Abstract
Objective
To determine the prevalence of subdural hematoma (SDH) in patients presenting with primary non-traumatic lobar intracerebral hemorrhage (ICH) and characteristics associated with the presence of SDH.
Design
Retrospective analysis of data collected in a prospective cohort study.
Setting
Hospital.
Patients
Consecutive sample of 200 patients with primary lobar ICH and 75 patients with deep hemispheric ICH.
Main Outcome Measures
Presence of SDH and mortality.
Results
Subdural hematoma was present in 40 of 200 patients (20%) with primary lobar ICH. By contrast, SDH was not present in any of 75 consecutive patients with deep hemispheric ICH (P<.001 for comparison with lobar ICH). Intracerebral hemorrhage volume higher than 60 cm3 was the only independent predictor of SDH (odds ratio [OR], 2.69; 95% confidence interval [CI], 1.14–6.34; P=.02). Subdural hematoma thickness more than 5 mm was an independent predictor of increased 30-day mortality (OR, 7.60; 95% CI, 1.86–30.99; P=.005) after controlling for other factors including ICH volume. Further analysis showed that the effect of SDH on mortality depended on ICH volume, with larger odds for mortality in those with low ICH volume (OR, 12.85; 95% CI, 2.42–68.23; P=.003 for those with ICH volume <30 cm3). Cerebral amyloid angiopathy was present in 8 of 9 patients with pathological specimens.
Conclusions
Nontraumatic SDH frequently accompanies primary lobar ICH and is associated with higher 30-day mortality, particularly when the ICH volume is relatively low. Rupture of an amyloid-laden leptomeningeal vessel, with extravasation into the brain parenchyma and subdural space, may be the pathogenic mechanism.
Primary lobar intracerebral hemorrhage (ICH), where the origin of the hemorrhage is in the cortex or corticosubcortical junction, accounts for 30% to 40% of all primary ICH.1,2 Cerebral amyloid angiopathy (CAA), characterized by amyloid deposition in the media and adventitia of small arteries and arterioles,3 is a major cause of lobar ICH but not deep hemispheric ICH.4 Risk factors for mortality following lobar ICH are similar to those for ICH in general and include size of the hematoma, intraventricular extension, depressed level of consciousness, and increased age.5,6
Acute subdural hematoma (SDH) has been proposed as part of the radiographic syndrome of lobar ICH caused by CAA,7 and SDH has occasionally been reported in association with CAA based on pathologic examination.3 However, the frequency with which SDH accompanies lobar ICH is uncertain. Furthermore, is it not known whether the syndrome of lobar ICH with SDH is associated with different risk factors or outcomes compared with lobar ICH without SDH. To determine the prevalence of SDH in lobar ICH, and its effect on 30-day mortality, we retrospectively reviewed radiographic and clinical data collected as part of an ongoing prospective single-center study of ICH.
METHODS
PATIENT SELECTION
Study subjects were drawn from an ongoing prospective longitudinal cohort study of spontaneous intracerebral hemorrhage.5,8 From this study, we selected consecutive patients with symptomatic lobar ICH 55 years or older presenting to the emergency department of Massachusetts General Hospital between January 2000 and December 2005. To determine whether the association between SDH and primary ICH was limited to those with lobar ICH location, we also selected a control group of 75 consecutive subjects with supratentorial deep hemispheric ICH, drawn from the same prospective cohort study and within the same interval (December 1, 2003, to July 1, 2005).
Patients were not enrolled if there was evidence of a secondary cause of ICH, such as vascular malformation, aneurysm, tumor, or infarction with hemorrhagic transformation. A history of head trauma prior to the onset of symptoms was specifically sought. Subjects with presumed traumatic ICH from brain contusion were excluded, including those with a history of antecedent high-velocity head trauma, evidence of head injury such as skull fracture, or the presence of contrecoup hemorrhage. Those with a history of collapse at the time of symptom onset were included if stroke symptoms were judged to be the cause of the fall and there was no evidence of major trauma to the skull or scalp. Patients found unresponsive without witnessed onset were considered eligible if the observed ICH was judged, by the study investigator, to be a sufficient explanation for the neurologic presentation and there was no evidence of major trauma to the skull, scalp, or body. Select patients underwent surgical hematoma resection at the discretion of the treating clinician. At our center, surgical resection is typically reserved for patients not in deep coma who develop acute deterioration from mass effect and have a superficial hematoma.
