Abstract
Microbial pathogens continue to cause widespread morbidity and mortality. Central to the pathogens' virulence is manipulation of the host cell's cytoskeleton, which facilitates microbial invasion, multiplication, and avoidance of the innate immune response. IQGAP1 is a ubiquitously expressed scaffold protein that integrates diverse signaling cascades. Research has shown that IQGAP1 binds to and modulates the activity of multiple proteins that participate in bacterial invasion. Here, we review data that support a role for IQGAP1 in infectious disease via its ability to regulate the actin cytoskeleton. In addition, we explore other mechanisms by which IQGAP1 may be exploited by microbial pathogens.
1. Introduction
Microbial pathogens are a major cause of morbidity and mortality worldwide. In the United States alone, an estimated 76 million foodborne illnesses (caused primarily by Salmonella enterica serovar typhimurium, Campylobacter jejuni, Shigella flexneri, Cryptosporidium parvum and Escherichia coli) occur annually, and account for an estimated treatment cost of up to 83 billion US dollars [1]. Despite considerable variation in the manner by which they produce disease, most microbial pathogens exert and sustain their effects by usurping a relatively limited number of signaling pathways inside the host cell. In particular, microbes frequently manipulate the cytoskeleton of the host cell, thereby facilitating their attachment and entry [2-4]. Microbial pathogens also employ several survival strategies, allowing them to migrate within the host cell and avoid bactericidal defense mechanisms [2-4]. Control of the host cell's cytoskeleton is also integral to each of these stages of infection, therefore proteins that govern cytoskeletal remodeling participate in microbial pathogenesis. In this review, we summarize recent evidence that strongly supports a role for the scaffold protein IQGAP1 in infectious disease.
2. IQGAP1: a key modulator of cytoskeletal function
IQGAP1 is a ubiquitously expressed 189-kDa scaffold protein that contains several protein-interacting domains. These include a calponin homology domain, a poly-proline binding region, four IQ motifs (IQ motifs bind calmodulin), and a region with significant sequence similarity to the catalytic domain of Ras GTPase-activating proteins (GAPs) [5]. Each of these domains serves to mediate the interaction of IQGAP1 with multiple distinct proteins [6]. By regulating the function of its binding partners, IQGAP1 participates in diverse cellular functions, ranging from small GTPase signaling to control of cell proliferation and motility [6,7]. Of particular relevance in the context of this article is the role of IQGAP1 in the maintenance of cytoskeletal architecture. IQGAP1 binds actin directly [8], enhances actin polymerization in vitro [9,10], and colocalizes with actin in lamellipodia [11]. Moreover, IQGAP1 stimulates actin assembly by forming complexes with N-WASP (neuronal Wiskott Aldrich Syndrome protein) and Arp2/3 (actin-related protein 2/3) [12]. By controlling the activity of the small GTPases Rac1 and Cdc42, IQGAP1 also modulates the cytoskeleton indirectly. (Note that, despite its name, IQGAP1 is not a GAP and actually stabilizes Rac1 and Cdc42 in their active forms [11,13].) The role of IQGAP1 in cellular signaling and cytoskeletal dynamics has been the focus of several excellent reviews [5-7,14,15]. Here, we focus only on those IQGAP1 functions germane to microbial pathogenesis.
3. IQGAP1 and microbial pathogenesis
Early evidence to implicate the involvement of IQGAP1 in microbial pathogenesis was derived by gene profiling. Microarray analysis revealed that <3.5% of 3500 genes in a human monocyte cell line, U937, had altered expression following infection with Mycobacteria. One of the genes identified was IQGAP1, which was downregulated 5.6-fold [16]. Proteomic analysis later showed reduced IQGAP1 expression in murine splenic tissue after infection by Yersinia pestis [17], suggesting that IQGAP1 may be a target for pathogen-induced changes in the host cell. Consistent with this postulate, IQGAP1 is known to interact with numerous proteins that functionally link pathogenic microbes to host cell invasion (Table 1). For example, IQGAP1 binding to Dia1, a Diaphanous-related formin that assembles actin filaments, is required for phagocytic cup formation [18], an essential step in microbial invasion into host cells [19]. IQGAP1 also binds directly to selected bacterial proteins with defined roles in pathogen invasion, including the E. coli-derived Tir and Ibe, and the Salmonella-derived SseI [20-22] (Table 1). The functional consequences of these interactions are discussed in more detail in the following paragraphs.
Table 1. Host- and pathogen-derived IQGAP1 binding partners relevant to microbial pathogenesis.
