SUMMARY
Ease of experimental gene transfer into viral and prokaryotic pathogens has made transgenesis a powerful tool for investigating the interactions of these pathogens with the host immune system. Recent advances have made this approach feasible for more complex protozoan parasites. By contrast, the lack of a system for heritable transgenesis in parasitic nematodes has hampered progress toward understanding the development of nematode-specific cellular responses. Recently, however, significant strides towards such a system have been made in several parasitic nematodes, and the possible applications of these in immunological research should now be contemplated. In addition, methods for targeted cell ablation have been successfully adapted from Caenorhabditis elegans methodology and applied to studies of neurobiology and behaviour in Strongyloides stercoralis. Together, these new technical developments offer exciting new tools to interrogate multiple aspects of the host–parasite interaction following nematode infection.
Keywords: biolistics, microinjection, model antigen, Strongyloides, transgenesis
INTRODUCTION
Robust systems for genetic manipulation, particularly transgenesis, in protozoan parasites such as Trypanosoma, Leishmania, Toxoplasma and Plasmodium (1–4) provide many potential avenues for investigating the biology of these parasites including their interactions with their hosts’ immune systems. These avenues have included the use of transgenic parasites expressing exogenous model antigens associated with T cells that express defined T-cell receptors specific for the model antigen to characterize parasite-specific cellular immune responses (5–8). In addition, genetically attenuated parasites have been employed to examine the effect of persistent subclinical infection on the development of effector and memory T cell responses in vivo (9–11). By contrast, transgenesis and related methods have been slower to develop for parasitic nematodes, making it impractical, until recently, to propose using genetically modified parasites to study molecular and cellular aspects of the immune response to nematode infections. Nevertheless, two current trends in research on parasitic nematodes indicate that momentum exists in this area and that this impediment may be surmounted in the near future.
The first of these promising trends is constituted by significant strides towards transgenesis in parasitic nematodes, some of which are amenable to detailed immunological study. The most substantial gains towards transgenesis have been made with nematodes in the superfamily Strongyloidoidea (12,13), where the first heritable DNA transformation of a mammalian parasitic nematode has been achieved with Parastrongyloides trichosuri using gonadal microinjection of plasmid-based reporter constructs followed by serial passage in vitro (14). In our own studies with Strongyloides stercoralis, we have developed reliable methods for gene transfer and a series of gfp-based reporter constructs that give tissue- and cell-specific transgene expression in developing F1 transformed larvae. Inheritance of transgene constructs in the F2 generation and beyond has been demonstrated in S. stercoralis, but in their present configuration these plasmid constructs are not expressed beyond the F1 generation (15,16). While we are unaware of directed efforts to achieve heritable transformation in other parasitic nematodes, transient transfer and expression of DNA constructs have been achieved in the filariae, Litomosoides sigmodontis (17) and Brugia malayi (18). Reliable methods for transfer and expression of both DNA and RNA constructs in the intestinal parasite, Ascaris suum are also in hand (19).
The second promising trend comprises significant advances in descriptive genomics in the past decade for a number of selected parasitic nematodes. Initially, partial sequencing of randomly selected cloned cDNAs to assemble expressed sequence tag (EST) databases has provided a number of valuable tools for comparative genomics. Such databases have been assembled for several parasitic nematodes, including several strongyloidoid species. Mitreva et al. (20) analysed 10 921 ESTs from L1 and infective L3 of S. stercoralis, and compared their developmental expression patterns to C. elegans. More recently, Thompson et al. (21) analysed the developmental expression patterns of 4156 EST clusters from S. ratti, representing an estimated 20% of this parasite’s genes. Currently, the EST databases at NCBI for S. stercoralis, S. ratti and P. trichosuri contain 11 335, 14 761 and 7963 accessions, respectively, and S. stercoralis and S. ratti have priority for future genome sequencing projects (22). The growing body of descriptive genomic data for strongyloidoid nematodes forms a sound basis for the use of recombinant parasite molecules and for the application of reverse genetic methods such as targeted gene ablation, gene silencing or transgenesis to immunological studies.
