Abstract
Objective
Cyclophilin A (CyPA, encoded by Ppia) is a pro-inflammatory protein secreted in response to oxidative stress in mice and humans. We recently demonstrated that CyPA increased angiotensin II (AngII)-induced reactive oxygen species (ROS) production in the aortas of Apoe−/− mice.
In this study we sought to evaluate the role of CyPA in AngII–induced cardiac hypertrophy.
Methods and Results
Cardiac hypertrophy was not significantly different between Ppia+/+ and Ppia−/− mice infused with AngII (1000 ng/min/kg for 4 weeks). Therefore, we investigated the effect of CyPA under conditions of high ROS and inflammation using the Apoe−/− mice. In contrast to Apoe−/− mice, Apoe−/− Ppia−/− mice exhibited significantly less AngII-induced cardiac hypertrophy. Bone marrow cell transplantation showed that CyPA in cells intrinsic to the heart plays an important role in the cardiac hypertrophic response. AngII-induced ROS production, cardiac fibroblast proliferation and migration were markedly decreased in Apoe−/− Ppia−/− cardiac fibroblasts. Furthermore, CyPA directly induced the hypertrophy of cultured neonatal cardiac myocytes.
Conclusions
CyPA is required for AngII-mediated cardiac hypertrophy by directly potentiating ROS production, stimulating the proliferation and migration of cardiac fibroblasts, and promoting cardiac myocyte hypertrophy.
Keywords: oxidative stress, cardiac remodeling, cardiac hypertrophy
Introduction
Cardiac hypertrophy is a fundamental response of cardiac cells to common clinical disorders such as arterial hypertension, valvular heart disease, myocardial infarction, cardiomyopathy, and congenital heart disease.1 Emerging data reveal that the communication between cardiac fibroblasts and myocytes plays a critical role in cell-cell signaling in the heart and it is implicated in the process of cardiac remodeling and overall heart function during development and cardiopathology.2–5 There are numerous lines of evidence indicating that cardiac fibroblasts and myocytes release into their local microenvironment proteins that regulate neighboring cells via paracrine mechanisms.2–5 Although multiple factors have been implicated in this intercellular crosstalk, the discovery of new hypertrophic players and a better understanding of the underlying mechanisms hold the key to successful therapy of hypertrophic heart disease.
Cyclophilin A (CyPA, encoded by Ppia) was originally found as a binding partner of cyclosporine A (CsA).6 Intracellular CyPA is a chaperone protein that has several functions including peptidyl-prolyl isomerase (PPIase) activity and protein trafficking such as nuclear translocation of ERK1/27 and apoptosis-inducing factor (AIF).8 We and others have provided evidence that CyPA is secreted in response to reactive oxygen species (ROS) from vascular smooth muscle cells (VSMC)9 and rat neonatal cardiac myocytes.10 Moreover, we have shown the involvement of CyPA in angiotensin II (AngII)-induced aortic aneurysms and oxidative stress.11, 12
AngII plays a key role in many physiological and pathological processes in cardiac cells, including cardiac hypertrophy.13 Therefore, understanding the molecular mechanisms responsible for AngII-mediated myocardial pathophysiology will be critical to developing new therapies for cardiac dysfunction.14 One important mechanism now recognized to be involved in AngII-induced cardiac hypertrophy is ROS production,15, 16 but the precise mechanism by which ROS cause hypertrophy remains unknown. Our recent study provides strong mechanistic evidence of synergy between CyPA and AngII to increase ROS generation.11
Since ROS stimulate myocardial hypertrophy, matrix remodeling, and cellular dysfunction,17 we tested the hypothesis that CyPA enhances AngII-induced cardiac ROS production, and therefore cardiac hypertrophy.
Methods
An expanded Methods section is available online at http://atvb.ahajournals.org.
Analysis of cardiac hypertrophy
AngII-infused models were employed to assess the effect of CyPA deficiency on cardiac hypertrophy.18 Five- to 6 week old male mice on a normal chow diet were infused with 1000 ng/min/kg AngII (MP Biomedicals, Solon, Ohio, USA) or saline vehicle for 4 weeks. AngII was dissolved in sterile saline and infused using Alzet osmotic pumps (Model 2004, DURECT Corp., Cupertino, California, USA). Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg). Pumps were placed into the subcutaneous space of anesthetized mice through a small incision in the back of the neck that was closed with suture. After 4 weeks of AngII infusion, the animals were again anesthetized with ketamine (80 mg/kg) and xylazine (5 mg/kg). The heart tissue was perfused with calcium-and magnesium-free phosphate-buffered saline (PBS) and then fixed with phosphate-buffered 10% formalin solution and subsequently prepared for histological analysis as previously described.19 Heart weight was measured, and the ratio of heart weight to body weight was calculated to determine an index of cardiac hypertrophy. Five sections were obtained from each heart and mounted on slides and stained with Masson’s Trichrome or H&E. To evaluate the perivascular fibrosis, short-axis images of coronary arteries were scanned at x200 magnification. The area of perivascular fibrosis (the ratio of the fibrosis area surrounding the vessel to the total vessel area) was calculated. To evaluate the extent of cardiac myocyte hypertrophy, cross-sectional images of cardiac myocytes were scanned at x400 magnification. Approximately 10 cross-sections of cardiac myocytes were analyzed in each heart. Average values for each heart were used for analysis.