| Host-derived IQGAP1 binding partner | Proposed function(s) of interaction with IQGAP1 | Relevance of binding partner to microbial pathogenesis | Reference(s) |
|---|---|---|---|
| Actin | Cross-links actin filaments | Intricately involved in many aspects of bacterial invasion | [8,26,74] |
| Arf6 | Links Arf6 to Rac1 activation and cell migration | Controls bacterial invasion by modulating host cell actin remodeling | [75] |
| Arp2/3 | Links growth factor signaling to actin assembly | Promotes host cell membrane ruffling; essential for S. typhimurium, S. flexneri and Rickettsia conorii invasion | [12] |
| c-Src | c-Src catalyzes tyrosine phosphorylation of IQGAP1 and bridges IQGAP1 to VEGFR2 | Promotes bacterial invasion | [76] |
| Calmodulin | Regulates IQGAP1 function | EPEC-induced actin pedestals are formed via a Ca2+/calmodulin-dependent pathway | [20,41] |
| CaMKII | Unknown; may be involved in the regulation of cell adhesion | Phosphorylates vimentin at Ser82, thereby positively regulating EPEC invasion | [77] |
| CD44 | Links hyaluronan to actin cytoskeleton | Recruited to the bacterial attachment site during EPEC infection | [78] |
| Cortactin | Necessary for hyperoxia-induced tyrosine phosphorylation of Src, and cortactin and ROS generation | Tyrosine phosphorylation of cortactin is required for invasion of biliary epithelium by Cryptosporidium parvum | [79] |
| ERK1/2 | MAPK Scaffold | Stimulated by Salmonella infection; involved in Campylobacter jejuni internalization | [53,71] |
| Exo70 | Necessary for correct localization of the exocyst; regulates protein synthesis, exocytosis and secretion | Recruitment of the exocyst to localized areas of the host cell membrane promotes S. typhimurium invasion | [80] |
| MEK1/2 | MAPK Scaffold | MAPK signaling targeted by cell-invasive bacteria; MEK activation required for efficient invasion of L. monocytogenes, S. typhimurium, Chlamydophila pneumonia and Pseudomonas aeruginosa | [51,54,55] |
| N-WASP | Activates N-WASP and stimulates actin assembly; necessary for N-WASP localization at lamellipodia | Promotes host cell actin polymerization; essential for EPEC and S. flexneri invasion | [12,81] |
| PtdIns(4,5)P2 | Maintains integrity of PIP2-containing lipid rafts | PIP2 is metabolized at sites of S. typhimurium invasion | [49] |
| PLD2 | Necessary for hyperoxia-induced tyrosine phosphorylation of Src, and cortactin and ROS generation | PLD activity correlates with Acinetobacter baumannii pathogenesis | [79] |
| Rac1/Cdc42 | Inhibits intrinsic GTPase activity, thereby stabilizing active form | Involved in host cell membrane ruffling; essential for S. typhimurium invasion | [74,82,83] |
| ShcA | Unknown; may be important in cytoskeletal reorganization in response to activation of growth factor receptors | Affects Salmonella adherence to host cell; involved in C. pneumoniae invasion | [84] |
| WAVE2 | Involved in lamellipodia formation in response to HGF | Required for invasion of L. monocytogenes; regulates invasion of S. typhimurium | [85] |
| Pathogen-derived IQGAP1 binding partnerΨ | Proposed function(s) of interaction with IQGAP1 | Relevance of binding partner to microbial pathogenesis | Reference(s) |
| Gag (M-MuLV) | Necessary for virus trafficking and replication | Promotes viral replication | [48] |
| Ibe (EPEC) | Appears to be necessary for Ibe function during infection | Promotes EPEC invasion | [21] |
| SseI (Salmonella) | Necessary for SseI inhibition of cell migration in primary macrophages and dendritic cells | Disables host macrophage function | [22] |
| Tir (EPEC) | Regulates actin pedestal formation by EPEC | Promotes EPEC invasion | [20] |
Abbreviations: Arf6, ADP-ribosylation factor 6; CaMKII, Ca2+/calmodulin-dependent protein kinase II; EPEC, enteropathogenic Escherichia coli; ERK, extracellular-regulated kinase; HGF, hepatocyte growth factor; Ibe, IQGAP1-binding effector protein; M-MuLV, moloney murine leukemia virus; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; PtdIns(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PLD2, Phospholipase D2; ROS, reactive oxygen species; Tir, translocated intimin receptor; VEGFR2, vascular endothelial growth factor receptor-2; WAVE, WASP family Verprolin-homologous protein.
The pathogen from which each protein is derived is indicated in parentheses.
4. IQGAP1 is a target for Salmonella pathogenesis
4.1. Regulation of IQGAP1 for Salmonella invasion
As is characteristic of many cell-invasive pathogens, Salmonella typhimurium employs an elaborate molecular apparatus, called a type III secretion system (T3SS), to facilitate its infection by injecting bacterial toxins directly into host cells [23]. Among the injected effectors are SopE and SopE2, which act as guanine nucleotide exchange factors (GEFs). In their catalytically inactive forms, Rac1 and Cdc42 are bound to guanosine diphosphate (GDP). GEFs catalyze the substitution of GDP for guanosine-5′-triphosphate (GTP), resulting in Rac1 and Cdc42 activation [24]. Once activated, Rac1 and Cdc42 activate N-WASP and the Arp2/3 complex, thereby promoting actin polymerization and actin filament elongation at the Salmonella-host cell interface [25]. These molecular events result in the formation of membrane ruffles that facilitate S. typhimurium internalization.
Recent published data indicate S. typhimurium modulates IQGAP1 to gain entry into host cells [26]. IQGAP1 is recruited to sites of S. typhimurium attachment to HeLa cells, and siRNA-mediated knockdown of IQGAP1 reduces ruffle formation and decreases S. typhimurium infection by 33%. The magnitude of this effect may be limited by residual IQGAP1 in the siRNA-treated cells, since S. typhimurium entry into IQGAP1-null mouse embryonic fibroblasts (MEFs) is reduced to 35% of that into control MEFs [26]. These data suggest that IQGAP1 is usurped by S. typhimurium to enter host cells. The molecular mechanisms underlying these observations have begun to be characterized. Overexpression of IQGAP1 increases the amount of active Rac1 and Cdc42 in cells, while reducing the amount of endogenous IQGAP1 markedly decreases the activity of both GTPases [13,26]. During S. typhimurium infection of HeLa cells, the levels of active Rac1 and Cdc42 increase >2-fold [26]. However, in IQGAP1-null MEFs, Rac1 and Cdc42 activation is abrogated and S. typhimurium invasion is decreased [26]. These findings imply that regulation of Rac1 and Cdc42 by IQGAP1 is important for S. typhimurium entry. Consistent with this hypothesis, S. typhimurium infection is increased in cells transfected with wild-type IQGAP1, but not in cells transfected with an IQGAP1 mutant that lacks Rac1 and Cdc42 binding [26]. Interestingly, an IQGAP1 mutant that does not bind actin (termed IQGAP1·G75Q [27]) also fails to promote S. typhimurium entry [26]. Moreover, in contrast to wild-type IQGAP1, IQGAP1·G75Q does not translocate to sites of S. typhimurium infection. Based on the data described above, S. typhimurium invasion into host cells appears contingent on IQGAP1 binding to both Rac1/Cdc42 and actin.
