Abstract
Leishmania, a parasitic protozoan, infects human macrophages, often causing severe morbidity and mortality. The pathogenic form of this parasite, the amastigote, lives inside the acidic phagolysosomes of infected macrophages. In our attempt to develop anti-miniexon phosphorothioate oligodeoxyribonucleotides (S-oligos) as an alternative chemotherapy against Leishmania, we found that intracellular as well as ‘axenic’ amastigotes were more susceptible to these S-oligos than were the cultured promastigotes. Lower pH (4.5) and elevated temperature (35°) of the medium were among the direct enhancing factors for killing. Addition of the cationic polypeptide poly-l-lysine (PLL) to the growth medium further enhanced the killing effect of the S-oligo at pH 4.5. The enhancement of specific ablation of mRNA expression was directly correlated to the increased leishmanicidal activity of the S-oligo. This was shown by the increased inhibition of luciferase activity expressed in transgenic Leishmania amazonensis promastigotes by anti-miniexon S-oligo or anti-luciferase S-oligo at acidic pHs and in the presence of PLL. The leishmanicidal effects of S-oligos at acidic pH and in the presence of PLL were related to increased uptake of the S-oligos under these conditions. The rate of S-oligo uptake was enhanced up to 15-fold at pH 4.5. The addition of PLL to the assay medium at acidic pH further enhanced the uptake of S-oligo up to 80-fold. RNase H is known to accentuate the antisense action of S-oligos. We found that at an elevated temperature RNase H activity in Leishmania cell extracts increased about 5-fold. Thus, enhanced uptake of S-oligos at the acidic pH of macrophage phagolysosomes and activation of RNase H may explain the efficient killing of the parasite in macrophages, both in tissue culture and in the animal model, by antisense miniexon oligonucleotide/PLL, when targeted directly to the parasite-containing phagolysosomes.
Keywords: Leishmania, Amastigote, Promastigote, Differentiation, Ribonuclease H, Antisense phosphorothioate oligodeoxyribonucleotide
1. Introduction
The etiological agent for leishmaniasis, a human parasitic disease, is the protozoon of the genus Leishmania. Leishmaniasis currently affects some 12 million people in 88 countries, all but 16 of which are in the developing world. The disease is transmitted by sandflies (e.g. Phlebotomus spp.), small biting insects that breed in moist soil, forest areas, caves, or the burrows of rodents and feed from soft plant tissues or animal reservoir hosts including humans. According to the latest epidemiological data from the World Health Organization [http://www.who.int/emc/diseases/leish/leis.html], the global annual incidence of leishmaniasis is 1.5 to 2 million new cases per year. During its life cycle, Leishmania alternates between the insect vector and a vertebrate host. The parasite lives as a motile, flagellated promastigote in the gut of various sandfly vectors, characterized by its elongated shape and a single long anterior flagellum [1]. When delivered into the mammalian body by the bite of an infected sandfly, the promastigotes are engulfed by the macrophages by a receptor-mediated process [1]. The promastigotes end up in the phagolysosomes of macrophages where they transform into non-motile, intracellular amastigotes [1]. Both the promastigotes and the amastigotes may be grown in axenic culture media [2,3]. Presently, there is no effective vaccine available for prophylactic purposes against leishmaniasis [http://www.cdc.gov/travel/diseases/leishmaniasis.html]. The Center for Disease Control recommends sodium stibogluconate or meglumine antimoniate as drugs of choice against leishmaniasis [http://www.cdc.gov/travel/diseases/leishmaniasis.html]. Also recommended are amphotericin B (including its lipid-encapsulated form), pentamidine isethionate, or paramomycin as alternatives. Other useful antileishmanial chemotherapeutic agents include ketoconazole and its derivatives [4]. The frequent toxic side-effects of these drugs and the potential of Leishmania to develop resistance to these drugs [5] suggest the need for more rational alternative chemotherapeutic agents against leishmaniasis.
Recently, we and others [6–10] have become interested in exploring the possibility of using antisense S-oligo as an alternative chemotherapy against leishmaniasis and as an alternative tool for targeted gene knockout of mRNAs in this and other similar cells. Using S-oligos, Ramazeilles et al. [6] targeted the miniexon sequence present at the 5′-end of every mRNA of Leishmania amazonensis. The complementary 16-mer (16PS) was able to kill amastigotes in murine macrophages in culture. Using 16PS linked to a palmitate chain, which enabled it to complex with low-density lipoproteins, improved the leishmanicidal efficiency on intracellular amastigotes, probably due to increased endocytosis [7]. The targeted miniexon segment of the mRNAs folds into a hairpin, and the antisense disrupts the stem region of this hairpin [8,9]. We targeted a 17-mer S-oligo antisense (ASM) to the 3′-end of the miniexon of Leishmania to the phagolysosomes of cultured murine macrophages infected with L. amazonensis using the macrophage scavenger receptor-mediated modality [10]. ASM was encapsulated into liposomes coated with MBSA, the artificial ligand for macrophage scavenger receptors. MBSA-coating of the liposomes allowed specific binding of the liposomes to the macrophages, their receptor-mediated uptake, and subsequent degradation inside the phagolysosomes to release the ASM [10]. When incubated with Leishmania-infected macrophages, MBSA-liposome-encapsulated ASM (10 µM) was able to kill >90% of the parasites within 5 hr as compared with 20% killing within this time period by free ASM. Similar anti-miniexon S-oligo encapsulated in cationic liposomes has also been shown to specifically reduce the amastigote burden within cultured macrophages [11].
We found that the phagolysosomal amastigotes of L. amazonensis are killed more efficiently by ASM than are the cultured promastigotes. To understand the underlying mechanism of this difference, we examined axenically grown amastigotes and found the same to be true. Thus, enhanced leishmanicidal efficacy of ASM against amastigotes may be macrophage-independent. Here, we report that the acidic pH of the amastigote growth medium allows them to efficiently pinocytose ASM and other oligos, resulting in enhanced ablation of target mRNA functions. Activation of RNase H at a higher temperature may aid in the enhancement of antileishmanial activity of ASM in amastigotes.
2. Materials and methods
2.1. Materials
L. amazonensis (MHOM/BR/72/LV79), L. donovani (MHOM/IN/80/DD8), L. major (MHOM/IL/80/FRIEDLIN), and mouse macrophage cells, J774G8, were obtained from Prof. K. P. Chang of the Chicago Medical School. Cell culture media and antibiotics were purchased from Life Technologies, Inc. S-oligos were custom synthesized, HPLC-purified, and analyzed by Trilink Biotechnology. 35S-oligos also were synthesized and purified by Trilink Biotechnology. Other radiolabeled compounds were purchased from Amersham Pharmacia Biotech. Some of the S-oligos were synthesized in-house in a Beckman 1000M Oligo Synthesizer, using a 1000 nmol scale Beckman column. Post-synthesis, the S-oligos were purified using RPHPLC (Beckman Ultrasphere) C-18 columns. Oligos for polymerase chain reaction were custom made by Life Technologies, Inc. PLL (MW 30,000 –70,000), BSA, DMPC, DMPE, cholesterol, dicetyl phosphate, maleic anhydride, dibutyl phthalate, dextran sulfate, phytic acid, wortmannin, dioleoyl glycerol, dipalmitoyl glycerol, and phorbol myristate acetate were purchased from the Sigma Chemical Co. Fetal bovine serum was purchased from Atlanta Biologicals. T4 DNA ligase was purchased from Promega. Other molecular biology reagents were procured from Life Technologies, Inc. The Leishmania/Escherichia coli shuttle vector was obtained from Prof. S. Beverley of Washington University. Plasmid isolation reagents were obtained from Qiagen. Nucleotide sequencing reagents were from PE Biosystems. All other chemicals were purchased from the Sigma Chemical Co.
2.2. Cell culture
Leishmania promastigotes were grown at 25° in Medium M199 or in Schneider’s Drosophila medium with 10% HIFBS [12]. The amastigotes of L. amazonensis and L. major were obtained from tail-base lesions of infected Balb/C mice [13]. The amastigotes were also isolated from infected, cultured macrophages [13]. Axenic amastigotes of all these Leishmania species were grown in cell-free medium at pH 4.5 and at 33–37° [2,3]. Amastigotes were allowed to transform into promastigotes at 25° in Medium M199 containing 10% HIFBS [13]. J774G8 cells were cultured in medium RPMI 1640 with 20% HIFBS at 35° [10]. J774G8 cells were infected with amastigotes following the standard protocol [14].
