Abstract
Herpes simplex virus entry into cells requires the binding of envelope glycoprotein D (gD) to an entry receptor. Depending on the cell, entry occurs by different mechanisms, including fusion at the cell surface or endocytosis. Here we examined the entry mechanism through a non-HSV receptor mediated by a soluble bi-specific adapter protein composed of recognition elements for gD and the EGF receptor (EGFR). Virus entered into endosomes using either EGF or an EGFR-specific single chain antibody (scFv) for receptor recognition. Infection was less efficient with the EGF adapter which could be attributed to its weaker binding to viral gD. Infection mediated by the scFv adapter was pH sensitive, indicating that gD-EGFR bridging alone was insufficient for capsid release from endosomes. We also show that the scFv adapter enhanced infection of EGFR-expressing tumor tissue in vivo. Our results indicate that adapters may retarget HSV infection without drastically changing the entry mechanism.
Keywords: HSV entry, bispecific adapter, EGF receptor, endocytosis, fusion
Introduction
HSV-1 entry into cells is initiated by the binding of envelope glycoprotein D (gD) to one of several entry receptors, mainly nectin-1, a cell adhesion molecule, and HVEM, a member of the TNF receptor family (Campadelli-Fiume et al., 2007; Spear, 2004; Spear, Eisenberg, and Cohen, 2000). The route of HSV entry can vary between cells even when the same entry receptor is used, suggesting that the membrane environment and associated cell properties are key determinants of the entry pathway. Vero cells expressing endogenous nectin-1 and HVEM are infected by viral envelope fusion with the plasma membrane, resulting in direct delivery of capsids into the cytoplasm (Spear, 1993). Chinese hamster ovary (CHO) cells are resistant to HSV-1 infection due to the absence of entry receptors, but ectopic expression of nectin-1 or HVEM allows infection via acidifying endosomes (Nicola, McEvoy, and Straus, 2003). Virus internalization by B78H1 mouse melanoma cells also involves endocytosis, but exposure to an acidic milieu is not required for penetration (Milne et al., 2005). In addition, a mechanism resembling phagocytosis has been described (Clement et al., 2006).
It was previously reported that replacement of the transmembrane and cytoplasmic regions of nectin-1 ectopically expressed in baby hamster kidney J1.1-2 cells with the corresponding regions of EGFR switched the HSV entry pathway from a pH-independent to a pH-dependent mechanism (Gianni, Campadelli-Fiume, and Menotti, 2004). While entry in this study involved virus binding to the nectin-1 ectodomain displayed on the cell surface, we have explored the mode of HSV entry when the virus binds to a non-HSV receptor, full-length EGFR, via a soluble bridging molecule (adapter).
We previously reported that an adapter composed of the gD-binding nectin-1 variable (V) domain and a single-chain antibody (scFv) recognizing the EGFR mediated efficient infection of EGFR-transduced CHO-K1 cells that lack normal gD receptors (Nakano et al., 2005). Here we show that adapter-mediated EGFR-dependent infection of both J1.1-2 and CHO-K1 cells takes place by low pH-dependent endocytosis. Thus indirect gD binding to a novel receptor has the same effect on the viral entry mechanism into J1.1-2 cells as direct gD interaction with chimeric nectin-1/EGFR. However, since HSV infection of nectin-1-transduced CHO-K1 cells occurs by low pH-dependent endocytosis, a change in entry mechanism is not obligatory for adapter-mediated infection. We also report that replacement of the scFv portion of the adapter with EGF weakened the adapter-gD interaction and reduced EGFR-dependent virus entry. Experiments to determine the effect of the scFv adapter in vivo demonstrated increased infection of EGFR-bearing tumor tissue. Our results suggest that adapters do not fundamentally change the mechanism of HSV infection and can be utilized for enhanced gene delivery to targeted cell populations in vivo.
Materials and Methods
Cell lines
Chinese hamster ovary cells (CHO-K1, ATCC, CCL-61) were maintained in Ham's F12K medium (Gibco-Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco-Invitrogen). CHO-gD (Kwon et al., 2006) and CHO-EGFR (a kind gift from H. Ebina, Tokushima University, Japan) are CHO-K1 derivatives constitutively expressing HSV-1 gD and the human EGFR, respectively. Both cell lines were grown in the above medium containing 400 μg/ml G418 (Gibco-Invitrogen). HSV-1-resistant J1.1-2 cells (kindly provided by G. Campadelli-Fiume, Bologna, Italy) were grown in Dulbecco's MEM (Gibco-Invitrogen) containing 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. J-EGFR cells were generated by transfection of J1.1-2 cells with a human EGFR expression plasmid (EGFR/pCx5 ori, a gift from I. Pastan, NIH, Bethesda, MD) and cloning of G418-resistant cells. Human lung cancer A549, glioblastoma U-87MG, and breast cancer MCF-7 cells were purchased from ATCC (Manassas, VA).
Virus
QOZHG is a replication-defective derivative of HSV-1 strain KOS (Chen et al., 2000). Briefly, QOZHG is deleted for the immediate early (IE or α) genes ICP4 and ICP27 and expresses the ICP22 and ICP47 IE genes from early (E or β) promoters. The virus contains an expression cassette for bacterial β-galactosidase in the UL41 locus and for enhanced green fluorescent protein (eGFP) in UL54 (ICP27). QOZHG was grown and titered on ICP4/ICP27-complementing 7b cells (Krisky et al., 1997).
