Abstract
Background
Patients receiving high-dose chemotherapy and hematopoietic stem cell transplantation (HSCT) experience considerable reductions in physical activity and deterioration of their health status.
Objective
The purpose of this pilot study was to test the effects of strength training compared to usual activity on physical activity, muscle strength, fatigue, health status perceptions, and quality of life following HSCT.
Interventions/Methods
Nineteen subjects were randomized to the exercise or control group. Moderate intensity strength training began following discharge from the hospital. Dependent variables included physical activity, muscle strength, fatigue, health status perceptions and quality of life. Variables were measured prior to admission to the hospital for HSCT, day 8 following HSCT, and six weeks following discharge from the hospital.
Results
Significant time effects were noted for many variables with anticipated declines in physical activity, muscle strength, fatigue, and health status perceptions immediately after HSCT with subsequent improvements six weeks following hospital discharge. One group effect was noted with subjects in the exercise group reporting less fatigue than subjects in the control group. Although no significant interactions were detected, the trends suggest that the exercise group may be more physically active following the intervention compared to the usual activity group.
Conclusions
This study demonstrates the potential positive effects of strength training on physical activity, fatigue, and quality of life in people receiving high-dose chemotherapy and HSCT.
Implications for Practice
Preliminary evidence is provided for using strength training to enhance early recovery following HSCT. Elastic resistance bands are easy to use and relatively inexpensive.
High-dose chemotherapy followed by hematopoietic stem cell transplantation is a potentially curative treatment for a variety of hematologic malignancies, such as acute myeloid leukemia and lymphoma.1 This intensive cancer therapy, however, is associated with significant acute complications, such as serious infections and graft versus host disease. People receiving this treatment often experience considerable deterioration of their health status, particularly during the first 100 days following the transplant.2, 3 Patients demonstrate a marked reduction in physical activity and report severe fatigue, inability to maintain their functional capacity and diminished quality of life.4-7 This deterioration in health status can affect multiple aspects of a person’s life, and the impact may be felt for years following treatment. Without intervention, these problems may lead to physical deconditioning, loss of muscle mass, and decreased strength and endurance, possibly resulting in long-term consequences in people who might otherwise be cured of their underlying malignancy.
The beneficial effects of exercise on physical and psychological health have been well established in people with cancer and include increased physical activity, diminished symptomatology, enhanced functional capacity, and improved quality of life.8-11 Commonly recommended exercises to increase physical activity consist of aerobic exercises, strength training, or a combination of both.12 While aerobic exercise primarily improves cardiorespiratory conditioning, strength training is more effective in minimizing skeletal muscle wasting associated with prolonged physical inactivity.13, 14 A strength training intervention that minimizes muscle wasting associated with prolonged physical inactivity following hematopoietic stem cell transplantation is particularly attractive if the end result is increased physical activity, decreased fatigue and improved health status perceptions and quality of life.
Although the beneficial effects of exercise have been well documented, relatively few exercise studies have been conducted in the hematopoietic stem cell transplant population, and even fewer in the immediate post-transplant period when people are recovering from the intensive cancer therapy.15 Most studies focused on aerobic training.16-18 or a combination of aerobic and strength training.19-21 When aerobic and strength training are combined, however, it is difficult to determine how the individual modality contributes to the overall effect. The ability to detect the individual effects of strength training versus aerobic exercise becomes particularly important when trying to maximize the benefits and minimize the burden associated with exercising. Only one study in the hematopoietic stem cell transplant literature evaluated strength training used alone.22 While beneficial effects were noted, the intervention lasted only 35 days, and information regarding the exercise intensity was not included. No studies found in the literature examined the impact of strength training on physical activity, muscle strength, fatigue, general health perceptions, and quality of life in people receiving hematopoietic stem cell transplants.
The overall purpose of this study was to determine the effects of a strength training program on physical activity, muscle strength, fatigue, health status perceptions, and quality of life in patients following high-dose chemotherapy and hematopoietic stem cell transplantation. This work was conducted in two phases. During the initial pilot study, the strength training intervention was evaluated to determine the acceptability and feasibility of the strength training intervention with respect to the appropriate time for initiating the intervention and the appropriate intensity of the strength training intervention (Hacker, E.D., Larson, J., & Peace, D. (in press). Oncol Nurs Forum). Based on these findings, revisions to the strength training intervention and study methodology were implemented in the second phase. The purpose of the current pilot study, the second phase, was to test the effects of strength training compared to usual activity on physical activity, muscle strength, fatigue, health status perceptions, and quality of life following hematopoietic stem cell transplantation. A secondary purpose was to further evaluate the feasibility of the revised strength training intervention by determining the week-to-week strength training frequency of study participants.
Conceptual Framework
Wilson and Cleary’s Revised Model of Patient Outcomes was used to guide the study, particularly the selection of outcome variables.23, 24 The model proposes the dominant, causal relationships between traditional, biological, and physiological variables to health-related quality of life. In the model, five types of health outcomes are proposed, including a) biological function; b) symptoms; c) functional status; d) general health perceptions; and e) quality of life. In this study, Wilson and Cleary’s model guided the selection of outcome variables, such as symptoms, functional ability, health perceptions, and overall quality of life.
Methods
Design
This pilot study used a two-group, randomized block design to test the effects of a strength training intervention compared to usual activity in people receiving high-dose chemotherapy and hematopoietic stem cell transplantation. Subjects were randomly assigned to strength training or usual activity, stratified by type of transplant: allogeneic or autologous. The strength training intervention, described in detail below, consisted of a comprehensive program of progressive resistance to strengthen the upper body, lower body, and abdominal muscles using elastic resistance bands (Therabands, Hygenic Corp., Akron, OH). Instruction began while the subjects were hospitalized. Subjects began moderate-intensity training immediately following hospital discharge. Training continued for six weeks after discharge from the hospital. The control group continued with their usual rest, physical activity, and exercise as recommended by their attending physicians.
Dependent variables included physical activity, muscle strength, fatigue, health status perceptions, and quality of life. Physical activity was measured using wrist actigraphy (Actiwatch-Scorel®, Phillips Respironics, Bend, OR). Four tests of muscle strength were performed: (1) timed stair climb; (2) hand grip strength; (3) 30-second chair-stand test; and, (4) time needed to stand up from bedrest exam. Fatigue was measured with a one-item fatigue intensity scale, using computerized ecological momentary assessment (real-time assessment) and the fatigue subscale of the European Organization for Research and Training, Quality of Life Questionnaire - Core 30, Version 3.0 (EORTC QLQ-C30).25 Health status perceptions and quality of life were measured with the EORTC QLQ-C3025 and the Quality of Life Index, respectively.26, 27 Variables were measured three times: (1) prior to admission to the hospital for the hematopoietic stem cell transplant (time 1); (2) eight days following hematopoietic stem cell transplantation (time 2); and (3) six weeks following discharge from the hospital (time 3). The full battery of tests was conducted at times 1 and 3. A limited number of tests were conducted at time 2, as it was expected that subjects would be experiencing profound neutropenia and other expected side effects from the high-dose chemotherapy. The study was approved by the Institutional Review Board.