2.3. Oligonucleotides used
The following S-oligos were used in this study: antiminiexon antisense oligo ASM, 5′-CTGATACTTATAT AGCG-3′ [10], the corresponding sense oligo SSM, 5′-CGCTATATAAGTATCAG-3′ [10], anti-luciferase antisense oligo LUAS, 5′-ATGCCCATACTGTTGAG-3′, and the corresponding sense oligo LUS, 5′-CTCAACAGTATGGGCAT-3′. Phosphorothioate oligos were radiolabeled either with 35S at the 2nd bond from the 5′-end during their chemical synthesis or uniformly with 3H after synthesis by an exchange reaction protocol [15]. The sense and antisense primers were designed using MacVector software (Oxford Molecular). Phosphorothioate derivatives of the oligonucleotides were used because these molecules are known to mediate their action in an RNase H-dependent manner as opposed to other types of chemical modifications, e.g. morpholino antisense oligomers, which mediate their actions via RNase H-independent mechanisms. The specificity of the antisense S-oligos used in this study has been documented using appropriate control S-oligos, which did not have any significant target ablation effects in our studies. We have previously documented the specificity of ASM against Leishmania cell growth using additional control oligonucleotides [10].
2.4. Preparation of MBSA-coated liposomes
BSA (Fraction V, Sigma) was maleylated with maleic anhydride [16]. Dipalmitoyl phosphatidylethanolamine (Sigma) (100 mg, dissolved in 0.15 M NaCl by ultrasonication) was coupled to MBSA (30 mg) by a 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (100 mg)-mediated reaction [16,17] in a total volume of 3 mL. DMPC was used to prepare liposomes for encapsulation of ASM or other oligonucleotides [16,17]. Briefly, DMPC (10 mg) was made into vesicles with cholesterol (9 µmol) and dicetyl phosphate (1.2 µmol) in 1 mL HBS (HEPES buffer, 25 mM; NaCl, 0.15 M; sometimes contained oligonucleotide, 50 µmol/mL). Typically by this procedure, 0.1 to 0.2 µmol ASM became trapped in 1 mg of liposomal phospholipids. The amount of phospholipids in each liposome preparation was assayed by estimating its phosphate content [10]. For coating the liposomes with phospholipid-tailed MBSA (which binds via the phospholipid moiety), 200 µg of the protein was incubated with 1 mg of liposomes in HBS for 20 hr at 4°. Bound MBSA was separated from unbound molecules by centrifugation at 100,000 g for 1 hr at 4°, as described [10].
2.5. Assay of leishmanicidal activity of ASM
To assay the leishmanicidal activities of ASM against promastigotes and axenic amastigotes, cells in mid-log phase were aliquoted (1 mL) into 24-well plates. SSM or ASM and the effector (if any) were added from 100× solutions. The plates were incubated in a humidified incubator on a rocker platform for 20 hr before counting the cells microscopically, using a hemocytometer [10]. To evaluate their effects on amastigotes inside the phagolysosomes of J774G8 cells, monolayers of J774G8 cells in 24-well plastic tissue culture plates were incubated at 37° with L. amazonensis promastigotes in medium RPMI 1640, with 20% heat-inactivated fetal bovine serum and a parasite-to-macrophage ratio of 10:1 [10]. The infected macrophages were incubated for 48 hr in the growth medium at 35° at which time the growth medium was replaced with fresh medium to which the test compound was added. After incubation of the parasite-laden macrophages at 35° for 16 hr, the number of amastigotes per 100–200 macrophages was counted microscopically, as described [10].
2.6. Development of stable transfectant of L. amazonensis
We constructed a plasmid for the permanent expression of firefly luciferase in L. amazonensis promastigotes. The firefly luciferase gene was cut out from pGL3-basic plasmid (Promega) with BglII and BamHI and subcloned into the BamHI site of pX63-NEO [18]. The recombinant clone, having the luciferase gene in the same orientation as the Neo gene, as was selected by BamHI/XbaI digestion of the plasmid, gave us the construct, pXNeo-Luci. The orientation of the luciferase gene insert was also confirmed by nucleotide sequencing with T3 primer using Big Dye Terminator reagents (PE Biosystem) and a 310 Genetic Analyzer (PE Biosystem). Plasmid DNA was isolated from recombinant cells using Qiagen midiprep reagents. Late-log phase promastigotes (4 × 107) of L. amazonensis were electroporated [19,20] with 25 µg of pXNeo-Luci, and stable transfectants were maintained at a G418 concentration of 500 µg/mL.
2.7. Assay for luciferase activity
The luciferase assay was performed using the Luciferase Reporter Assay System (Promega). Cells were incubated with the given concentrations of the anti-miniexon or antiluciferase oligonucleotides overnight (16 hr) at pH 7.5/4.5 with or without PLL at 10 µg/mL. Cells were washed with ice-cold PBS and lysed with Passive Lysis Buffer (Promega, 100 µL/107 cells) at room temperature for 15 min on a rocker platform. Lysate was centrifuged at 16,000 g for 10 min at 22°. An aliquot (20 µL) of the supernatant was added to substrate LARII (Promega, 100 µL) and mixed by trituration, and luminescence was assayed for 10 sec in a Turner Designs model 20/20 Luminometer [21].
2.8. Assay for S-oligo uptake by Leishmania
The uptake of 35S- or 3H-labeled phosphorothioate S-oligos (ASM, SSM, LUAS, and LUS) was assayed following a protocol described by Seyfang et al. [22]. Late-log phase promastigotes were suspended (5 × 107/mL) in serum-free culture medium. The pH of the culture medium was adjusted with 1 M HCl. The radiolabeled S-oligo was dissolved in the same medium as the cells. Uptake was measured at 25 or 37° by incubating 100 µL of cell suspension with 100 µL of S-oligo solution (0–20 µM) for up to 2 hr on a cushion of 100 µL dibutyl phthalate [22]. Incubation was carried out for the required time intervals, and cells were pelleted by centrifugation (12,000 g) for 2 min at room temperature. Microfuge tubes were snap-frozen in liquid N2. The tip of the tube containing the pellet was chopped off, and the contents were dissolved in 250 µL of 1% SDS solution [22]. Liquid scintillation fluid (2 mL, ScintiSafe Gel, Fisher Scientific) was added to the SDS solution, and the radioactivity of the solution was assessed by liquid scintillation counting. The uptake of oligo was computed from the cell-associated radioactivity and was expressed as picomoles of radioactive oligo associated with 106 Leishmania per hour.
2.9. Assay for RNase H activity in Leishmania cell extracts
[32P]Poly (rA):poly(dT) was used as the substrate of RNase H [23,24]. E. coli RNA polymerase (6 µL, 10 U/µL, Amersham Pharmacia Biotech) was added to a mixture containing 5 A260 units poly(dT), 5% glycerol, 50 mM Tris–HCl (pH 8.0), 5 mM MgCl2, 1 mM MnCl2, 4 mM dithiothreitol, 30 µM ATP, and 100 µCi [α-32P]ATP (3000 Ci/mmol, Amersham) in a total volume of 3 mL [23,24]. The reaction mixture was extracted twice with an equal volume of water-saturated phenol (GIBCO-BRL). The pooled phenol phases were extracted with an equal volume of 50 mM Tris–HCl (pH 8.0). The pooled aqueous extracts then were extracted with an equal volume of chloroform: isoamyl alcohol (24:1). Absolute ethanol was added to the aqueous phase to a final concentration of 35%, and the resulting solution was loaded onto a 2-mL column of cellulose (CF11, Sigma). The column was pre-equilibrated with a 35:65 mixture of ethanol:‘buffer A’ [50 mM Tris– HCl (pH 7.5)/0.1 M NaCl, 50 mM EDTA]. After sample loading, the column was washed extensively with this ethanol:‘ buffer A’ mixture (~50 mL) until all unbound radioactivity was removed (<1000 cpm/10 µL wash). The bound nucleic acid polymer was then eluted from the column with 5 mL of buffer A, and 500-µL fractions were collected in microfuge tubes. Fractions with high radioactivity (generally tubes 3–7) were pooled, and radioactivity was measured by scintillation counting [25]. A typical preparation usually gave ~125,000 cpm/10 µL. The hybrid solution was aliquoted, and stored at –20° for later use. Washed (in PBS) Leishmania promastigotes or amastigotes were lysed in Passive lysis buffer (Promega, 108 cells/mL) for 15 min in ice, and cleared by centrifugation at 4° for 10 min at 16,000 g; the supernatant was assayed for RNase H activity. Proteins in Leishmania cell extracts were determined colorimetrically using Bradford’s reagent and BSA as a standard [21]. For the RNase H solution assay, cell lysate (1 µg protein) was incubated at 37° for 30 min in 10 mM Tris–HCl (pH 8.0), 10 mM MgCl2, 50 mM KCl, and 0.1 mM dithiothreitol with the substrate (5 µL) in a total volume of 25 µL. The reaction was terminated by the addition of 50 mM (final concentration) EDTA and glycogen (1 µg/µL, final concentration). The unreacted substrates and proteins were precipitated by the addition of trichloroacetic acid (5%, final concentration for 10 min at 4°) followed by centrifugation at 16,000 g for 10 min at 4°. The radioactivity in the supernatant was determined by liquid scintillation counting [25], and RNase H activity was expressed as nanograms of the radiolabeled substrate degraded into acid-soluble fragments per microgram protein per hour. Bovine pancreatic RNase A (Type 1A, Sigma Chemical Co.) and E. coli RNase H (Life Technologies, Inc.) were used as negative and positive controls, respectively, to verify the efficacy and specificity of the substrate. While E. coli RNase H cleaved the substrate into acid-soluble radioactive products, RNase A did not cleave the substrate under the conditions described. The specific presence of RNase H activity in an ~64-kDa protein molecule in Leishmania cell extract was further documented by zymogram analysis using this substrate (Bennett JR and Chaudhuri G, unpublished data), as described [23, 24].