Adapter proteins
1) Plasmid constructs
The bacterial expression construct pRA-V-528LH for production of the scFv adapter was previously described (Nakano et al., 2005). An expression construct for the EGF adapter, termed pRA-V-EGF, was derived from pRA-V-528LH by replacing the XhoI-SacII fragment spanning the scFv region with the complete EGF open reading frame (Fig. 1A). The EGF fragment was obtained by PCR using a human EGF cDNA plasmid (kindly provided by I. Dmitriev, University of Alabama) as template and a pair of primers that added a XhoI site immediately upstream of the open reading frame and a SacII site immediately downstream. The primer sequences were as follows: forward, 5′-GGGGCT CGAGAATAGTGACTCTGAATGTCCCCTGTCC-3′; reverse, 5′-CTGCAGCCGCGGAG CGCAGTTCCCACCACTTCAGGTCTCG-3′.
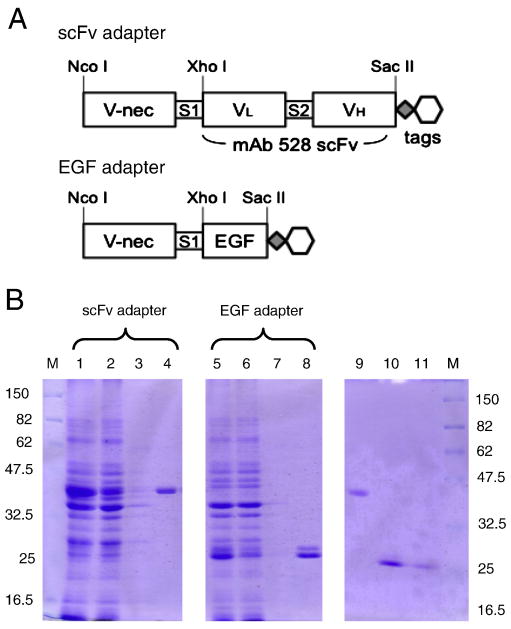
Figure 1. Adapter structures and production.
(A) Structure of the scFv and EGF adapters. V-nec, nectin-1 V-domain; S1 and S2, spacers; VL, light chain variable region of anti-EGFR monoclonal antibody 528; VH, heavy chain variable region of mAb 528. Both proteins have a C-terminal c-Myc (diamond) and His6 (hexagon) tag. (B) Coomassie-stained SDS-PAGE gels of bacterial protein samples at different stages of adapter purification. Lanes (1, 5), solubilized intracellular insoluble fraction (sICIS) prepared as in Nakano et al., 2005; (2, 6), Ni2+ column flow-through; (3, 7), column wash; (4, 8), column eluate; (9), refolded scFv adapter; (10), refolded EGF adapter; (11) refolded EGF adapter, half the amount of lane 11. Size markers (M) are in kDa.
2) Production of adapter proteins
Adapter proteins were produced as previously described (Nakano et al., 2005). Briefly, E. coli BL21 (DE3) cells were transformed with pRA-V-EGF or pRA-V-528LH and grown at 28°C in 2xYT broth supplemented with 100 μg/mL ampicillin. Expression of recombinant proteins (EGF- or scFv-adapter, respectively) was induced by addition of 1 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG). After overnight culture, bacterial pellets were collected by centrifugation (2,000 × g, 35 min) and solubilized with 10 ml of 6 M guanidinium hydrochloride (Sigma-Aldrich, St. Louis, MO) in PBS (Gu-HCl/PBS) overnight at 4°C. Protein samples were purified on a 2 ml TALON metal affinity column (Clontech, Palo Alto, CA) by elution with Gu-HCl/PBS containing 250mM imidazole. The recovered protein was refolded by sequential dialysis against Gu-HCl/PBS and PBS (pH 7.9), centrifuged at 4,500 × g for 20 min at 4°C, and sterilized by filtration through a 0.22 μm ultrafiltration membrane (Millipore, Tokyo, Japan).
HSV-1 entry assays
Cells were grown to confluence in 96-well plates (5 × 104 cells/well) in medium containing 10% FBS. QOZHG virus and adapters were inoculated with the cells in 40 μL of serum-free medium for 2 h at 37°C. The cells were washed once with PBS, overlaid with fresh 10% FBS medium, and incubated for 16 h at 37°C. Entry was evaluated as previously described (Nakano et al., 2005), including: (i) visual inspection of monolayers for GFP reporter gene expression under a fluorescent microscope; (ii) staining of fixed cultures with β-galactosidase colorimetric substrate, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal, 0.2 mg/ml; Sigma-Aldrich); or (iii) ONPG assay to determine β-galactosidase levels in triplicate-infected cultures. ONPG assays involved cell lysis in 150 μl of 1% NP-40, 1 mM MgCl2, 50 mM β-mercaptoethanol and 4 mg/ml β-galactosidase substrate o-nitrophenyl-β-D-galactopyranoside (ONPG; Sigma-Aldrich), incubation at 37 °C for 15-20 min until a faint yellow color developed, and measurement of absorbance at 405 nm.
Effect of blocking agents on adapter-mediated infection
Stock solutions of ammonium chloride, bafilomycin A1, amiloride, cytochalasin D, dansylcadaverine (all from Sigma-Aldrich), sucrose, and filipin (WAKO, Osaka, Japan) were prepared in de-ionized water or ethanol and adjusted to pH 7.4 just before use. Cells were pre-incubated in F12K or DMEM media in triplicate with different concentrations of the drugs or sucrose for 30 min, infected with QOZHG (MOI=3) and scFv adapter for 2 h in the continued presence of the inhibitor, cultured in fresh media for 16 h, and examined for HSV entry by ONPG assay.