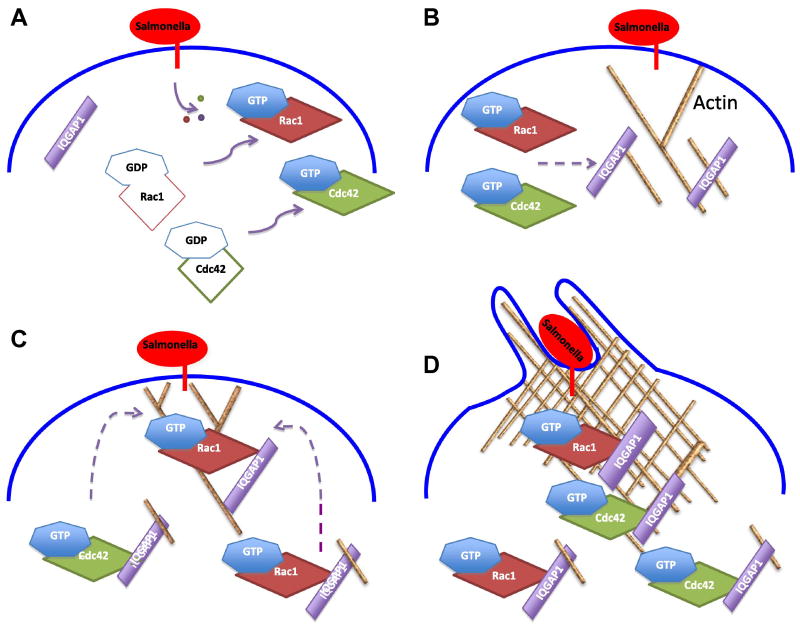
Based on research from our laboratory, we propose a model that integrates the observations described above (Figure 1). As previously highlighted, S. typhimurium attachment to the host cell results in the injection of effectors via the T3SS. Following their injection, SopE and SopE2 catalyze the exchange of GDP on Rac1 and Cdc42 for GTP. This facilitates actin polymerization. IQGAP1 bound to actin translocates to the site of S. typhimurium attachment on the host cell. Here, IQGAP1 binds to active Rac1 and Cdc42, maintaining them in their GTP-bound form. IQGAP1 binding to Rac1-GTP and Cdc42-GTP enhances actin polymerization, and results in the recruitment of additional IQGAP1 (which is also bound to active Rac1 and Cdc42) to the site of S. typhimurium attachment. The presence of increased Rac1-GTP and Cdc42-GTP bound to IQGAP1 further augments actin polymerization, inducing the formation of membrane ruffles and facilitating S. typhimurium internalization. IQGAP1 therefore functionally links Rac1 and Cdc42 to actin, augments actin polymerization, and promotes bacterial invasion.
Figure 1. Salmonella targets IQGAP1 to invade host cells.
A. Salmonella binds to the host cell membrane and injects effector proteins (small circles) that catalyze the activation of Rac1 and Cdc42. B. Active (GTP-bound) Rac1 and Cdc42 promote localized actin polymerization at the Salmonella-host cell interface. IQGAP1 bound to actin translocates to the site of Salmonella attachment. C. IQGAP1 binds Rac1-GTP and Cdc42-GTP, stabilizing the GTPases in their active GTP-bound forms. The active GTPases promote further local actin polymerization. D. Ongoing actin assembly induces the accumulation of additional IQGAP1, with attached active Rac1 and Cdc42, at the phagocytic cup. This process promotes further actin polymerization, augmenting formation of the phagocytic cup that engulfs the Salmonella. E. Salmonella is internalized within a Salmonella-containing vacuole (SCV).
4.2. Salmonella targets IQGAP1 to establish chronic infection
An important aspect of Salmonella pathogenicity is the ability of certain species to establish chronic, long-term infection in the host by evading the host's immune response [28,29]. The capacity of S. typhimurium to survive and replicate in macrophages is therefore of major importance to the ability of the pathogen to cause disease. Macrophages are key participants in cell-mediated immunity as they destroy foreign pathogens and function as antigen-presenting cells [30]. The Salmonella pathogenicity island 2 (SPI2)-coded T3SS injects proteins that enable S. typhimurium to create a Salmonella-containing vacuole (SCV) in which it can replicate [31]. Moreover, SPI2-secreted effectors allow intracellular bacteria to avoid the bactericidal properties of macrophages and dendritic cells, and to interfere with antigen presentation to T-cells [32]. One such Salmonella effector, SseI (also known as SrfH), impedes the normal migration of macrophages and dendritic cells, thus severely impairing their bactericidal abilities [22]. Consequently, mice infected with wild-type S. typhimurium continue to exhibit increased systemic levels of bacteria up to 45 days post-infection, while mice infected with SseI-deficient S. typhimurium clear the pathogen more rapidly [22]. Importantly, IQGAP1 may contribute to the ability of S. typhimurium to circumvent the host's immune response and establish chronic infection. IQGAP1 binds SseI directly in vitro, and colocalizes with SseI in Salmonella-infected macrophages [22]. Moreover, the inhibitory effect of SseI on macrophage migration is contingent on IQGAP1 expression; SseI does not impair migration of macrophages lacking IQGAP1 [22]. The Salmonella effector SseI therefore exploits IQGAP1 to reduce macrophage motility, thereby suppressing host immunity and promoting a chronic infective state.
5. IQGAP1 is a target for E. coli pathogenesis
Like Salmonella, enteropathogenic Escherichia coli (EPEC) utilizes a T3SS to establish infection in the host [23]. Evidence suggests that this is a multi-step process [33]. First, EPEC injects translocated intimin receptor (Tir) into the host cell. Tir is inserted into the host cell plasma membrane, where it binds intimin, which is embedded in the bacterial outer membrane [34]. Tir-intimin adhesion leads to Tir clustering, which recruits the host protein Nck. Binding to intimin also catalyzes phosphorylation of Tir by the tyrosine kinase c-Fyn, resulting in N-WASP and Arp2/3 recruitment to the cell-microbe interface [35,36]. N-WASP mediates actin polymerization, thereby creating an “actin pedestal”. This structure, which is an essential hallmark of E. coli pathogenesis [33], elevates and supports the EPEC above the surface of the epithelial cell.
