Abstract
We investigated playmate and play style preference in children with congenital adrenal hyperplasia (CAH) (26 females, 31 males) and their unaffected siblings (26 females, 17 males) using the Playmate and Play Style Preferences Structured Interview (PPPSI). Both unaffected boys and girls preferred same-sex playmates and sex-typical play styles. In the conflict condition where children chose between a same-sex playmate engaged in an other-sex activity or an other-sex playmate engaged in a same-sex activity, boys (both CAH and unaffected brothers) almost exclusively chose playmates based on the preferred play style of the playmate as opposed to the preferred gender label of the playmate. By contrast, unaffected girls used play style and gender label about equally when choosing playmates. Girls with CAH showed a pattern similar to that of boys: their playmate selections were more masculine than unaffected girls, they preferred a boy-typical play style and, in the conflict condition, chose playmates engaged in a masculine activity. These findings suggest that prenatal androgen exposure contributes to sex differences in playmate selection observed in typically-developing children, and that, among boys and girls exposed to high levels of androgens prenatally, play style preferences drive sex segregation in play.
Keywords: androgen, CAH, hormones, playmate, play style, rough-and-tumble, sex differences, sex segregation
Introduction
Childhood sex segregation is a robust phenomenon and the developmental trajectory of same-sex affiliation has been well documented (Fabes, Martin, & Hanish, 2003; Jacklin & Maccoby, 1978; LaFreniere, Strayer, and Gauthier, 1984; Maccoby and Jacklin, 1987; Martin and Fabes, 2001; Ruble & Martin, 1998; Wasserman & Stern, 1978). The magnitude of the preference for same- versus other-sex affiliation is quite large, increases at least into middle childhood, and appears to be resistant to change (Maccoby and Jacklin, 1987; Powlishta, Serbin, and Moller, 1993; Serbin, Tonick, and Sternglanz, 1977). However, while the behavioral features of children’s sex-typed playmate preferences are generally understood, the underlying mechanisms are not.
The extant literature on childhood sex segregation in general implicates cognitive, social, and biological processes with the general consensus that the full explanation integrates these theoretical perspectives. Independent effects of gender labels and play styles in children’s playmate selections have also been investigated with some researchers hypothesizing that the sex differences in play style themselves probably contribute to children’s preferences for same-sex play partners. For example, it has been demonstrated that boys and girls differ in toy choices, activity levels, and rough-and-tumble play. Compared to boys, girls play more with dolls and doll furnishings, are less active, and are less interested in rough, outdoor play. By contrast, compared to girls, boys play more with construction and transportation toys, are more active and are more interested in rough, outdoor play (Berenbaum & Hines, 1992; DiPietro, 1981; Eaton & Enns, 1986; Hines & Kaufman, 1994; Maccoby & Jacklin, 1974; Pasterski, et al., 2005; Pasterski, et al., 2007; Ruble & Martin, 1998; see Hines, 2009 for review). Furthermore, research with non-human primates and other mammals has shown similar sex differences. Male rats and monkeys show more rough-and-tumble play than females of the species (Meaney & Stewart, 1981; Ward & Stehm, 1991) and two studies (Alexander & Hines 2002; Hassett, Siebert, & Wallen, 2008) have found sex differences in toy choices among vervet and rhesus monkeys which parallel those of children. Given the clear sex differences in preference for play styles, it is not surprising that children engaging in sex-typed activities generally do so with others of the same sex.
However, even when the activity or toy is neutral, sex-segregation is still observed (Jacklin & Maccoby, 1978), suggesting that play style cannot fully account for children’s preferences for same-sex play partners. Cognitive-developmental theory suggests that sex-segregation occurs as children begin to understand gender and grow to value and prefer members of their own gender group (Maccoby, 1988; Maccoby & Jacklin, 1987), and that playmate selection is based on the gender label of the playmate rather than the play style. Accordingly, this theory suggests that the ability to correctly label oneself and others by gender underlies the formation of gender schemas (Martin & Halverson, 1981). These schemas allow children to recognize attributes about the other sex while distinguishing and adopting behaviors characteristic of their own sex. As children’s schemas grow, so does their repertoire of sex-typed behaviors, including preference for same-sex partners.
Social learning theory suggests that, as they develop, children acquire knowledge regarding socially approved behavior (Mischel, 1966) including play with same-sex peers. As they grow to accurately identify their own sex, they apply this knowledge to their own behavior. There is ample evidence that children receive reinforcements from their peers for sex-appropriate behavior (Fagot & Hagan, 1991), and they appear to be more responsive to reactions by their own sex than by the other sex (Fagot, 1985). According to this theory, then, sex-segregation is presumed to result from socialization by peers (in addition to parents and others) (Maccoby, 1988).
With respect to inborn influences on sex segregation, the most prominent explanation proposes an influence of prenatal hormones (primarily androgens) on neurobehavioral development. Evidence for such an influence comes mostly from studies of girls with congenital adrenal hyperplasia (CAH; Hines, 2004). This disorder involves an enzymatic deficiency resulting in an overproduction of adrenal androgens, beginning prenatally. Girls affected with this disorder are usually born with virilized genitalia and, beginning in early childhood, typically show masculinized patterns of sex-typed behavior such as toy and activity preferences (Collaer & Hines, 1995; Ehrhardt & Baker, 1974; Pasterski, et. al., 2005).