Transmission electron microscopy
QOZHG (MOI=10) and adapters (30 μg/mL) were adsorbed to cells at 4°C for 1 h and entry was initiated by raising the temperature to 37°C for 0-30 min, as above. The cells were then washed with cold phosphate buffer (pH 7.2) and fixed with 2.5% glutaraldehyde/1% osmium tetraoxide. Samples were embedded in epoxy resin (Oken, Japan) and ultra thin sections (70 nm) were double stained with uranyl acetate and lead citrate for observation under a transmission electron microscope (H-7000, Hitachi, Japan) (Ishikawa et al., 2006).
Flow cytometry
To compare the affinities of the scFv and EGF adapters for EGFR or HSV-gD, the adapters (30 μg/mL in F12K/HEPES buffer) were adsorbed to cells expressing EGFR or gD (2×105 cells/24-well plate) at 4°C for 1 h. The cells were washed, re-suspended in PBS, and incubated with anti-c-Myc antibody at 4°C for 1 h and secondary PE-conjugated anti-mouse IgG for 30 min. After washing with BD FACSFlow solution (BD Biosciences Pharmingen), adapter binding to the cells was evaluated by BD FACSCalibur flow cytometry.
Tumor infection in vivo
EGFR-overexpressing A549 lung cancer cells (5×106 cells/animal) were implanted in the flanks of BALB/c nude mice. When tumors reached a diameter of approximately 8 mm, QOZHG virus (1×107 pfu) was injected intratumorally with or without scFv adapter at 25 μg/100 μL. Two days later, tumors were surgically removed and stored at -80°C. Tissue sections (12 μm thickness) were prepared by cryostat and processed for DAPI (Invitrogen) staining and detection of GFP fluorescence to visualize the extent of infection.
Results
Adapter construction
Unlike EGF, the scFv used in our previous EGFR targeting adapter is not a signaling ligand (Gill et al., 1984), and thus it was of interest to determine whether replacement of the scFv with EGF would provide an effective adapter. As illustrated in Fig. 1A, the two adapters contained the same gD-binding variable domain of human nectin-1 (V-nec) followed by a spacer (S1) at the amino terminus and the same epitope tags at the carboxyl terminus, but differed in the EGFR-interacting sequences linking these elements. Both adapters were produced by bacterial expression as previously described (Nakano et al., 2005). Fig. 1B shows a Coomassie-stained gel of samples of the purification steps. Both purified adapters were readily detected by immunoblotting using an antibody directed against the c-Myc tag (data not shown).
HSV entry-mediating activities
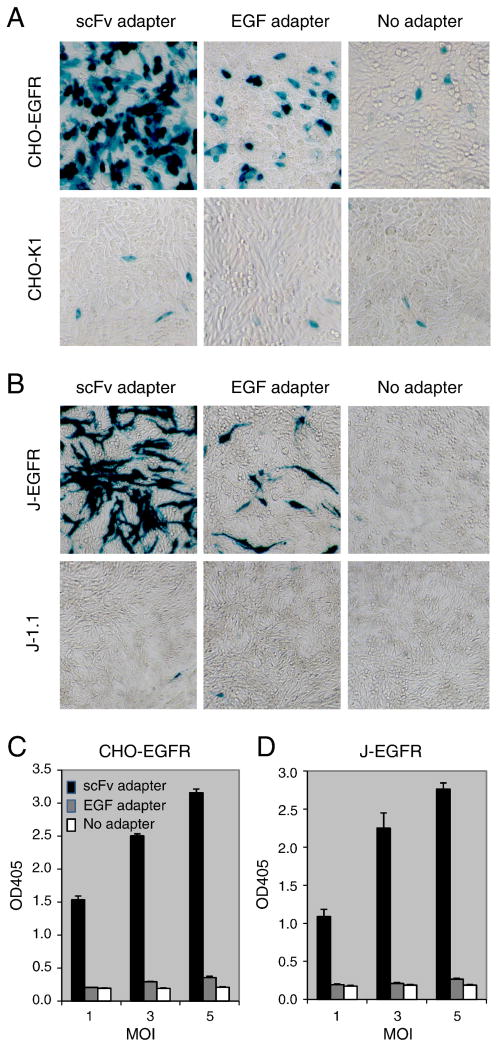
The abilities of the two adapters to mediate HSV entry into CHO-EGFR and J-EGFR cells, which lack receptors for gD but stably express human EGFR, were examined by reporter-gene assays using a replication-defective, dual reporter gene (lacZ and eGFP) recombinant virus, QOZHG (Chen et al., 2000). Based on our previous determination that 30 μg/ml of scFv adapter is saturating for QOZHG entry into CHO-EGFR cells at an MOI of 3 (Nakano et al., 2005), we used these conditions to compare the entry-mediating activities of the two adapters. Visualization of β-galactosidase (LacZ) activity at 16 h post-infection (p.i.) showed that both adapters mediated EGFR-dependent infection of CHO cells (Fig. 2A) and J cells (Fig. 2B). Quantitative measurement of LacZ activity by ONPG assay (see Materials and Methods) demonstrated that virus entry was MOI-dependent and that the scFv adapter was approximately 15-fold more effective than the EGF adapter at MOIs where EGF adapter-mediated entry above background was detectable (Fig. 2C, D).
Figure 2. Comparison of EGF- and scFv-adapter-mediated EGFR-dependent HSV infection of HSV-resistant cells.
