.
N-(4-Cyano-tetrahydro-2H-pyran-4-yl)-1-(2-phenylphenyl)-4-methyl-5-phenyl-1H-pyrazole-3-carboxamide (9k)
The procedure described for the synthesis of 9a was applied to 8g and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9k (44% from acid) as a white solid. Mp 98–100 °C; 1H NMR δ 7.62 (d, J = 7.6, 1H), 7.51–7.47 (m, 2H), 7.29–7.02 (m, 8H), 6.51 (d, J = 7.6 Hz, 2H), 6.47 (d, J = 7.6 Hz, 2H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.87 (td, J = 2.0 and 12.4 Hz, 2H), 2.56 (d, J = 12.8 Hz, 2H), 2.41 (s, 3H), 2.08–2.04 (m, 2H); HRMS (tof): [M+Na]+, calc’d for C29H26N4NaO2, 463.2129; found, 463.2107, error 4.7 ppm; Anal. calc’d for C29H26N4O2: C 75.3, H 5.7, N 12.1; found C 74.9, H 5.8, N 11.7.
N-(4-Cyano-tetrahydro-2H-pyran-4-yl)-1-(2-fluorophenyl)-5-(4-methoxyphenyl)-4-methyl-1H-pyrazole-3-carboxamide (9l)
The procedure described for the synthesis of 9a was applied to 8i and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9l (59% from acid) as a white solid. Mp 210–212 °C; δ 7.41–7.34 (m, 2H), 7.22 (t, J = 7.2 Hz, 1H), 7.10–7.05 (m, 4H), 6.85 (d, J = 8.4 Hz, 2H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.88 (td, J = 2.0 and 12.4 Hz, 2H), 3.79 (s, 3H), 2.57 (d, J = 12.8 Hz, 2H), 2.35 (s, 3H), 2.11–2.04 (m, 2H); HRMS (tof): [M+Na]+ calc’d for C24H23FN4NaO3, 457.1646; found, 457.1831, error 3.4 ppm; Anal. calc’d for C24H23FN4O3˙0.3 H2O: C 65.5, H 5.4, N 12.7; found C 65.5, H 5.4, N 12.7.
1-(2-Bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-5-(4-methoxyphenyl)-4-methyl-1H-pyrazole-3-carboxamide (9m)
The procedure described for the synthesis of 9a was applied to 8o and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to give 9m (87 mg, 34% from acid) as a white solid. Mp 208–210 °C; 1H NMR δ 7.62 (dd, J = 6.7 and 1.0 Hz, 1H), 7.37–7.23 (m, 3H), 7.09 (s, 1H), 7.08 (dt, J = 8.8 and 1.6 Hz, 2H), 6.82 (dt, J = 8.8 and 1.6 Hz, 2H), 3.99 (d, J = 8.1 Hz, 2H), 3.85 (t, J = 10.6 Hz, 2H), 3.77 (s, 3H), 2.54 (d, J = 13.4 Hz, 2H), 2.38 (s, 3H), 2.06 (t, J = 10.0 Hz, 3H); 13C NMR δ 162.41, 159.70, 144.30, 143.16, 133.60, 131.07, 130.81, 130.02, 128.08, 122.10, 120.88, 119.03, 113.89, 63.81, 55.21, 49.15, 35.65, 9.48; HRMS (m/z) [M+H]+, calc’d for C24H2479BrN4O3, 495.1032; found 495.1029, error −0.6 ppm; Anal. calc’d for C24H23BrN4O3: C 58.2, H 4.7, N 11.3; found C 58.2, N 4.8, H 11.2.
1-(2-Bromophenyl)-N-(1-cyanocyclohexyl)-5-(4-methoxyphenyl)-4-methyl-1H-pyrazole-3-carboxamide (9n)
The procedure described for the synthesis of 9a was applied to 8o to afford 9n (36% from acid) as a white solid. Mp = 220–222 °C; 1H NMR δ 7.61 (dd, J = 6.7 and 1.0 Hz, 1H), 7.35–7.24 (m, 3H), 7.07 (dt, J = 8.8 and 1.6 Hz, 2H), 7.01 (s, 1H), 3.77 (s. 3H), 2.51 (bs, 2H), 2.39 (s, 3H), 1.77–1.71 (m, 8H), 1.15 (bs, 1H); 13C NMR δ 162.22, 158.67, 144.12, 143.49, 138.98, 133.56, 132.84, 131.06, 130.70, 130.06, 128.05, 122.14, 119.90, 117.99, , 114.56, 68.74, 51.39, 35.64, 24.84, 22.15, 9.55; HRMS (m/z) [M+H]+, calc’d for C25H2679Br N4O2, 493.1239; found 493.1235, error −0.8 ppm; Anal. calc’d for C25H25BrN4O2: C 60.9, H 5.1, N 11.4; found C 60.8, H 5.4, N 11.2.