In addition to toy and activity preferences, playmate preferences have also been studied in this particular population. Specifically, five studies have investigated the influence of prenatal androgens on children’s playmate preferences (Berenbaum & Snyder, 1995; Dittmann, et al., 1990; Ehrhardt & Baker, 1974; Hines & Kaufman, 1994; Servin, Nördenstrom, Larsson, & Bohlin, 2003) by studying girls with CAH; however, the findings from these studies are contradictory, with three reporting effects of androgen (Ehhardt & Baker, 1974; Hines & Kaufman, 1994; Servin, Nördenstrom, Larrson, & Bohlin, 2003) and two reporting no effects (Berenbaum & Snyder, 1995; Dittmann, et al., 1990). Furthermore, those who do report an effect do not point to a mechanism by which androgens influence playmate selection. There are two possible modes of action of prenatal androgens on playmate preferences. First, androgen may directly influence peer preferences. Alternatively, androgen may influence components of play style which then influence playmate selection. Behavioral compatibility hypotheses, for example, suggest that children choose playmates who are compatible, e.g. they have similar toy and activity preferences (Maccoby & Jacklin, 1987; Moller & Serbin, 1996; Pellegrini, 2004). From this perspective, one might consider that hormone influences on toy and/or activity preference may underlie sex-typical playmate selection.
Because play styles covary with sex, it is difficult to disentangle the relative contributions of play styles and gender labels to children’s playmate preferences. To address the issue, Alexander and Hines (1994) developed a structured interview to assess play style preference, playmate preference, and the preference for one over the other in a conflict condition. The Playmate and Play Style Preferences Structured Interview (PPPSI), requires the child to indicate preferences for feminine or masculine activities (play style preference), for female targets or male targets (playmate preference), and for female targets engaging in masculine activities or male targets engaging in feminine activities (conflict condition). With respect to the conflict condition, for boys, a consistent preference for female targets engaging in masculine activities would indicate playmate selection on the basis of play style and a consistent preference for male targets engaging in feminine activities would indicate conflict resolution based on gender labels. Likewise, for females, a consistent preference for male targets engaging in feminine activities would indicate conflict resolution on the basis of play style and a consistent preference for female targets engaging in masculine activities would indicate conflict resolution on the basis of gender labels. Alexander and Hines (1994) found that boys made most of their selections based on the play style of the targets and that, overall, girls did not show a selection bias based on the play style or the gender label of the target. This led them to conclude that the processes that led to children’s sex segregation differed to some extent for girls and boys.
The relative importance of play style in gender-typical boys’ preference for boys as playmates is consistent with the observation that boys are more stereotyped in their play style preferences than are girls (Eisenberg, Murray, & Hite, 1982). Although this sex difference may be explained in part by stronger social pressures for sex-appropriate play for boys than for girls, biological, particularly hormonal, influences may also be partially responsible. The purpose of the current study was to investigate the potential role of prenatal androgen in playmate selection with respect to the mechanisms underlying sex segregation in playmate selection. To do so, we assessed preferences for gender labels and play styles, including when the two are in conflict, in a cohort of children exposed prenatally to excess androgen, due to CAH, as compared to unaffected siblings.
Hypotheses for the current investigation were as follows: (1) compared to unaffected girls, unaffected boys will choose more male targets as playmates and more masculine activities; (2) compared to unaffected boys, unaffected girls will choose more female targets as playmates and more feminine activities; and (3) compared to unaffected girls, girls with CAH will choose more male targets as playmates and more masculine activities. Given the sex difference in conflict resolution found by Alexander and Hines (1994), we predicted further that: (4); unaffected boys will choose playmates based more on the play style than on the gender label of the target; (5) unaffected girls will not show a preference for play style or gender label of the target when choosing playmates; and (6) girls with CAH will choose playmates based more on the play style than on the gender label of the target. We had no specific hypotheses for boys with CAH since previous findings suggest they generally do not differ from unaffected boys in play style or playmate preference.
Method
Participants
One hundred 3- to 10-year-old children (26 females, 31 males with CAH; 26 unaffected sisters, 17 unaffected brothers) completed the PPPSI as part of a larger study (Pasterski, 2005:2007). The mean age of the children was 86.2 month (SEM = 3), and the mean ages (with SEMs) of the four groups were 83.1 ± 5 for girls with CAH; 78.6 ± 5 for unaffected girls; 88.4 ± 5 for boys with CAH; and 98.6 ± 3 for unaffected boys. A one-way analysis of variance (ANOVA) for age indicated that the four groups were not significantly different, F(1, 96) = 2.02. Fifty-three (95%) of the children with CAH had the more severe salt-wasting form of the disorder, and 3 (5%) had the simple virilizing form of the disorder.
Thirty-three of the children were recruited through pediatric endocrinologists in Los Angeles, California, and participated at the University of California Los Angeles (UCLA), and 67 children were recruited in the United Kingdom through pediatric endocrinologists in London or through a CAH support group. Forty-seven percent of the Los Angeles sample was Hispanic, 38% was White, and 15% was Black. The majority of the London sample was white (of British or other European decent); 2 participants (brothers) were not and were of mixed race (black/white).