EGFR-positive or -negative CHO-K1 (A, C) and J1.1-2 cells (B, D) were incubated for 2 h at 37°C with scFv adapter, EGF adapter, or no adapter and a replication-defective HSV-1 recombinant virus expressing LacZ from the immediate-early ICP0 promoter (QOZHG; MOI=3). Cells were washed after 2 h, incubated with fresh media for 16 h, and examined for LacZ activity by X-gal staining (A, B) or quantitative ONPG assay (average of triplicate samples) (C, D).
Comparative adapter binding to the EGFR and HSV gD
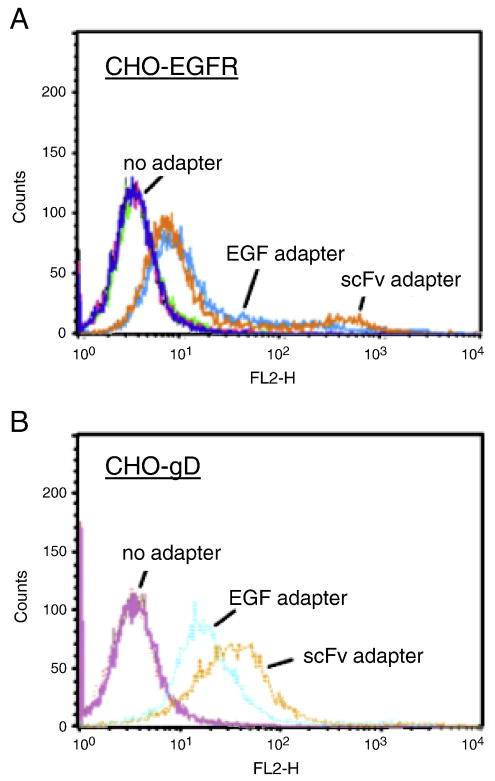
To identify differences between the two adapters that could be responsible for the difference in their entry-mediating efficiencies, we examined the binding of the adapters to the EGFR and HSV-1 gD expressed on separate CHO cell lines (CHO-EGFR and CHO-gD, respectively). The cells were incubated for 90 min at 4°C with the same adapter concentrations as used in the entry assays and immunofluorescent staining was performed with an antibody directed against the c-Myc tag. Flow cytometry demonstrated similar binding of the two adapters to CHO-EGFR cells (Fig. 3A), but greater binding of the scFv adapter than the EGF adapter to CHO-gD cells (Fig. 3B). While surprising since both adapters had the same gD recognition domain, this result indicated that the affinity of the scFv adapter for gD was higher than that of the EGF adapter, possibly due to differential effects of the EGFR-recognition component of the adapters on the refolding efficiency of the bacterially produced proteins or the refolded conformation of the nectin portion. Given this binding difference, we abandoned the EGF adapter for the remainder of this study except where used as a negative control.
Figure 3. Adapter binding to EGFR and HSV gD.
CHO-EGFR (A) and CHO-gD cells (B) were incubated at 4°C with or without adapter, washed extensively, and examined by flow cytometry with anti-c-Myc tag monoclonal antibody (mAb). Light-blue, EGF adapter; orange, scFv adapter.
Mechanism of adapter-mediated virus entry
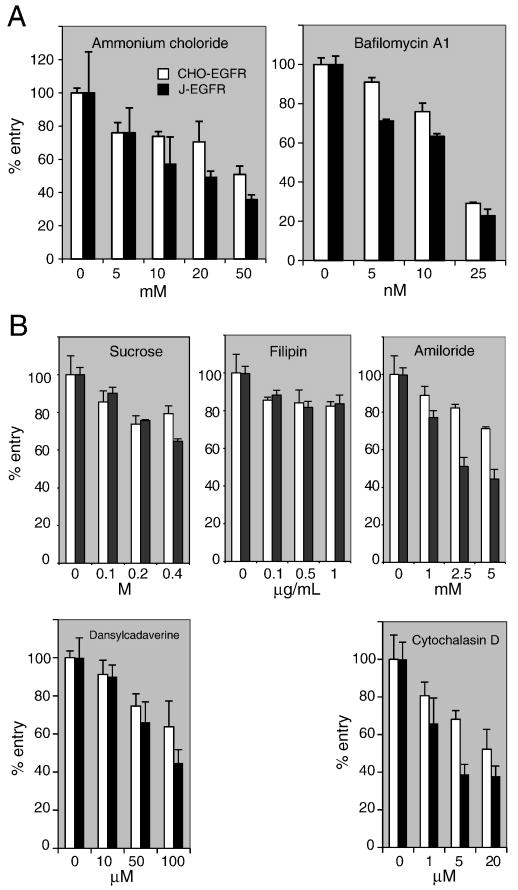
To determine the pathway of scFv adapter-mediated entry, we first tested the sensitivity of virus infection to lysosomotropic agents that block endosome acidification. On pre-treatment of CHO-EGFR or J-EGFR cells with ammonium chloride or bafilomycin A1 (BFLA), dose-dependent inhibition of adapter-mediated QOZHG infection was observed (Fig. 4A). We used a relatively low MOI to avoid potential artifacts due to overloading of the cells and removed the drugs at 2 h post-infection to avoid drug-associated toxicity. These conditions precluded definitive determination whether all infection involved virus passage through acidified endosomes or whether some degree of entry (20-25% based on the BFLA results) might take place by a pH-independent pathway.
Figure 4. Sensitivity of scFv-mediated infection of CHO-EGFR and J-EGFR cells to endocytic pathway inhibitors.