Bone marrow transplantation
Bone marrow transplantation was performed as described.20 Briefly, recipient mice were lethally irradiated and received an intravenous injectionof 5×106 donor bone marrow cells suspended in 100 μl PBS with 2% fetal bovineserum (FBS). After transplantation, the mice were placed on a regular chow diet for 6 weeks followed by infusion of 1000 ng/kg/min AngII for 4 weeks. Transgenic mice ubiquitously expressing green fluorescentprotein (GFP) were obtained from Jackson Laboratory (Bar Harbor, Me). The chimeric rate assessed by GFP+ cells in the peripheral blood was more than 99% by fluorescence-activated cell sorter analysis (FACS CantoII, Becton Dickinson, San Jose, CA).
ROS analysis
The evaluation of ROS production in response to AngII was performed as described before.21 After treatment with AngII (1 μM), cardiac fibroblasts were washed with PBS and loaded with 2,7-dichlorofluoresceine diacetate (H2DCF-DA) (5 μM; Molecular Probes) for 30 min. Hearts were perfused with PBS (pH 7.4) at 100 mmHg for 5 minutes at 4°C. Heart tissue was harvested, and embedded in OCT (Tissue-Tek; Miles Inc., Elkhart, Illinois, USA) and snap-frozen. Dihydroethidine hydrochloride (5μM, Molecular Probes) was topically applied to the freshly cut frozen heart sections (10μm) for 30 min at 37°C. DCF and DHE fluorescence was revealed by confocal microscopy (Olympus, FLUOVIEW).22
Lucigenin assay was performed as previously described with some modifications.23 Briefly, control cells or cells treated with Ang II for 4 hours were harvested and pelleted by centrifugation (1200 rpm, 4°C, 5 minutes). To start the assay, cells were resuspended in a Hank’s balanced salt solution (cellegro, VA) containing lucigenin (final concentration, 500, μmol/L). Photon emission was measured every 15 seconds for 20 minutes in a luminometer (Wallac Plate Reader, model 1420). A buffer blank (<5% of the cell signal) was subtracted from each reading before transformation of the data.
[3H]-Leucine incorporation study
The effect of conditioned medium from AngII-stimulated cardiac fibroblasts for protein synthesis in cardiac myocytes was determined by [3H]-Leucine incorporation as previously described.24 Briefly, neonatal rat cardiac myocytes were plated at a density of 250,000 cells/well in 12-well plates and maintained in DMEM supplemented with ITS, 5% Horse Serum, 5% NCS, 50 units/ml penicillin, and 50 μg/ml streptomycin for 24 hours. The cells were then starved in serum-free DMEM for 48 hours. Cardiac myocytes were stimulated with cardiac fibroblast-derived conditioned medium (CM) for 24 hours, following incubation with [3H]-Leucine (2 μCi/ml) for 24 hours. To precipitate proteins, ice-cold 10% trichloroacetic acid (TCA) was added to the wells. After 30 min of incubation on ice, the myocytes were lysed with 0.5 N NaOH and incubated for 10 min on ice. All samples were mixed with scintillation cocktail (Biosafe-II) before counting.
Statistical analysis
Quantitative results are expressed as mean ± SD. Comparisons of parameters among 2 groups were made by the unpaired Student’s t-test. Comparisons of parameters among >2 groups were made by one-way analysis of variance (ANOVA), and comparisons of different parameters between the 2 genotypes were made by two-way analysis of variance (ANOVA), followed by a post hoc analysis using the Bonferroni test. Statistical significance was evaluated with StatView (StatView 5.0, SAS Institute Inc. Cary, NC). A value of *P <0.05 was considered to be statistically significant.
Results
CyPA augments AngII-induced cardiac hypertrophy in vivo
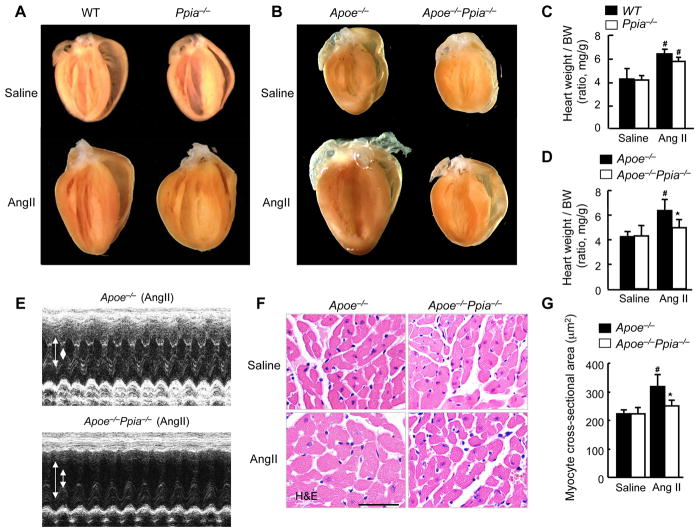
To define the role of CyPA in cardiac hypertrophy, we studied the Ppia−/− (knockout) and wild-type (WT) mice following AngII infusion for 4 weeks. AngII significantly increased heart weight/body weight (BW) in both Ppia−/− and WT mice (Figure 1A and 1C). However, there were no significant differences in heart weight/BW (Figure 1A and 1C), systolic blood pressure, and interventricular septum (IVS) thickness (Supplemental Table I) between Ppia−/− and WT mice before or after AngII treatment. While there was an apparent increase in echo-estimated LV mass in the WT compared to Ppia−/−, when gravimetric heart was normalized to the BW the relative increase in heart weight/BW did not differ (1.25 fold versus 1.20 fold).
Figure 1.