Procedures, Materials, and Scoring
Playmate and Play Style Preferences Structured Interview (PPPSI)
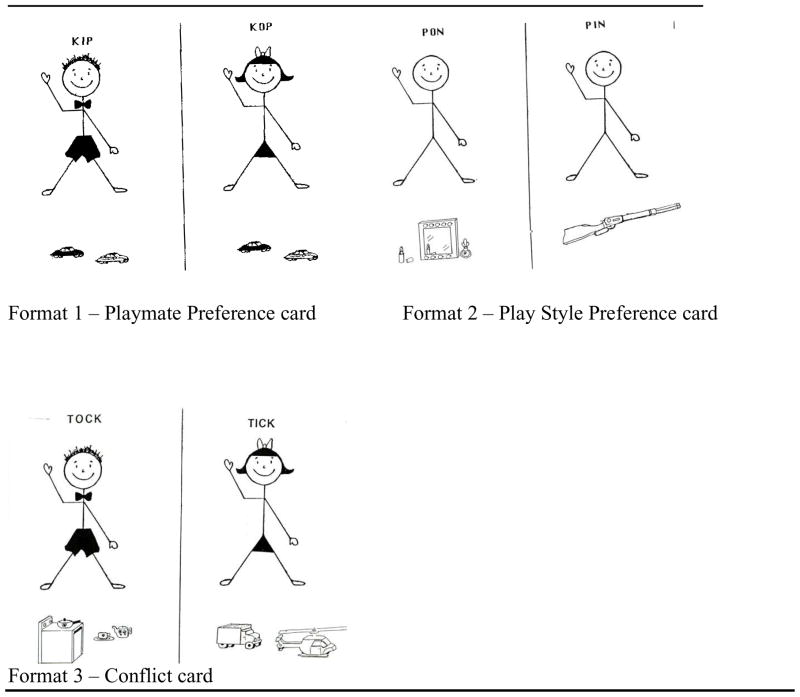
The PPPSI (Alexander and Hines, 1994) was administered to each child individually and rendered independent preference scores for gender label and play style (play style consisted of 3 subscales, toy choice, activity level, rough-and-tumble play). The interview measure consisted of 38 cards (20 x 28cm), each containing two figures drawn on either side of a central line (See Figure 1). Two cards from the original stimuli developed by Alexander and Hines (1994) depict a preference for social group rather than for a toy or activity, so they were not included in the current study. Above each figure in the 38 cards was written the figure’s name. For each pair of figures the names were nonsense words with either the initial consonant or central vowel changed (e.g., Dippy and Doppy or Miff and Piff). These were selected to minimize gender association. A toy (e.g., a cosmetics kit or a gun) or an activity (e.g., playing ring-around-the-rosie or playing games where you jump on top of other children) was depicted below each figure. The stimuli comprised 3 basic formats differing in the gender of the figure (male, female, gender neutral) and the toy or the activity associated with the figures (stereotypical masculine, stereotypical feminine). Format 1 consisted of 14 playmate preference cards and assessed the playmate preferences of the child independent of play styles. Format 2 consisted of 12 play style cards, four each for toy preferences, activity level, and rough-and-tumble play. Each of these cards showed neutral figures, one engaged in a masculine play style, and the other engaged in a feminine play style. Format 3 consisted of 12 cards and assessed the relative contributions of gender label and play style to playmate choice by putting the two into conflict. Each card showed a male target above a feminine play dimension on one side of the central line and a female target above a masculine play dimension on the other.
Figure 1.
Playmate and Play Style Preferences Structured Interview (PPPSI) stimuli.
For all playmate and play style items, a preference for the female target or the feminine play style was scored as “1” and a preference for the male target or the masculine play style was scored as “0”. Hence, higher scores indicate a female-typical pattern of responding. Scores ranged from 0 to 14 for the measure of playmate preference. Scores for the measure of play style ranged from 0 to 12 and included three sub-scores (toy preference, activity level and rough-and-tumble play), each ranging from 0 to 4. For the conflict condition, a preference for the female target engaging in the masculine play style was scored as “1” and a preference for the male target engaging in the feminine play style was scored as “0”. Scores for the conflict condition range from 0 to 12.
Results
Playmate and play style
Table 1 shows means and effect sizes (d; Cohen, 1988) for playmate and play style preferences by sex and CAH status. While there were no group differences in age, age correlated significantly with both playmate (r (98) = −.30, p = .002) and play style (r (98) = −.36, p < .001) and two of the three sub-components (r (98) = −.39 for toy choice and −.27 for activity level; both p<.01). Therefore we included age as a covariate to control for error variance for playmate and play style preferences analysis of variance. As such, Multivariate analysis of covariance (MANCOVA), with age as the covariate, was performed to assess sex and CAH status effects for playmate and play style sub-components. To test simple effects where there were interactions, we used F-tests including age as a covariate, where relevant, to make specific group-wise comparisons. Where a priori predictions were made, one-tailed significance values are reported. These included: 1) unaffected girls compared to boys with and without CAH; and 2) unaffected girls compared to girls with CAH.