EPEC manipulates IQGAP1 to mediate infection. Specifically, EPEC induces the translocation of IQGAP1 to actin pedestals where IQGAP1 is necessary for pedestal formation [20]. Moreover, actin polymerization induced by EPEC in IQGAP1-null MEFs is significantly less that that in control cells, and reconstitution of IQGAP1 into IQGAP1-null MEFs rescues EPEC infection. Interestingly, the molecular mechanisms underlying these observations are different to those of Salmonella. In contrast to Salmonella, which promotes the binding of IQGAP1 to Rac1 and Cdc42 [26], EPEC inhibits the interaction of IQGAP1 with the Rho GTPases [20]. Another important difference between Salmonella and EPEC is that the latter modulates association of IQGAP1 and calmodulin [20]. Calmodulin, a 16.7-kDa Ca2+-binding protein, is an important transducer of Ca2+ signaling [37]. Ca2+ signaling is necessary for actin remodeling near the cell surface [38], and increases in intracellular free Ca2+ concentrations ([Ca2+]i) are required for EPEC-induced actin pedestal formation [39,40]. Importantly, treatment with a cell-permeable calmodulin antagonist (CGS9343B) or a cell-permeable Ca2+ chelator (1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA/AM)) abrogates actin pedestal formation in wild-type but not IQGAP1-null MEFs [20]. These data indicate that Ca2+/calmodulin signaling by EPEC to induce actin pedestal formation is mediated entirely through IQGAP1. Thus, although both Salmonella and E. coli usurp IQGAP1 function, the bacteria employ different molecular mechanisms and exploit distinct IQGAP1 binding partners to infect host cells.
Additional insight into how EPEC manipulates IQGAP1 is derived from research from both our laboratory and other investigators. IQGAP1 interacts directly with EPEC-derived effector proteins that are injected into the host cell and facilitate infection [20,21]. For example, IQGAP1 binds Tir in vitro, and confocal microscopy reveals that IQGAP1 colocalizes with Tir at EPEC-induced actin pedestals [20]. Subsequently, another bacterial protein, termed IQGAP1-binding effector protein (Ibe), was shown to regulate Tir phosphorylation and actin pedestal formation [21]. Like Tir, IQGAP1 binds Ibe in vitro, and colocalizes with Ibe at actin pedestals. Although the mechanism underlying the contribution of Ibe to EPEC pathogenesis remains to be defined, these data provide further evidence that IQGAP1 is a critical component of E. coli infection.
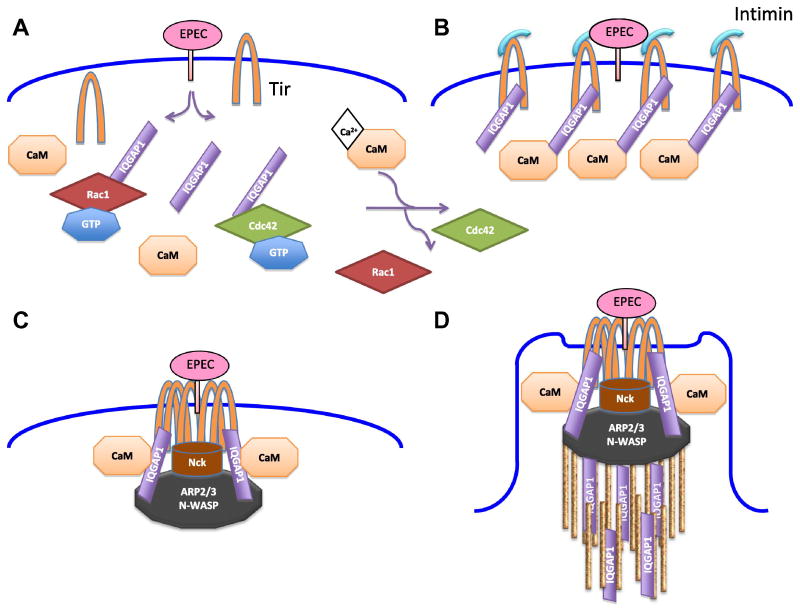
Research carried out in our laboratory integrates the findings discussed above (Figure 2). In this model, EPEC binds to the surface of cells and injects effectors via its T3SS. One of the effectors injected, Tir, associates with intimin and is retained at the site of infection. IQGAP1, presumably via its interaction with Tir, also accumulates at the EPEC injection site. Simultaneously, EPEC induces an increase in [Ca2+]i, thereby enhancing the association of IQGAP1 with calmodulin, which reduces IQGAP1 binding to Rac1 and Cdc42 [41]. Because it is bound to IQGAP1, calmodulin accumulates at the site of bacterial adhesion. Recruitment of Nck induces clustering of N-WASP and Arp2/3 at the cell-microbe interface, thereby promoting polymerization of actin. The presence of IQGAP1 and calmodulin, and the interaction of IQGAP1 with Tir, augments this localized actin polymerization, contributing to pedestal formation. Additionally, IQGAP1 bundles actin to ensure that an ordered structure of parallel filaments is formed.
Figure 2. IQGAP1 and Ca2+/calmodulin are required for actin pedestal formation by EPEC.
A. EPEC binds to the surface of the host cell and injects effector proteins, including Tir, thereby promoting a transient increase in [Ca2+]i. B. IQGAP1 binds Tir directly and is recruited to the site of EPEC adhesion. The increase in [Ca2+]i promotes the interaction of IQGAP1 with calmodulin (CaM), with a concomitant disruption of its association with Rac1 and Cdc42. C. Following its binding to intimin, Tir is clustered and tyrosine-phosphorylated, inducing the recruitment of Nck to the site of EPEC attachment. Nck directs N-WASP and the Arp2/3 complex to clustered Tir, where together they form an IQGAP1-containing complex that promotes actin polymerization. D. IQGAP1 contributes to ongoing actin polymerization, resulting in the formation of a pedestal structure that supports EPEC. Additionally, IQGAP1 bundles actin to ensure that an ordered structure of parallel filaments is formed.