Sample and Setting
Consecutively eligible adult patients electing to undergo a hematopoietic stem cell transplant at a Midwestern academic medical center were invited to participate. Initial eligibility criteria included: (1) patients scheduled to receive a hematopoietic stem cell transplant; (2) ability to speak English; (3) ability to comprehend the purpose of the study; and (4) no history of a psychiatric illness. Subjects eligible to receive a hematopoietic stem cell transplant undergo extensive medical work-up prior to transplantation. All of the pretesting procedures are standard of care and were not considered part of the research. Pretesting included a history and physical, MUGA scans to assess heart function, pulmonary function tests to assess pulmonary function, various blood tests to assess exposure to viruses and kidney, liver, and blood cell function, chest x-rays, urinalysis, and a dental exam. The treating physicians reviewed all the pretests and provided approval for subjects to participate in this study.
Twenty-nine subjects were eligible to participate in the study. Seven subjects declined. Twenty-two subjects enrolled in the study. Two subjects did not receive a hematopoietic stem cell transplant and were no longer eligible to participate. One additional subject was withdrawn from the study because of inability to collect any data during time 1. The subject was an inpatient from the time of informed consent to the time of stem cell transplantation, preventing collection of any time 1 data. Nineteen subjects were randomly assigned to the strength training (n = 9) or usual activity group (n = 10). The demographic data presented below are for the 19 study participants who were randomly assigned to a group and received a hematopoietic stem cell transplant. The sample ranged in age from 20 to 67 (mean = 46.26; sd = 16.23). The subjects were racially diverse; composed of African Americans (n = 11); Caucasians (n = 7) and one Hispanic. Fourteen males and five females participated in the study. The majority were married (n = 9) and completed some college as their highest level of education (n = 10). The majority of subjects (n = 10) reported income levels below $40,000. All subjects received a hematopoietic stem cell transplant for treatment of an underlying malignancy. Thirteen subjects received an autologous transplant (strength training group = 6; control group = 7) and six subjects received an allogeneic transplant (strength training group = 3; control group = 3). Even though the sample size is small, it is considered sufficient for providing pilot study data.
All study participants were outpatients during the pretransplant data collection phase (data collection period: time 1), inpatients during the immediate post-transplant period (data collection period: time 2), and outpatients during the data collection period six weeks following discharge from the hospital (data collection period: time 3). Two subjects, one in the strength training group and one in the control group, expired during the course of the study as a result of their underlying medical condition. The subject in the strength training group that expired did not begin participation in the strength training intervention.
Strength Training Intervention
The strength training intervention consisted of a comprehensive program of progressive resistance to strengthen the upper body, lower body, and abdominal muscles using elastic resistance bands (Therabands®, Hygenic Corp., Akron, OH) and body weight for resistance. Subjects received instruction while they were hospitalized for the hematopoietic stem cell transplant and began moderate-intensity training immediately following discharge from the hospital. Training continued for six weeks. Hospital discharge was chosen as the time to initiate moderate-intensity strength training as HSCT patients are generally considered medically stable at hospital discharge; thus, it is a common safe point to initiate the intervention. The exercise prescription was tailored to the individual’s capabilities. The Borg Rating of Perceived exertion (RPE) scale, a 20-point scale, was used to estimate the intensity of the resistance used.28 The moderate-intensity strength training prescription was based on a rating of somewhat hard (Borg scale 13). Instruction and training were conducted by the principal investigator or one trained member of the research team.
The strength training intervention consisted of 11 pre-selected exercises with concentric and eccentric muscle contractions: (a) eight exercises using elastic resistance bands (chest fly, biceps curl, triceps extension, shoulder shrug, shoulder upright row, shoulder lateral raise, knee flexion, and knee extension) and (b) three exercises that used body weight as resistance (wall push-ups, squats, and bed sit-ups). Subjects were prescribed as many exercises as they could perform, starting with the easiest exercises and advancing to the most difficult. Subjects who were not strong enough to use elastic resistance bands for strength training participated in the study by performing the exercises (concentric/eccentric contractions of muscles) without bands.
A combination supervised/unsupervised approach was used for implementing the strength training. Subjects were seen once or twice a week during their regularly scheduled clinic visit to exercise under the supervision of research staff. They exercised in the clinic exam rooms. Changes to the exercise prescription, mostly advancements, were made at that time, and this facilitated progression with the exercise prescription. Subjects were encouraged to exercise on their own one or two more times during the week for a total of three times per week. Progression of the exercise prescription was structured to first increase the number of sets from 1 to 2 sets of 8-10 repetitions and then to increase the resistance level of elastic bands. Subjects provided return demonstrations of the exercises to ensure proper form and reduce the chance of injury. Subjects documented completion of each individual exercise, including information about the number of repetitions and sets, on preprinted exercise logs. Subjects were queried regarding exercise adherence and tolerance to the exercise prescription during weekly clinic visits.
Usual Activity
In this study, subjects in the usual activity group received recommendations regarding rest, physical activity, and exercise from their attending HSCT physician. Subjects were not told that they could not exercise as this would be considered unethical given the widespread media coverage of the benefits of exercise.
Instrumentation
Physical Activity
Physical activity was objectively measured using a wrist-worn accelerometer, the Actiwatch-Score® (Phillips Respironics, Bend, OR). The accelerometer monitors the occurrence, speed and degree of motion. Successful use of wrist actigraphy to quantify physical activity and sleep disturbances has been reported in several recent studies involving people with cancer, including hematopoietic stem cell transplant patients.6, 29-31
Muscle strength
Timed stair climb
The timed stair climb was used to assess functional quadriceps femoris muscle strength.32 Subjects were timed with a digital stopwatch as they ascended a flight of nine steps. Results are reported in seconds to reach the top of the flight of stairs. The timed stair climb has excellent test-retest reliability (r = 0.94).33 The results for timed stair climbs have been significantly correlated with isokinetic strength measurement of knee flexors and extensors.34
Handgrip strength
Handgrip strength was assessed using a Jamar dynamometer (TEC, Clifton, NJ). Testing was performed with subjects standing with feet hip-distance apart and the shoulder adducted and neutrally rotated. The elbow was flexed at 90 degrees with the forearm in a neutral position and the wrist between 0 and 30 degrees and between 0 and 15 degrees ulnar deviation, according to recommendations.35 The results are reported as the mean of the three trials for the dominant hand.36 Reported test-retest reliability coefficients for handgrip strength are 0.91 for men and 0.94 for women.37 Handgrip strength has been validated as an indicator of upper extremity strength.38, 39
30-second chair-stand test
The 30-second chair-stand test involves recording the number of stands (sitting in a chair to standing erect) that a subject can complete in 30 seconds. A practice trial of one repetition was conducted to ensure proper form. All tests were timed with a stopwatch. The total number of stands that were correctly and fully executed within the 30-second timeframe were used for scoring purposes. If the subject was more than halfway up at the end of the 30-second test, this was scored as a stand. Good stability reliability for the 30-second chair stand has been reported in community-residing older.40 In the same study, the test was validated as an indicator of lower body strength, with moderate correlations between the chair-stand test and the 1-RM (repetition maximum) weight-adjusted leg-press performance.40 Chair-stand performance discriminated between less-active participants and more-active participants, providing support for construct validity through contrasted groups.