Table 1.
Group comparisons for playmate and play style preferences
| Variables | Males | Females | Sex difference | |||||
|---|---|---|---|---|---|---|---|---|
| Unaffected M (SD) | CAH M (SD) | Unaffected M (SD) | CAH M (SD) | p | d | p | d | |
| N=17 | N=31 | N=26 | N=26 | |||||
| Playmate preference a | 2.18 (2.48) | 2.71 (3.47) | 10.96 (3.11) | 7.15(4.27) | .001 | 1.03 | .000 | 3.07 |
| Play style preferencesb | 2.41 (1.91) | 3.32 (1.97) | 7.58 (2.56) | 4.89 (2.44) | .000 | 1.08 | .000 | 2.24 |
| Toy choicec | 0.18 (0.39) | 0.29 (0.64) | 2.77 (1.24) | 1.07 (1.30) | .000 | 1.34 | .000 | 2.87 |
| Activity levelc | 1.47 (1.07) | 1.61 (.096) | 2.15 (0.97) | 1.78 (0.80) | .075 | 0.42 | .089 | 0.67 |
| Rough-and-tumblec | 0.76 (0.97) | 1.42 (1.12) | 2.65 (1.20) | 2.04 (1.22) | .036 | 0.50 | .000 | 1.70 |
Note: Higher scores are more female-typical; P values are for simple effects F tests, one-tailed according to directional hypotheses, with age as a covariate; Effect sizes (d) do not control for age. The sex differences reported here are for unaffected children only.
Range 0 – 14
Range 0 – 12
Range 0 – 4
Group-wise comparisons
Playmate preference
The 2 (sex) × 2 (CAH status) MANCOVA for playmate preference revealed a significant sex × CAH status interaction, F(1, 96) = 7.90, p = .006. Simple effects analysis showed that unaffected girls chose more female targets as playmates than did unaffected boys, F(1, 40) = 79.66, p < .001, boys with CAH, F(1, 54) = 82.94, p < .001, or girls with CAH, F(1, 50) = 13.37, p = .001. However, girls with CAH chose more female targets than did either unaffected boys, F(1,41) = 14.90, p < .001 or boys with CAH, F(1, 55) = 18.33, p < .001. There was no difference in playmate preference between boys with and without CAH, F(1, 45) < 1.
Play style dimensions
Toy choice
The 2 (sex) × 2 (CAH status) MANCOVA for toy choice also revealed a significant sex × CAH status interaction, F(1, 96) = 17.39, p < .001, such that unaffected girls preferred girls’ toys more frequently than either unaffected boys, F(1, 40) = 54.26, p < .001, boys with CAH, F(1, 54) = 88.69, p < .001, or girls with CAH, F(1, 50) = 24.19, p < .001. Girls with CAH chose girls’ toys more frequently than boys with CAH, F(1, 55) = 8.37, p = .005, and slightly more frequently than unaffected boys, F(1, 41) = 3.94, p < .10. There was no difference in preference for sex-typed toys between boys with and without CAH, F (1, 45) < 1.
Activity level
The 2 (sex) × 2 (CAH status) MANCOVA for activity level revealed a main effect of age, F(1, 96) = 4.72, p = .032, such that younger children preferred a higher level of activity, r = −.26, p = .009, but there were no other significant main effects or interactions.
Rough-and-tumble play
The 2 (sex) × 2 (CAH status) MANCOVA for rough-and-tumble play revealed a significant sex × CAH status interaction, F(1, 96) = 6.80, p = .011, such that unaffected girls preferred a less rough-and-tumble play style than unaffected boys, t(41) = 5.43, p = .001, boys with CAH, t(55) = 4.02, p = .000, or girls with CAH, t(50) = −2.10, p = .020. In addition, girls with CAH preferred a less rough-and-tumble play style than unaffected boys, t(41) = 3.47, p = .001, but not less than boys with CAH, t(55) = 1.78. Boys with CAH preferred a less rough-and-tumble play style than unaffected boys, t(46) = 2.03, p = .048.
One-sample t-tests and chi square
In addition to evaluating sex and CAH group differences as a function of mean scores on the playmate and play styles preferences scales, we conducted two further analyses. First, we used one-sample t-tests within each group to determine whether their means for playmate and play style preferences were shifted significantly from the midpoint, i.e., whether or not they preferred feminine versus masculine playmates and play styles. For playmate preferences, unaffected girls and boys with and without CAH showed preferences which were shifted significantly from the midpoint of 7 in the expected directions (p < .001 for each of the three groups). Unaffected girls as a group preferred female over male playmates and boys with and without CAH preferred male over female playmates. The mean score for girls with CAH (M = 7.15) did not differ significantly from the midpoint (p = .963), indicating a pattern of choosing playmates that is shifted in the male-typical direction, i.e., between female-typical and male-typical responding. For the analysis of play style a composite of the two sub-scores that showed sex differences, i.e., toy choice and rough-and-tumble play, was used. All four groups were shifted significantly from the midpoint of 4 in regard to sex-typed play style (p = .003 for unaffected girls; p = .006 for girls with CAH; p < .001 for boys with and without CAH). Boys with and without CAH as well as girls with CAH chose a masculine over a feminine play style, whereas unaffected girls chose a feminine over masculine play style.