2.10. Treatment of mouse tail-base lesions of L. amazonensis with MBSA-liposome encapsulated S-oligo and PLL
Balb/C mice (6- to 8-weeks-old) were infected at the tail base with L. amazonensis (LV78, clone 12–1) or L. major (Friedlin) amastigotes from donor mice tail bases. After 8 weeks, the mice were injected intramuscularly (100 µL) near the lesion with MBSA-liposome-encapsulated S-oligo (SSM or ASM, 10 µmol/kg) with PLL (10 mg/kg) on days 1–4 [17]. Control animals received the same volume of MBSA-liposome without any S-oligo or PLL. The size of the lesion was measured with a dial caliper [17]. The volume of the lesion before injection on day 1 was taken as 100% (206 ± 20 mm3).
2.11. Statistical procedures
Each data set is presented as means ± SEM (N = 5 or more). Statistical significance of a difference between two series of data was tested by determining the P value [26]. If the P value was less than 0.05, the difference was considered to be significant [26].
3. Results
3.1. Enhanced susceptibility of axenic amastigotes to ASM-mediated killing compared with that of promastigotes
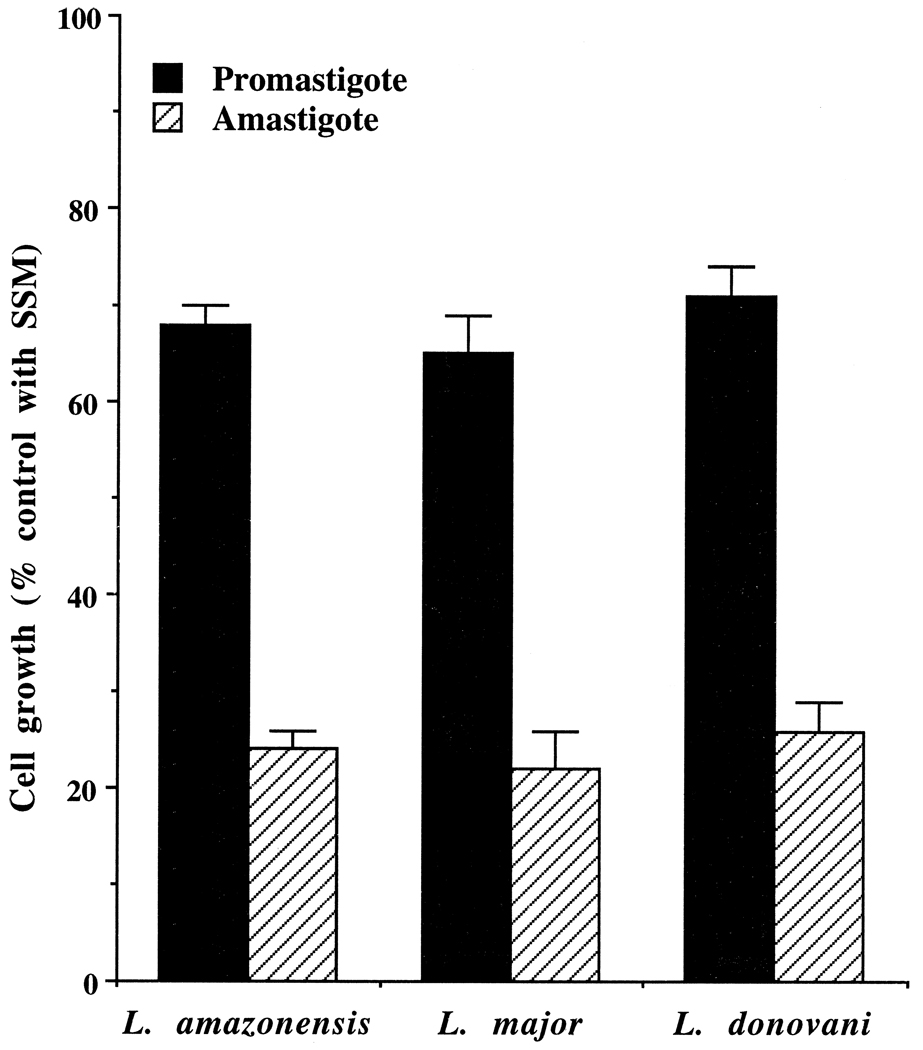
We previously found that L. amazonensis amastigotes inside macrophage phagolysosomes are more susceptible to ASM-mediated killing than are the cultured promastigotes [10]. We did not know whether some macrophage factor was contributing to that susceptibility or whether the physico-chemical environment of the phagolysosomes was enhancing the effect of ASM. We cultured the amastigotes of L. amazonensis, L. major, and L. donovani axenically without macrophages at 35°, pH 4.5, and tested the efficacy of ASM against them. These axenic amastigotes showed higher susceptibility to ASM-mediated killing in the growth medium than the promastigotes cultured in the same medium but at 25°, pH 7.5 (Fig. 1). This effect was concentration- and time-dependent (data not shown). These data suggested that besides the potential contributing factors in the amastigotes, pH and temperature of the growth medium may be determining factors for the enhancement.
Fig. 1.
Antileishmanial activity of ASM against axenic amastigotes and promastigotes of L. amazonensis, L. donovani, and L. major. Growing cultures of the cells (~5 × 106 cells/mL) were incubated with 20 µM SSM (control) or ASM for 20 hr at 25°, pH 7.5 (promastigotes) or at 35°, pH 4.5 (axenic amastigotes). SSM had less than a 2% effect on the growth of the cells at this concentration. Results are means ± SEM (N = 6). The differences of the antisense activities of ASM between the promastigotes and the amastigotes for each species were statistically significant (P < 0.01).