6. Other bacteria regulate IQGAP1
In addition to being manipulated by S. typhimurium and EPEC, IQGAP1 is exploited by other pathogens as part of their infective mechanisms. For example, a fundamental aspect of Shigella pathogenesis is the intercellular spread of bacteria within epithelial tissues [42]. Initial data indicate that intercellular spread of S. flexneri is significantly enhanced in IQGAP1-null MEFs, suggesting that IQGAP1 may be targeted during S. flexneri infection (R. Lu, D. B. Sacks, M. B. Goldberg, unpublished observations). Other findings concern the regulation of IQGAP1 during infection of host cells by Pseudomonas aeruginosa and Helicobacter pylori. In HL60 leukemia cells, IQGAP1 is recruited to sites of P. aeruginosa attachment [43]. Moreover, infection of human antral epithelial cells with H. pylori increases IQGAP1 mRNA and induces translocation of IQGAP1 protein from the cytoplasm to intracellular tubulovesicular structures [44]. Collectively, these findings implicate IQGAP1 as a host cell target for infection by several bacteria. Further work is likely to identify additional bacteria that manipulate IQGAP1 to facilitate host cell infection.
7. IQGAP1 as a target for viral pathogenesis
Viruses are strictly dependent on host cells for the transcription of their genomes [45]. Invasion of host cells is therefore an important determinant of viral pathogenesis. Gag proteins are major virulence factors produced by retroviruses since they promote viral binding to the host cell membrane [46]. The matrix protein domains of Gag are particularly important for the intracellular trafficking of viral proteins [47]. Evidence suggests that IQGAP1 may also be a target of viral pathogenesis. The Gag matrix protein of the Moloney murine leukemia virus (M-MuLV) binds IQGAP1, and this binding is essential for viral replication [48]. Moreover, viruses encoding mutant, non-IQGAP1-binding matrix proteins are replication deficient, and M-MuLV replication is reduced when IQGAP1 is knocked down in host cells with siRNA. Additional studies are needed to determine whether other viruses also require IQGAP1 for replication, and/or whether IQGAP1 participates in viral infection in other ways.
8. Pathogenic microbes may target other IQGAP1-associated pathways
We have reviewed evidence which reveals that IQGAP1 interacts with numerous proteins known to be directly involved in host cell infection and/or microbial survival. Nevertheless, other IQGAP1-interacting partners, such as phosphoinositides [49], microtubules [50] and mitogen-activated protein kinases (MAPKs) [51-53], are targeted by microbial pathogens [54-57] (Table 1). Conceivably, these IQGAP1 binding molecules may also be modulated during microbial pathogenesis in an IQGAP1-regulated manner. Although no experimental evidence has been published to date, we suggest possible contributions of these IQGAP1 binding partners to infectious disease.
8.1 IQGAP1, phosphoinositides and microbial pathogenesis
Phosphoinositides are cell membrane-based signaling molecules that are critical for cytoskeletal remodeling and intracellular trafficking processes [58]. The seven known phosphoinositides are derived from phosphorylation of the precursor molecule, phosphatidylinositol (PI). PI is phosphorylated on the inositol ring by PI kinases to produce several distinct phosphoinositides. These signaling molecules have multiple functions, including regulation of cytoskeletal rearrangement, which is required for invasion by microbes. For example, E. coli invades human brain endothelial cells in a PI3-kinase-dependent manner [59]. Similarly, PI3-kinase is targeted by Listeria monocytogenes during invasion of Vero cells [60] and by S. typhimurium during entry into fibroblasts [55]. Like IQGAP1, the phosphoinositide PI(4,5)P2 (PtdIns(4,5)P2) promotes actin assembly by activating N-WASP and the Arp2/3 complex [61], and PtdIns(4,5)P2 is enriched in areas undergoing active actin polymerization (such as the Salmonella-host cell interface) [62]. Importantly, a functional interaction between IQGAP1 and PtdIns(4,5)P2 has been established. IQGAP1 colocalizes with PtdIns(4,5)P2 at the leading edge of growth factor-stimulated cells, and knockdown of IQGAP1 results in fragmentation of PtdIns(4,5)P2-enriched lipid rafts [49]. Based on these data, it is tempting to speculate that PtdIns(4,5)P2 activity at the cell membrane may be regulated by IQGAP1, and that this interaction may play a role in microbial invasion of the host cell.
8.2 IQGAP1 and microtubules in bacterial invasion
Microtubules are elements of the cytoskeleton and are essential for cell division, cell migration, vesicle transport and cell polarity [63]. Some pathogenic bacteria, including Salmonella, E. coli, Shigella, L. monocytogenes and Campylobacter jejuni, exploit host microtubule networks [55,64-67]. For example, pretreatment of fibroblasts with microtubule-depolymerizing agents such as colchicine or nocodazole impairs S. typhimurium invasion, suggesting that intact microtubule networks are required for entry of the bacteria [55]. Importantly, IQGAP1 regulates microtubule function through its interaction with CLIP-170 and mDia1 [50,68]. Moreover, siRNA-mediated knockdown of IQGAP1 results in decreased stability and increased dynamics of microtubules [68]. Additional work is necessary to establish whether microbial pathogens target the microtubule-stabilizing functions of IQGAP1 during host cell entry.