Second, we used chi square analysis to determine whether the numbers of children in each group who showed a clear preference for either same- or other-sex playmates and play styles were significantly different. As with the analyses above, the play style composite consisted of the two sub-scores that showed sex differences, i.e., toy choice and rough-and-tumble play, was used. Also, we considered a child to show a preference if s/he made a same- or other-sex selection on more than half of the trials (see table 2). Chi square analysis indicated that the groups were significantly different in terms of choosing playmates and play styles, x2(3) = 6.94, p = .012 and x2(3) = 10.11, p = .002, respectively. Further analyses showed that more girls with CAH chose more cross-sex playmates and play styles than children in the other groups: x2(1) = 13.07, p = .001 and x2(1) = 13.28, p = .001, respectively, compared to unaffected girls; x2(1) = 7.86, p = .020 and x2(1) = 23.72, p < .001, respectively, compared to unaffected boys; and x2(1) = 11.39, p = .003 and x2(1) = 31.93, p < .001, respectively, compared to boys with CAH. However, responses for all four groups of children were normally distributed and the variance in scores did not differ from one group to another, showing that androgen exposure did not alter the shape of the distribution of scores.
Table 2.
Percentages of children from each group who showed a preference same- or other-sex playmates and play styles
| Playmate* | Play style** | |||||
|---|---|---|---|---|---|---|
| same-sex | cross-sex | no pref. | same-sex | cross-sex | no pref. | |
| CAH girls (n=26) | 12 (46%) | 13 (50%) | 1 (4%) | 5 (19%) | 17 (65%) | 4 (15%) |
| Relative girls (n=26) | 24 (92%) | 2 (8%) | 1 (4%) | 18 (69%) | 6 (23%) | 2 (8%) |
| CAH boys (n=31) | 26 (84%) | 3 (10%) | 2 (6%) | 27 (87%) | 0 (0%) | 4 (13%) |
| Relative boys (n=17) | 15 (88%) | 2 (12%) | 0 (0%) | 16 (94%) | 0 (0%) | 1 (6%) |
Chi square p < .05, CAH girls compared to 3 other groups.
Chi square p < .01, CAH girls compared to 3 other groups.
Conflict condition
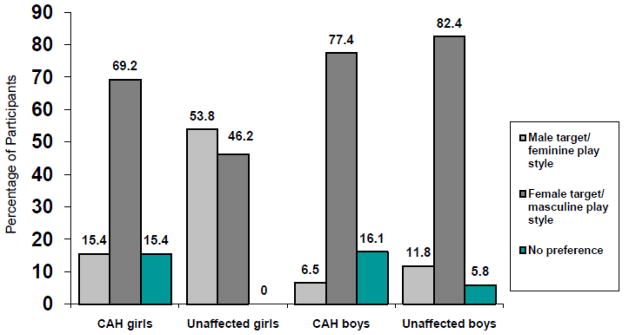
Children were asked to choose between a female target engaged in a masculine activity or a male target engaged in a feminine activity. For children who showed a pattern of sex-typed preferences on the playmate and play style dimensions, a preference in the conflict condition for the same-sex target engaged in other-sex activity would indicate a choice based on the gender label of the playmate; and a preference for an other-sex target engaged in same-sex activity would indicate a choice based on the play style of the playmate. We calculated the numbers of children in each group who chose a female target engaged in a masculine activity on the majority of trials versus a male target engaged in a feminine activity on the majority of trials, as well as those who showed no preference (see Figure 2). Chi square analysis suggested that numbers of children in the respective groups were significantly different, x²(3) = 17.03, p = .001. Further comparisons showed that unaffected boys and girls differed significantly, x²(1) = 7.18, p = .008, and that girls with CAH differed from unaffected girls, x²(1) = 5.30, p = .021, but not from unaffected boys, x²(1) = 0.27, p = .495, or boys with CAH, x²(1) = 1.97, p = .161. Roughly equal numbers of unaffected girls showed preferences for the two alternatives (46.2% compared to 53.8%). The majority of boys, with and without CAH, chose the female target engaged in a masculine activity as opposed to the male target engaged in a feminine activity (82.4% compared to 17.6% for boys with CAH and 77.4% compared to 22.6% for unaffected boys, respectively). Girls with CAH showed a pattern similar to boys: the majority chose the playmate engaged in a masculine activity (69.2% of participants). Figure 2 shows the percentages of children in each group who made selections based on the play style versus gender label of the playmate.
Figure 2.
Conflict condition: Percentages of children who preferred male targets engaged in a feminine play style, female targets engaged in a masculine play style, and those who showed no preference.