(A) Sensitivity to inhibitors of endosome acidification. Cells were pre-incubated with increasing concentrations of ammonium chloride or bafilomycin A1 for 30 min and infected with QOZHG (MOI=3) plus scFv adapter in the continued presence of inhibitor for 2 h at 37°C. The media was replaced and the cells were harvested for ONPG assay 16 h later. (B) Sensitivity to inhibitors of endocytic uptake. Cells were tested with sucrose and dansylcadaverine for clathrin-dependent uptake, filipin for caveolar uptake, and amiloride and cytochalasin D for macropinocytosis. Conditions were as in (A). Values are averages of triplicate infections.
We next examined whether scFv adapter-mediated virus entry involved clathrin-coated pits, caveolae, or macropinocytosis. Dose-dependent decreases in infection were observed on J-EGFR and, less pronounced, CHO-EGFR cells treated with sucrose or dansylcadaverine (inhibitors of clathrin-dependent uptake) or with amiloride or cytochalasin D (inhibitors of micropinocytosis) (Mercer and Helenius, 2009), whereas filipin had a small, dose-independent effect (Fig. 4B). These results suggested that scFv adapter-mediated virus entry into both cell lines occurred at least in part by clathrin-dependent endocytosis, the dominant mechanism of ligand-induced EGFR internalization, and macropinocytosis, a clathrin-independent mechanism observed at high EGF and/or EGFR levels (Sorkin and Goh, 2008). The consistently smaller effects of the different inhibitors on CHO-EGFR than on J-EGFR cells may be due to differences between the two lines in their uptake or intracellular clearance of the inhibitory agents. We did not test combinations of the different drugs due to increased toxicity at the effective concentrations of the individual drugs. Moreover, we note that Mercer and Helenius (2009) list additional criteria for macropinocytosis that have not been examined here.
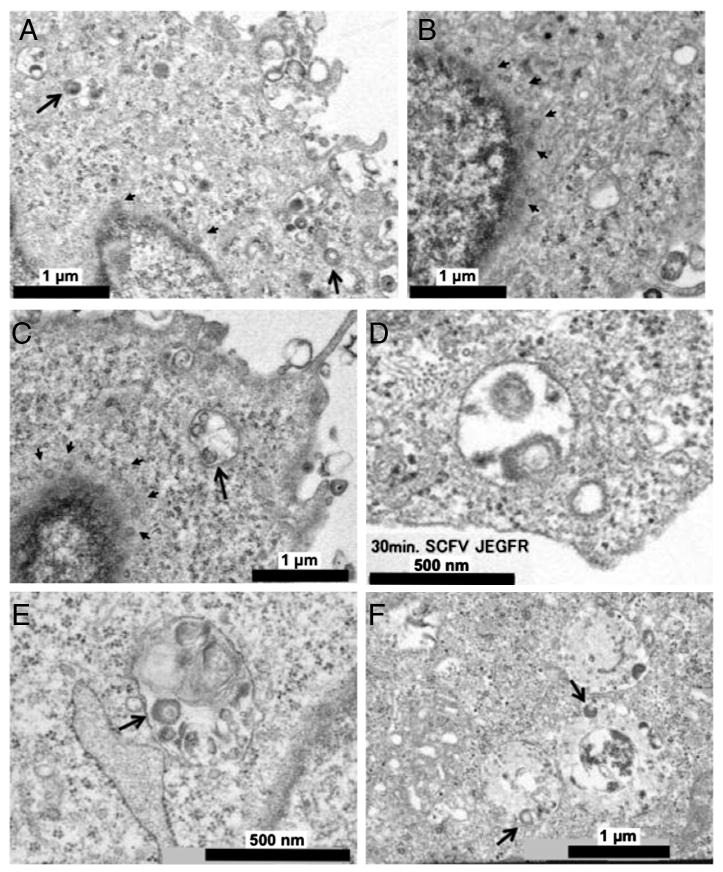
Transmission electron microscopy was performed on cells incubated for 15 or 30 min at 37°C following virus adsorption. At 15 min, we saw virions in cytoplasmic vesicles of CHO-EGFR cells in the presence of the scFv adapter, some intimately associated with the vesicle membrane (Fig. 5A, large arrows), but obtained no evidence of fusion at the cell surface. In addition, capsids could be distinguished in the perinuclear area (Fig. 5A, B, small arrows). At 30 min, more capsids were observed near the nucleus along with an occasional large cytoplasmic vesicle containing deformed virions not clearly associated with the vesicle membrane (Fig. 5C). Particles in the process of fusing with the vesicle membrane were seen at 30 min in J-EGFR cells (Fig. 5D), but no clear examples were captured in CHO-EGFR cells, perhaps indicating that this is a rapid event in CHO-EGFR cells. These findings were consistent with endocytic HSV uptake by both cell lines in the presence of the scFv adapter and subsequent capsid release into the cytoplasm. The presence of degrading virions in large vesicles at 30 min in CHO-EGFR cells may suggest that some virus particles may have been insufficiently complexed with the adapter to escape from endosomes, similar to the reported fate of HSV incubated with receptor-deficient CHO cells (Nicola, McEvoy, and Straus, 2003). In addition, it is possible that some of these particles represented endocytosed defective virions present in the vector stock. As expected, infection in the presence of the EGF adapter showed no evidence of cytoplasmic capsids or membrane fusion in CHO-EGFR and J-EGFR cells at 15 min (data not shown) or 30 min (Fig. 5E, F). Intact and degrading virus particles were observed in vesicles, consistent with endosomal uptake without subsequent fusion-mediated release. Together, these observations indicated that the scFv adapter enabled envelope fusion with the endosomal membrane in both CHO-EGFR and J-EGFR cells to deliver capsids into the cytoplasm, whereas the EGF adapter lacked this ability most likely as a result of its reduced association with virion gD.