Time needed to stand up from bed rest exam
The time needed to stand up from bed rest began with the subject lying supine in bed. Subjects were instructed to stand up as quickly as possibly from the lying position to a standing position with hands at their sides using any independent method of their choice. Two tests were timed with a digital stopwatch. The test with the fastest time was used in the data analysis. This test is reported to have stable test-retest correlations at 1-minute and 1-year intervals.41 Significant correlations between maximum isometric strength of the quadriceps muscle and the time needed to stand up from bed rest in healthy elderly adults provide support for convergent validity.41
Fatigue
Fatigue was measured in real-time using a one-item fatigue intensity scale and the fatigue subscale of the EORTC QLQ-C30.25 The subjective event marker of the Actiwatch-Score®, described above, was programmed to be used as a single-item, global, self-report scale to measure real-time fatigue intensity. The fatigue intensity scores ranged from 1 (no fatigue) to 10 (worst fatigue). Real-time fatigue assessment has been used successfully in people with cancer, including hematopoietic stem cell transplant patients.42, 43 The second measure of fatigue, the fatigue subscale of the EORTC QLQ-C30, consists of three items measuring physical fatigue using a four-point Likert scale. The fatigue subscale of the EORTC QLQ-C30 has been used extensively in people with cancer and has established reliability and validity.44
Health Status Perceptions
The EORTC QLQ-C30, Version 3.0, a 30-item tool, was used to measure the subjects’ perceptions of health status. This is a well-established instrument consisting of five functional scales, a global QOL/health status scale, three multi-item symptom scales, and a number of single-item questions.44 Items on the multi-item subscales are averaged and then converted to a scale with a range of 0 to 100. Higher scores on the five functional scales and the global QOL/health status scale represent a higher level of functioning. Higher scores on the symptom scales and the single-item questions indicate a higher degree of symptomatology, and thus a poorer QOL. The EORTC QLQ-C30 is a widely used, well-established instrument in cancer clinical trials, and the psychometric properties have been previously reported.25, 45
In this study, Cronbach’s alpha coefficients were computed for all multi-item subscales. Adequate reliability (alpha coefficient ≥ .70) was established for most of the subscales (physical functioning = 0.81; role functioning = 0.80; social functioning = 0.85; global QOL/health status = 0.84; fatigue = 0.75; and pain subscale = 0.91). The emotional functioning and cognitive functioning subscales did not demonstrate adequate reliability (Cronbach’s alpha = 0.63 and 0.62, respectively). The means and standard deviations of this subscale are reported for comparison purposes only, and their meaning must be interpreted with caution. Internal consistency estimates for nausea/vomiting was not calculated due to restricted variance at time 1; no subjects reported vomiting at time 1.
Quality of Life
The Ferrans and Powers Quality of Life Index (QLI) was used to measure life satisfaction.26, 27 The QLI is a two-part, 70-item instrument that measures satisfaction with various aspects of life (part 1) as well as the relative importance of each specific aspect to the individual (part 2). The QLI produces an overall QOL score and subscale scores relative to four specific domains: (a) health and functioning; (b) social and economic; (c) psychological/spiritual; and (d) family. Possible scores range from 0 to 30. Higher scores indicate greater satisfaction with life. The QLI is a well-established instrument that has been used extensively in a variety of populations, including cancer, bone marrow, and stem cell transplant patients.26, 46, 47 The psychometric properties have been previously reported.26, 27, 48, 49
In this study, Cronbach’s alpha coefficients were computed for the total QLI and the multi-item subscales. Adequate reliability was established for the total QLI, health and functioning, social and economic, and psychological/spiritual subscales (Cronbach’s alphas = .93, .85, .66, and .82, respectively). The family subscale did not demonstrate adequate reliability. The means and standard deviations for this subscale are reported for comparison purposes only, and their meaning must be interpreted with caution.
Data Collection Schedule and Procedures
Prior to hospitalization for hematopoietic stem cell transplantation (time 1)
Wrist actigraphs were placed on the patient’s nondominant hand to measure physical activity. The subjects were instructed to leave the device in place for the next five days. Because of the expected variability in time associated with placing and removing the wrist actigraph on the first and fifth day, only the middle 72 hours of complete, continuous data were analyzed. Subjects were instructed to carry on with normal activities, including bathing and showering, as the Actiwatch-Score is water-resistant. Subjects rated their fatigue intensity on a 1 (no fatigue) to 10 (worst fatigue) scale three times during the course of the day (10:00 am, 2:00 pm, and 6:00 pm). An alarm on the wrist actigraph sounded at these times to remind patients to complete the data. Subjects were instructed to enter the rating directly into the subjective event marker of the wrist actigraph. At the end of the 5-day period, subjects completed the four muscle strength tests and the questionnaires to measure health status perceptions and quality of life. The questionnaires were administered via interview. The muscle strength tests took approximately 20 minutes to complete, and the questionnaires took approximately 15 minutes.
During hospitalization for stem cell transplantation (day 4–day 8) (time 2)
The procedures for placing and removing the wrist actigraph were the same as described for time 1. The device was placed on the fourth day following the hematopoietic stem cell transplant and removed on day 8. Subjects rated the intensity of fatigue three times per day as described for time 1. The tests for general health perceptions and quality of life were administered via interview on day 8 following the transplant. In addition, subjects completed two tests for muscle strength (30-second chair-stand test, and time needed to stand up from bed rest).
Six weeks following hospital discharge (time 3)
The procedures for data collection six weeks post-hospital discharge were the same as described in time 1, prior to hospitalization for hematopoietic stem cell transplantation.