Discussion
The findings from the current study elucidate a potential underlying mechanism responsible for the observed pattern of childhood sex segregation. For boys and for girls with CAH, playmate selection relates mostly to the play style of the playmate, irrespective of the playmate’s gender. Playstyle and playmate preferences of girls with CAH were both shifted significantly in the masculine direction compared to unaffected girls. In addition, for girls with CAH play style was more important than gender label in choosing playmates, as evidenced by their selecting female playmates engaged in a masculine activity when these two dimensions were put into conflict. As noted above, play style is influenced by prenatal androgen exposure. Girls with CAH show masculinized play styles (Hines, 2009) and amniotic fluid testosterone, as well as maternal testosterone during pregnancy, correlates with male-typical play styles in healthy girls (Auyeung, et al., 2009; Hines, et al., 2002). Thus, playmate preference may be a secondary effect of the influence of androgen on play style. Although social sanctions may also contribute to boys’ rejection of feminine play styles, this is unlikely for girls with CAH who appear not to be subjected to such sanctions. In fact, girls with CAH have been shown to receive increased parental encouragement of girl-typical play (see Pasterski, et al., 2005).
Regarding normative sex differences in preferences for same-sex playmates and overall play styles, our study replicated findings from the original report by Alexander & Hines (1994) as well as one further study which reports on the PPPSI (Fridell, et al. 2006), in addition to resembling findings from other studies using other methodologies (e.g., Pasterski, et al, 2005). Compared to unaffected girls, the unaffected boys in our study preferred male targets as playmates (d = 3.07) and an overall male-typical play style (d = 2.50). In terms of CAH effects on play styles, results from the current study also are consistent with findings from previous studies utilizing other methodologies, e.g., observation and interviews, where girls with CAH have been found to show play styles that are more male-typical than those of other girls. Results for playmate preferences agree with the three prior studies finding enhanced preferences for boys as playmates in girls with CAH, and strengthen evidence that sex-segregation in play is influenced by prenatal androgen exposure. Compared to unaffected sisters, girls with CAH preferred boys as playmates (d = 1.01), and a masculinized play style (d = 1.25). With respect to the specific play style dimensions, we found the expected sex and CAH effects for toy choice (d = 2.87 and 1.34, respectively) and rough-and-tumble play (d = 1.70 and 0.50, respectively). We also found that boys with CAH preferred a less rough-and-tumble play style compared to unaffected boys (d = 1.07). We had not predicted this effect, because most prior studies of boys with CAH have found that their sex-typed toy and activity preferences do not differ from those of other boys. Our finding is consistent, however, with the one prior study that looked specifically at rough and tumble play in boys with CAH (Hines and Kaufman, 1994). In that study too, boys with CAH showed reduced rough-and-tumble play. Thus, these boys may show a specific demasculinization of behavior in this one area.
We did not find sex or CAH differences in activity level. By contrast, in another study of children with CAH, we found that both unaffected boys and girls with CAH were reported by their mothers to be more active than unaffected girls (Pasterski, et. al., 2007). The discrepancy in findings may be due to differences in measurement. The PPPSI inquires about activity in general terms, e.g., ‘running around’ compared to ‘reading a book,’ whereas the measure used in the Pasterski, et al. (2007) study, developed by Zucker and Bradley (1995), assessed specific types of physical activity and in various contexts. Although the PPPSI was designed to tap sexually differentiated aspects of childhood play, the activity dimension did not show a significant sex difference in the current study, perhaps explaining the lack of CAH effects.
With respect to normative sex differences in the conflict resolution condition, again, our findings are consistent with those of the two previous studies using the PPPSI. The unaffected boys in our study primarily chose playmates engaged in a masculine activity. These boys would rather play with a girl who has similar interests than a boy engaged in cross-sex behavior. By contrast, unaffected girls did not show a preference. They were as likely to choose a girl engaged in a male-typical activity as a boy engaged in a female-typical activity. This is also consistent with previous studies which have suggested that boys are more sex-typed than are girls in their toy preferences (Eisenberg, Murray, & Hite, 1982).
Furthermore, our hypothesis that girls with CAH would show a pattern similar to that of boys in the conflict condition was also supported. The majority of girls with CAH chose the female target engaged in the masculine activity. Regardless of the gender of the play partner, most girls with CAH chose the masculine play style. This is in contrast to unaffected girls who chose based on play style and the gender of the play partner in roughly equal numbers. In this respect, girls with CAH resemble boys and cross-sexed girls (referred for gender identity disorder, GID) who also have been found to select based on the preferred play style of the target rather than the preferred gender label (Fridell, et al., 2006).
A component of GID in children is a strong preference for cross-gendered play styles and playmates, and about 10% of girls with CAH show such extreme cross-sex interests that they would qualify for a GID diagnosis (Slijper et al). It might, therefore, be of interest to compare the size of the difference between girls with and without CAH to the size of the difference between girls with and without GID, by comparing our data to those reported elsewhere for GID referred children and healthy controls (Fridell et al., 2006). This comparison suggests that the average girl with CAH, though markedly more male-typical than unaffected female relatives in playmate and play style preference (d = 1.03 and 1.08, in order), appears not to be as dramatically male-typical in these respects as the average girl referred for evaluation of GID compared to healthy controls (d = 1.91 and 2.78, in order).