8.3 IQGAP1 and MAPKs in bacterial invasion
MAPKs transmit extracellular signals to a diverse array of nuclear and cytoplasmic targets [69,70]. Stimulation of cell surface receptors, such as the epidermal growth factor receptor, activates the small GTPase Ras. Ras stimulates B-Raf kinase activity, which catalyzes the sequential phosphorylation of MEKs (or MAPK kinases) and extracellular-regulated kinases (ERKs). In addition to being a pivotal determinant of eukaryotic gene expression, cell differentiation and cell migration [70], the MEK/ERK pathway is also involved in microbial pathogenesis. For example, MEK and ERK activity are increased in cells infected with S. typhimurium [71]. Moreover, pretreatment of cells with the MEK inhibitor PD98059 curtails invasion in some cell types by several organisms, including L. monocytogenes [54], S. typhimurium [55], Chlamydia pneumonia [72] and P. aeruginosa [73]. Importantly, IQGAP1 is a scaffold in the MAPK pathway. IQGAP1 binds to and regulates the function of multiple components of the MAPK cascade [51-53]. For example, knockdown of IQGAP1 by siRNA abrogates the activation of MEK [51] and ERK [53] by epidermal growth factor. Moreover, stimulation of B-Raf requires IQGAP1 as B-Raf kinase activity is not induced by growth factors in IQGAP1-null MEFs [52]. These data reveal that IQGAP1 is necessary for activation of MAPK signaling. Based on the evidence outlined above, it is likely that IQGAP1 is important for MAPK-driven microbial invasion.
9. Perspectives
Recent evidence strongly supports the concept that selected microbial pathogens regulates the host cell's cytoskeleton, at least in part by usurping IQGAP1 function. These observations raise several intriguing questions. For example, are the IQGAP1-dependent molecular mechanisms underlying the entry of other cell-invasive pathogens, such as S. flexneri and P. aeruginosa, analogous to those of S. typhimurium? What is the contribution of other molecules that bind IQGAP1, such as Ibe, phosphoinositides, microtubules and MAPKs, to the cytoskeletal rearrangements elicited by bacterial infection? The current literature indicates that IQGAP1 may be modulated at different stages during the infectious disease process. It is likely that comparing infection of wild-type and Iqgap1-/- mice will therefore yield insight into the development and clinical progression of microbial pathogenesis. We look forward to the findings from these and other investigations, which will enhance our comprehension of pathogen biology and evaluate the feasibility of targeting IQGAP1 for the prevention and treatment of infectious disease.
Acknowledgments
The authors thank all members of the Sacks laboratory for insightful discussions. This work was supported by the Canadian Institutes of Health Research (to H.K.) and the National Institutes of Health (to D.B.S.).
References
- 1.Nyachuba DG. Foodborne illness: is it on the rise? Nutr Rev. 2010;68:257–69. doi: 10.1111/j.1753-4887.2010.00286.x. [DOI] [PubMed] [Google Scholar]
- 2.Gruenheid S, Finlay BB. Microbial pathogenesis and cytoskeletal function. Nature. 2003;422:775–81. doi: 10.1038/nature01603. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri JT, Riese MJ, Aktories K. Bacterial toxins that modify the actin cytoskeleton. Annu Rev Cell Dev Biol. 2002;18:315–44. doi: 10.1146/annurev.cellbio.18.012502.134748. [DOI] [PubMed] [Google Scholar]
- 4.Rottner K, Stradal TE, Wehland J. Bacteria-host-cell interactions at the plasma membrane: stories on actin cytoskeleton subversion. Dev Cell. 2005;9:3–17. doi: 10.1016/j.devcel.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–4. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006;16:242–9. doi: 10.1016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.White CD, Brown MD, Sacks DB. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–24. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, King ME, Bloom GS. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem. 2002;277:12324–33. doi: 10.1074/jbc.M109535200. [DOI] [PubMed] [Google Scholar]
- 9.Erickson JW, Cerione RA, Hart MJ. Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J Biol Chem. 1997;272:24443–24447. doi: 10.1074/jbc.272.39.24443. [DOI] [PubMed] [Google Scholar]
- 10.Fukata M, et al. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem. 1997;272:29579–83. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- 11.Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a RasGAP-related domain, is a potential effector for Cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 12.Bensenor LB, Kan HM, Wang N, Wallrabe H, Davidson LA, Cai Y, Schafer DA, Bloom GS. IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J Cell Sci. 2007;120:658–69. doi: 10.1242/jcs.03376. [DOI] [PubMed] [Google Scholar]
- 13.Swart-Mataraza JM, Li Z, Sacks DB. IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J Biol Chem. 2002;277:24753–63. doi: 10.1074/jbc.M111165200. [DOI] [PubMed] [Google Scholar]
- 14.Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–92. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 15.Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–23. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGarvey JA, Wagner D, Bermudez LE. Differential gene expression in mononuclear phagocytes infected with pathogenic and non-pathogenic mycobacteria. Clin Exp Immunol. 2004;136:490–500. doi: 10.1111/j.1365-2249.2004.02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers JV, Choi YW, Giannunzio LF, Sabourin PJ, Bornman DM, Blosser EG, Sabourin CL. Transcriptional responses in spleens from mice exposed to Yersinia pestis CO92. Microb Pathog. 2007;43:67–77. doi: 10.1016/j.micpath.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007;178:193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel JC, Galan JE. Manipulation of the host actin cytoskeleton by Salmonella - all in the name of entry. Curr Opin Microbiol. 2005;8:10–5. doi: 10.1016/j.mib.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Brown MD, Bry L, Li Z, Sacks DB. Actin pedestal formation by enteropathogenic Escherichia coli is regulated by IQGAP1, calcium, and calmodulin. J Biol Chem. 2008;283:35212–22. doi: 10.1074/jbc.M803477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buss C, Muller D, Ruter C, Heusipp G, Schmidt MA. Identification and characterization of Ibe, a novel type III effector protein of A/E pathogens targeting human IQGAP1. Cell Microbiol. 2009;11:661–77. doi: 10.1111/j.1462-5822.2009.01284.x. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin LM, et al. The Salmonella SPI2 effector SseI mediates long-term systemic infection by modulating host cell migration. PLoS Pathog. 2009;5:e1000671. doi: 10.1371/journal.ppat.1000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coburn B, Sekirov I, Finlay BB. Type III secretion systems and disease. Clin Microbiol Rev. 2007;20:535–49. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 25.Hayward RD, Koronakis V. Direct modulation of the host cell cytoskeleton by Salmonella actin-binding proteins. Trends Cell Biol. 2002;12:15–20. doi: 10.1016/s0962-8924(01)02183-3. [DOI] [PubMed] [Google Scholar]