Baseline clinical information was obtained from interview of the subject or an informant by study personnel and review of the medical record, as previously described.8 Follow-up data on 30-day mortality were obtained through telephone interviews of consenting proxies.8 The Social Security Death Index was queried to confirm the presence or absence of death at 30 days.9
Surgical and autopsy specimens were reviewed by a pathologist. The presence or absence of CAA was confirmed by Congo red staining or immunohistochemical stains for β-amyloid using anti–β-amyloid antibody clone 6F/3D (Dako, Carpinteria, California) or Ab-1 (Neomarkers/Laboratory Vision, Freemont, California). For immunohistochemical stains, positive and negative controls were prepared and viewed with each case to ensure the validity of the staining. In autopsy cases, the dura mater was removed and reviewed for evidence of gross pathologic features but not microscopic pathologic features, as per departmental protocol.
The likelihood of underlying CAA was determined using the validated Boston criteria.10 These criteria are used to assign the probability of CAA-related hemorrhage based on pathologic evidence and the number of lobar brain hemorrhages without other cause. The category “probable CAA” is assigned when there are multiple lobar hemorrhages, including magnetic resonance imaging (MRI) microbleeds, or pathologic evidence of some degree of CAA from a biopsy or surgical specimen. “Possible CAA” is assigned when there is a single lobar brain hemorrhage. “Definite CAA” is assigned when severe CAA is present on autopsy.
All study procedures were approved by the local institutional review board.
RADIOLOGIC DATA
The presence or absence of SDH on computed tomographic (CT) scan was determined by the consensus of 2 of us (P.V.P. and E.E.S.). All reviewed CT scans were obtained prior to intracranial surgical procedures, such as placement of an intracranial monitor or ventriculostomy drain, or craniotomy. The CT scans were viewed on a digital PACS viewer using individually optimized window settings for SDH. The SDH thickness was measured, perpendicular to the skull table, at the region of maximal thickness on axial images.11 Midline shift was measured as the maximal displacement of the septum pellucidum, measured from a line connecting the anterior and posterior portions of the falx cerebri, per a previously published method.12 The SDH was considered acute if portions of the SDH were hyperdense relative to the adjacent brain parenchyma and subacute or chronic if the SDH was entirely isodense or hypodense. Manual segmentation of the ICH on the admission CT was performed by study personnel using Alice software (Parexel Corporation, Waltham, Massachusetts) and was used to determine ICH volume.13 The number of hemorrhages, including asymptomatic microbleeds, was determined on MRI gradient echo sequences.14 All CT and MRI interpretations and measurements were performed without knowledge of clinical information, including radiology reports.
STATISTICAL ANALYSIS
Characteristics associated with the presence of SDH were determined using Fisher exact test, Wilcoxon rank sum test, or t test as appropriate. The ICH volume, Glasgow Coma Scale score, mid-line shift, and maximum SDH thickness were nonnormally distributed and were therefore analyzed with nonparametric tests. Logistic regression models were used to determine the independent predictors of SDH and whether SDH was a predictor of increased 30-day mortality. Candidate variables for the models were those with a trend for an association with the outcome in univariate analyses (P<.20). Backward elimination was used to eliminate nonsignificant variables (P>.05) from the final models. Age, ICH volume, and intraventricular hemorrhage were forced into the mortality models based on their known association with ICH outcome.5,6 The ICH volume was categorized in a similar fashion as other studies (0–30 cm3, 31–60 cm3, or >60 cm3).6 Glasgow Coma Scale score, midline shift, and withdrawal of life-sustaining measures were not included as candidate variables because they were considered to be consequences of ICH or SDH. Because many of the SDHs were small and therefore unlikely, a priori, to increase mortality, we chose to evaluate whether SDH with a maximum thickness greater than the median was a risk factor for mortality. We hypothesized that the relationship between SDH and mortality might be dependent on ICH volume and tested this hypothesis using an interaction term.
RESULTS
There were 212 consecutive admissions for primary lobar hemorrhage, of whom 12 were excluded because there was no baseline CT scan or the baseline CT scan was performed after an intracranial procedure, such as placement of a ventriculostomy catheter. Therefore, 200 patients with lobar ICH were included. The prevalence of SDH was 40 in 200 (20%) (Figure). All SDHs were acute; there were no chronic SDHs observed. By contrast, there were no SDHs identified on CT of 75 consecutive patients with deep hemispheric ICH (P<.001 for comparison with prevalence of SDH in lobar ICH).