In addition to transgenesis, the ability to alter the sensory or developmental potential of nematode parasites by means of targeted cell ablation may also provide an important experimental tool for understanding the host–parasite relationship and the nature of the host immune response to these organisms. This approach, primarily involving cell ablation by laser microsurgery (23), has been used to elucidate the neuronal basis of many behaviours, notably mechano- and chemosensory responses, in C. elegans (24–26). Schad and colleagues, exploiting the morphological similarities between chemosensory neurones in C. elegans and S. stercoralis, have adapted this approach to extensive study of the neuronal control of specific behaviours constituting the infective process (27). This work has emphasized the roles of amphidial chemosensory neurones in regulating developmental events and behaviours such as host finding, skin penetration and resumption of development by infective third stage larvae (L3i) of S. stercoralis. As they are in physical contact with the external environment, amphidial neurones identified as mediating early developmental events in the host may constitute natural targets of immune attack and hence offer potential as future vaccine candidates. Moreover, worms subjected to cell ablation might be rendered deficient in their ability to undertake such developmental steps. Such worms, if viable, would be attenuated in a reproducible fashion and therefore useful in investigating the efficacy of attenuated nematode parasites in promoting long-term immunity to re-infection. In the present paper, we begin by discussing existing techniques for transgenesis in parasitic nematodes, with particular emphasis on Strongyloides and its relatives. We also review the development and use of targeted cell ablation to study neuronal function in S. stercoralis. We conclude by projecting how these approaches to modifying parasite genetics, behaviour and development can be applied to analyse helminth-specific immune responses in vivo (5,28–32).
METHODS FOR TRANSGENESIS IN STRONGYLOIDES AND OTHER PARASITIC NEMATODES
Modes of gene transfer
Gonadal microinjection
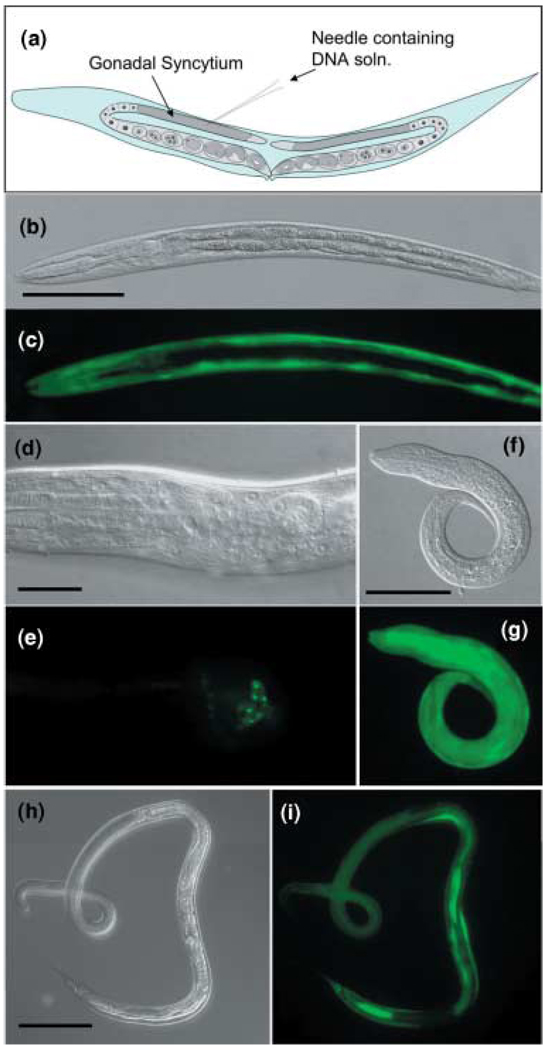
This method was pioneered over two decades ago in C. elegans (33–36) and takes advantage of the fact that a significant span of each arm of the long double re-curved gonad in adult hermaphrodites (Figure 1a) comprises a syncytium of nuclei orientated around a core of common cytoplasm. Plasmid DNA solution infused into this region using a finely drawn glass needle is incorporated into the nuclei of developing oocytes as they are enclosed in plasma membranes and core cytoplasm in more distal regions of the reproductive tract (37,38). DNA delivered in this manner is inherited in several possible forms in subsequent generations of worms, most frequently in the form of extrachromosomal arrays, less frequently as random integrations into the chromosome and rarely as homologous chromosomal integrations (37).