Data Analysis
All data analyses were performed using SPSS. Descriptive statistics were calculated for all measures for the total sample and for each time period. Internal consistency estimates (Cronbach’s alpha) were assessed for all multi-item Likert items (EORTC QLQ-C30 and QLI). Split plot ANOVA (3 × 2) was used to examine the effects of group assignment (strength training compared to usual activity), time (before hematopoietic stem cell transplantation – time 1, during stem cell transplant – time 2, and six weeks following hospital discharge – time 3), and the group × time interaction on physical activity, muscle strength, fatigue, health status perceptions and quality of life. For those variables that were measured only at time 1 and time 3 (timed stair climb and hand grip strength), 2 × 2 split plot ANOVA was used. No missing data were imputed.
Results
Physical Activity
Means and standard deviations for physical activity are presented in Table 1. No group or interaction effects were observed for physical activity, but there were significant time effects (p < .001). Physical activity significantly decreased during hospitalization for the hematopoietic stem cell transplant, followed by significant increases in physical activity six weeks post hospital discharge. Subjects in the strength training group experienced a 47% reduction in physical activity immediately following the transplant compared to 41% in the usual activity group. Following the strength training intervention, however, physical activity increased by 116% in the strength training group compared to only 88% in the usual activity group.
Table 1.
Means (Standard Deviations) of Physical Activity and Muscle Strength for the Strength Training and Usual Activity Group.
| Time 1 Mean (SD) | Time 2 Mean (SD) | Time 3 Mean (SD) | Group Effect | Time Effect | Time 1 to Time 2 | Time 2 to Time 3 | Time 1 to Time 3 | Interaction Effect | |
|---|---|---|---|---|---|---|---|---|---|
| Physical Activity | ns | p < .001 | p < .001 | p < .001 | ns | ns | |||
| Strength training (n = 6) | 215 (66) | 113 (17) | 245 (80) | ||||||
| Usual activity (n = 8) | 167 (40) | 98 (36) | 184 (62) | ||||||
| Physical Activity | ns | p < .001 | ns | ||||||
| a Strength training (n = 8) | 114 (33) | 257 (80) | |||||||
| Usual activity (n = 8) | 98 (36) | 184 (62) | |||||||
| Muscle Strength | |||||||||
| Timed Stair Climb | ns | ns | ns | ||||||
| Strength training (n = 8) | 5.79 (2.23) | 5.77 (2.39) | |||||||
| Usual Activity (n = 9) | 5.53 (1.07) | 5.44 (1.31) | |||||||
| Hand grip strength | ns | p < .05 | ns | ||||||
| Strength training (n = 8) | 37.4 (11.1) | 34.8 (11.0) | |||||||
| Usual activity (n = 9) | 30.3 (8.4) | 29.0 (8.7) | |||||||
| 30 Second chair stand test | ns | p < .05 | ns | p < .05 | ns | ns | |||
| Strength training (n = 8) | 10.38 (4.54) | 9.06 (5.82) | 10.50 (5.46) | ||||||
| Usual Activity (n = 9) | 12.06 (3.65) | 10.61 (4.95) | 13.11 (4.57) | ||||||
| Time needed to stand up from bedrest | ns | p < .01 | ns | p < .01 | ns | ||||
| Strength training (n = 7) | 3.44 (1.92) | 4.03 (1.97) | 2.79 (1.16) | ||||||
| Usual Activity (n = 9) | 3.59 (2.30) | 3.99 (1.95) | 2.86 (.82) |
Physical activity reported as average physical activity count per minute.
Timed stair climb reported as time in seconds.
Hand grip strength reported as kilograms of force.
30-second chair-stand test reported as the number of sit/stand cycles in 30 seconds.
Time needed to stand up from bedrest exam reported as time in seconds.
A separate, 2 × 2 split plot ANOVA was conducted comparing the exercise and usual activity group at time 2 and time 3 only. Two subjects assigned to the strength training group did not provide five continuous days of physical activity data at time 1 and were not included in the 3 × 2 split plot ANOVA.
A separate, 2 × 2 split plot ANOVA was conducted comparing the exercise and usual activity group at time 2 and time 3 only. Two subjects assigned to the strength training group did not provide five continuous days of physical activity data at time 1 and were not included in the 3 × 2 split plot ANOVA. This may have influenced the above physical activity results. As expected, there was a significant time effect, with physical activity improving six weeks following discharge from the hospital (p < .001). No significant group or group × time effects were found.
Muscle Strength
The means and standard deviations for the timed stair climb, hand grip strength, 30-second chair-stand test, and the time needed to stand up from bedrest exam are presented in Table 1. No group or interaction effects were observed for any of the four muscle tests, but there were significant time effects for hand grip strength (p < .05), 30-second chair-stand test (p < .05), and the time needed to stand up from bedrest (p < .01). The specific time effects are noted in Table 1. The observed time effects were in the expected direction of declining muscle strength immediately following the hematopoietic stem cell transplantation, with either no change from baseline (Time 1) or improvement six weeks following discharge from the hospital.
Fatigue
Means and standard deviations for the fatigue subscale of the EORTC QLQ-C30 are displayed in Table 2. A significant (p < .01) group effect was observed, with the strength training group reporting less fatigue than the usual activity group. A significant time effect (p < .001) was also observed. Subjects reported significantly increased fatigue during hospitalization for the stem cell transplant, with significant improvement six weeks after discharge from the hospital. The interaction effect (group × time) was nonsignificant.
Table 2.
Mean Health Status Perceptions Scores for the Strength Training (n=8) and Usual Activity Group (n=9).