CyPA deficiency prevents AngII-induced cardiac hypertrophy in Apoe−/− mice. (A) Representative photographs showing macroscopic features of cardiac hypertrophy induced by AngII infusion in Ppia−/− mice versus WT mice. AngII-induced cardiac hypertrophy is not prevented in Ppia−/− mice compared with WT mice. (B) Representative photographs of the hearts of Apoe−/− and Apoe−/− Ppia−/− mice infused with AngII or saline for 4 weeks. AngII-induced cardiac hypertrophy is prevented in Apoe−/− Ppia−/− mice compared with Apoe−/− mice. (C) AngII-induced cardiac hypertrophy (heart weight per body weight ratio, HW/BW) was not significantly decreased in Ppia−/− (n = 5) compared with WT (n = 6). # equals P< 0.05 in Saline versus AngII; *equals P< 0.05 in WT versus Ppia−/− mice. (D) AngII-induced cardiac hypertrophy (heart weight per body weight ratio) was significantly reduced in Apoe−/− Ppia−/− mice (n = 11) compared with Apoe−/− mice (n = 15). No significant differences were found in the control groups (saline infusion) of Apoe−/− and Apoe−/− Ppia−/− mice (n=4, respectively). (E) Representative M-mode images of cardiac hypertrophy assessed by echocardiography after 4 weeks of AngII infusion. Arrows show the diastolic left ventricle (LV) cavity and systolic LV cavity. (F) H&E staining of representative cross-sections of cardiac myocytes of Apoe−/− and Apoe−/− Ppia−/− mice 4 weeks after AngII infusion. (G) Myocyte cross-sectional area was significantly reduced in Apoe−/− Ppia−/− mice (n = 7) compared with Apoe−/− mice (n = 9). Results are mean ± SD. # equals P< 0.05 in Saline versus AngII; *equals P< 0.05 in Apoe−/− versus Apoe−/− Ppia−/− mice.
Because CyPA is a pro-inflammatory cytokine secreted in response to oxidative stress, we hypothesized that the role of CyPA in cardiac hypertrophy would require a situation in which there was increased ROS generation or inflammation. Previously it was demonstrated that the hearts of Apoe−/− mice exhibit increased ROS production.11 Since we showed that ROS generation stimulates secretion of CyPA from VSMC, we compared the secretion of CyPA from WT and Apoe−/− cardiac fibroblasts in response to AngII. Secretion of CyPA was barely detected in conditioned media (CM) from WT fibroblasts (Supplemental Figure I). In contrast, there was abundant CyPA secretion from AngII-treated Apoe−/− cardiac fibroblasts. We attempted similar experiments in cultured adult cardiac myocytes. However, substantial cell death ensued upon culture in the required serum free medium.
The above data suggest that the Apoe−/− mice would provide an ideal model to study the role of CyPA in AngII-induced cardiac hypertrophy. Consistent with previous findings,18 we showed that AngII infusion for 4 weeks significantly increased cardiac hypertrophy in Apoe−/− mice (Figure 1B and 1D). Despite equal increases in blood pressure (Supplemental Figure IIA), Apoe−/− Ppia−/− mice had significantly smaller increases in heart weight after treatment with AngII compared to Apoe−/− mice (Figure 1B and 1D). Echocardiography showed no significant difference in LV mass and IVS thickness between Apoe−/− and Apoe−/− Ppia−/− mice before AngII treatment (Supplemental Table II). However, after AngII infusion, Apoe−/− Ppia−/− mice had significantly smaller increases in LV mass and IVS thickness compared to Apoe−/− mice (Figure 1E, Supplemental Figure II, B–C). Moreover, in Apoe−/− mice, AngII significantly increased cardiac myocyte size compared with control (saline-infused mice) (Figure 1F and 1G). In contrast, in Apoe−/− Ppia−/− mice, AngII–induced cardiac myocyte hypertrophy was significantly reduced (Figure 1F and 1G). These results suggest that CyPA is required for cardiac hypertrophy induced by AngII in Apoe−/− mice.
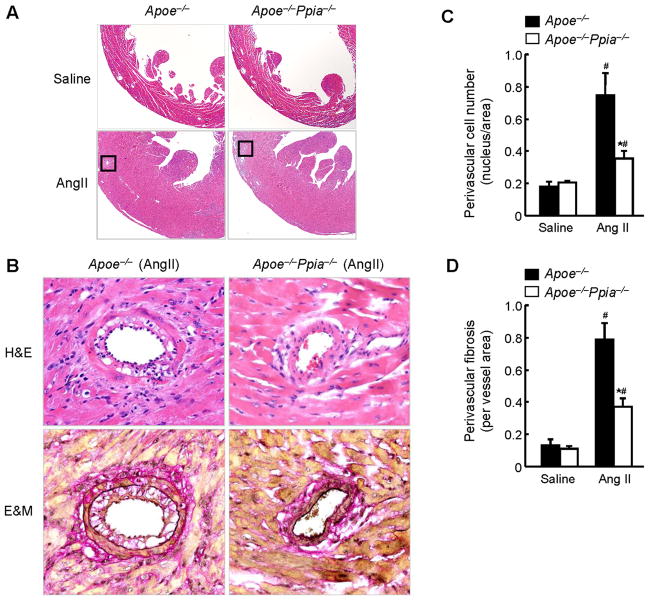
Morphologically, the hearts of saline-infused Apoe−/− Ppia−/− mice did not differ from the hearts of Apoe−/− mice as shown by H&E staining (Figure 2A). In response to AngII, LV wall thickness significantly increased in Apoe−/− and Apoe−/− Ppia−/− mice, although to a lesser extent in Apoe−/− Ppia−/− mice (Figure 2A). Most impressively there was an obvious reduction in perivascular cell number, suggesting decreased proliferation or migration of cells to the perivascular area in the Apoe−/− Ppia−/− mice (Figure 2B and 2C). There was also a decrease in collagen content in the perivascular area as shown by Elastica & Masson (E&M) staining (Figure 2B) in the Apoe−/− Ppia−/− mice after AngII treatment. Perivascular fibrosis area was markedly decreased in the coronary arteries of Apoe−/− Ppia−/− mice (Figure 2D). These data suggest that CyPA contributes to cardiac hypertrophy and perivascular fibrosis in Apoe−/− mice.