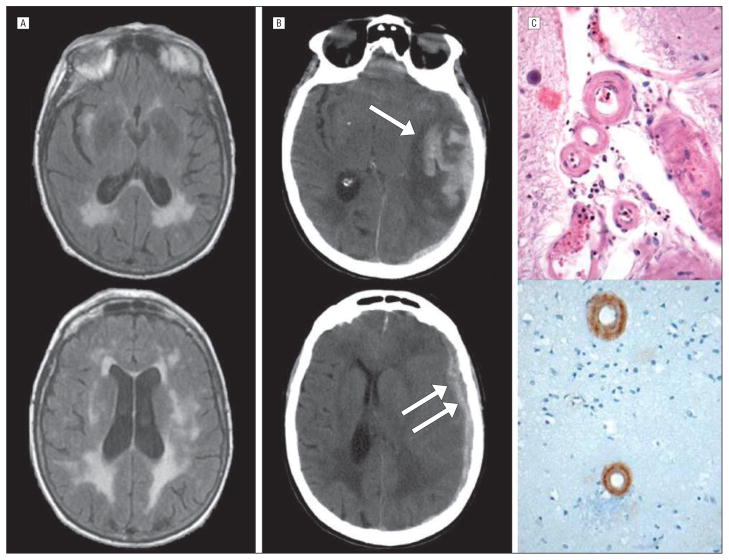
Figure.
Example of primary lobar intracerebral hemorrhage (ICH) with concurrent subdural hematoma (SDH). An 87-year-old woman was hospitalized for depression. Magnetic resonance fluid-attenuated inversion recovery images obtained the day prior to ICH showed extensive white matter hyperintensity and no evidence of subdural collections (A). The following morning, a routine nursing check found her in bed with aphasia and a right hemiparesis; there was no evidence of a fall or trauma. Head computed tomography showed a large temporal ICH (B, top panel) with an adjacent SDH that extended superiorly over the hemisphere (B, bottom panel). The patient died; an autopsy showed eosinophilic thickening of the media of small arteries and arterioles of the cortex and leptomeninges (C, top panel). Immunostaining for β-amyloid was positive in the affected vessels (C, bottom panel).
There were 37 with SDH on initial CT scan, and 3 who had SDH appear on a follow-up CT scan done less than 48 hours after admission. Four subjects had 2, apparently separate, SDHs each. Median maximal SDH thickness was 5.0 mm (interquartile range, 2.0–8.5 mm); 9of 40 SDHs (23%) were 6 to 10 mm thick and 7 of 40 SDHs (18%) were more than 10 mm thick. The SDH appeared contiguous with the lobar ICH in most cases (32 of 40; 80%) and all were in the hemisphere ipsilateral to the ICH. Four patients with acute SDH had a previous CT or MRI performed within 30 days prior to presentation with ICH; none showed evidence for preexisting SDH or other subdural collection.
Clinical characteristics are shown in Table 1. Those with SDH had lower Glasgow Coma Scale scores, higher ICH volumes, and more midline shift. There was a trend toward fewer lobar microbleeds in those with SDH, although the proportion with probable or possible CAA did not differ according to the presence of SDH. The distribution of the number of microbleeds in patients with probable or possible CAA was highly right-skewed, as observed in other studies,15 and ranged from 0 to 136. Presentation with collapse at the onset of stroke symptoms or being found unresponsive was more common in those with SDH (Table 1). Fall at the onset of symptoms or being found unresponsive was no longer associated with SDH, however, after controlling for ICH volume (P=.16). A multivariable logistic regression model, with backward elimination of nonsignificant variables, showed that the only independent predictor of SDH was ICH volume. Compared with the reference group with an ICH volume of 0 to 30 cm3, patients with an ICH volume of 31 to 60 cm3 had an odds ratio (OR) for SDH of 1.43 (95% confidence interval [CI], 0.55–3.74; P=.46) and patients with an ICH volume more than 60 cm3 had an OR for SDH of 2.69 (95% CI, 1.14–6.34; P=.02).
Table 1.