3.2. Enhanced killing of Leishmania promastigotes by ASM at acidic pH and/or elevated temperature and in the presence of PLL
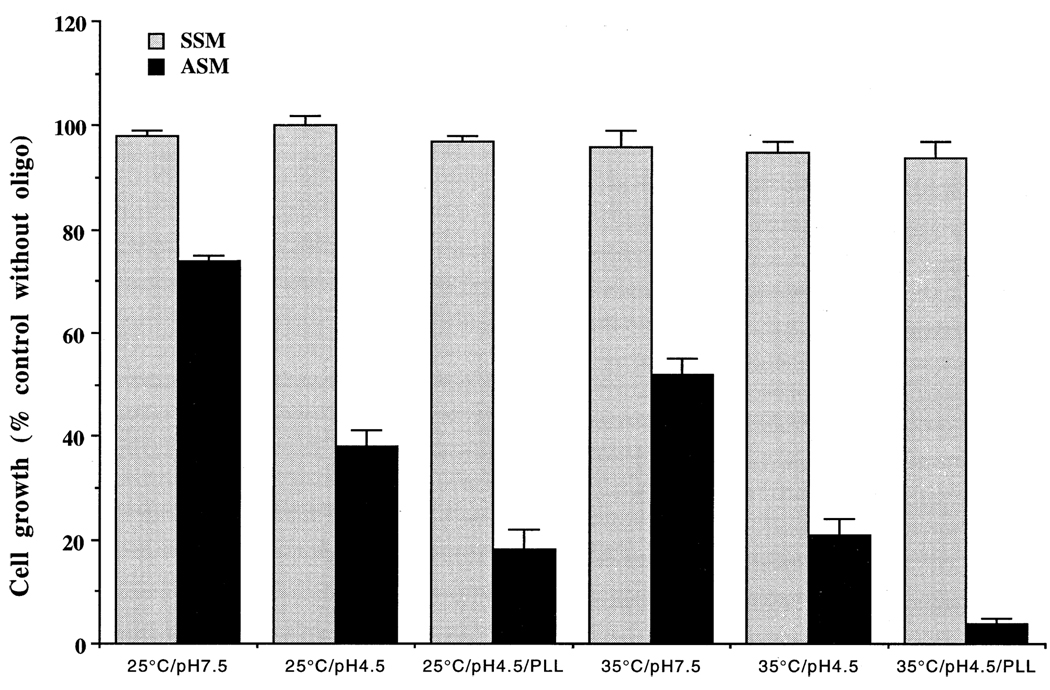
Promastigotes grow at neutral to alkaline pHs. We tested whether the pH of the growth medium had any effect on the efficiency of killing of the promastigotes by ASM. Our data (Fig. 2) show that, indeed, the medium pH had a significant effect on the efficacy of ASM to kill the parasite cells. More L. amazonensis promastigotes were killed by ASM at pH 4.5 than at pH 7.5 (Fig. 2). The inability of SSM to kill the parasite cells indicates that the antileishmanial effect of ASM is sequence specific, as reported previously [10]. In accordance with the enhancing effects of lowered pH, elevation of the temperature of the medium from 25 to 35° also had a significant effect on the killing of the parasites by ASM (Fig. 2). Lowering of the pH of the medium and/or elevation of the temperature for up to 20 hr was not lethal to the promastigotes. Although the morphology of the cells became stumpy and their motility was reduced, they were able to revert to motile promastigotes when re-incubated at 25° and pH 7.5. We tested the effects of several cationic and anionic macromolecules such as PLL, MBSA, dextran sulfate, and phytic acid on the efficacy of ASM to inhibit the growth of L. amazonensis. As shown in Fig. 2, addition of PLL to the growth medium enhanced the effect on the killing of the parasite cells by ASM at pH 4.5. PLL alone had no significant effect on the growth of the promastigotes at the concentrations tested (data not shown). In contrast to PLL, identical concentrations of polyanions such as dextran sulfate, MBSA, or phytic acid had no effect on the efficiency of killing of L. amazonensis promastigotes by ASM (data not shown). Classical pinocytosis effectors like wortmannin, dioleoyl glycerol, dipalmitoyl glycerol and phorbol myristate acetate had neither a significant effect on the growth of the promastigotes, nor an effect on the efficacy of ASM at acidic or neutral pHs, with or without PLL (data not shown).
Fig. 2.
Effects of changes in the pH and/or temperature of the growth medium on the leishmanicidal activity of ASM against promastigotes. Growing cultures of the L. amazonensis promastigotes (~1 × 107 cells/ mL) were incubated with 20 µM SSM or ASM for 20 hr at 25° or at 35°, pH 7.5 or 4.5, with or without PLL (10 µg/mL). Results are means ± SEM (N = 6). Similar results were obtained with L. major and L. donovani promastigotes. PLL alone at 10 µg/mL had a marginal effect on growth at pH 7.5. The differences of antileishmanial activities between ASM and SSM were statistically significant (P < 0.05 at pH 7.5 and P < 0.01 at pH 4.5).
3.3. Efficient killing of axenic amastigotes of Leishmania by ASM in the presence of PLL
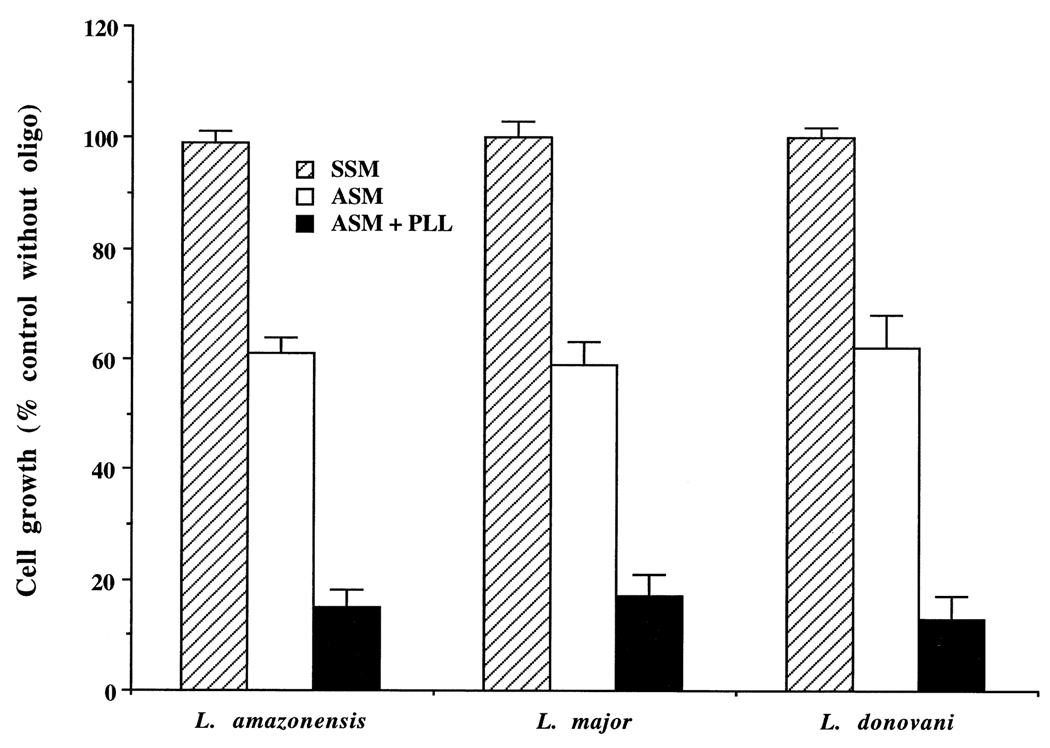
Axenic amastigotes were grown in vitro in axenic culture medium at 35°, pH 4.5 (see “Materials and methods”). We found that PLL also had an enhancing effect in the killing of axenic amastigotes by ASM in the growth medium (Fig. 3). As with the promastigotes, PLL alone had no effect on the growth of axenic amastigotes. Likewise, as with promastigotes, polyanions or the classical pinocytic effectors mentioned above had no effect on the inhibition of growth of axenic amastigotes.
Fig. 3.
Enhancement of leishmanicidal activity of ASM by PLL in axenic amastigotes. Growing cultures of the axenic amastigotes (~5 × 106 cells/mL) were incubated with 10 µM SSM or ASM for 20 hr with or without PLL (10 µg/mL). Results are means ± SEM (N = 6). In the presence of PLL (10 µg/mL), 20 µM ASM completely lysed the amastigotes of all three species. The differences between SSM and ASM, between SSM and ASM + PLL, and between ASM and ASM + PLL were statistically significant (P < 0.01).
3.4. Enhanced killing of L. amazonensis amastigotes inside macrophage phagolysosomes by ASM in the presence of PLL
We previously described the killing of L. amazonensis amastigotes inside the macrophage phagolysosomes by ASM when targeted via a scavenger receptor packaged inside MBSA-coated liposomes [10]. We employed a similar strategy to test whether the cationic peptide PLL can enhance the leishmanicidal effect of ASM inside the acidic phagolysosomes of L. amazonensis-infected macrophages. We encapsulated PLL inside DMPC-liposomes, with SSM or ASM, and then coated the liposomes with phosphatidylethanolamine-tagged MBSA, which is the ligand for the scavenger receptor on macrophages. These coated liposomes were then added to the growth medium of Leishmania-infected cultured macrophages. The leishmanicidal effect of ASM against the amastigotes was enhanced when PLL was included (Fig. 4), whereas PLL (10 µg/mL) alone did not have any significant effect on the viability of amastigotes.
Fig. 4.
Enhancement of leishmanicidal activity of ASM by PLL in L. amazonensis-infected macrophages. L. amazonensis-infected J77G8 cells were exposed to MBSA-coated liposomes containing the S-oligo with or without PLL (10 µg/mL) at 35° for 16 hr, and the number of the amastigotes per 100–200 macrophages was counted microscopically [12]. The final concentrations of the S-oligo in the medium are plotted on the x-axis. Results are means ± SEM (N = 5). The differences between ASM and ASM + PLL were statistically significant (P < 0.01).