Figure 2.
CyPA deficiency reduces AngII-induced perivascular cell number and fibrosis. (A) Representative H&E staining of hearts from Apoe−/− and Apoe−/− Ppia−/− mice infused with saline or AngII for 4 weeks. (B) Representative H&E and Elastica Masson (E&M) staining of coronary arteries from Apoe−/− and Apoe−/− Ppia−/− mice infused with AngII for 4 weeks. Elastic fibers stain black and collagen fibers stain red. (C) Statistical analysis of the number of cells in the perivascular area in Apoe−/− (n = 9) and Apoe−/− Ppia−/− (n = 7) mice. (D) Statistical analysis of the perivascular fibrotic area per total vascular area in Apoe−/− (n = 9) and Apoe−/− Ppia−/− (n = 7) mice. Results are mean ± SD. # equals P< 0.05 in Saline versus AngII; *equals P< 0.05 in Apoe−/− versus Apoe−/− Ppia−/− mice.
CyPA deficiency prevents AngII-induced ROS production in cardiac tissue
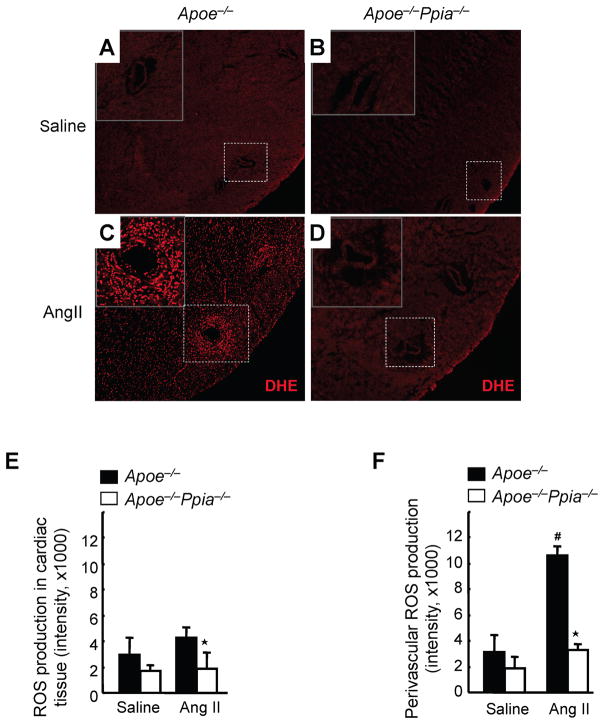
Because ROS are key mediators of AngII action, we next investigated whether CyPA altered the redox state of the heart after AngII treatment. Heart sections were incubated with dihydroethidium (DHE), which in the presence of superoxide anions is transformed to fluorescent oxy-ethidium. In the saline-infused heart, ROS production (red fluorescence) was low in both Apoe−/− and Apoe−/− Ppia−/− mice (Figure 3A, 3B and 3E). After AngII treatment, in the whole heart, oxy-ethidium fluorescence was ~2-fold greater in Apoe−/− mice compared with Apoe−/− Ppia−/− mice (Figure 3C, 3D and 3E). In the perivascular area of Apoe−/− mice, ROS production was increased by 4-fold after AngII-treatment (Figure 3C and 3F). In contrast, in Apoe−/− Ppia−/− mice, the perivascular increase in ROS was markedly reduced (Figure 3D and 3F). These data suggest that CyPA is a key determinant for AngII-mediated ROS production.
Figure 3.
CyPA is crucial for cardiac ROS formation. (A–D) Representative in situ dihydroethidium (DHE) staining of hearts. The hearts from Apoe−/− and Apoe−/− Ppia−/− mice infused with saline or AngII for 7 days were used for analysis. Images were obtained using the same magnification (x 100) and shutter speed. (E–F) Densitometric analysis of oxy-ethidium (red fluorescence) in the cardiac tissue (E) and the perivascular area (F). Results are mean ± SD (n = 4 in each group). # equals P< 0.05 in Saline versus AngII; *equals P< 0.05 in Apoe−/− versus Apoe−/− Ppia−/− mice.