Comparison of Subjects With Lobar ICH With and Without SDH
| Characteristic | % |
P Value | |
|---|---|---|---|
| With SDH (n=40) | Without SDH (n=160) | ||
| Age, y, mean (SD) | 78.6 (8.7) | 75.1 (8.7) | .03 |
| Female | 60 | 49 | .29 |
| Hypertension | 68 | 73 | .56 |
| History of ICH | 3 | 9 | .20 |
| Boston criteria diagnosisa | .42 | ||
| Possible CAA | 60 | 49 | |
| Probable CAA | 30 | 35 | |
| Warfarin use | 28 | 23 | .54 |
| Antiplatelet agent use | 43 | 46 | .72 |
| Fall at onset/found unresponsive | 38 | 21 | .04 |
| Glasgow Coma Scale score, median (25th percentile, 75th percentile) | 9 (6, 14.5) | 14 (9, 15) | .004 |
| ICH volume, cm3, median (25th percentile, 75th percentile) | 61.5 (32, 90) | 39 (15.5, 63.5) | .01 |
| Lobar location | .19 | ||
| Frontal | 13 | 26 | |
| Parietal | 8 | 11 | |
| Temporal | 20 | 16 | |
| Occipital | 13 | 5 | |
| Multipleb | 48 | 41 | |
| Multiple simultaneous acute ICH | 5 | 4 | .99 |
| Adjacent to pial surface | 93 | 81 | .10 |
| Intraventricular hemorrhage | 53 | 43 | .29 |
| Midline shift, mm, median (25th percentile, 75th percentile) | 7 (3.5, 14.5) | 3 (0, 7) | .001 |
| Surgical evacuation | 25 | 9 | .01 |
| No. of lobar microbleeds,c median (25th percentile, 75th percentile) | 1 (1, 3) | 2 (1, 4) | .06 |
| 30-d mortality | 55 | 31 | .009 |
Abbreviations: CAA, cerebral amyloid angiopathy; ICH, intracerebral hemorrhage; SDH, subdural hematoma.
Based on previously published validated criteria.10
This includes 9 patients with separate simultaneous acute lobar ICH in different lobes, as well as 76 patients where a single lobar hemorrhage traversed 2 or more lobes.
In 87 patients (14 with SDH, 73 without SDH) who had possible or probable CAA by Boston criteria and underwent magnetic resonance imaging with gradient echo sequence.
Characteristics associated with 30-day mortality are shown in Table 2. Subdural hematoma was associated with higher 30-day mortality. Withdrawal of life-sustaining measures was common in those who died and was present in all who died with SDH (22 of 22 who died with SDH had withdrawal of life-sustaining measures, compared with 43 of 50 who died without SDH; P=.09). The presence of SDH with more than a 5-mm maximum thickness (SDH>5 mm) was independently associated with increased 30-day mortality in a multivariable model (Table 3) (OR, 7.60; 95% CI, 1.86–30.99; P=.005). When analyzed as the presence or absence of any SDH, which includes many smaller SDHs, there was no longer an independent relationship (OR, 1.74; 95% CI, 0.73–4.13; P=.21). Model interaction terms between SDH and ICH volume categories were significant (P<.05), indicating that the effect of SDH on mortality differed according to ICH volume, however. Further analysis showed that SDH was associated with an increased risk of mortality in those with an ICH volume lower than 30 cm3 (OR, 12.85; 95% CI, 2.42–68.23; P=.003) but not in those with an ICH volume 30 to 59 cm3 (OR, 1.19; 95% CI, 0.28–5.11; P=.82) or an ICH volume of 60 cm3 or higher (OR, 0.82; 95% CI, 0.24–2.74; P=.74).
Table 2.