1-(3-Bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-5-(4-methoxyphenyl)-4-methyl-1H-pyrazole-3-carboxamide (9o)
The procedure described for the synthesis of 9a was applied to 8j and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9o (53% from acid) as a white solid. Mp 160–162 °C; 1H NMR δ 7.58 (bs, 1H), 7.45 (d, J = 8.4 Hz, 1H), 7.15–7.11 (m, 2H), 7.09 (d, J = 8.8 Hz, 2H), 7.00 (d, J = 8.4 Hz, 1H), 6.92 (d, J = 8.4 Hz, 2H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.88–3.83 (m, 5H), 3.79 (s, 3H), 2.57 (d, J = 12.8 Hz, 2H), 2.35 (s, 3H), 2.10–2.04 (m, 2H); HRMS (tof): [M+H]+, calc’d for C24H2479BrN4O3, 495.1028; found, 495.1020, error 1.4 ppm; Anal. calc’d for C24H23BrN4O3: C 58.2, H 4.7, N 11.3; found C 58.2, H 4.8, N 11.2.
4-Cyano-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-1-(2-iodophenyl)-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxamide (9p)
The procedure described for the synthesis of 9a was applied to 8q and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9p (68% from acid) as a white solid. Mp 232–214 °C; 1H NMR δ 7.95 (dd, J = 1.2 and 8.0 Hz, 1H), 7.52 (td, J = 1.2 and 7.2 Hz, 1H), 7.38 (dd, J = 1.6 and 7.6 Hz, 1H), 7.29–7.22 (m, 3H), 6.95 (s, 1H), 6.88 (dt, J = 2.8 and 9.2 Hz), 4.02 (dt, J = 3.2 and 12.8 Hz, 2H), 3.86 (td, J = 2.0 and 12.4 Hz, 2H), 2.58 (d, J = 12.8 Hz, 2H), 2.09–2.04 (m, 2H); HRMS (tof): [M+Na]+, calc’d for C24H20IN5NaO3, 576.0503; found, 576.0483, error 3.5 ppm; Anal. calc’d for C24H20IN5O3: C 52.1, H 3.6, N 12.7; found C 52.0, H 3.7, N 12.7.
4-Bromo-1-(3-bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxamide (9q)
The procedure described for the synthesis of 9a was applied to 8l and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9q (56% from acid) as a solid. Mp 182–184 °C; 1H NMR δ 7.58 (t, J = 1.6 Hz, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.21–7.15 (m, 3H), 7.07 (s, 1H), 7.02 (d, J = 8.0 Hz, 1H), 6.94 (dt, J = 2.8 and 8.8 Hz, 2H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.88–3.83 (m, 5H), 3.79 (s, 3H), 2.57 (d, J = 12.8 Hz, 2H), 2.35 (s, 3H), 2.10–2.04 (m, 2H); HRMS (tof): [M+Na]+, calc’d for C23H2079Br2N4NaO3, 580.9794; found, 580.9774, error 3.5 ppm; Anal. calc’d for C23H20Br2N4O3: C 49.3, H 3.6, N 10.0; found C 49.2, H 3.6, N 9.8.
1-(2-Bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-5-(4-fluorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (9r)
The procedure described for the synthesis of 9a was applied to 8m and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9r (54%) as a solid. Mp 188–190 °C; 1H NMR δ 7.62 (d, J = 6.4 Hz, 1H), 7.40–7.28 (m, 3H), 7.15–7.11 (m, 2H), 7.09 (s, 1H), 7.02 (t, J = 9.6 Hz, 2H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.88–3.83 (m, 5H), 3.79 (s, 3H), 2.56 (d, J = 12.8 Hz, 2H), 2.38 (s, 3H), 2.07–2.00 (m, 2H); HRMS (tof): [M+H]+, calc’d for C23H2179BrFN4O2, 483.0826; found, 483.0821, error 1.2 ppm; Anal. calc’d for C23H20BrFN4O2) C 57.2, H 4.2, N 11.6; found C 57.2, H 4.2, N 11.4.