Cardiac-derived CyPA promotes recruitment of bone marrow-derived cells
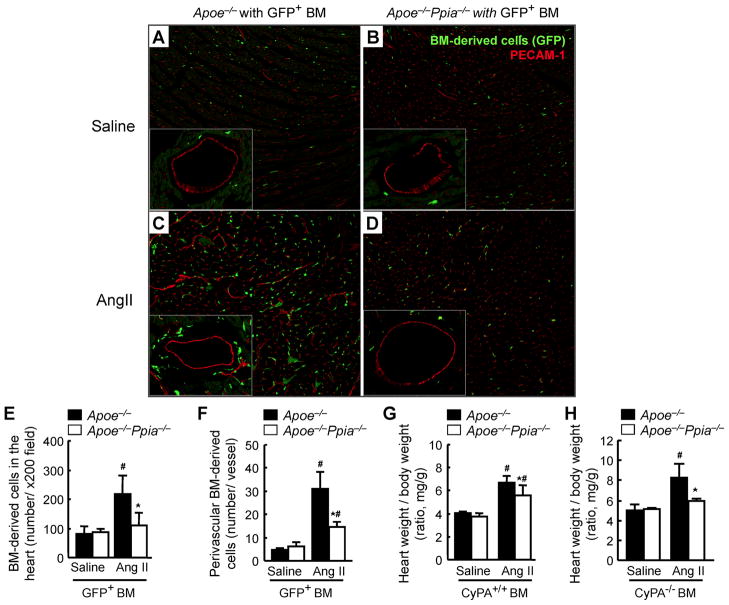
We have shown that CyPA has direct chemotactic effects on bone marrow-derived cells and promotes vascular cell proliferation and remodeling.19 To determine whether cardiac CyPA promotes recruitment of bone marrow-derived cells and mediates AngII-induced cardiac hypertrophy, GFP+ bone marrow cells (Ppia+/+) were transplanted into irradiated Apoe−/− or Apoe−/− Ppia−/− mice. After 42 days, these chimeric mice were treated with AngII for 4 weeks. There was no significant difference in the blood pressure between chimeric mice before and after AngII infusion (data not shown). AngII dramatically increased the number of bone marrow-derived cells (GFP+ cells) present in the cardiac tissue in the Apoe−/− recipient mice (Figure 4C and 4E). Most significant was the accumulation of bone marrow-derived cells in the perivascular area of Apoe−/− recipient mice (Figure 4C and 4F), which was significantly reduced in the perivascular area of Apoe−/− Ppia−/− recipient mice (Figure 4D and 4F). Since many of these bone marrow-derived cells are inflammatory cells, and inflammation is associated with increased ROS, it is possible that they will contribute to the elevated ROS observed in the perivascular area.
Figure 4.
Bone marrow reconstitution shows a strong effect of cardiac CyPA for recruitment of bone marrow-derived cells in response to AngII, and development of cardiac hypertrophy. GFP+ bone marrow cells (Ppia+/+) were transplanted into irradiated Apoe−/− or Apoe−/− Ppia−/− mice. After 6 weeks, these chimeric mice with GFP+ bone marrow were infused with saline (A–B) or AngII for 4 weeks (C–D). (A–D) Representative PECAM-1 staining (red) of hearts from Apoe−/− and Apoe−/− Ppia−/− recipient mice with GFP+ bone marrow (green). Statistical analysis of the number of migrating GFP+ bone marrow in the cardiac tissue (E) or perivascular area (F) in the hearts of Apoe−/− (n = 9) and Apoe−/− Ppia−/− (n = 8) mice. (G) AngII-induced cardiac hypertrophy (heart weight per body weight ratio) was significantly less in Apoe−/− Ppia−/− recipient mice (n = 9) compared with Apoe−/− recipient mice (n = 8) with Ppia+/+ bone marrow. (H) Heart weight per body weight ratio after AngII infusion for 4 weeks, was significantly less in ApoE−/− Ppia−/− (n = 6) compared to ApoE−/− (n = 6) chimeric mice with Ppia−/− bone marrow. Results are mean ± SD. # equals P< 0.05 in Saline versus AngII; *equals P< 0.05 in Apoe−/− versus Apoe−/− Ppia−/− mice.
After transplantation of Ppia+/+ bone marrow cells to the Apoe−/− Ppia−/− mice, cardiac hypertrophy was still significantly less in Apoe−/− Ppia−/− recipient mice compared to Apoe−/− recipient mice (Figure 4G). These data suggest that Ppia+/+ inflammatory cells are not important in AngII-induced cardiac hypertrophy. We next prepared chimeric mice transplanted with Ppia−/− bone marrow to investigate the role of recipient environment. There was no significant difference in the heart weight/BW of saline-infused groups. However, after AngII infusion for 4 weeks, there was still significantly greater heart weight/BW in the ApoE−/− compared to the ApoE−/− Ppia−/− chimeric mice even after transplantation with Ppia−/− bone marrow (Figure 4H). These data further demonstrate that cardiac-derived CyPA, not bone marrow-derived CyPA, is most important in AngII-induced cardiac hypertrophy.
CyPA augments AngII-induced ROS production and proliferation in cultured cardiac fibroblasts
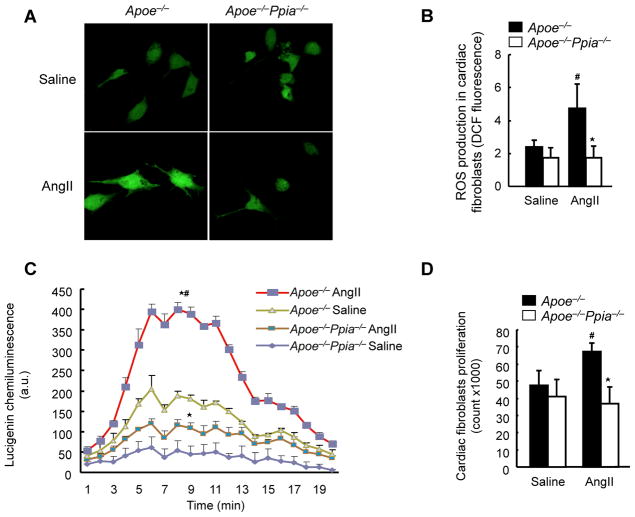
Based on the above results we next evaluated the effects of CyPA on cells present in cardiac tissue. We first studied cardiac fibroblasts because of the increase in perivascular collagen (Figure 2B) and large increase in CyPA secretion (Supplemental Figure I) in response to AngII. We isolated cardiac fibroblasts from Apoe−/− and Apoe−/− Ppia−/− mice and measured ROS by dichlorofluorescein (DCF) staining. In response to 1 μmol/L AngII there was a two-fold increase in ROS production in Apoe−/−(Figure 5A–B). There was a dramatic reduction in AngII-induced ROS production in Apoe−/− Ppia−/− fibroblasts (Figure 5A–B), suggesting that CyPA plays an autocrine role in cardiac fibroblast ROS generation, similar to our findings in VSMC.11 To verify the results with DCF, we also measured ROS by lucigenin chemiluminescence. As shown in Figure 5C, AngII induced ROS production was significantly decreased by ~ 50% in Apoe−/− Ppia−/− fibroblasts compared to Apoe−/− fibroblasts, similar to results with DCF.