Univariate Predictors of 30-Day Mortality
| Characteristic | % |
P Value | |
|---|---|---|---|
| Died (n=72) | Survived (n=128) | ||
| Age, y, mean (SD) | 78.5 (7.3) | 74.2 (9.2) | <.001 |
| Female | 54 | 50 | .66 |
| Hypertension | 76 | 70 | .33 |
| History of ICH | 13 | 5 | .10 |
| Warfarin use | 32 | 20 | .06 |
| Antiplatelet agent use | 49 | 44 | .56 |
| Glasgow Coma Scale score, median (25th percentile, 75th percentile) | 9 (4, 12) | 15 (14, 15) | <.001 |
| ICH volume, cm3, median (25th percentile, 75th percentile) | 79.5 (51, 110) | 28 (11, 46) | <.001 |
| Lobar location | .10 | ||
| Frontal | 15 | 28 | |
| Parietal | 10 | 11 | |
| Temporal | 17 | 17 | |
| Occipital | 4 | 7 | |
| Multiplea | 54 | 36 | |
| Multiple simultaneous acute ICH | 6 | 4 | .72 |
| Adjacent to pial surface | 85 | 83 | .84 |
| Intraventricular hemorrhage | 63 | 34 | <.001 |
| Midline shift, mm, median (25th percentile, 75th percentile) | 9 (4, 15) | 2 (0, 4) | <.001 |
| Any SDH | 31 | 14 | .009 |
| SDH > 5 mm | 17 | 3 | .002 |
| Surgical evacuation | 8 | 14 | .27 |
| Withdrawal of life-sustaining measures | 90 | 0 | <.001 |
Abbreviations: ICH, intracerebral hemorrhage; SDH, subdural hematoma.
This includes 9 patients with separate simultaneous acute lobar ICH in different lobes, as well as 76 patients where a single lobar hemorrhage traversed 2 or more lobes.
Table 3.
Logistic Regression Model of the Predictors of 30-Day Mortalitya
| Model Term | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| ICH volume, cm3 | ||
| 0–30 | 1 [Reference] | |
| 31–60 | 3.38 (1.24–9.23) | .02 |
| >60 | 16.63 (6.17–44.83) | <.001 |
| Age, y | ||
| <70 | 1 [Reference] | |
| 70–79 | 2.70 (1.03–7.13) | .04 |
| ≥80 | 3.46 (1.29–9.28) | .01 |
| Intraventricular hemorrhage | 1.72 (0.80–3.73) | .17 |
| SDH >5 mm | 7.60 (1.86–30.99) | .005 |
Abbreviations: ICH, intracerebral hemorrhage; SDH, subdural hematoma.
Lobar location and history of ICH were entered but removed from the model, by backward elimination, for P>.05.
Surgical evacuation was more common in those with SDH (10 of 40; 25%) than those without SDH (14 of 160; 9%; P=.01). Among the 24 patients who underwent surgery, mortality was higher in those who had SDH (5 of 10; 50%) compared with those who did not have SDH (1 of 14; 7%; P=.05). Pathologic examination of surgical resection specimens from the 10 patients with SDH revealed CAA in 6 of 7 cases where blood vessels could be identified within the specimen. An additional 2 patients who died with lobar ICH and SDH were autopsied, and both had parenchymal CAA (Figure).
COMMENT
Our systematic evaluation of consecutive patients with nontraumatic ICH shows that concurrent acute SDH is present in 20% of primary lobar ICH. By contrast there were no SDHs identified in 75 consecutive patients with deep ICH. The presence of SDH cannot be considered an unimportant epiphenomena of lobar ICH because when SDHs were relatively large (>5 mm) or present in the setting of a smaller parenchymal hematoma (<30 cm3), they were associated with higher mortality.
Rupture of a leptomeningeal artery adjacent to the dura, with extravasation into both the brain parenchyma as well as the subdural space, is the most likely explanation for the syndrome of SDH and nontraumatic primary lobar ICH. This explanation is consistent with observations that most SDHs were immediately adjacent to the lobar ICH, and all were in the same hemisphere. A leptomeningeal arterial origin of SDH, identified during surgery, has been described in the literature16–22 and is a common cause of spontaneous SDH without evident trauma.23 In our study, craniotomy or autopsy did not reveal the site of origin of the bleeding, probably because of the destructive nature of the ICH. Therefore, we cannot completely exclude that other local factors, such as shift of brain contents with secondary tearing of subdural bridging veins, played a role in the pathogenesis of SDH, particularly in the 20% of SDHs that did not appear directly adjacent to the lobar ICH.