Figure 5. Ultrastructural analysis of adapter-mediated HSV entry into CHO-EGFR and J-EGFR cells.
Cells were incubated with QOZHG (MOI=10) plus scFv or EGF adapter at 4°C for 1 h followed by 15 or 30 min at 37°C. After fixation, embedding, and staining with uranyl acetate and lead citrate, the samples were observed under a transmission electron microscope. CHO-EGFR cells at 15 (A, B) or 30 (C) min of incubation with QOZHG and scFv adapter. Large arrows, virions in vesicles; small arrows, viral capsids in the perinuclear area. (D) 30 min incubation of J-EGFR cells with QOZHG and scFv adapter. (E) CHO-EGFR and (F) J-EGFR cells at 30 min incubation with QOZHG and EGF adapter. Arrows point to degrading virions in multivesicular structures and endosome-like vesicles.
Adapter-mediated infection in vivo
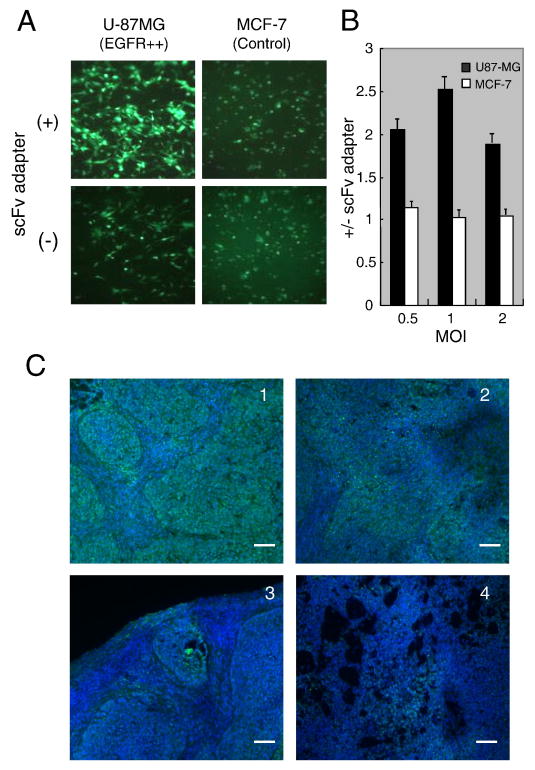
Since adapters are readily re-engineered, they may provide a flexible method to introduce replication-defective HSV gene transfer vectors into specific cell populations in vivo as a means to study these cells in their natural environment. We assessed the feasibility of this approach using a tumor model since tumors represent a relatively homogeneous population of target cells. In vitro infection assays showed that the scFv adapter augmented QOZHG infection of EGFR-overexpresssing U-87MG glioblastoma cells, but not of control MCF-7 breast cancer cells that express low levels of the receptor (Reilly and Gariepy, 1998) (Fig. 6A, B), indicating that the scFv adapter can increase infection of cells that express both HSV receptors and EGFR. We then used EGFR-overexpressing, HSV-susceptible A549 cells to form subcutaneous tumors and assessed infection 2 days after intratumoral injection of QOZHG with or without adapter. The results demonstrated widespread GFP fluorescence in the presence of the adapter compared to limited fluorescence in its absence (Fig. 6C), suggesting that the scFv adapter can be used to enhance HSV-mediated gene delivery to targeted cell populations even if the target cells express authentic HSV entry receptors. While our data do not show infection exclusively through the targeted receptor, they suggest that this may be achievable with suitable modifications to the vector and/or adapter, as recently discussed (Baek et al., 2010).
Figure 6. The scFv adapter mediates increased infection of EGFR-overexpressing tumor cells in vitro and tumors in vivo.
(A) Representative eGFP expression in U-87MG glioblastoma and MCF-7 breast cancer cells at 16 h post infection with QOZHG (MOI=3) in the presence (+) or absence (-) of the scFv adapter. (B) QOZHG infection of U-87MG and MCF-7 cells at MOIs of 0.5-2 with or without scFv adapter was measured by ONPG assay. The results of triplicate infections are expressed as the ratios of the average ONPG readings with and without adapter. (C) 5×106 A549 lung tumor cells were implanted in the flanks of BALB/c nude mice. Tumors were grown to ∼8 mm in diameter and injected with 1×107 pfu of QOZHG virus in the presence or absence of 25 μg scFv adapter. Two days later, tumors were surgically removed and processed for GFP detection and DAPI nuclear staining. Each section is from a different animal treated with virus plus adapter (panels 1, 2) or virus alone (panels 3, 4). Scale bars: 100 μm.
Discussion
HSV infection has long been considered to occur exclusively via pH-independent viral envelope fusion with the plasma membrane (Spear, 1993). However, several reports in the past few years have demonstrated that the virus can use other mechanisms to gain entry into cells. Nicola and co-workers first presented evidence of pH-dependent endocytic HSV entry into HeLa cells and CHO cells modified to express human nectin-1 or HVEM (Nicola, McEvoy, and Straus, 2003). Subsequently, pH-independent endocytic infection was described for murine melanoma cells expressing human nectin-1 (Milne et al., 2005). It has been suggested that endocytic virus uptake by keratinocytes, HeLa and CHO cells involves macropinocytosis (Mercer and Helenius, 2009; Nicola et al., 2005), while evidence of a phagocytosis-like mechanism has been reported for both CHO-nectin-1 and corneal fibroblast cells (Clement et al., 2006). In all of these instances, interaction of HSV gD with an entry receptor is essential for membrane fusion resulting in viral capsid delivery to the cytoplasm.