| Time 1 Mean (SD) | Time 2 Mean (SD) | Time 3 Mean (SD) | Group Effect | Time Effect | Time 1 to Time 2 | Time 2 to Time 3 | Time 1 to Time 3 | Interaction Effect | |
|---|---|---|---|---|---|---|---|---|---|
| Global quality of life/health status | ns | p < .05 | ns | ns | ns | ns | |||
| Strength training | 61.5 (15.4) | 56.2 (13.1) | 76.0 (12.2) | ||||||
| Usual Activity | 58.3 (23.6) | 49.0 (23.0) | 58.3 (19.5) | ||||||
| Functional Subcales | |||||||||
| Physical | ns | p < .05 | ns | ns | ns | ns | |||
| Strength training | 82.5 (16.7) | 75.0 (19.4) | 85.8 (13.1) | ||||||
| Usual Activity | 77.8 (22.1) | 70.4 (28.5) | 83.7 (23.1) | ||||||
| Emotional | ns | p < .01 | p < .05 | p < .01 | ns | ns | |||
| Strength training | 87.5 (12.6) | 66.7 (27.8) | 88.5 (10.9) | ||||||
| Usual Activity | 74.1 (15.3) | 63.9 (8.3) | 85.2 (21.2) | ||||||
| Role | ns | p < .001 | p < .01 | p < .001 | ns | ns | |||
| Strength training | 66.7 (38.8) | 18.8 (25.9) | 75.00 (17.8) | ||||||
| Usual Activity | 59.3 (38.3) | 24.1 (27.8) | 70.4 (35.1) | ||||||
| Cognitive | ns | p < .01 | p = .08 | p < .01 | ns | ns | |||
| Strength training | 95.8 (7.7) | 83.3 (12.6) | 95.8 (7.7) | ||||||
| Usual Activity | 81.5 (25.6) | 70.4 (21.7) | 85.2 (21.2) | ||||||
| Social | ns | p < .001 | p < .01 | p < .001 | ns | ns | |||
| Strength training | 62.5 (27.8) | 18.8 (20.8) | 81.3 (22.6) | ||||||
| Usual Activity | 66.7 (31.2) | 46.3 (32.0) | 77.8 (34.4) | ||||||
| Symptom Subscales | |||||||||
| Fatigue | p < .01 | p < .001 | p < .001 | p < .001 | ns | ns | |||
| Strength training | 30.6 (15.4) | 72.2 (17.8) | 23.6 (13.9) | ||||||
| Usual Activity | 45.7 (18.8) | 82.7 (13.7) | 46.9 (24.1) | ||||||
| Pain | ns | p < .001 | p < .01 | p < .05 | ns | ns | |||
| Strength training | 20.8 (24.8) | 39.5 (28.1) | 18.8 (20.8) | ||||||
| Usual Activity | 25.9 (25.2) | 75.9 (23.7) | 35.2 (36.8) | ||||||
| Nausea/Vomiting | ns | p < .001 | p < .001 | p < .001 | ns | ns | |||
| Strength training | 2.1 (5.9) | 50.0 (37.8) | 0.0 (0.0) | ||||||
| Usual Activity | 3.7 (7.4) | 33.3 (26.4) | 1.9 (5.6) | ||||||
| Single Items | |||||||||
| Appetite loss | ns | p < .001 | p < .001 | p < .01 | ns | ns | |||
| Strength training | 16.7 (25.2) | 62.5 (37.5) | 12.5 (17.3) | ||||||
| Usual Activity | 3.70 (11.1) | 63.0 (38.9) | 18.5 (24.2) | ||||||
| Constipation | ns | ns | ns | ||||||
| Strength training | 8.3 (15.4) | 25.0 (38.8) | 4.2 (11.8) | ||||||
| Usual Activity | 3.70 (11.11) | 3.70 (11.11) | 0.00 (0.00) | ||||||
| Dyspnea | ns | ns | ns | ||||||
| Strength training | 16.7 (17.8) | 16.7 (17.82) | 12.5 (17.3) | ||||||
| Usual Activity | 18.5 (17.6) | 22.2 (37.3) | 11.1 (16.7) | ||||||
| Diarrhea | ns | p < .001 | p < .001 | p < .001 | ns | ns | |||
| Strength training | 4.2 (11.8) | 62.5 (37.5) | 0.0 (0.0) | ||||||
| Usual Activity | 7.4 (22.2) | 51.9 (47.5) | 11.1 (23.6) | ||||||
| Financial Stress | ns | ns | ns | ||||||
| Strength training | 54.2 (39.6) | 54.2 (35.7) | 50.0 (35.6) | ||||||
| Usual Activity | 33.3 (37.3) | 37.0 (38.9) | 29.6 (30.9) | ||||||
| Sleep Disturbances | ns | p < .001 | p < .01 | p < .001 | ns | ns | |||
| Strength training | 29.2 (33.0) | 62.5 (37.5) | 25.0 (23.6) | ||||||
| Usual Activity | 22.2 (23.6) | 63.0 (38.9) | 18.5 (17.6) |
The real-time fatigue intensity ratings were classified as mild (rating of 1-3), moderate (4-6), or severe (7-10), based on recommendations from the National Comprehensive Cancer Network.50 The nine fatigue intensity ratings (three times per day/3 days) were averaged for every patient for each data collection period. Only subjects who completed 7 of the 9 assessments during the specific data collection period were included in the analysis. The real-time fatigue data are displayed in Figure 1. An equal number of subjects rated their fatigue as mild (47%) or moderate (47%) prior to transplant. Following transplant, most subjects rated their fatigue as moderate (41%) or severe (41%). After discharge from the hospital, 69% rated their fatigue as mild. There was little difference in fatigue intensity ratings, particularly at time 3 where five out of eight in the exercise group and six of nine in the usual activity group reported their fatigue as mild.
Figure 1.
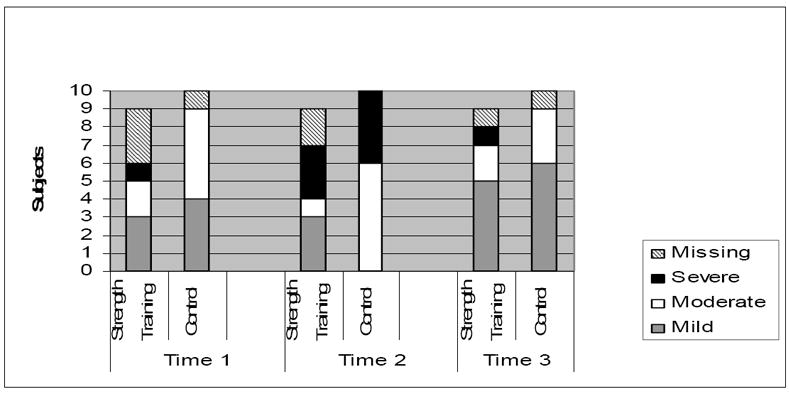
Real-time Fatigue Data for the Strength Training (n = 9) and Usual Activity Groups (n = 10).
Time 1: Missing Data
Strength Training: Two subjects were not included in the analysis due to inability to collect 5 full days of continuous data. One subject did not complete 7 of the required 9 assessments.
Control: One subject did not complete 7 of the required 9 assessments.
Time 2 Missing Data
Strength Training: One subject did not complete 7 of the required 9 assessments. One subject was unable to provide self-report assessments because of acuity of illness.
Time 3 Missing Data
Strength Training: One patient expired.
Control: One patient expired.