Our findings are consistent with previous observations of an association between CAA and SDH.3,7 The majority of our patients with lobar ICH and SDH had either probable or possible CAA according to the validated Boston criteria, but there was a trend toward fewer lobar microbleeds among those with SDH. Pathologic evidence of CAA was found in 8 of 9 patients with specimens that included visible arteries or arterioles. The remaining patient, whose pathologic material came from hematoma resection, either did not have CAA or had CAA but not in the vessels visible in the resection specimen because of sampling error. Cerebral amyloid angiopathy is known to involve the leptomeningeal arteries and has previously been associated with subarachnoid hemorrhage.24–26 It is therefore possible that CAA-related vascular rupture of these same leptomeningeal arteries could cause both lobar ICH and SDH. Our data suggest that CAA-related vascular rupture is another cause of SDH of arterial origin. It is possible that CAA may be the cause of some spontaneous SDHs in the absence of lobar ICH, although this is speculative.
Subdural hematoma is most commonly caused by tearing of subdural bridging veins as a result of acceleration forces imparted to the head by trauma.27 There are several reasons why occult trauma is unlikely to have been the cause of lobar ICH and SDH in our population. First, study personnel prospectively screened subjects to exclude ICH caused by trauma. Collapse at the onset of symptoms, or being found unresponsive, was more common when SDH was present but this association was explained by larger ICH volumes resulting in more severe stroke symptoms. Second, there were 4 patients admitted to the hospital where SDH only became apparent on repeated imaging (Figure). These patients were under medical supervision at the time when SDH become apparent and therefore antecedent trauma, at least in the 24 hours prior, can be reliably excluded. Third, a plausible alternate explanation for ICH, CAA, was found in most patients who contributed pathologic specimens, including both who underwent autopsy. Finally, it is unlikely that SDH resulted from head trauma after collapse caused by primary ICH because a similar portion of patients with deep hemispheric ICH and lobar ICH presented with collapse at symptom onset or were found unresponsive (data not shown) but only lobar ICH was accompanied by SDH. Although trauma is unlikely for the aforementioned reasons, we cannot completely exclude that minor events, such as rapid head movement or a minor blow to the head, triggered CAA-related vascular rupture with subsequent lobar ICH and SDH.
Larger SDH, and those occurring in patients with an ICH volume lower than 30 cm3, were associated with higher mortality. There was a trend toward more frequent withdrawal of life-sustaining measures in those who died of SDH, suggesting that the presence of SDH was considered a marker of worse prognosis by the treating clinicians. The decreased effect of SDH on mortality in those with higher ICH volumes probably reflects the poor prognosis conferred by the large ICH. Our data suggest that SDH may be of greater clinical relevance in patients with smaller ICH volumes who are not necessarily destined for poor outcomes based on ICH size alone. Subdural hematomas are relatively accessible by surgery; however, in our study, half of patients with SDH who had surgery died. Whether the observed high mortality rate is because surgery is relatively ineffective for this syndrome, or because prognosis is poor regardless of surgery, cannot be determined in this observational study.
Our data suggest that the presence or absence of SDH should be systematically assessed in patients with lobar ICH as one of the predictors of prognosis and should prompt consideration of underlying CAA when present. Further studies from other centers are needed to determine whether the prevalence and risk factors for SDH are similar in other populations. The role of surgical evacuation of SDH in the setting of ICH is uncertain; however, we suggest that it warrants consideration, particularly in patients with smaller ICH volumes who otherwise appear to have a reasonable chance of survival.
Acknowledgments
Funding/Support: This work was supported by grant funding from the National Institute of Neurological Disorders and Stroke (Drs Smith and Rosand), National Institute of Aging (Dr Greenberg), and American Heart Association (Dr Rosand).
Footnotes
Financial Disclosure: None reported.
Author Contributions: The study authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Patel, Rosand, and Smith. Acquisition of data: Patel, FitzMaurice, Nandigam, Auluck, Viswanathan, Goldstein, Rosand, and Smith. Analysis and interpretation of data: Patel, Nandigam, Greenberg, and Smith. Drafting of the manuscript: Patel, Rosand, and Smith. Critical revision of the manuscript for important intellectual content: FitzMaurice, Nandigam, Auluck, Viswanathan, Goldstein, Rosand, Greenberg, and Smith. Statistical analysis: Smith. Obtained funding: Rosand and Greenberg. Administrative, technical, and material support: Patel and Goldstein. Study supervision: Rosand, Greenberg, and Smith.