Thus, whatever processes lead to GID in girls appear to be more powerfully masculinizing than those caused by CAH. Non-hormonal factors, such as genes, or social experiences, may be primary causes of GID, although there is, as yet, no evidence linking specific genetic or socialization processes to GID. Alternatively, because most girls with CAH are typically born with partially, and not completely, virilized genitalia, their prenatal androgen exposure appears to be less extensive that that of a typical boy. Girls with GID generally do not show evidence of genital virilization, but there are many mechanisms downstream from androgen itself that are involved in neurobehavioral sexual differentiation, such as enzymes, hormone receptors, and co-factors (Hines, 2009; McCarthy, et al., 2009). Abnormalities related to these mechanisms could be involved in GID, without causing the genital virilization associated with androgen exposure. If so, the abnormality in these downstream mechanisms could be more substantial or of longer duration than the hormone abnormality associated with CAH. A final possibility, and probably the most likely, is that GID, like many other clinical psychological conditions, results from a combination of factors, such as genetic predispositions, combined with subsequent hormone-related or socialization experiences. Similarly, GID in girls with CAH may occur only when the hormonal abnormality is combined with as yet unidentified genetic factors or factors related to social experiences that relate to GID.
Summary and Conclusions
This study demonstrates that not only are there sex and CAH-related differences in play styles, but also in the extent to which play style matters when choosing a playmate. We found that the majority of boys and girls with CAH chose playmates based on the preferred play style of the playmate rather than the gender label of the playmate. By contrast, the group of unaffected girls chose playmates based on the playmate’s gender and play style roughly equally. Although there are stronger social sanctions on cross-gender play for boys than there are for girls, these stronger sanctions are unlikely to account for the male-typical pattern displayed by girls with CAH since they are not subject to the sanctions. The implication is that prenatal androgen influences not only preferences for play styles, but also makes these play styles of greater importance when choosing a playmate. The findings also suggest that, although prenatal androgen exposure influences both children’s play style preferences and their playmate preferences, the shift in the masculine direction for playmate preference may be indirect, via the altered preference for a masculine play style. Thus, both in boys and in girls with CAH, prenatal exposure to high levels of androgen may make certain toys and activities so appealing that they drive playmate selection.
Acknowledgments
We thank all of the families in Los Angeles and London whose participation made this study possible. We also thank Dr. Evangelia Charmandari for referring patients to this study as well as those involved in the CAH Support Group in the United Kingdom for their time and efforts. The study was supported by USPHS Grant HD24542 to Melissa Hines, and by funds from Cambridge University. Some of the data were submitted by Vickie Pasterski as part of the requirements for the Ph.D. at City University (London).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vickie Pasterski, University of Cambridge, University of Warwick.
Mitchell E. Geffner, University of Southern California and Children’s Hospital, Los Angeles
Caroline Brain, University College London Hospitals.
Peter Hindmarsh, Charles Brook, University College London.
Charles Brook, University College London.
Melissa Hines, University of Cambridge.
References
- Alexander GM, Hines M. Gender labels and play styles: Their relative contributions to children’s selection of playmates. Child Development. 1994;65:869–879. [PubMed] [Google Scholar]
- Alexander GM, Hines M. Sex differences in response to children’s toys in nonhuman primates (Cercopithecus aethiops sabaeus) Evolution and Human Behavior. 2002;23:467–479. [Google Scholar]
- Auyeung B, Baron-Cohen S, Knickmeyer R, Taylor K, Hackett G, Hines M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychological Science. 2009;20(2):144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychological Science. 1992;3(3):203–206. [Google Scholar]
- Berenbaum SA, Snyder E. Early hormonal influences on childhood sex-typed activity and playmate preferences: Implications for the development of sexual orientation. Developmental Psychology. 1995;31(1):31–42. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Collaer ML, Hines M. Human behavioral sex differences: A role for Gonadal hormones during early development? Psychological Bulletin. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- DiPietro JA. Rough and tumble play: A function of gender. Developmental Psychology. 1981;17:50–58. [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, Börger D, Stegner H, Willig R, Wallis H. Congenital adrenal hyperplasia I: Gender-related behavior and attitudes in female patients and sisters. Psychoneuroendocrinology. 1990;15:401–420. doi: 10.1016/0306-4530(90)90065-h. [DOI] [PubMed] [Google Scholar]
- Eaton WO, Enns LR. Sex differences in human motor activity. Psychological Bulletin. 1986;100:19–28. [PubMed] [Google Scholar]
- Ehrhardt AA, Baker SW. Fetal androgens, human central nervous system differentiation and behavior sex differences. In: Friedman RC, Richardt RM, Vande Wiele RL, editors. Sex differences in behavior. New York: Wiley; 1974. [Google Scholar]