Health Status Perceptions
Means and standard deviations for the EORTC QLQ-C30 are displayed in Table 2. There were no significant group or group × time interaction effects for any of the subscales or single-item questions. Significant time effects were noted for all functional subscales (physical, p < .05; role, p < .001; emotional, p < .005; cognitive, p < .01; and social functioning, p < .001), global QOL/health status (p < .05), all multi-item symptom subscales (fatigue reported previously, p < .001; pain, p < .001; and nausea/vomiting, p < .001), and three single-item symptom questions (appetite loss, p < .001; diarrhea, p < .001; and sleep disturbance, p < .001). Subjects reported significant declines in role, emotional, and cognitive functioning during hospitalization for the stem cell transplant. This decline in functioning coincided with significant increases in symptomatology. Patients reported increased fatigue, pain, nausea/vomiting, appetite loss, sleep disturbance, and diarrhea in the immediate post-transplant period. Six weeks following discharge from the hospital, subjects reported significant improvements in physical, role, emotional, cognitive and social functioning. This coincided with improvements in symptomatology, with subjects reporting decreased fatigue, pain, nausea/vomiting, appetite loss, sleep disturbance, and diarrhea six weeks following hospital discharge. Global quality of life/health status improved six weeks following the transplant compared to during hospitalization.
Quality of Life (Life Satisfaction)
Means and standard deviations for overall quality of life and subscales are displayed in Table 3. No group, time or interaction effects were observed.
Table 3.
Mean Quality of Life for the Strength Training (n = 8) and Usual Activity Groups (n = 9).
| Time 1 | Time 2 | Time 3 | |
|---|---|---|---|
| Overall Quality of Life | |||
| Strength Training Group | 22.9 (2.3) | 23.3 (4.6) | 23.3 (4.2) |
| Usual Activity Group | 20.4 (5.3) | 21.0 (4.3) | 22.3 (4.9) |
| Health and functioning | |||
| Strength Training Group | 21.4 (2.8) | 20.4 (5.9) | 22.6 (6.0) |
| Usual Activity Group | 17.9 (7.5) | 17.8 (6.2) | 20.3 (7.3) |
| Psychological/Spiritual | |||
| Strength Training Group | 25.2 (3.6) | 25.9 (3.6) | 26.4 (3.2) |
| Usual Activity Group | 21.8 (5.0) | 22.5 (3.6) | 22.6 (5.2) |
| Social and Economic | |||
| Strength Training Group | 21.0 (5.5) | 24.6 (9.9) | 19.6 (4.7) |
| Usual Activity Group | 20.9 (5.8) | 22.2 (5.2) | 22.6 (4.7) |
| Family | |||
| Strength Training Group | 26.6 (2.0) | 25.8 (2.9) | 26.4 (2.1) |
| Usual Activity Group | 25.0 (2.3) | 26.4 (2.8) | 26.8 (2.2) |
Feasibility of the Strength Training Program
The feasibility of the strength training intervention was evaluated to determine if modifications to the strength training program were required. Feasibility was evaluated by determining the week-to-week strength training frequency of study participants (see Figure 2). By week 2, all subjects in the strength training group exercised at least 1-2 times per week, and most met the strength training prescription of exercising 3 times per week by week 3. All of the subjects exercised at least 1-2 times per week for at least 5 of the 6 weeks.
Figure 2.
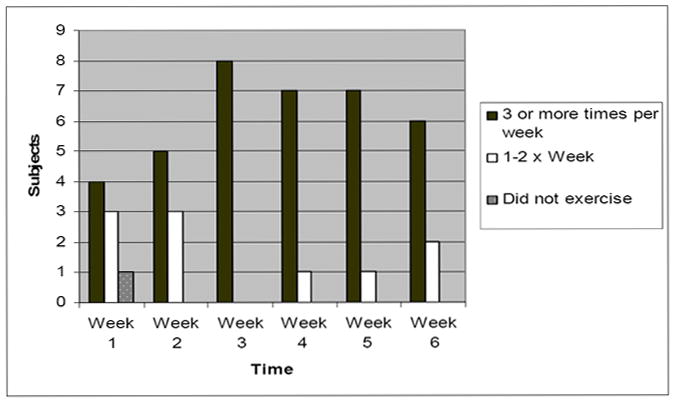
Strength Training Frequency (n = 8).
Discussion
The link between exercise and improved physical and psychological health has been well established in people with cancer.8-11 Questions still remain, however, about the type of exercise that is best for achieving specific health outcomes across the cancer care trajectory. This pilot study is unique in that a strength training intervention was tested in people who received high-dose chemotherapy and hematopoietic stem cell transplantation, a cancer treatment associated with significant morbidity and mortality, and the subjects were very early in their recovery. The findings from this pilot study demonstrate the potential positive effects of strength training on physical activity and fatigue, and these preliminary findings warrant further investigation in a larger randomized controlled study. This pilot study further demonstrates that strength training using elastic resistance bands is both acceptable and feasible in this population when considered in conjunction with our phase 1 results (Hacker, E.D., Larson, J., & Peace, D. (in press). Oncol Nurs Forum).
Significant time effects were found for almost all of the variables of interest, with anticipated declines in physical activity, muscle strength, fatigue, and health status perceptions following high-dose chemotherapy and hematopoietic stem cell transplantation. Six weeks after hospital discharge, subjects in both groups experienced subsequent improvements in many of these same variables. This trajectory of recovery was expected and is consistent with our previous work, as well as the work of others.4, 5, 47
With respect to differences between the exercise and usual activity groups, only one finding was statistically significant; subjects in the strength training group reported less fatigue compared to subjects in the usual activity group. Although not statistically significant, the subjects in the exercise group trended toward increased physical activity. These preliminary data are promising in that they provide support for further investigation into the effects of strength training on fatigue and physical activity in a full-scale, sufficiently powered study.
The most important data collection point for this study was time 3, six weeks following hospital discharge. It was hypothesized that the strength training intervention would have resulted in a group × time interaction effect (see Figure 3), with the strength training group exhibiting enhanced recovery compared to the usual activity group, specifically at time 3. Although no significant interaction effects were detected for any of the dependent variables, the trends suggest that subjects in the strength training group may be more physically active six weeks after hospital discharge compared to the usual activity group. These data are displayed in Figure 4. This pilot study was not powered for statistical significance but the trends provide support for continued investigation.
Figure 3.
Expected Data Trends for Variables.
Figure 4.
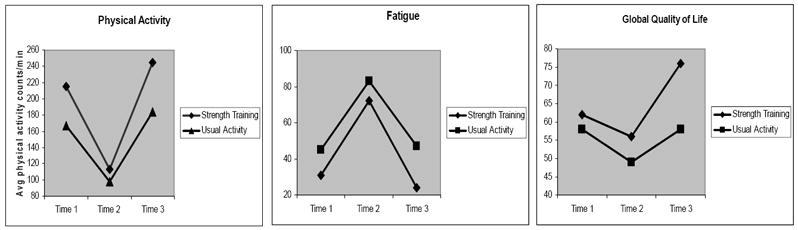
Data trends for physical activity, fatigue, and quality of life.