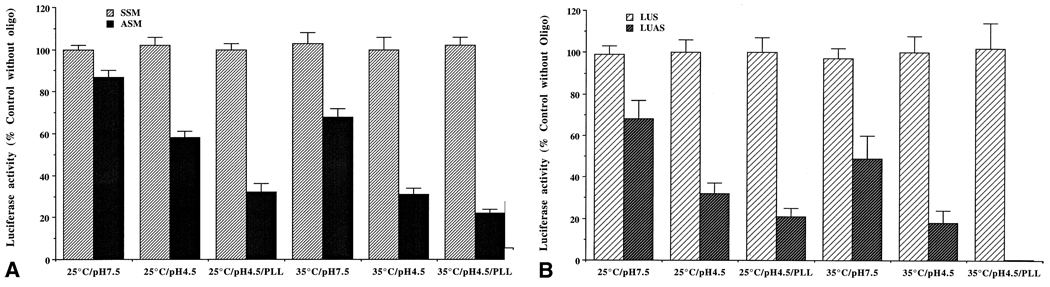
3.5. Enhanced inhibition of luciferase expression by ASM and anti-luciferase S-oligo in stably transfected Leishmania promastigotes at acidic pH and in the presence of PLL
To evaluate whether the observed enhancement of leishmanicidal activity of antisense phosphorothioate oligos at acidic pH and in the presence of PLL is due to enhanced inhibition of target gene expression, we developed a model system. We constructed a plasmid (pXNeo-Luci) that has the G418-resistance-conferring gene and the firefly luciferase gene behind leishmanial transplicing signals ([18,19]; see “Materials and methods”). This plasmid was introduced inside Leishmania promastigotes by electroporation, and recombinants were selected with G418. Recombinant G418-resistant L. amazonensis promastigotes expressed high levels of luciferase activities. The expression of luciferase activity in these cells was inhibited by ASM (Fig. 5A), as well as by anti-luciferase antisense phosphorothioate oligo (LUAS) (Fig. 5B). Inhibition of luciferase expression by ASM and LUAS was enhanced significantly when the pH of the medium was lowered from 7.5 to 4.5 (Fig. 5, A and B). Addition of PLL further enhanced the effect of LUAS on the luciferase expression by the recombinant L. amazonensis promastigotes at pH 4.5 and 35° (Fig. 5, A and B). We also tested the polyanions or the classical pinocytic effectors, mentioned above, on LUAS-mediated inhibition of luciferase activity of recombinant L. amazonensis. Those compounds had no significant effect on this process, as expected (data not shown). LUAS or PLL had no significant effect on the enzymatic activity of luciferase when directly added to the cell extract during the assay, suggesting that the inhibition is at the level of luciferase mRNA activity.
Fig. 5.
Effects of S-oligos on the expression of luciferase activity in stably transfected Leishmania promastigotes at different pHs and in the presence of PLL. (A) Effect of SSM and ASM on luciferase expression. (B) Effect of LUS and LUAS on luciferase expression. LUAS: anti-luciferase S-oligo; LUS: S-oligo complementary and anti-parallel to LUAS. The control luciferase activities varied from 4321 ± 187 (N = 8) to 4155 ± 241 (N = 8) light units per 106 cells depending upon the treatment. Results are means ± SEM (N = 8). The influence of acidic pH and/or PLL on the inhibitory effects of ASM or LUAS on the luciferase activity in the cell extracts was statistically significant (P < 0.05).
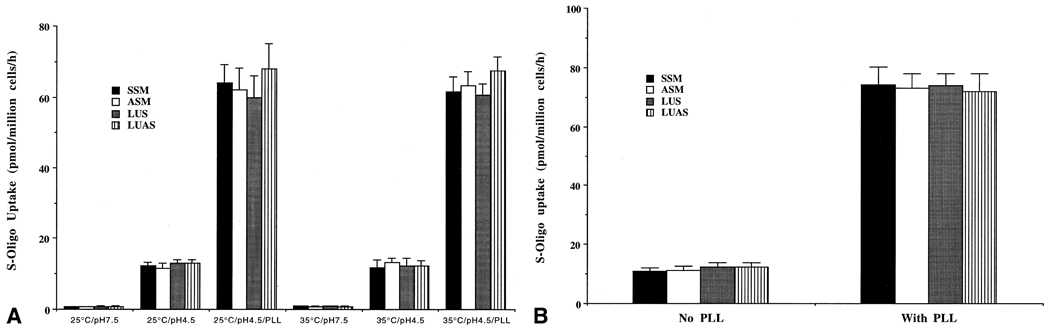
3.6. Enhanced uptake of phosphorothioate oligos by Leishmania promastigotes at acidic pHs and in the presence of PLL
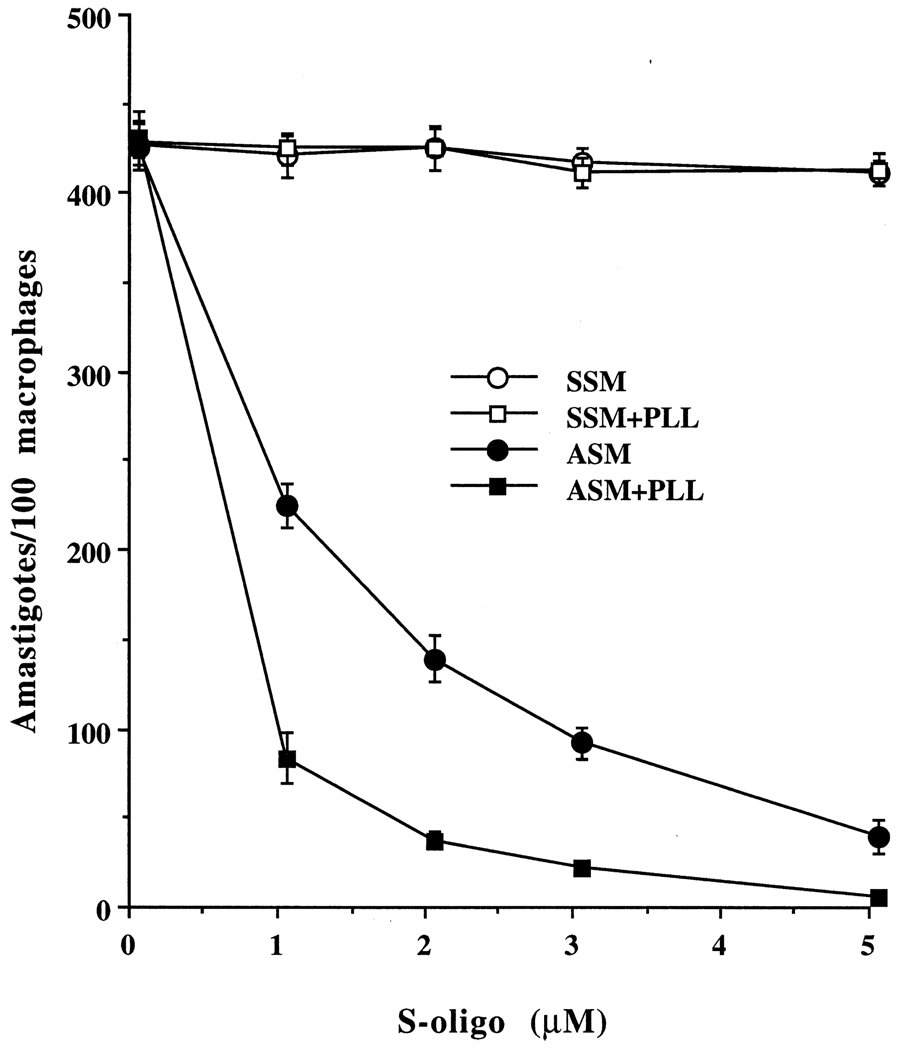
One of the possible mechanisms of enhancement of the efficacy of antisense phosphorothioate oligos in Leishmania at acidic pHs and with PLL is enhancement of the uptake of S-oligos under those conditions. We used 3H- or 35S-labeled S-oligos for the uptake study with L. amazonensis promastigotes. We incubated the cells with 0–20 µM radiolabeled S-oligo for 0–120 min and assayed for cell-bound radioactivity as the amount of S-oligo taken up by these cells under the specified conditions (see “Materials and methods” for details). Uptake of S-oligos by L. amazonensis promastigotes was enhanced at acidic pHs and with PLL, irrespective of temperature (Fig. 6A). The uptake was up to 15-fold higher at pH 4.5 than at pH 7.5, and this pattern was true for L. amazonensis, L. major, and L. donovani promastigotes (Fig. 6A). Addition of PLL to the assay medium at acidic pH further enhanced the uptake of S-oligo up to 80-fold (Fig. 6A). Similar observations with PLL were made with the axenic amastigotes (Fig. 6B). The uptake of ASM by any of these cells was not affected by polyanions or the classical pinocytic effectors mentioned above (data not shown).
Fig. 6.
Effects of PLL (10 µg/mL) on the level of cell-associated S-oligo in the promastigotes (A) and the axenic amastigotes (B) of L. amazonensis when incubated with radioactive S-oligos (10 µM). The level of S-oligos in the cells is expressed as picomoles of radioactive S-oligos associated with 106 Leishmania per hour. Results are means ± SEM (N = 6). Similar data were obtained with L. major and L. donovani promastigotes and axenic amastigotes (not shown). The influence of acidic pH and/or PLL was statistically significant (P < 0.01).