- 26.Brown MD, Bry L, Li Z, Sacks DB. IQGAP1 regulates Salmonella invasion through interactions with actin, Rac1, and Cdc42. J Biol Chem. 2007;282:30265–72. doi: 10.1074/jbc.M702537200. [DOI] [PubMed] [Google Scholar]
- 27.Mataraza JM, Li Z, Jeong HW, Brown MD, Sacks DB. Multiple proteins mediate IQGAP1-stimulated cell migration. Cell Signal. 2007;19:1857–65. doi: 10.1016/j.cellsig.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J. Pathogenic bacterial proteins and their anti-inflammatory effects in the eukaryotic host. Antiinflamm Antiallergy Agents Med Chem. 2009;8:214–227. doi: 10.2174/187152309789151986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon MA. Salmonella infections in immunocompromised adults. J Infect. 2008;56:413–22. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prost LR, Sanowar S, Miller SI. Salmonella sensing of anti-microbial mechanisms to promote survival within macrophages. Immunol Rev. 2007;219:55–65. doi: 10.1111/j.1600-065X.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 32.Cheminay C, Mohlenbrink A, Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J Immunol. 2005;174:2892–9. doi: 10.4049/jimmunol.174.5.2892. [DOI] [PubMed] [Google Scholar]
- 33.Campellone KG. Cytoskeleton-modulating effectors of enteropathogenic and enterohaemorrhagic Escherichia coli: Tir, EspFU and actin pedestal assembly. FEBS J. 2010;277:2390–402. doi: 10.1111/j.1742-4658.2010.07653.x. [DOI] [PubMed] [Google Scholar]
- 34.Yi CR, Goldberg MB. Enterohemorrhagic Escherichia coli raises the I-BAR. Proc Natl Acad Sci U S A. 2009;106:6431–2. doi: 10.1073/pnas.0902773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frankel G, Phillips AD. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell Microbiol. 2008;10:549–56. doi: 10.1111/j.1462-5822.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 36.Caron E, Crepin VF, Simpson N, Knutton S, Garmendia J, Frankel G. Subversion of actin dynamics by EPEC and EHEC. Curr Opin Microbiol. 2006;9:40–5. doi: 10.1016/j.mib.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–8. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–6. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 39.Baldwin TJ, Ward W, Aitken A, Knutton S, Williams PH. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ide T, Michgehl S, Knappstein S, Heusipp G, Schmidt MA. Differential modulation by Ca2+ of type III secretion of diffusely adhering enteropathogenic Escherichia coli. Infect Immun. 2003;71:1725–32. doi: 10.1128/IAI.71.4.1725-1732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joyal JL, Annan RS, Ho YD, Huddleston ME, Carr SA, Hart MJ, Sacks DB. Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry. J Biol Chem. 1997;272:15419–25. doi: 10.1074/jbc.272.24.15419. [DOI] [PubMed] [Google Scholar]
- 42.Goosney DL, Knoechel DG, Finlay BB. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg Infect Dis. 1999;5:216–23. doi: 10.3201/eid0502.990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bridge DR, Novotny MJ, Moore ER, Olson JC. Role of host cell polarity and leading edge properties in Pseudomonas type III secretion. Microbiology. 2010;156:356–73. doi: 10.1099/mic.0.033241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conlin VS, Curtis SB, Zhao Y, Moore ED, Smith VC, Meloche RM, Finlay BB, Buchan AM. Helicobacter pylori infection targets adherens junction regulatory proteins and results in increased rates of migration in human gastric epithelial cells. Infect Immun. 2004;72:5181–92. doi: 10.1128/IAI.72.9.5181-5192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohr I. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 2006;119:89–99. doi: 10.1016/j.virusres.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Ono A, Demirov D, Freed EO. Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding. J Virol. 2000;74:5142–50. doi: 10.1128/jvi.74.11.5142-5150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan X, Yu X, Lee TH, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–94. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung J, Yueh A, Appah FS, Jr, Yuan B, de los Santos K, Goff SP. Interaction of Moloney murine leukemia virus matrix protein with IQGAP. EMBO J. 2006;25:2155–66. doi: 10.1038/sj.emboj.7601097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golub T, Caroni P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol. 2005;169:151–65. doi: 10.1083/jcb.200407058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukata M, et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–85. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 51.Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25:7940–52. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren JG, Li Z, Sacks DB. IQGAP1 modulates activation of B-Raf. Proc Natl Acad Sci U S A. 2007;104:10465–9. doi: 10.1073/pnas.0611308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy M, Li Z, Sacks DB. IQGAP1 binds ERK2 and modulates its activity. J Biol Chem. 2004;279:17329–37. doi: 10.1074/jbc.M308405200. [DOI] [PubMed] [Google Scholar]