Mean differences between groups on the EORTC QLQ-30 multi-item subscales were compared to previously published criteria,51, 52 particularly at time 3. Data trends similar to physical activity, albeit not statistically significant, were found for fatigue and global quality of life, with the largest difference between the two groups occurring after the strength training intervention (Figure 4). Subjects in the strength training group trended toward less fatigue and better quality of life compared to the usual activity group six weeks following discharge from the hospital. Although these results must be interpreted with caution, the consistent trends for physical activity, fatigue, and quality of life suggest that strength training in a clinic or home setting may be a viable option to enhance recovery for hematopoietic stem cell transplant patients.
While there were statistically significant changes over time for three of the four muscle strength tests (hand grip strength, 30-second chair-stand test, and the time needed to stand up from bedrest exam), the exercise group compared to the usual activity group did not perform significantly better on any of the four tests six weeks following hospitalization. A number of possible reasons for this exist, such as the small sample size or diffusion of the treatment across groups. One major challenge associated with testing an exercise intervention in a randomized clinical trial setting is deciding upon an appropriate control group. In this study, subjects in the usual activity were instructed to carry on with their usual activities as prescribed by the attending physician. Subjects were not explicitly informed that they could not exercise, as this would have been considered inappropriate given the potential benefits of exercise that are frequently reported in the lay media. While most subjects in the control group did not participate in any structured exercise activity, two subjects chose to engage in strength training exercises on their own starting approximately four weeks following hospital discharge, possibly creating a confounding effect.
Researchers evaluating interventions to reduce fatigue rely on self-report mechanisms to collect information regarding the hematopoietic stem cell transplant patients’ experience with this distressing and long lasting symptom. This study used two methods for collecting fatigue data: real-time fatigue assessments and a traditional, retrospective, questionnaire (the fatigue subscale of the EORTC QLQ C-30). Incorporating real-time fatigue assessments into the study helps minimize problems with recall biases (fatigue remembered to be better or worse than actually experienced). Recall biases may be particularly problematic in people experiencing multiple symptoms simultaneously, such as hematopoietic stem cell transplant patients. Additionally, real-time fatigue assessment allows data to be collected in the subject’s natural environment reducing the problem with artificial contexts. Conversely, collecting fatigue data via the EORTC QLQ C-30’s fatigue subscale, a well-established and frequently employed questionnaire, allows fatigue data to be compared across multiple studies, including those testing other potentially effective interventions for fatigue.
Nursing Implications
In order to increase the likelihood of successful patient outcomes, deciding upon an appropriate exercise intervention requires detailed knowledge of the cancer treatment and expected recovery period. This intervention was designed to dovetail seamlessly into clinical practice if proven to be effective. This study provides some preliminary evidence for the use of strength training using elastic resistance bands following high-dose chemotherapy and hematopoietic stem cell transplantation. Elastic resistance bands were chosen because the bands are portable, easy to use, and relatively inexpensive compared to hand weights or free-standing machines, thus facilitating translation into clinical practice. A clinic- or home-based exercise program is particularly appealing in the early phase of recovery following high-dose chemotherapy and hematopoietic stem cell transplantation, due to physical or time restraints that preclude most patients’ ability to exercise in a gym or health fitness facility. The opportunity to take advantage of scheduled clinic visits to implement a strength training program in patients who require frequent clinic visits makes sense from a patient-centered point of view.
Conclusions
Conducting pilot work, such as this strength training study, must be undertaken prior to initiating large-scale investigations in order to avoid costly mistakes while potentially improving quality and efficiency--important considerations given shrinking health care budgets. This study demonstrates the potential positive effects of strength training on physical activity, fatigue, and quality of life in people receiving high-dose chemotherapy and hematopoietic stem cell transplantation. The ability of the subjects in the strength training group to participate provides further support for this type of exercise program. While the findings from this study are preliminary, they do provide support for continued investigation on the effects of strength training on physical activity, fatigue and global quality of life.
Acknowledgments
This work was supported with funding from the National Institutes of Health, National Institute of Nursing Research (PI, E. Hacker; K01 NR009375).
References
- 1.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: Part I CIBMTR summary slides. CIBMTR Newsletter [serial online] 2009;15:7–11. [Google Scholar]
- 2.Bevans MF, Mitchell SA, Marden S. The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT) Support Care Cancer. 2008 Nov;16(11):1243–1254. doi: 10.1007/s00520-008-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson KO, Giralt SA, Mendoza TR, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. Bone Marrow Transplant. 2007 Jun;39(12):759–766. doi: 10.1038/sj.bmt.1705664. [DOI] [PubMed] [Google Scholar]
- 4.Sherman AC, Simonton S, Latif U, Plante TG, Anaissie EJ. Changes in quality-of-life and psychosocial adjustment among multiple myeloma patients treated with high-dose melphalan and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2009 Jan;15(1):12–20. doi: 10.1016/j.bbmt.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Andersson I, Ahlberg K, Stockelberg D, Brune M, Persson LO. Health-related quality of life in patients undergoing allogeneic stem cell transplantation after reduced intensity conditioning versus myeloablative conditioning. Cancer Nurs. 2009 Jul-Aug;32(4):325–334. doi: 10.1097/NCC.0b013e31819b5c81. [DOI] [PubMed] [Google Scholar]
- 6.Hacker ED, Ferrans C, Verlen E, et al. Fatigue and physical activity in patients undergoing hematopoietic stem cell transplant. Oncol Nurs Forum. 2006 May;33(3):614–624. doi: 10.1188/06.ONF.614-624. [DOI] [PubMed] [Google Scholar]
- 7.Mosher CE, Redd WH, Rini CM, Burkhalter JE, DuHamel KN. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology. 2009 Feb;18(2):113–127. doi: 10.1002/pon.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999 Spring;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 9.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005 Feb 1;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 10.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005 Jun 1;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 11.Stevinson C, Lawlor DA, Fox KR. Exercise interventions for cancer patients: systematic review of controlled trials. Cancer Causes Control. 2004 Dec;15(10):1035–1056. doi: 10.1007/s10552-004-1325-4. [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007 Aug;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 13.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998 Jun;30(6):975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 14.American College of Sports Medicine Progression Models in Resistance Training for Healthy Adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 15.Wiskemann J, Huber G. Physical exercise as adjuvant therapy for patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008 Feb;41(4):321–329. doi: 10.1038/sj.bmt.1705917. [DOI] [PubMed] [Google Scholar]