1-(2-Bromophenyl)-N-(4-cyano-tetrahydro-2H-pyran-4-yl)-5-(3-fluorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (9s)
The procedure described for the synthesis of 9a was applied to 8n and 4-amino-tetrahydro-2H-pyran-4-carbonitrile to yield 9s (62% from acid) as a solid. Mp 204–206 °C; 1H NMR δ 7.64 (d, J = 8.0 Hz, 1H), 7.41–7.25 (m, 4H), 7.09 (s, 1H), 7.04 (td, J = 2.4 and 8.4 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 6.88 (dt, J = 2.0 and 9.6 Hz, 1H), 4.01 (dt, J = 3.2 and 12.8 Hz, 2H), 3.88–3.83 (m, 5H), 3.79 (s, 3H), 2.56 (d, J = 12.8 Hz, 2H), 2.38 (s, 3H), 2.07–2.00 (m, 2H); HRMS (tof): [M+Na]+, calc’d for C23H2079BrFNaN4O2, 505.0646; found, 505.0623, error 4.6 ppm; Anal. calc’d for C23H20BrFN4O2: C 57.2, H 4.2, N 11.6; found C 57.3, H 4.3, N 11.6.
Computation of cLogD
The lipophilicity of each synthesized ligand, expressed as the partition of the compound between n-octanol and buffer at pH 7.4 (cLogD) was computed with Pallas 3.70 software (Compudrug, South San Francisco, CA).
Receptor assays
Ligands 9a–s were tested for affinity (Ki values) at GABAA-Bz site, TSPO, and CB1, and CB2 receptors by the National Institute of Mental Health Psychoactive Drug Screening Program. Detailed protocols are available on-line for all binding assays at NIMH-PDSP web site (http://pdsp.med.unc.edu).
Receptor screening
Ligands 9m and 9n were screened for binding to a wide range of receptors and transporters by National Institute of Mental Health Psychoactive Drug Screening Program. Detailed protocols are available on-line for all binding assays at NIMH-PDSP web site (http://pdsp.med.unc.edu).
Acknowledgements
VWP and SRD were supported by the Intramural Research Program of NIH (NIMH) (project # Z01-MH-002795); specifically SRD was initially supported by the National Institute of Mental Health (NIMH), through a studentship to SRD under the NIH-Karolinska Institutet Graduate Partnership in Neuroscience. SRD was subsequently supported by Hoffmann–La Roche and The Johns Hopkins PET Center through a postdoctoral fellowship. We are grateful to the NIMH Psychoactive Drug Screening Program (PDSP) for pharmacological assays and screens. The PDSP is directed by Bryan L. Roth, PhD with project officer Jamie Driscol (NIMH), at the University of North Carolina at Chapel Hill (contract # NO1MH32004).
Footnotes
Abbreviations: CB1, cannabinoid type-1, CB2, cannabinoid type-2; D, dopamine; DAT, dopamine transporter; DCM, dichloromethane; DIPEA, diisopropylethylamine; DMF, dimethylformamide; DOR, δ opiate receptor; GABAA-Bz site, γ-aminobutyric acidA-benzodiazepine site; GPCR, G-protein coupled receptor; H, histamine; 5-HT, 5-hydroxytryptamine; KOR, κ opiate receptor; MeCN, acetonitrile; MOR, μ opiate receptor; NBS, N-bromosuccinimide; NCA, no-carrier added; NET noradrenaline transoporter; PET, positron emission tomography; PBR, peripheral benzodiazepine receptor; rt, room temperature; SERT, serotonin transporter; THF tetrahydrofuran; Δ9-THC, Δ9-tetrahydrocannabinol.