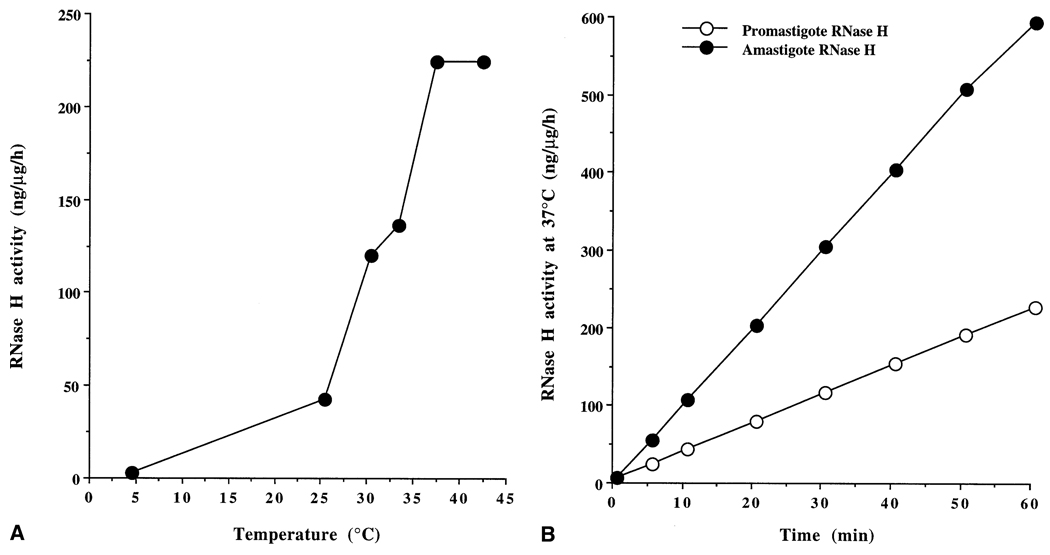
3.7. Increased RNase H activity in amastigote cell extracts
RNase H is known to mediate the action of antisense phosphorothioates in other cells [27]. Although likely, RNase H activity in Leishmania cell extracts has not been documented. Here, we report the detection of strong RNase H activity in the cell extracts of L. amazonensis amastigotes and promastigotes. One of the mechanisms of the enhanced effect of ASM against Leishmania could involve the activation of RNase H under specific environmental physicochemical conditions. Therefore, we studied RNase H activity in soluble cell extracts of L. amazonensis amastigotes and promastigotes. RNase H activity in the promastigotes exposed to acidic pH with or without PLL treatment was not altered significantly. RNase H in the promastigote or amastigote extracts was optimally active at 37° (Fig. 7A). Thus, at the elevated temperature, the RNase H in Leishmania worked ~5-fold faster than at 25°. In addition, there was about a 3-fold increase in the level of RNase H activity in the cell extracts from axenic amastigotes when assayed at 37° (Fig. 7B).
Fig. 7.
RNase H activity in the cell extracts of L. amazonensis amastigotes and promastigotes. (A) Effects of temperature on RNase H activity in promastigote cell extract. (B) Comparison of the specific activities (nanograms of substrate degraded to acid-soluble fragments per microgram protein per hour) of RNase H in amastigote and promastigote cell extracts. Results are the means from five independent assays for each point. Similar observations were made with extracts from L. major and L. donovani promastigote and axenic amastigote extracts.
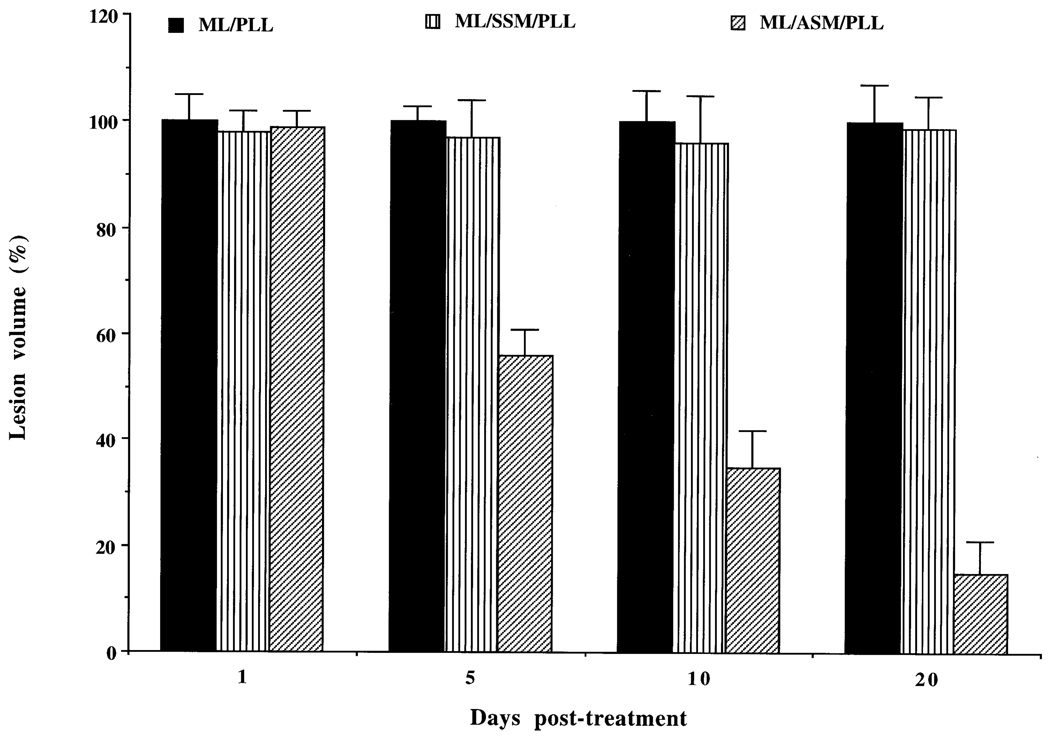
3.8. Leishmanolytic activity of MBSA-liposome encapsulated ASM/PLL in infected mice
We encapsulated SSM or ASM with or without PLL inside MBSA-coated liposomes and injected them intramuscularly near the mouse tail-base lesion (see “Materials and methods” for details). Liposomes with ASM, but not those with SSM, significantly inhibited the progression of the tail-base lesion in L. amazonensis-infected Balb/C mice (Fig. 8). The effect of ASM alone was 40–50% less than when both ASM and PLL were used together. SSM or PLL alone did not have any significant effect on the progression of the mouse tail-base lesion. There was no visual sign of gross toxicity or change in behavior of the animal after the drug treatment. We did not perform any biochemical or toxicological analyses to evaluate toxicity at the fine level.
Fig. 8.
Treatment of mouse tail-base lesions of L. amazonensis with MBSA-liposome (ML)/PLL, ML/SSM/PLL, ML/ASM/PLL, or simply PBS. Results are expressed as a mean percentage of lesion volume ± SEM (with PBS alone) for six animals per group. The effect of ASM/PLL/MBSA-liposome was statistically significant except for the 1-day post-treatment (P < 0.01).
4. Discussion
Leishmania is an intracellular parasite of mammalian macrophages. The overall goal of our study was to understand, optimize, and evaluate the feasibility of the antisense approach against the expression of leishmanial genes and to employ this technology in therapeutic as well as in investigative research applications with Leishmania and other related protozoa. We developed a targeted delivery modality for the delivery of oligonucleotides to macrophage phagolysosomes [10] where Leishmania lives and proliferates. Delivered this way, ASM seems to be far more effective in killing the phagolysosomal amastigotes than promastigotes cultured in vitro [10]. This observation was puzzling because although the targeting modality will raise the S-oligo concentration inside the phagolysosomes quickly, there is no apparent mechanism to keep the S-oligos inside the phagolysosomes. Our hypothesis was that the S-oligos under the physico-chemical conditions of macrophage phagolysosomes are taken up by the amastigotes quickly, and some cellular factor aids in the mode of action of the antisense S-oligo molecules to accentuate their activity.