Figure 5.
CyPA activates cardiac fibroblasts by enhancing ROS production. (A) Representative DCF staining of mouse cardiac fibroblasts. AngII-induced ROS generation after 4 hours was decreased in CyPA-deficient cardiac fibroblasts. (B) Densitometric analysis of DCF fluorescence in response to AngII shows significant reduction in Ppia−/− cardiac fibroblasts at 4 hours (n = 8 in each group). (C) Superoxide production in cardiac fibroblasts exposed to lucingenin for 4 hours. Results are mean ± SD of three independent experiments performed in triplicate. # equals P< 0.05 in Saline versus AngII; *equals P< 0.05 in Apoe−/− versus Apoe−/− Ppia−/− mice. (D) Proliferation of cardiac fibroblasts. Apoe−/− and Apoe−/− Ppia−/− fibroblasts were treated with saline or AngII. After 48 hours of incubation, cells were counted (n = 3 in each group). Results are mean ± SD. # equals P< 0.05 in Saline versus AngII; *equals P< 0.05 in Apoe−/− versus Apoe−/− Ppia−/− cardiac fibroblasts.
Excessive fibroblast proliferation induces myocardial stiffening, an important component of pathologic cardiac hypertrophy.25, 26 To evaluate whether CyPA is important in fibroblast proliferation, cell number in response to AngII was assessed. Proliferation was significantly augmented by AngII in Apoe−/− cardiac fibroblasts, whereas there was no change in Apoe−/− Ppia−/− fibroblasts (Figure 5D). These results suggest that CyPA-mediated cardiac fibroblast proliferation contributes to the perivascular cellular response.
Extracellular CyPA promotes proliferation and migration of cultured cardiac fibroblasts
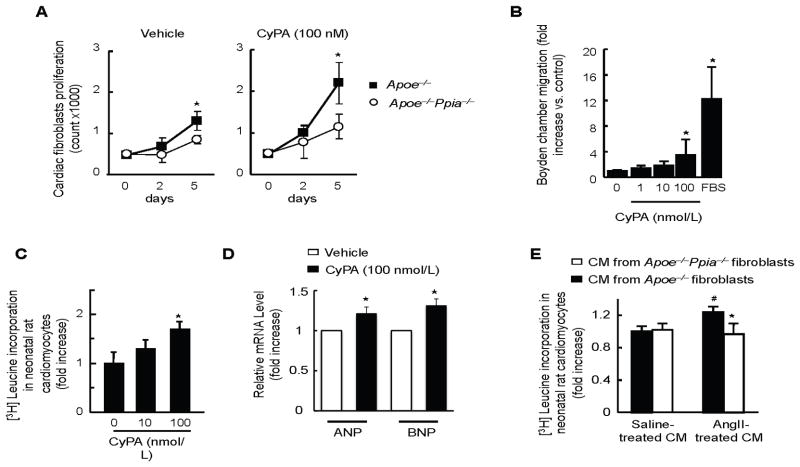
We next evaluated the effect of extracellular CyPA on fibroblast proliferation. Interestingly, there was a small but significantly greater growth rate of Apoe−/− fibroblasts compared to Apoe−/− Ppia−/− fibroblasts treated with vehicle measured by cell number (Fig 6A). Addition of recombinant CyPA (100 nmol/L) significantly increased proliferation of Apoe−/− fibroblasts (Figure 6A), which was significantly reduced Apoe−/− Ppia−/− cardiac fibroblasts. (Figure 6A).
Figure 6.
CyPA promotes proliferation and migration of cardiac fibroblasts and hypertrophy of cardiac myocytes. (A) After starvation for 24 hours, cardiac fibroblasts from Apoe−/− and Apoe−/− Ppia−/− mice were stimulated with 100 nmol/L CyPA or vehicle for 5 days. Medium was changed at day 2 and cells were counted at day 2 and day 5. Data are mean ± SD. *P<0.01. n = 4 in each group. (B) Recombinant CyPA promotes cardiac fibroblasts migration in a dose-dependent manner. Data are mean ± SD. *P<0.01 compared with control. n = 6 in each group. (C–D) Neonatal rat cardiac myocytes were treated with recombinant CyPA (0, 10, 100 nmol/L) for 24 hours. Hypertrophy was assessed by [3H]-leucine incorporation method (C) and by measuring ANP and BNP mRNA levels (D). Results are mean ± SD. *P < 0.05. (E) Cardiac myocytes were stimulated with conditioned medium (CM) prepared from cardiac fibroblasts that were treated with saline or AngII for 12 hours. Hypertrophy of neonatal rat cardiac myocytes was determined by means of [3H]-leucine incorporation. Data were normalized to myocytes stimulated by CM prepared from saline-treated Apoe−/− fibroblasts. n = 9 in each group. Results are mean ± SD. # equals P< 0.05 in Saline treated CM versus AngII; *equals P< 0.05 in Apoe−/− versus Apoe−/− Ppia−/− CM.