- 16.Dimeo F, Bertz H, Finke J, Fetscher S, Mertelsmann R, Keul J. An aerobic exercise program for patients with haematological malignancies after bone marrow transplantation. Bone Marrow Transplant. 1996 Dec;18(6):1157–1160. [PubMed] [Google Scholar]
- 17.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997 Nov 1;90(9):3390–3394. [PubMed] [Google Scholar]
- 18.Carlson LE, Smith D, Russell J, Fibich C, Whittaker T. Individualized exercise program for the treatment of severe fatigue in patients after allogeneic hematopoietic stem-cell transplant: a pilot study. Bone Marrow Transplant. 2006 May;37(10):945–954. doi: 10.1038/sj.bmt.1705343. [DOI] [PubMed] [Google Scholar]
- 19.Coleman EA, Coon S, Hall-Barrow J, Richards K, Gaylor D, Stewart B. Feasibility of exercise during treatment for multiple myeloma. Cancer Nurs. 2003 Oct;26(5):410–419. doi: 10.1097/00002820-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Hayes S, Davies PS, Parker T, Bashford J. Total energy expenditure and body composition changes following peripheral blood stem cell transplantation and participation in an exercise programme. Bone Marrow Transplant. 2003 Mar;31(5):331–338. doi: 10.1038/sj.bmt.1703867. [DOI] [PubMed] [Google Scholar]
- 21.Jarden M, Baadsgaard MT, Hovgaard DJ, Boesen E, Adamsen L. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Transplant. 2009 May;43(9):725–737. doi: 10.1038/bmt.2009.27. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham BA, Morris G, Cheney CL, Buergel N, Aker SN, Lenssen P. Effects of resistive exercise on skeletal muscle in marrow transplant recipients receiving total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1986 Nov-Dec;10(6):558–563. doi: 10.1177/0148607186010006558. [DOI] [PubMed] [Google Scholar]
- 23.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995 Jan 4;273(1):59–65. [PubMed] [Google Scholar]
- 24.Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. J Nurs Scholarsh. 2005;37(4):336–342. doi: 10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 25.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 Mar 3;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 26.Ferrans C. Development of a quality of life index for patients with cancer. Oncol Nurs Forum. 1990;17(3 suppl):15–19. [PubMed] [Google Scholar]
- 27.Ferrans CE, Powers MJ. Quality of life index: development and psychometric properties. ANS Adv Nurs Sci. 1985 Oct;8(1):15–24. doi: 10.1097/00012272-198510000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Borg G. Borg’s perceived exertion and pain scales. Stockholm: Human Kinetics; 1998. [Google Scholar]
- 29.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998 Jan-Feb;25(1):51–62. [PubMed] [Google Scholar]
- 30.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999 May;17(5):320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 31.Sarna L, Conde F. Physical activity and fatigue during radiation therapy: a pilot study using actigraph monitors. Oncol Nurs Forum. 2001 Jul;28(6):1043–1046. [PubMed] [Google Scholar]
- 32.Sowers M, Jannausch ML, Gross M, et al. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoarthritis. Am J Epidemiol. 2006 May 15;163(10):950–958. doi: 10.1093/aje/kwj109. [DOI] [PubMed] [Google Scholar]
- 33.Nyland J, Frost K, Quesada P, et al. Self-reported chair-rise ability relates to stair-climbing readiness of total knee arthroplasty patients: a pilot study. J Rehabil Res Dev. 2007;44(5):751–759. doi: 10.1682/jrrd.2006.11.0146. [DOI] [PubMed] [Google Scholar]
- 34.Salem GJ, Wang MY, Young JT, Marion M, Greendale GA. Knee strength and lower- and higher-intensity functional performance in older adults. Med Sci Sports Exerc. 2000 Oct;32(10):1679–1684. doi: 10.1097/00005768-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Mathiowetz V, Rennells C, Donahoe L. Effect of elbow position on grip and key pinch strength. J Hand Surg [Am] 1985 Sep;10(5):694–697. doi: 10.1016/s0363-5023(85)80210-0. [DOI] [PubMed] [Google Scholar]
- 36.Trossman PB, Suleski KB, Li PW. Test-retest reliability and day to day variability of an isometric grip strength test using the work simulator. Occup Ther J Research. 1990;10:266–279. [Google Scholar]
- 37.Reddon JR, Stefanyk WO, Gill DM, Renney C. Hand dynamometer: effects of trials and sessions. Percept Mot Skills. 1985 Dec;61(3 Pt 2):1195–1198. doi: 10.2466/pms.1985.61.3f.1195. [DOI] [PubMed] [Google Scholar]
- 38.Balogun JA, Akomolafe CT, Amusa LO. Grip strength: effects of testing posture and elbow position. Arch Phys Med Rehabil. 1991 Apr;72(5):280–283. [PubMed] [Google Scholar]
- 39.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990 May;45(3):M82–88. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- 40.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999 Jun;70(2):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 41.Maeda A, Yuasa T, Nakamura K, Higuchi S, Motohashi Y. Physical performance tests after stroke: reliability and validity. Am J Phys Med Rehabil. 2000 Nov-Dec;79(6):519–525. doi: 10.1097/00002060-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Curran SL, Beacham AO, Andrykowski MA. Ecological momentary assessment of fatigue following breast cancer treatment. J Behav Med. 2004 Oct;27(5):425–444. doi: 10.1023/b:jobm.0000047608.03692.0c. [DOI] [PubMed] [Google Scholar]
- 43.Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007 Mar;33(3):267–275. doi: 10.1016/j.jpainsymman.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Aaronson NK, Cull AN, Kaasa S, Sprangers MA. Quality of life and pharmacoeconomics. 2. Philadelphia: Lipponcott-Raven.; 1996. [Google Scholar]
- 45.Osoba D, Zee B, Pater J, Warr D, Kaizer L, Latreille J. Psychometric properties and responsiveness of the EORTC quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual Life Res. 1994 Oct;3(5):353–364. doi: 10.1007/BF00451727. [DOI] [PubMed] [Google Scholar]
- 46.Belec RH. Quality of life: perceptions of long-term survivors of bone marrow transplantation. Oncol Nurs Forum. 1992 Jan-Feb;19(1):31–37. [PubMed] [Google Scholar]
- 47.Hacker ED, Ferrans CE. Quality of life immediately after peripheral blood stem cell transplantation. Cancer Nurs. 2003 Aug;26(4):312–322. doi: 10.1097/00002820-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Hughes KK. Psychosocial and functional status of breast cancer patients. The influence of diagnosis and treatment choice. Cancer Nurs. 1993 Jun;16(3):222–229. [PubMed] [Google Scholar]
- 49.Anderson JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome. J Nerv Ment Dis. 1997 Jun;185(6):359–367. doi: 10.1097/00005053-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Mock V, Atkinson A, Barsevick A, et al. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology (Huntingt) 2000 Nov;14(11A):151–161. [PubMed] [Google Scholar]
- 51.King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996 Dec;5(6):555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 52.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998 Jan;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]