Taking advantage of this understanding of the central role of gD and its receptors in HSV entry, several strategies have been pursued to enable HSV infection via alternate receptors. One strategy involves virus engineering to abolish gD recognition of its natural receptors (detargeting) and incorporate a new ligand into gD for interaction with the new target receptor (retargeting) (Menotti et al., 2009; Zhou and Roizman, 2006). A second strategy does not require virus re-engineering but instead employs bi-specific adapters that can function as an attachment bridge for the virus to the target receptor; without modifications (van Beusechem et al., 2003; Verheije et al., 2006), this method is restricted to a single round of virus entry and is therefore primarily useful for targeted transgene delivery by replication-defective vectors.
We previously demonstrated that an EGFR-specific adapter employing the gD-binding V domain of nectin-1 and an anti-EGFR scFv was effective in mediating virus infection through the EGFR (Nakano et al., 2005). In the present study, we explored the cellular pathway utilized by the virus for entry via this adapter and determined whether an alternate adapter designed to target the same receptor through a natural ligand could be effective as well. Although EGFR recognition was similar between the scFv and EGF adapters, we found that the EGF adapter was much less effective in mediating virus infection, likely because of its weak binding to viral gD limiting stable virus-adapter complex formation; without stably bound adapter, internalized virions were expected to remain trapped in endosomes as previously reported (Nicola and Straus, 2004) and observed here. In contrast, we propose that stable association of the scFv adapter with the targeted receptor and viral gD resulted in endocytic co-internalization of the complex allowing membrane fusion in acidifying endosomes and capsid release into the cytoplasm. This suggestion is based on the evidence that scFv adapter-mediated entry involves low pH-dependent endocytosis along with our previous observation that the process requires binding of the adapter to both the targeted receptor and viral gD (Nakano et al., 2005).
We previously reported that the V domain of nectin-1 can mediate efficient HSV entry as a soluble molecule not anchored in or attached to the cell membrane (Kwon et al., 2006). In addition, we showed that virion attachment to cell-surface proteoglycans is essential in this instance. Our experiments in the present study allowed for virus attachment to proteoglycans, raising the question why adapter-mediated infection required a gD receptor. It is possible that the conformation of the nectin-1 portion of the receptor is altered by its fusion to the scFv region, precluding gD activation without additional changes induced by EGFR binding and an acidic environment. The reduced gD binding activity of the EGF adapter is consistent with the suggestion that the conformation of the nectin-1 V domain is sensitive to C-terminal additions, as also suggested by our previous finding that the soluble V domain blocks HSV infection of nectin-1 expressing cells while the scFv adapter does not (Nakano et al., 2005).
The evidence presented in this study that the scFv adapter was able to increase the area of infection in the relatively homogeneous target cell population provided by a tumor supports the feasibility of using adapters for HSV-mediated gene transfer to specific populations of cells in vivo, such as neuronal subtypes in the brain that can be differentially targeted based on their unique receptor profiles (Anliker et al., 2010). This would involve the use of replication-defective vectors like QOZHG that enter a latent-like state upon infection, leaving cellular gene expression essentially unaltered while allowing short- or long-term transgene expression from different promoters (Craft et al., 2008; Krisky et al., 1998; Puskovic et al., 2004; Wu et al., 1996). Such a system could be used to label specific cell populations with reporter genes and/or to examine the effect of adding or blocking certain gene functions exclusively in these cells. Our current adapters rely on the ability of gD to bind to either nectin-1 (Nakano et al., 2005) or HVEM (Baek et al., 2010) and can be used in combination with our recently described nectin-1- or HVEM-restricted mutant vectors (Uchida et al., 2009) to reduce infection through the natural HSV receptors present on a broad range of cells. Ultimately, adapter-vector pairs can be designed that no longer require gD binding to one of its cognate receptors and such combinations will allow virus entry exclusively via the targeted receptor. Adapters provide greater flexibility than currently described retargeted HSV vectors since adapter specificity can be readily changed by incorporation of a different scFv (Baek et al., 2010) while the ground rules for efficient HSV retargeting via ligand or scFv incorporation into gD remain to be firmly established. Adapters may therefore be a practical and broadly applicable tool to study and modify the function of specific cells in mixed cell populations.