We next studied the effect of CyPA on migration of fibroblasts. There was a concentration dependent increase in migration, which was significant at 100 nmol/L CyPA (Figure 6B). These findings highlight the importance of extracellular and intracellular CyPA in cardiac fibroblast proliferation and migration.
Extracellular CyPA has a direct effect on cardiac myocyte hypertrophy
To evaluate whether CyPA could be a direct hypertrophic factor, we investigated the effect of extracellular CyPA on protein synthesis of isolated rat neonatal cardiac myocytes. Treatment with recombinant CyPA significantly increased [3H]-leucine incorporation in cardiac myocytes, suggesting the critical role of extracellular CyPA for the hypertrophic response (Figure 6C). To confirm further our data, we measured ANP and BNP mRNA expression, two accepted markers of cardiac hypertrophy. We found that CyPA upregulated ANP and BNP in neonatal cardiac myocytes (Figure 6D).
Cardiac fibroblasts isolated from Apoe−/− micesecreted substantial amounts of CyPA in response to AngII (Supplemental Figure I). Therefore, we investigated the role of fibroblast-derived secreted CyPA in mediating AngII-induced hypertrophic response in cardiac myocytes. We treated rat neonatal cardiac myocytes with conditioned medium (CM) prepared from AngII-stimulated cardiac fibroblasts. Protein synthesis, as measured by [3H]-leucine incorporation in myocytes, was used as a parameter of hypertrophy. CM prepared from AngII-stimulated Apoe−/− fibroblasts significantly augmented [3H]-leucine incorporation in cardiac myocytes (Figure 6E). In contrast, there was no effect when stimulated with CM prepared from the Apoe−/− Ppia−/− fibroblasts (Figure 6E). These results suggest that CyPA secreted from cardiac fibroblasts can act as a hypertrophic factor on cardiac myocytes. However, we cannot exclude that CyPA is involved in secretion of another factor, either from fibroblasts or myocytes which in turn induces cardiac hypertrophy based on these data. Together, these results indicate that both intracellular and extracellular CyPA play critical roles in the mechanisms that contribute to cardiac hypertrophy.
Discussion
The major finding of the present study is that CyPA is a novel mediator of cardiac hypertrophy. We characterized four important pathologic mechanisms by which CyPA promotes cardiac hypertrophy (Supplemental Figure III): 1) CyPA is a key determinant for ROS generation; 2) Secretion of CyPA in the Apoe−/− background is characterized by increased oxidative stress and inflammation; 3) CyPA has a direct hypertrophic effect on cardiac myocytes; and 4) CyPA stimulates proliferation of cardiac fibroblasts that also secrete CyPA, which indirectly stimulates cardiac myocyte hypertrophy. Together these direct and indirect effects of CyPA contribute to cardiac hypertrophy in Apoe−/− mice in response to AngII.
To examine the involvement of CyPA in the process of the cardiac hypertrophy, we used the AngII-infusion approach, a well-established mouse model to study cardiac hypertrophy. Here, we observed no significant differences in the magnitude of the hypertrophy induced by AngII between WT and Ppia−/− mice on C57Bl/6 background. Therefore, we investigated the effect of CyPA in Apoe−/− mice because they are characterized by high ROS and inflammation 27. We have recently shown that extracellular CyPA promotes vascular ROS production, which will further induce CyPA secretion and enhance ROS generation.11 The fact that the CyPA effect was most apparent in the setting of Apoe deficiency supports our concept that CyPA plays a key role under conditions in which ROS production contributes to cardiac hypertrophy. However, it is also conceivable that the hyperlipidemia and/or heightened inflammation characteristic of this model could have contributed to the observed effects.
We anticipated that the Apoe−/− background would greatly exacerbate the CyPA-mediated hypertrophic response because AngII-induced CyPA secretion is significantly elevated in these mice.11 Consistent with this concept, we found that CyPA secretion from cardiac fibroblasts isolated from WT mice was dramatically less compared to Apoe−/− fibroblasts when stimulated withAngII.
CyPA has important roles in the immune system and is a well described regulator of T lymphocyte functions.28 It is relevant to note that the primary sources of CyPA responsible for cardiac hypertrophy are likely cells in the heart and not inflammatory cells, because transplantation with Ppia+/+ bone marrow cells still resulted in less cardiac hypertrophy in Apoe−/− Ppia−/− compared to Apoe−/− mice (Figure 4). In addition, transplantation of Apoe−/− Ppia−/− bone marrow into Apoe−/− did not prevent cardiac hypertrophy in response to AngII (Fig 4H). These data suggest the importance of cardiac derived CyPA for recruitment of bone marrow-derived cells to perivascular tissues to create an environment that is pro-hypertrophic.
The interaction between cardiac myocytes and cardiac fibroblasts is a key event during AngII-induced cardiac hypertrophy.2, 5, 24, 29, 30 In particular, several studies have shown that cardiac myocyte hypertrophy was stimulated by growth factors and/or cytokines secreted from cardiac fibroblasts.2–4, 31, 32 To prove that CyPA could be one of these factors that in a paracrine fashion ultimately induces cardiac myocyte hypertrophy, we first showed that CyPA is released from cardiac fibroblasts after AngII treatment, and then we proved that extracellular CyPA stimulates cardiac hypertrophy. However, we cannot exclude the involvement of CyPA produced by VSMC and/or cardiac myocytes in the enhancement of hypertrophy and fibrosis.