References
- 1.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33(5):1190–1195. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- 2.Smith EE, Rosand J, Greenberg SM. Hemorrhagic stroke. Neuroimaging Clin N Am. 2005;15(2):259–272. doi: 10.1016/j.nic.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Vinters HV. Cerebral amyloid angiopathy: a critical review. Stroke. 1987;18(2):311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 5.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164(8):880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 7.Chao CP, Kotsenas AL, Broderick DF. Cerebral amyloid angiopathy: CT and MR imaging findings. Radiographics. 2006;26(5):1517–1531. doi: 10.1148/rg.265055090. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342(4):240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 9.Hill ME, Rosenwaike I. The Social Security Administration’s Death Master File: the completeness of death reporting at older ages. Soc Secur Bull. 2001;64 (1):45–51. [PubMed] [Google Scholar]
- 10.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56(4):537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 11.Zumkeller M, Behrmann R, Heissler HE, Dietz H. Computed tomographic criteria and survival rate for patients with acute subdural hematoma. Neurosurgery. 1996;39(4):708–712. doi: 10.1097/00006123-199610000-00011. discussion 712–713. [DOI] [PubMed] [Google Scholar]
- 12.Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60(9):1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- 13.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63(6):1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology. 1996;46(6):1751–1754. doi: 10.1212/wnl.46.6.1751. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35(6):1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 16.Koç RK, Pasaoglu A, Kurtsoy A, Oktem IS, Kavuncu I. Acute spontaneous subdural hematoma of arterial origin: a report of five cases. Surg Neurol. 1997;47(1):9–11. doi: 10.1016/s0090-3019(96)00150-4. [DOI] [PubMed] [Google Scholar]
- 17.McDermott M, Fleming JF, Vanderlinden RG, Tucker WS. Spontaneous arterial subdural hematoma. Neurosurgery. 1984;14(1):13–18. doi: 10.1227/00006123-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Tokoro K, Nakajima F, Yamataki A. Acute spontaneous subdural hematoma of arterial origin. Surg Neurol. 1988;29(2):159–163. doi: 10.1016/0090-3019(88)90076-6. [DOI] [PubMed] [Google Scholar]
- 19.Shenkin HA. Acute subdural hematoma: review of 39 consecutive cases with high incidence of cortical artery rupture. J Neurosurg. 1982;57(2):254–257. doi: 10.3171/jns.1982.57.2.0254. [DOI] [PubMed] [Google Scholar]
- 20.Nizzoli V, Brambilla P, Tonnarelli GP. Acute subdural hematoma: spontaneous forms of arterial origin. Eur Neurol. 1981;20(1):4–8. doi: 10.1159/000115196. [DOI] [PubMed] [Google Scholar]
- 21.Byun HS, Patel PP. Spontaneous subdural hematoma of arterial origin: report of two cases. Neurosurgery. 1979;5(5):611–613. doi: 10.1227/00006123-197911000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Depreitere B, Van Calenbergh F, van Loon J. A clinical comparison of non-traumatic acute subdural haematomas either related to coagulopathy or of arterial origin without coagulopathy. Acta Neurochir (Wien) 2003;145(7):541–546. doi: 10.1007/s00701-003-0020-7. discussion 546. [DOI] [PubMed] [Google Scholar]
- 23.Akioka N, Fukuda O, Takaba M, Kameda H, Saito T, Endo S. Clinical investigation of acute spontaneous subdural hematoma cases. J Stroke Cerebrovasc Dis. 2007;16(3):109–113. doi: 10.1016/j.jstrokecerebrovasdis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Yamada M, Itoh Y, Otomo E, Hayakawa M, Miyatake T. Subarachnoid haemorrhage in the elderly: a necropsy study of the association with cerebral amyloid angiopathy. J Neurol Neurosurg Psychiatry. 1993;56(5):543–547. doi: 10.1136/jnnp.56.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda S, Yamazaki K, Miyakawa T, et al. Subcortical hematoma caused by cerebral amyloid angiopathy: does the first evidence of hemorrhage occur in the subarachnoid space? Neuropathology. 2003;23(4):254–261. doi: 10.1046/j.1440-1789.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohshima T, Endo T, Nukui H, Ikeda S, Allsop D, Onaya T. Cerebral amyloid angiopathy as a cause of subarachnoid hemorrhage. Stroke. 1990;21(3):480–483. doi: 10.1161/01.str.21.3.480. [DOI] [PubMed] [Google Scholar]
- 27.Graham DI, Gennarelli TA, McIntosh TK. Trauma. In: Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. 7. Vol. 1. London, England: Arnold; 2002. [Google Scholar]