References
- 1.Lambert DM. Medical use of cannabis through history. J. Pharm. Belg. 2001;56:111–118. [PubMed] [Google Scholar]
- 2.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. [Google Scholar]
- 3.Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 4.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;364:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 5.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 6.Wilson R, Nicoll R. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 7.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J. Pharmacol. Exp. Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 8.Black SC. Cannabinoid receptor antagonists and obesity. Curr. Opin. Investig. Drugs. 2004;5:389–394. [PubMed] [Google Scholar]
- 9.Serra G, Fratta W. A possible role for the endocannabinoid system in the neurobiology of depression. Clin. Prac. Epidem. Ment. Health. 2007;3:25–36. doi: 10.1186/1745-0179-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neurosci. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 11.Horti AG, Van Laere K. Development of radioligands for in vivo imaging of type 1 cannabinoid receptors (CB1) in human brain. Curr. Pharm. Design. 2008;14:3363–3383. doi: 10.2174/138161208786549380. [DOI] [PubMed] [Google Scholar]
- 12.Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara T, Soubrié P, Breliere JC, Le Fur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 13.Lange JHM, Krause CG. Medicinal chemsitry strategies to CB1 cannabinoid receptor antagonists. Drug Discovery Today. 2005;10:693–702. doi: 10.1016/S1359-6446(05)03427-6. [DOI] [PubMed] [Google Scholar]
- 14.Pike VW. Positron-emitting radioligands for studies in vivo – probes for human psychopharmacology. J. Psychopharmacol. 1993;7:139–158. doi: 10.1177/026988119300700202. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003;5:376–389. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Laruelle M, Slifstein M, Huang Y. Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol. Imaging Biol. 2003;5:363–375. doi: 10.1016/j.mibio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Patel S, Gibson R. In vivo site-directed radiotracers; a mini-review. Nucl. Med. Biol. 2008;35:805–815. doi: 10.1016/j.nucmedbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Pike VW. PET Radiotracers: crossing the blood-brain barrier and surviing metabolism. Trends in Pharmacological Science. 2009;30:431–440. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevera M, Ye WG, Dannals RF, Ravert HT, Nandi A, Rahmin A, Ming JE, Grachev I, Roy C, Casella N. Quantification of cerebral cannabinoid receptors subtype 1(CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 2010;52:1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donohue SR, Krushinski JH, Pike VW, Chernet E, Phebus L, Chesterfield AK, Felder CC, Halldin C, Schaus JM. Synthesis, ex vivo evaluation and radiolabeling of potent 1,5-diphenyl-pyrrolidin-2-one cannabinoid subtype-1 (CB1) receptor ligands as candidates for in vivo imaging. J. Med. Chem. 2008;51:5833–5842. doi: 10.1021/jm800416m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry GE, Liow J-S, Zoghbi SS, Hirvonen J, Farris AG, Lerner A, Tauscher JT, Schaus JM, Phebus L, Felder CC, Morse CL, Hong JS, Pike VW, Halldin C, Innis RB. Quantitation of cannabinoid CB1 receptors in healthy human brain using positron emission tomography and an inverse agonist radioligand. NeuroImage. 2009;48:362–370. doi: 10.1016/j.neuroimage.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terry GE, Hirvonen J, Liow J-S, Zoghbi SS, Gladding R, Tauscher JT, Schaus JM, Phebus L, Felder CC, Morse CL, Donohue SR, Pike VW, Halldin C, Innis RB. Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brain using 18F-labeled inverse agonist radioligands. J. Nucl. Med. 2010;51:112–120. doi: 10.2967/jnumed.109.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns HD, Van Laere K, Sanabria-Bohórquez S, Hamill TG, Bormans G, Eng W, Gibson R, Ryan C, Connolly B, Patel S, Krause S, Vanko A, Van Hecken A, Dupont P, De Lepeleire I, Rothenberg P, Stoch SA, Cote J, Hagmann WK, Jewell JP, Lin LS, Liu P, Goulet MT, Gottesdiener K, Wagner JA, de Hoon J, Mortelmans L, Fong TM, Hargreaves RJ. Proc. Natl. Acad. Sci. (USA) 2007;104:9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming FF, Yai L, Ravikumar PC, Funk L, Shook BC. Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. J. Med. Chem. 2010;53:7902–7917. doi: 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q, Ma P, Wang W, Cole RB, Wang G. In vitro metabolism of diarylpyrazoles, a novel group of cannabinoid receptor liagnds. Drug Metab. Disp. 2005;33:508–517. doi: 10.1124/dmd.104.001974. [DOI] [PubMed] [Google Scholar]