The precise mechanism by which CyPA directly enhances cardiac hypertrophy remains to be elucidated, in part because the CyPA receptor has not been identified. Nonetheless, the present study suggests that inhibition of CyPA may be a useful therapeutic strategy to attenuate cardiac hypertrophy in patients who experience high oxidative stress such as smoking, hypertension, and hyperlipidemia.
Supplementary Material
Acknowledgments
We thank Dr. Alan Smrcka and Nancy Ward for the preparation of recombinant CyPA. We are grateful to the Aab Cardiovascular Research Institute members for useful suggestions and Robert Winterkorn, Anna T. Paxhia, and Dmitriy Migdalovich for technical assistance.
Sources of Funding
This work was supported by NIH grant HL49192 (to B.C. Berk), HL8487 and HL089885 (to B.C. Blaxall), grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (to K. Satoh), and Internal Grant of the University of Salerno (to P. Nigro).
Footnotes
Disclosures
None.
References
- 1.Izumo S, Aoki H. Calcineurin--the missing link in cardiac hypertrophy. Nat Med. 1998;4:661–662. doi: 10.1038/nm0698-661. [DOI] [PubMed] [Google Scholar]
- 2.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 2010;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowers SL, Borg TK, Baudino TA. The dynamics of fibroblast-myocyte-capillary interactions in the heart. Ann N Y Acad Sci. 2010;1188:143–152. doi: 10.1111/j.1749-6632.2009.05094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffre F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, Maroteaux L. Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ Res. 2009;104:113–123. doi: 10.1161/CIRCRESAHA.108.180976. [DOI] [PubMed] [Google Scholar]
- 6.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 7.Pan H, Luo C, Qiao A, Zhang L, Mines M, Nyanda AM, Zhang J, Fan GH. Cyclophilin A is required for CXCR4-mediated nuclear export of hnRNP A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2007;283:623–637. doi: 10.1074/jbc.M704934200. [DOI] [PubMed] [Google Scholar]
- 8.Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, Neagu MR, Nilsson M, Eriksson PS, Hagberg H, Luban J, Kroemer G, Blomgren K. Cyclophilin A participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med. 2007;204:1741–1748. doi: 10.1084/jem.20070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 10.Seko Y, Fujimura T, Taka H, Mineki R, Murayama K, Nagai R. Hypoxia followed by reoxygenation induces secretion of cyclophilin A from cultured rat cardiac myocytes. Biochem Biophys Res Commun. 2004;317:162–168. doi: 10.1016/j.bbrc.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med. 2009;361:1114–1116. doi: 10.1056/NEJMcibr0905244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 14.Sadoshima JI, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 16.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 18.Wang YX, da Cunha V, Martin-McNulty B, Vincelette J, Li W, Choy DF, Halks-Miller M, Mahmoudi M, Schroeder M, Johns A, Light DR, Dole WP. Inhibition of Rho-kinase by fasudil attenuated angiotensin II-induced cardiac hypertrophy in apolipoprotein E deficient mice. Eur J Pharmacol. 2005;512:215–222. doi: 10.1016/j.ejphar.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Satoh K, Matoba T, Suzuki J, O’Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, Yan C, Abe J, Berk BC. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh K, Kagaya Y, Nakano M, Ito Y, Ohta J, Tada H, Karibe A, Minegishi N, Suzuki N, Yamamoto M, Ono M, Watanabe J, Shirato K, Ishii N, Sugamura K, Shimokawa H. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation. 2006;113:1442–1450. doi: 10.1161/CIRCULATIONAHA.105.583732. [DOI] [PubMed] [Google Scholar]
- 21.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 22.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- 23.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activation in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 24.Kim NN, Villarreal FJ, Printz MP, Lee AA, Dillmann WH. Trophic effects of angiotensin II on neonatal rat cardiac myocytes are mediated by cardiac fibroblasts. Am J Physiol. 1995;269:E426–437. doi: 10.1152/ajpendo.1995.269.3.E426. [DOI] [PubMed] [Google Scholar]
- 25.Zannad F, Dousset B, Alla F. Treatment of congestive heart failure: interfering the aldosterone-cardiac extracellular matrix relationship. Hypertension. 2001;38:1227–1232. doi: 10.1161/hy1101.099484. [DOI] [PubMed] [Google Scholar]
- 26.Weber KT, Brilla CG, Janicki JS, Reddy HK, Campbell SE. Myocardial fibrosis: role of ventricular systolic pressure, arterial hypertension, and circulating hormones. Basic Res Cardiol. 1991;86 (Suppl 3):25–31. doi: 10.1007/978-3-662-30769-4_3. [DOI] [PubMed] [Google Scholar]
- 27.Niu XL, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause KH, Humphries J, Smith A, Burnand KG, Runge MS. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation. 2010;121:549–559. doi: 10.1161/CIRCULATIONAHA.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A, Luban J. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21:189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–363. doi: 10.1016/s0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 30.Jaffe IZ, Mendelsohn ME. Angiotensin II and Aldosterone Regulate Gene Transcription Via Functional Mineralocortocoid Receptors in Human Coronary Artery Smooth Muscle Cells. Circ Res. 2005 doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 31.Long CS, Henrich CJ, Simpson PC. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul. 1991;2:1081–1095. doi: 10.1091/mbc.2.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eid H, de Bold ML, Chen JH, de Bold AJ. Epicardial mesothelial cells synthesize and release endothelin. J Cardiovasc Pharmacol. 1994;24:715–720. doi: 10.1097/00005344-199424050-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.