- Eisenberg N, Murray E, Hite T. Children’s reasoning regarding sex-typed toy choices. Child Development. 1982;53:81–86. [Google Scholar]
- Fabes RA, Martin CL, Hanish LD. Young children’s play qualities in same-, other-, and mixed-sex peer groups. Child Development. 2003;74:921–932. doi: 10.1111/1467-8624.00576. [DOI] [PubMed] [Google Scholar]
- Fagot BI. Changes in thinking about early sex role development. Developmental Review. 1985;5(1):83–98. [Google Scholar]
- Fagot BI, Hagan R. Observations of parent reaction to sex-stereotyped behaviors. Child Development. 1991;52:617–628. doi: 10.1111/j.1467-8624.1991.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Fridell SR, Owen-Anderson A, Johnson LL, Bradley SJ, Zucker KJ. The playmate and play style preferences structured interview: A comparison of children with gender identity disorder and controls. Archives of Sexual Behavior. 2006;35:729–737. doi: 10.1007/s10508-006-9085-8. [DOI] [PubMed] [Google Scholar]
- Hassett JM, Siebert ER, Wallen K. Sex differences in rhesus monkey toy preferences parallel those of children. Hormones and Behavior. 2008;54:359–364. doi: 10.1016/j.yhbeh.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. Gonadal hormones and sexual differentiation of human brain and behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2009. [Google Scholar]
- Hines M. Sex and sex differences. In: Zelazo PD, editor. The Oxford Handbook of Developmental Psychology. Oxford: Oxford University Press; 2009. [Google Scholar]
- Hines M, Kaufman FR. Androgen and the development of human sex-typical behavior: Rough-and-tumble play and sex of preferred playmates in children with congenital adrenal hyperplasia (CAH) Child Development. 1994;65:1042–1053. [PubMed] [Google Scholar]
- Hines M, Golombok S, Rust J, Johnston KJ, Golding J. Testosterone during pregnancy and gender role behavior of preschool children: A longitudinal, populations study. Child Development. 2002;73(6):1678–1687. doi: 10.1111/1467-8624.00498. [DOI] [PubMed] [Google Scholar]
- Jacklin CN, Maccoby EE. Social behavior at 33 months in same-sex and mixed-sex dyads. Child Development. 1978;49:557–569. [Google Scholar]
- LaFreniere P, Strayer FF, Gauthier R. The emergence of same-sex affiliative preferences among preschool peers: A developmental/ethological perspective. Developmental Psychology. 1984;55:1958–1965. [Google Scholar]
- Maccoby EE. Gender as a social category. Developmental Psychology. 1988;24:755–765. [Google Scholar]
- Maccoby EE, Jacklin CN. The psychology of sex differences. Stanford, CA: Stanford University Press; 1974. [Google Scholar]
- Maccoby EE, Jacklin CN. Gender segregation in childhood. In: Hayne WR, editor. Advances in child development and behavior. Orlando, FL: Academic Press; 1987. [DOI] [PubMed] [Google Scholar]
- Martin CL, Fabes AF. The stability and consequences of young children’s same-sex peer interactions. Developmental Psychology. 2001;37(3):431–446. [PubMed] [Google Scholar]
- Martin CL, Halverson C. A schematic processing model of sex typing and stereotyping in children. Child Development. 1981;52:1119–1134. [Google Scholar]
- McCarthy MM, De Vries GJ, Forger NJ. Sexual differentiation of the brain: mode, mechanisms, and meaning. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2009. [Google Scholar]
- Meaney MJ, Stewart J. Neonatal androgens influence the social play of prepubescent rats. Hormones and Behavior. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Mischel W. A social learning view of sex differences in behavior. In: Maccoby EE, editor. The development of sex differences. Stanford, CA: Stanford University Press; 1966. [Google Scholar]
- Moller LC, Serbin LA. Antecedents of toddler gender segregation: Cognitive consonance, gender-typed preferences and behavioral compatibility. Sex Roles. 1996;35(7–8):445–460. [Google Scholar]
- Pasterski VL, Geffner M, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Development. 2005;76(1):264–278. doi: 10.1111/j.1467-8624.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Pasterski VL, Geffner M, Brain C, Hindmarsh P, Brook C, Hines M. Increased aggression and activity level in 3- to 11-year old girls with congenital adrenal hyperplasia (CAH) Hormones and Behavior. 2007;52:368–74. doi: 10.1016/j.yhbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini A. Sexual segregation in childhood: a review of evidence for two hypotheses. Animal Behaviour. 2004;68:435–443. [Google Scholar]
- Powlishta KK, Serbin LA, Moller LC. The stability of individual differences in gender typing: Implications for understanding gender segregation. Sex Roles. 1993;29:723–737. [Google Scholar]
- Ruble DN, Martin CL. Gender development. In: Eisenberg N, editor. Handbook of child psychology: Vol. 3. Personality and social development. New York, NY: Wiley; 1998. [Google Scholar]
- Servin A, Nordenström A, Larsson A, Bohlin G. Prenatal androgens and gender-typed behavior: A study of girls with mild and severe forms of congenital adrenal hyperplasia. Developmental Psychology. 2003;39(3):440–450. doi: 10.1037/0012-1649.39.3.440. [DOI] [PubMed] [Google Scholar]
- Serbin LA, Tonick IJ, Sternglanz SH. Shaping cooperative cross-play. Child Development. 1977;48:924–929. [Google Scholar]
- Ward IL, Stehm KE. Prenatal stress feminizes juvenile play patterns in male rats. Physiology and Behavior. 1991;50(3):601–605. doi: 10.1016/0031-9384(91)90552-y. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Stern DN. An early manifestation of differential behavior toward children of the same and opposite sex. The Journal of Genetic Psychology. 1978;133:129–137. [Google Scholar]
- Zucker KJ, Bradley SJ. Gender identity disorder and psychosexual problems in children and adolescents. New York: Guilford Press; 1995. [DOI] [PubMed] [Google Scholar]