Figure 1.
Transgenesis in Strongyloides stercoralis. (a) Diagrammatic representation of the syncytial ovary as microinjection site for DNA transfer. (b–i) F1 progeny of free-living female S. stercoralis microinjected as shown in panel (a) with reporter transgene constructs, all containing the Ss era-1 3′ UTR as a terminator. (b,c) DIC and fluorescence images, respectively, of a first-stage larva (L1) expressing gfp under the Ss act-2 promoter; note body wall localization (Scale bar in (b) = 50 µm). (d,e) DIC and fluorescence images, respectively, of an L1 expressing gfp under the Ss gpa-3 promoter; note localization in ampidial neurones (Scale bar in (d) = 10 µm). (f,g) DIC and fluorescence images, respectively, of an L1 expressing gfp under the Ss rps-21 promoter; note ubiquitous pattern of expression (Scale bar in (f) = 50 µm). (h,i) DIC and fluorescence images, respectively, of a parasitic female expressing the Ss rps-21 reporter, recovered from the gerbil intestine at necropsy (Scale bar in (h) = 200 µm).
The structural similarity of the ovaries of free-living female Strongyloides spp. and Parastrongyloides. spp. to the gonads of C. elegans hermaphrodites has made it relatively straightforward to adapt the technique of gonadal microinjection of DNA for routine gene delivery to these parasites (Figure 1a) (14–16). Grant et al. (14) have devised a system of heritable transgenesis in P. trichosuri using this approach, and we have developed reliable methods for gene transfer into S. stercoralis by gonadal microinjection (15,16), observing tissue- and cell-specific expression of several different gfp-based reporter constructs in developing F1 transformant larvae (Figure 1b – g) (16,39).
Biolistics
This method, also called particle bombardment, involves the transfer of nucleic acids into target cells adsorbed onto high-density microparticles of gold or tungsten. Gold is currently the preferred material because, compared to tungsten, microparticles of this metal are more uniform in size and shape, and are chemically inert and therefore non-toxic to cells (40). Early versions of this technology involved accelerating microparticles into target cells by means of explosive charges or electrical discharge while contemporary instruments use highly pressurized helium (40). Biolistic transfer of nucleic acids has been used to create transient transformants in several parasitic nematode species, generally in larger or more resistant parasites such as adult filariae (17,18) and A. suum embryos, which have the additional advantage of availability in exceedingly high numbers (19). Along with electroporation, particle bombardment is a preferred mode of gene transfer into schistosomes (19,41–43).
Biolistic gene transfer has also been used to create stable transgenic lines in C. elegans (17,44,45). Transgenes introduced by the biolistic route are integrated spontaneously into the chromosomes of C. elegans at a higher frequency than those transferred by gonadal microinjection, a method that favours assembly of transgenes as tandem repeats in extrachromosomal arrays (37,38,44). The tendency of biolistic transfer to give chromosomal integration of transgene sequences in C. elegans makes it advantageous for generating gene knockouts by homologous recombination (46). It has also been incorporated as a component of a new high-throughput ‘recombineering’ system for parallel functional study of multiple genes in this worm (47). The success of biolistic gene transfer as a means of heritable transformation in C. elegans depends upon the practicality of culturing large numbers of individuals in vitro, the availability of genetically based systems for selection of transformants and the ease of deriving transgenic lines from small numbers of founders. Although the frequency of integrative transformation following biolistic gene transfer is relatively high, the overall frequency of transformation per se using this method (5·3 × 10−5) is quite low relative to gonadal microinjection. In one study (48), bombardment of 19 000 young adult C. elegans was required to generate a single independent transformant and bombardment of over 40 000 such individuals, on average, was necessary to derive a single integrated line. As a consequence, the rare F1 transformants that give rise to these transformant lines must be selected from an exceedingly large number of nontransformed worms and their progeny. Powerful selectable markers, such as the unc-119 rescue system used in the study just described (48), make it feasible to use biolistic gene transfer to establish both extrachromosomal array and integrated lines of C. elegans, even with this relatively low level of transformation efficiency. Preliminary trials in our laboratory, involving bombardment of 5–7 × 104 free living S. stercoralis adults with a reporter construct fusing gfp to the Ss act-2 promoter (Ss act-2::gfp::Ss era-1 3′ UTR, Figure 1b,c) failed to yield green fluorescent protein (GFP)-expressing transformants (A. Junio, unpublished data). Therefore, although it is practical to grow almost 105 free living adult S. stercoralis in charcoal co-proculture (49), adaptation of particle bombardment as a practical method of gene transfer in this species awaits the development of an efficient selectable marker system similar to those available for C. elegans.