- 54.Tang P, Sutherland CL, Gold MR, Finlay BB. Listeria monocytogenes invasion of epithelial cells requires the MEK1/ERK2 mitogen-activated protein kinase pathway. Infect Immun. 1998;66:1106–12. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aiastui A, Pucciarelli MG, Garcia-del Portillo F. Salmonella enterica serovar typhimurium invades fibroblasts by multiple routes differing from the entry into epithelial cells. Infect Immun. 2010;78:2700–13. doi: 10.1128/IAI.01389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steele-Mortimer O. The Salmonella-containing vacuole: moving with the times. Curr Opin Microbiol. 2008;11:38–45. doi: 10.1016/j.mib.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brumell JH, Grinstein S. Role of lipid-mediated signal transduction in bacterial internalization. Cell Microbiol. 2003;5:287–97. doi: 10.1046/j.1462-5822.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 58.Drecktrah D, Knodler LA, Steele-Mortimer O. Modulation and utilization of host cell phosphoinositides by Salmonella. Infect Immun. 2004;72:4331–5. doi: 10.1128/IAI.72.8.4331-4335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reddy MA, Prasadarao NV, Wass CA, Kim KS. Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J Biol Chem. 2000;275:36769–74. doi: 10.1074/jbc.M007382200. [DOI] [PubMed] [Google Scholar]
- 60.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–2. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 61.Sechi AS, Wehland J. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P2 influences cytoskeletal protein activity at the plasma membrane. J Cell Sci. 2000;113:3685–95. doi: 10.1242/jcs.113.21.3685. [DOI] [PubMed] [Google Scholar]
- 62.Terebiznik MR, et al. Elimination of host cell PtdIns(4,5)P2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4:766–73. doi: 10.1038/ncb854. [DOI] [PubMed] [Google Scholar]
- 63.Gundersen GG. Evolutionary conservation of microtubule-capture mechanisms. Nat Rev Mol Cell Biol. 2002;3:296–304. doi: 10.1038/nrm777. [DOI] [PubMed] [Google Scholar]
- 64.Oelschlaeger TA, Guerry P, Kopecko DJ. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci U S A. 1993;90:6884–8. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuhn M. The microtubule depolymerizing drugs nocodazole and colchicine inhibit the uptake of Listeria monocytogenes by P388D1 macrophages. FEMS Microbiol Lett. 1998;160:87–90. doi: 10.1111/j.1574-6968.1998.tb12895.x. [DOI] [PubMed] [Google Scholar]
- 66.Dhakal BK, Mulvey MA. Uropathogenic Escherichia coli invades host cells via an HDAC6-modulated microtubule-dependent pathway. J Biol Chem. 2009;284:446–54. doi: 10.1074/jbc.M805010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida S, Sasakawa C. Exploiting host microtubule dynamics: a new aspect of bacterial invasion. Trends Microbiol. 2003;11:139–43. doi: 10.1016/s0966-842x(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 68.Wickstrom SA, et al. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell. 2010;19:574–88. doi: 10.1016/j.devcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pullikuth AK, Catling AD. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell Signal. 2007;19:1621–32. doi: 10.1016/j.cellsig.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–37. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 71.Procyk KJ, Kovarik P, von Gabain A, Baccarini M. Salmonella typhimurium and lipopolysaccharide stimulate extracellularly regulated kinase activation in macrophages by a mechanism involving phosphatidylinositol 3-kinase and phospholipase D as novel intermediates. Infect Immun. 1999;67:1011–7. doi: 10.1128/iai.67.3.1011-1017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coombes BK, Mahony JB. Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of HEp2 cells. Cell Microbiol. 2002;4:447–60. doi: 10.1046/j.1462-5822.2002.00203.x. [DOI] [PubMed] [Google Scholar]
- 73.Evans DJ, Maltseva IA, Wu J, Fleiszig SM. Pseudomonas aeruginosa internalization by corneal epithelial cells involves MEK and ERK signal transduction proteins. FEMS Microbiol Lett. 2002;213:73–9. doi: 10.1111/j.1574-6968.2002.tb11288.x. [DOI] [PubMed] [Google Scholar]
- 74.Ho YD, Joyal JL, Li Z, Sacks DB. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J Biol Chem. 1999;274:464–70. doi: 10.1074/jbc.274.1.464. [DOI] [PubMed] [Google Scholar]
- 75.Hu B, Shi B, Jarzynka MJ, Yiin JJ, D'Souza-Schorey C, Cheng SY. ADP ribosylation factor 6 regulates glioma cell invasion through the IQ-domain GTPase-activating protein 1-Rac1-mediated pathway. Cancer Res. 2009;69:794–801. doi: 10.1158/0008-5472.CAN-08-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer RD, Sacks DB, Rahimi N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS One. 2008;3:e3848. doi: 10.1371/journal.pone.0003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi K, Suzuki K. Regulation of protein phosphatase 2A-mediated recruitment of IQGAP1 to β1 integrin by EGF through activation of Ca2+/calmodulin-dependent protein kinase II. J Cell Physiol. 2006;208:213–9. doi: 10.1002/jcp.20657. [DOI] [PubMed] [Google Scholar]
- 78.Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling leading to actin binding, Elk-1/estrogen receptor transcriptional activation and ovarian cancer progression. J Biol Chem. 2005;280:11961–72. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- 79.Usatyuk PV, Gorshkova IA, He D, Zhao Y, Kalari SK, Garcia JG, Natarajan V. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem. 2009;284:15339–15352. doi: 10.1074/jbc.M109.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rittmeyer EN, Daniel S, Hsu SC, Osman MA. A dual role for IQGAP1 in regulating exocytosis. J Cell Sci. 2008;121:391–403. doi: 10.1242/jcs.016881. [DOI] [PubMed] [Google Scholar]
- 81.Le Clainche C, et al. IQGAP1 stimulates actin assembly through the N-WASP-Arp2/3 pathway. J Biol Chem. 2007;282:426–35. doi: 10.1074/jbc.M607711200. [DOI] [PubMed] [Google Scholar]
- 82.Mataraza JM, Briggs MW, Li Z, Frank R, Sacks DB. Identification and characterization of the Cdc42 binding site of IQGAP1. Biochem Biophys Res Commun. 2003;305:315–21. doi: 10.1016/s0006-291x(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 83.Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271:23363–7. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- 84.Smith MJ, et al. The PTB domain of ShcA couples receptor activation to the cytoskeletal regulator IQGAP1. Embo J. 2010;29:884–96. doi: 10.1038/emboj.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi K, Suzuki K. Requirement of kinesin-mediated membrane transport of WAVE2 along microtubules for lamellipodia formation promoted by hepatocyte growth factor. Exp Cell Res. 2008;314:2313–22. doi: 10.1016/j.yexcr.2008.04.009. [DOI] [PubMed] [Google Scholar]