Acknowledgments
We thank Dr. Yasushi Kawaguchi and Ms. Setsuko Nakayama (Tokyo) for technical assistance and Drs. William Goins and Hiroaki Uchida (Pittsburgh) for helpful discussions. This work was supported by NIH grants NS040923 and CA119298 to J.C.G. and by a grant from the Japanese Ministry of Education, Culture, Science, Sports and Technology to K.N. (Grants-in-Aid for Scientific Research #21390374).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anliker B, Abel T, Kneissl S, Hlavaty J, Caputi A, Brynza J, Schneider IC, Munch RC, Petznek H, Kontermann RE, Koehl U, Johnston IC, Keinanen K, Muller UC, Hohenadl C, Monyer H, Cichutek K, Buchholz CJ. Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat Methods. 2010;7(11):929–35. doi: 10.1038/nmeth.1514. [DOI] [PubMed] [Google Scholar]
- Baek H, Uchida H, Jun K, Kim JH, Kuroki M, Cohen JB, Glorioso JC, Kwon H. Bispecific Adapter-Mediated Retargeting of a Receptor-Restricted HSV-1 Vector to CEA-Bearing Tumor Cells. Mol Ther. 2010 Oct 5; doi: 10.1038/mt.2010.207. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G, Amasio M, Avitabile E, Cerretani A, Forghieri C, Gianni T, Menotti L. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev Med Virol. 2007;17(5):313–26. doi: 10.1002/rmv.546. [DOI] [PubMed] [Google Scholar]
- Chen X, Li J, Mata M, Goss J, Wolfe D, Glorioso JC, Fink DJ. Herpes simplex virus type 1 ICP0 protein does not accumulate in the nucleus of primary neurons in culture. J Virol. 2000;74(21):10132–41. doi: 10.1128/jvi.74.21.10132-10141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174(7):1009–21. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft AM, Krisky DM, Wechuck JB, Lobenhofer EK, Jiang Y, Wolfe DP, Glorioso JC. Herpes simplex virus-mediated expression of Pax3 and MyoD in embryoid bodies results in lineage-Related alterations in gene expression profiles. Stem Cells. 2008;26(12):3119–29. doi: 10.1634/stemcells.2008-0417. [DOI] [PubMed] [Google Scholar]
- Gianni T, Campadelli-Fiume G, Menotti L. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J Virol. 2004;78(22):12268–76. doi: 10.1128/JVI.78.22.12268-12276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, McLeod C, Mendelsohn J. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984;259(12):7755–60. [PubMed] [Google Scholar]
- Ishikawa F, Shimazu H, Shultz LD, Fukata M, Nakamura R, Lyons B, Shimoda K, Shimoda S, Kanemaru T, Nakamura K, Ito H, Kaji Y, Perry AC, Harada M. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. Faseb J. 2006;20(7):950–2. doi: 10.1096/fj.05-4863fje. [DOI] [PubMed] [Google Scholar]
- Krisky DM, Marconi PC, Oligino T, Rouse RJ, Fink DJ, Glorioso JC. Rapid method for construction of recombinant HSV gene transfer vectors. Gene Ther. 1997;4(10):1120–5. doi: 10.1038/sj.gt.3300497. [DOI] [PubMed] [Google Scholar]
- Krisky DM, Wolfe D, Goins WF, Marconi PC, Ramakrishnan R, Mata M, Rouse RJ, Fink DJ, Glorioso JC. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Ther. 1998;5(12):1593–603. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- Kwon H, Bai Q, Baek HJ, Felmet K, Burton EA, Goins WF, Cohen JB, Glorioso JC. Soluble V domain of Nectin-1/HveC enables entry of herpes simplex virus type 1 (HSV-1) into HSV-resistant cells by binding to viral glycoprotein D. J Virol. 2006;80(1):138–48. doi: 10.1128/JVI.80.1.138-148.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menotti L, Nicoletti G, Gatta V, Croci S, Landuzzi L, De Giovanni C, Nanni P, Lollini PL, Campadelli-Fiume G. Inhibition of human tumor growth in mice by an oncolytic herpes simplex virus designed to target solely HER-2-positive cells. Proc Natl Acad Sci U S A. 2009;106(22):9039–44. doi: 10.1073/pnas.0812268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11(5):510–20. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol. 2005;79(11):6655–63. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Asano R, Tsumoto K, Kwon H, Goins WF, Kumagai I, Cohen JB, Glorioso JC. Herpes simplex virus targeting to the EGF receptor by a gD-specific soluble bridging molecule. Mol Ther. 2005;11(4):617–26. doi: 10.1016/j.ymthe.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79(12):7609–16. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77(9):5324–32. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78(14):7508–17. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskovic V, Wolfe D, Goss J, Huang S, Mata M, Glorioso JC, Fink DJ. Prolonged biologically active transgene expression driven by HSV LAP2 in brain in vivo. Mol Ther. 2004;10(1):67–75. doi: 10.1016/j.ymthe.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Reilly RM, Gariepy J. Factors influencing the sensitivity of tumor imaging with a receptor-binding radiopharmaceutical. J Nucl Med. 1998;39(6):1036–43. [PubMed] [Google Scholar]
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008;314(17):3093–106. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PG. Entry of alphaherpesviruses into cells. Sem Virol. 1993;4:167–180. [Google Scholar]
- Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6(5):401–10. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275(1):1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- Uchida H, Shah WA, Ozuer A, Frampton AR, Jr, Goins WF, Grandi P, Cohen JB, Glorioso JC. Generation of herpesvirus entry mediator (HVEM)-restricted herpes simplex virus type 1 mutant viruses: resistance of HVEM-expressing cells and identification of mutations that rescue nectin-1 recognition. J Virol. 2009;83(7):2951–61. doi: 10.1128/JVI.01449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusechem VW, Mastenbroek DC, van den Doel PB, Lamfers ML, Grill J, Wurdinger T, Haisma HJ, Pinedo HM, Gerritsen WR. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Ther. 2003;10(23):1982–91. doi: 10.1038/sj.gt.3302103. [DOI] [PubMed] [Google Scholar]
- Verheije MH, Wurdinger T, van Beusechem VW, de Haan CA, Gerritsen WR, Rottier PJ. Redirecting coronavirus to a nonnative receptor through a virus-encoded targeting adapter. J Virol. 2006;80(3):1250–60. doi: 10.1128/JVI.80.3.1250-1260.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Watkins SC, Schaffer PA, DeLuca NA. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70:6358–6368. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Roizman B. Construction and properties of a herpes simplex virus 1 designed to enter cells solely via the IL-13alpha2 receptor. Proc Natl Acad Sci U S A. 2006;103(14):5508–13. doi: 10.1073/pnas.0601258103. [DOI] [PMC free article] [PubMed] [Google Scholar]