S-oligos are negatively charged molecules at pH 7.5. Such polyanionic molecules do not cross cell membranes easily [27–30]. Thus, the transport of these molecules through the negatively charged membrane of Leishmania is slow. Lowering of the pH of the medium to 4.5 will almost neutralize S-oligo molecules, thus making them more permeable through the membrane by fluid-phase or adsorptive endocytosis [30]. Chemical modification in the oligonucleotide backbone structure to neutralize the molecules (e.g. methylphosphonate, morpholino) was found to enhance their uptake by mammalian cells [30]. Elevated temperature may also boost the uptake of the neutral oligo molecules [30], although we did not observe this increase with Leishmania promastigotes or amastigotes. In addition, neutralization of the negative charge of the phospholipid membrane with cationic molecules such as PLL was shown to be beneficial in enhancing the uptake of neutral oligonucleotide molecules [30]. Acceleration of S-oligo uptake by Leishmania amastigotes or promastigotes by inclusion of PLL at acidic pH may testify to that hypothesis. Classical pinocytosis effectors like wortmannin, dioleoyl glycerol, dipalmitoyl glycerol and phorbol myristate acetate [31] had neither a significant effect on the growth of the promastigotes nor an effect on the efficacy of antileishmanial activity of ASM at acidic or neutral pHs, with or without PLL. In Leishmania, vesicular transport appears to occur solely through the flagellar pocket membrane [32]. Whether the pinocytic effectors used in this study also work in the flagellar pocket membrane in Leishmania is not known.
To understand whether the accelerated killing of Leishmania by ASM at pH 4.5 in the presence of PLL is due to some unknown non-antisense effects of the test S-oligo molecules inside the cells or whether the ablation of target mRNA function is also accelerated, we studied the effect of ASM or anti-luciferase phosphorothioate S-oligo (LUAS) under these conditions on the expression of luciferase activity in a transgenic Leishmania. Our data suggested that the acceleration was due to the increased inhibition of target function and may not be due to non-antisense action, as respective control S-oligos had no significant effect. One interesting point is that although LUAS and ASM were taken up by Leishmania at a very similar rate, the inhibition of luciferase activity by LUAS seemed to be higher than that with ASM. One explanation could be that LUAS binds with higher affinity to the target than the ASM (predicted from the Tm). A second possibility is that there are numerous ASM targets because all mRNAs have the miniexon sequence, and, thus, the binding of ASM to luciferase mRNA is competed out by other mRNAs. Other possibilities include greater accessibility of the LUAS binding site than the ASM binding site in the luciferase mRNA (determined by possible secondary structures in the mRNA) or perhaps not all translated luciferase mRNAs are transpliced, thus lacking an ASM binding site. We checked the last possibility by reverse transcription–polymerase chain reaction and could not detect any unspliced luciferase mRNA in the cell (data not shown). Luciferase pre-mRNA transcribed from pXNeo-Luci plasmid should have a strong transplicing signal (a long polypyrimidine stretch followed by numerous AG sequences before the translation start codon), and thus all these pre-mRNAs should be transpliced with miniexon.
Although elevating the temperature of incubation accelerated growth inhibition and luciferase expression by the antisense S-oligos used, this factor had only a marginal effect on the uptake of the S-oligos by Leishmania cells. One explanation could be the stimulation of the mechanism of antisense action inside Leishmania at a higher temperature. This enhancement of luciferase inhibition at 35° did not happen when an equivalent concentration of a methylphosphonate derivative of LUAS was used instead of the phosphorothioate derivative (Bennett JR, Mishra M, and Chaudhuri G, unpublished data), indicating that RNase H may have a role in this process because the methylphosphonate derivative of S-oligo does not activate this enzyme [30]. Indeed, we found that there is a correlation between the activation of RNase H in Leishmania promastigotes treated at 35° and the efficacy of phosphorothioate S-oligos in the cells. Thus, notwithstanding many other possibilities, a simple explanation of the increased efficacy of ASM against Leishmania amastigotes is increased permeability of the S-oligo at pH 4.5 and enhanced degradation of the target mRNA after binding with the S-oligo due to stimulated RNase H activity inside the amastigotes. Although assayed in crude extract, the degradation of the RNA/DNA duplex substrate was RNase H-specific. The higher RNase H activity of the crude cell lysates at elevated temperatures was not due to denaturation of the substrate and its degradation by an RNase, as was verified using RNase A under similar conditions. RNase H has long been implicated as a mediator of the mode of action of antisense phosphorothioate oligonucleotides [33].
Antisense oligonucleotides show great promise as sequence specific agents and are able to down-regulate the expression of targeted genes [34–37]. In this capacity, they have advanced not only to clinical trials but also to clinical practice [38–41]. They also have been proven to be very useful as research tools [34]. Antisense oligonucleotides of different chemistries and conjugations have been tried both in vitro and in vivo, and each presented varying degrees of advantages and disadvantages over the others [34,42]. Here, we used S-oligos because these molecules are known to mediate their action in an RNase H-dependent manner as opposed to other types of chemical modifications, e.g. morpholino antisense oligomers, which mediate their actions via RNase H-independent mechanisms [43,44]. The specificity of the antisense S-oligos used in this study has been documented using appropriate control S-oligos that did not have any significant target ablation effects in our studies. We have previously documented the specificity of ASM against Leishmania cell growth using additional control oligonucleotides [10].
Finally, we employed the ASM transport-promoting effect of PLL in killing the amastigotes of L. amazonensis in the tail-base lesions of mice. PLL alone may have cytotoxic effects. However, under the conditions of the assay, the concentration of PLL used did not show any visible toxicity. The combination of PLL with the control S-oligo (SSM) did not show the antileishmanial effect shown by the PLL/ASM combination, strongly suggesting that the anti-parasitic effect was due to the antisense effect of ASM and to the potential toxicity of PLL. PLL may have many other effects in a mouse system; thus, whether this combination can be used for actual chemotherapy is far from clear. Testing other positively charged macromolecules such as histones [45] and/or a pinocytic enhancer such as suramin [46] along with the S-oligo may be more effective. Identification of an antisense S-oligo with high affinity for its target, which is essential for parasite growth, will bring much improvement to the antisense approach against leishmaniasis.
Acknowledgments
We thank Prof. Scott Landfear of Oregon State University for advice on the oligonucleotide transport assays, Prof. K.P. Chang of the Chicago Medical School for the suggestions on the growth of amastigotes in the lesions of the Balb/C mouse tail base, and Mrs. Angelika K. Parl for technical assistance. This work was supported by the National Institutes of Health, USA Grants R01AI42327–03 and 2S06GM08037–24 to G.C.
Abbreviations
- S-oligo
phosphorothioate oligodeoxyribonucleotide
- ASM
anti-miniexon S-oligo
- SSM
S-oligo complementary and anti-parallel to ASM
- LUAS
anti-luciferase S-oligo
- LUS
S-oligo complementary and anti-parallel to LUAS
- MBSA
maleylated BSA
- DMPC
dimyristoyl phosphatidylcholine
- DMPE
dimyristoyl phosphatidylethanolamine
- PLL
poly-l-lysine
- HIFBS
heat (56°)-inactivated fetal bovine serum
References
- 1.Alexander J, Satoskar AR, Russell DG. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 2.Ephros M, Waldman E, Zilberstein D. Pentostam induces resistance to antimony and the preservative chlorocresol in Leishmania donovani promastigotes and axenically grown amastigotes. Antimicrob Agents Chemother. 1997;41:1064–1068. doi: 10.1128/aac.41.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callahan HL, Portal AC, Devereaux R, Grogl M. An axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41:818–822. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balana-Fouce R, Reguera RM, Cubria JC, Ordonez D. The pharmacology of leishmaniasis. Gen Pharmacol. 1998;30:435–443. doi: 10.1016/s0306-3623(97)00268-1. [DOI] [PubMed] [Google Scholar]