- 26.Albert JJ, Li W, Behnia K, Zhang LM, Johnghar S, Humphreys WG, Zadjura L, Davis CD, Santone KS, Hunag S, Liu X, Kang L, Carlson KE, Wu ST, Shu Y-Z. In vitro and in vivo metabolism of rimonabant (SR-141716), a cannabinoid (CB1) receptor antagonist. Drug Metab. Rev. 2003;35 Suppl. 2:38–38. [Google Scholar]
- 27.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang M, Gavish M. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Li ZB, Conti PS. Radiopharmaceutical chemistry for positron emission tomography. Adv. Drug Delivery Rev. 2010;62:1031–1051. doi: 10.1016/j.addr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 29.White WA, Weingarten H. A versatile new enamine synthesis. J. Org. Chem. 1967;32:213–214. [Google Scholar]
- 30.Donohue SR, Halldin C, Pike VW. A facile and regioselective synthesis of rimonabant through an enamine-directed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2008;49:2789–2791. [Google Scholar]
- 31.Bourguignon J-J. Endogenous and synthetic ligands of mitochondrial benzodiazepine receptors: structure–affinity relationships. In: Giesen-Crouse E, editor. Peripheral Benzodiazepine Receptors. Ch. 3. London: Academic Press Ltd.; 1983. pp. 59–85. [Google Scholar]
- 32.Schweitzer PJ, Fallon BA, Mann JJ, Kumar JSD. PET tracers for the peripheral benzodiazepine receptor and uses thereof. Drug Discovery Today. 2010;15:933–942. doi: 10.1016/j.drudis.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Campiani G, Nacci V, Fiorini I, De Fillippis MP, Garofalo A, Ciani SM, Greco G, Novellino E, Williams DC, Zisterer DM, Woods MJ, Mihai C, Manzoni C, Mennini T. Synthesis, biological activity and SARs of pyrrolobenzoxazepine derivatives, a new class of specific ‘peripheral-type’ benzodiazepine receptor ligands. J. Med. Chem. 1996;39:3435–3450. doi: 10.1021/jm960251b. [DOI] [PubMed] [Google Scholar]
- 34.Primofiore G, Da Settimo F, Taliani S, Simorini F, Patrizi MP, Novellino E, Greco G, Abignente E, Costa B, Chelli B, Martini C. N,N-Dialkyl-2-phenylindol-3-ylglyoxylamides. A new class of potent and selective ligands at the peripheral benzodiazepine receptor. J. Med. Chem. 2004;47:1852–1855. doi: 10.1021/jm030973k. [DOI] [PubMed] [Google Scholar]
- 35.Da Settimo F, Simorini F, Taliani S, La Motta C, Marini AM, Salerno S, Bellandi M, Novellino E, Greco G, Cosimelli B, Da Pozzo E, Simola N, Morelli M, Martini C. Anxiolytic effects of N,N-dialkyl-2-phenylindol-3-ylglyoxylamides by modulation of translocator protein promoting neurosteroid biosynthesis. J. Med. Chem. 2008;51:5798–5806. doi: 10.1021/jm8003224. [DOI] [PubMed] [Google Scholar]
- 36.Pike VW, Taliani S, Lohith TG, Owen DRJ, Pugliesi I, Da Pozzo E, Hong J, Zoghbi SS, Gunn RN, Parker CA, Rabiner EA, Fujita M, Innis RB, Martini C, Da Settimo F. Evaluation of novel N1-methyl-2-phenylindol-3-ylglyoxylamides as a new chemotype of 18 kDa translocator protein-selective ligand suitable for the development of positron emission tomography radioligands. J. Med. Chem. 2011;54:366–373. doi: 10.1021/jm101230g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan H, Ravert HT, Holt DP, Dannals RF, Horti AG. Synthesis of 1-(2,4-dichlorophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide ([11C]JHU75528) and 1-(2-bromophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-N-piperidin-1-yl)-1H-pyrazole-3-carboxamide ([11C]JHU75575) as potential radioligands for PET imaging of cerebral cannabinoid receptor. J. Label. Compd. Radiopharm. 2006;49:1021–1036. [Google Scholar]
- 38.Donohue SR, Halldin C, Pike VW. Synthesis and structure-activity relationships (SARs) of 1,5-diarylpyrazole cannabinoid (CB1) receptor ligands for potential use in molecular imaging. Bioorg. Med. Chem. 2006;14:3712–3720. doi: 10.1016/j.bmc.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 39.Donohue SR, Varnäs K, Jia Z, Gulyás B, Pike VW, Halldin C. Synthesis and in vitro autoradiographic evaluation of a novel high-affinity radioiodinated ligand for imaging brain cannabinoid subtype-1 receptors. Bioorg. Med. Chem. Lett. 2009;19:6209–6212. doi: 10.1016/j.bmcl.2009.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]