- 5.Cotrim PC, Garrity LK, Beverley SM. Isolation of genes mediating resistance to inhibitors of nucleoside and ergosterol metabolism in Leishmania by overexpression/selection. J Biol Chem. 1999;274:37723–37730. doi: 10.1074/jbc.274.53.37723. [DOI] [PubMed] [Google Scholar]
- 6.Ramazeilles C, Mishra RK, Moreau S, Pascolo E, Toulme JJ. Antisense phosphorothioate oligonucleotides: selective killing of the intracellular parasite Leishmania amazonensis. Proc Natl Acad Sci USA. 1994;91:7859–7863. doi: 10.1073/pnas.91.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra RK, Moreau C, Ramazeilles C, Moreau S, Bonnet J, Toulme JJ. Improved leishmanicidal effect of phosphorothioate antisense oligonucleotides by LDL-mediated delivery. Biochim Biophys Acta. 1995;1264:229–237. doi: 10.1016/0167-4781(95)00145-7. [DOI] [PubMed] [Google Scholar]
- 8.Compagno D, Lampe JN, Bourget C, Kutyavin IV, Yurchenko L, Lukhtanov EA, Gorn VV, Gamper HB, Toulme JJ. Antisense oligonucleotides containing modified bases inhibit in vitro translation of Leishmania amazonensis mRNAs by invading the miniexon hairpin. J Biol Chem. 1999;274:8191–8198. doi: 10.1074/jbc.274.12.8191. [DOI] [PubMed] [Google Scholar]
- 9.Compagno D, Toulme JJ. Antisense effects of oligonucleotides complementary to the hairpin of the Leishmania mini-exon RNA. Nucleosides Nucleotides. 1999;18:1701–1704. doi: 10.1080/07328319908044827. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri G. Scavenger receptor-mediated delivery of antisense mini-exon phosphorothioate oligonucleotide to Leishmania-infected macrophages: selective and efficient elimination of the parasite. Biochem Pharmacol. 1997;53:385–391. doi: 10.1016/s0006-2952(96)00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty R, Dasgupta D, Adhya S, Basu MK. Cationic liposome-encapsulated antisense oligonucleotide mediates efficient killing of intracellular Leishmania. Biochem J. 1999;340:393–396. [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhuri G, Chaudhuri M, Pan A, Chang K-P. Surface acid proteinase (gp63) of Leishmania mexicana: A metallo-enzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J Biol Chem. 1989;264:7483–7489. [PubMed] [Google Scholar]
- 13.Chang KP, Hendricks LD. Laboratory cultivation and maintenance of Leishmania. In: Chang KP, Bray RS, editors. Leishmaniasis. New York: Elsevier; 1985. pp. 213–244. [Google Scholar]
- 14.Chang KP. Human cutaneous Leishmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980;209:1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- 15.Graham MJ, Freier SM, Crooke RM, Ecker DJ, Maslova RN, Lesnik EA. Tritium labeling of antisense oligonucleotides by exchange with tritiated water. Nucleic Acids Res. 1993;21:3737–3743. doi: 10.1093/nar/21.16.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhuri G, Mukhopadhyay A, Basu SK. Selective delivery of drugs to macrophages through a highly specific receptor: an efficient chemotherapeutic approach against leishmaniasis. Biochem Pharmacol. 1989;38:2995–3002. doi: 10.1016/0006-2952(89)90007-5. [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay A, Chaudhuri G, Arora SK, Sehgal S, Basu SK. Receptor-mediated drug delivery to macrophages in chemotherapy of leishmaniasis. Science. 1989;244:705–707. doi: 10.1126/science.2717947. [DOI] [PubMed] [Google Scholar]
- 18.Cruz A, Coburn CM, Beverley SM. Double-targeted gene replacement for creating null mutants. Proc Natl Acad Sci USA. 1991;88:7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beverley SM, Clayton C. Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol Biol. 1993;21:333–348. doi: 10.1385/0-89603-239-6:333. [DOI] [PubMed] [Google Scholar]
- 20.Seay MB, Heard PL, Chaudhuri G. Surface Zn-proteinase as a defense molecule for the survival of Leishmania mexicana amazonensis promastigotes from cytolysis inside macrophage phagolysosomes. Infect Immun. 1996;64:5129–5137. doi: 10.1128/iai.64.12.5129-5137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharan C, Hamilton NM, Parl AK, Singh PK, Chaudhuri G. Identification and characterization of a transcriptional silencer upstream of the human BRCA2 gene. Biochem Biophys Res Commun. 1999;265:285–290. doi: 10.1006/bbrc.1999.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyfang A, Kavanaugh MP, Landfear SM. Aspartate 19 and glutamate 121 are critical for transport function of the myo-inositol/H+ symporter from Leishmania donovani. J Biol Chem. 1997;272:24210–24215. doi: 10.1074/jbc.272.39.24210. [DOI] [PubMed] [Google Scholar]
- 23.Cazenave C, Mizrahi V, Crouch RJ. Methods-rnh gene and ribonuclease H activity analysis. In: Crouch RJ, Toulme JJ, editors. Ribonuclease. H. Paris: INSERM; 1998. pp. 251–265. [Google Scholar]
- 24.Cerritelli SM, Crouch RJ. Cloning, expression, and mapping of ribonuclease H of human and mouse related to bacterial RNase H1. Genomics. 1998;53:300–307. doi: 10.1006/geno.1998.5497. [DOI] [PubMed] [Google Scholar]
- 25.Ausubel M, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York: John Wiley; 1994. [Google Scholar]
- 26.Campbell MJ, Machin D. Medical statistics: a commonsense approach. 2nd ed. New York: John Wiley; 1994. [Google Scholar]
- 27.Tidd DM. A potential role for antisense oligonucleotide analogues in the development of oncogene targeted cancer chemotherapy. Anticancer Res. 1990;10:1169–1182. [PubMed] [Google Scholar]
- 28.Mirabelli CK, Crooke ST. Antisense oligonucleotide in the context of modern molecular drug discovery and development. In: Crooke ST, Lebleu B, editors. Antisense research and applications. New York: CRC Press; 1993. pp. 7–35. [Google Scholar]
- 29.Stein CA, Cheng YC. Antisense oligonucleotides as therapeutic agents–is the bullet really magical? Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- 30.Kregenow D, Ratajczak MZ, Gewirtz AM. Disrupting the flow of genetic information with antisense oligodeoxynucleotides: research and therapeutic applications. In: Akhtar S, editor. Delivery strategies for antisense oligonucleotide therapeutics. New York: CRC Press; 1995. pp. 1–15. [Google Scholar]
- 31.Li G, D’Souza-Schorey C, Barbieri MA, Cooper JA, Stahl PD. Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J Biol Chem. 1997;272:10337–10340. [PubMed] [Google Scholar]
- 32.Sengupta S, Tripathi J, Tandon R, Raje M, Roy RP, Basu SK, Mukhopadhyay A. Hemoglobin endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J Biol Chem. 1999;274:2758–2765. doi: 10.1074/jbc.274.5.2758. [DOI] [PubMed] [Google Scholar]
- 33.Crooke ST. Molecular mechanisms of antisense drugs: RNase H. Antisense Nucleic Acid Drug Dev. 1998;8:133–134. doi: 10.1089/oli.1.1998.8.133. [DOI] [PubMed] [Google Scholar]
- 34.Stein CA, Krieg AM. Applied antisense oligonucleotide technology. New York: Wiley-Liss; 1998. [Google Scholar]
- 35.Gewirtz AM, Sokol DL, Ratajczak MZ. Nucleic acid therapeutics: state of the art and future prospects. Blood. 1998;92:712–736. [PubMed] [Google Scholar]
- 36.Crooke ST. Antisense therapeutics. Biotechnol Genet Eng Rev. 1998;15:121–157. doi: 10.1080/02648725.1998.10647954. [DOI] [PubMed] [Google Scholar]
- 37.Crooke ST. An overview of progress in antisense therapeutics. Antisense Nucleic Acid Drug Dev. 1998;8:115–122. doi: 10.1089/oli.1.1998.8.115. [DOI] [PubMed] [Google Scholar]
- 38.Dean NM, McKay RA, Holmlund J. Antisense oligonucleotides as inhibitors of genes that regulate AP-1: pharmacology and clinical development. Antisense Nucleic Acid Drug Dev. 1998;8:147–151. doi: 10.1089/oli.1.1998.8.147. [DOI] [PubMed] [Google Scholar]
- 39.Stone TW, Jaffe GJ. Reversible bull’s-eye maculopathy associated with intravitreal fomivirsen therapy for cytomegalovirus retinitis. Am J Ophthalmol. 2000;130:242–243. doi: 10.1016/s0002-9394(00)00495-5. [DOI] [PubMed] [Google Scholar]
- 40.Bochot A, Couvreur P, Fattal E. Intravitreal administration of antisense oligonucleotide: potential of liposomal delivery. Prog Retin Eye Res. 2000;19:131–147. doi: 10.1016/s1350-9462(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 41.Galderisi U, Cascino A, Giordano A. Antisense oligonucleotides as therapeutic agents. J Cell Physiol. 1999;181:251–257. doi: 10.1002/(SICI)1097-4652(199911)181:2<251::AID-JCP7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 42.Schmajuk G, Sierakowska H, Kole R. Antisense oligonucleotides with different backbones: modification of splicing pathways and efficacy of uptake. J Biol Chem. 1999;274:21783–21789. doi: 10.1074/jbc.274.31.21783. [DOI] [PubMed] [Google Scholar]
- 43.Summerton J, Weller D. Morpholino antisense oligomers: design, preparation and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 44.Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 45.Haberland A, Knaus T, Zaitsev SV, Stahn R, Mistry AR, Coutelle C, Haller H, Bottger M. Calcium ions as efficient cofactor of polycation-mediated gene transfer. Biochim Biophys Acta. 1999;1445:21–30. doi: 10.1016/s0167-4781(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 46.Davies DE, Lloyd JB. Quantitation of pinocytosis in human monocytes during in vitro maturation into macrophages. J Immunol Methods. 1990;132:111–117. doi: 10.1016/0022-1759(90)90404-j. [DOI] [PubMed] [Google Scholar]