Configurations of delivered nucleic acids
The majority of cited studies of transgenesis in parasitic nematodes have involved expression of reporter genes under the control of various promoters encoded in circular plasmid DNA. In C. elegans, gonadal microinjection of single or mixed circular plasmids favours formation of large extra-chromosomal elements termed episomal arrays. These arrays contain multiple, tandem copies of transgene DNA. Spontaneous chromosomal integrations of microinjected transgene DNA occur, but only rarely (34,36–38). This episomal mode of transgene incorporation and inheritance has not been strictly proven to occur in Strongyloides or Parastrongyloides, but the recent demonstration that co-injected plasmids are incorporated together into F1 transgenic progeny of S. stercoralis (39) is consistent with it. Linearized plasmid DNA appears to be incorporated with less efficiency into episomes following microinjection in C. elegans, and the resulting low-copy number transformations may be used to advantage when toxic effects of over-expression from long, multicopy arrays is problematic (36–38). The configuration of injected DNA may also be manipulated to increase the frequency of low-copy chromosomal integration of transgenes in C. elegans. Mello et al. (36) discovered that co-injection of random single-stranded oligonucleotides along with plasmid-encoded transgenes greatly increased the frequency of spontaneous integrations and formation of stable integrated lines. This approach complements others involving the use of γ- or UV-irradiation to induce chromosomal breaks and thereby facilitate chromosomal integration (37,38). These and other methods that promote chromosomal integration of transgenes may be important in addressing the problem of transgene silencing following host passage of F1 transformants in S. stercoralis (16) and other parasites. Finally, pioneering work on A. suum, indicates that, in addition to plasmid encoded transgenes, embryos of this parasite subjected to particle bombardment will express a firefly luciferase reporter encoded on in vitro transcribed RNA bearing a m7GpppG cap analogue as well (19).
Requirements for regulated transgene expression in Strongyloides stercoralis
Our attempts at transformation of S. stercoralis involved gonadal microinjection of GFP-encoding plasmid vectors based on standard ones from the C. elegans vector kit originally prepared and distributed to the research community by A. Fire and colleagues (50,51), and currently distributed by a nonprofit corporation (Addgene, Cambridge, MA; <http://www.addgene.org>). Vectors containing both 5′ and 3′ regulatory sequences derived from C. elegans or one of several conserved S. stercoralis promoters paired with a C. elegans 3′ UTR, resulted only in dysregulated expression of gfp in nonviable embryos (15). Regulated, tissue-appropriate transgene expression in developing F1 transformants was not achieved for S. stercoralis until both 5′ and 3′ regulatory sequences derived from the parasite were incorporated into reporter gene constructs (Table 1). In this case, expression of the gfp reporter flanked by the promoter and 3′ UTR for the S. stercoralis gene Ss era-1 predominated in intestinal cells of F1 transformant larvae (16). This expression pattern approximates that of the gene’s orthologue, cdc-48·2, in C. elegans (52). Subsequently, we demonstrated that the Ss era-1 3′ UTR can act as a multipurpose terminator, giving tissue-appropriate expression of numerous S. stercoralis promoter-gfp fusions (39). We are still in the process of comparing the structure of the Ss era-1 3′ UTR to previously used elements from C. elegans, such as the unc-54 3′ UTR. However, inspection of these two sequences reveals that, whereas, the C. elegans unc-54 3′ UTR contains a single consensus polyadenylation signal along with numerous variant signals, the S. stercoralis element contains three such consensus motifs and an even higher density of variant signals (16), possibly indicating that the number, structure or position of polyadenylation signals in the C. elegans 3′ UTRs present in the standard vector constructs are not capable of efficiently terminating messages in this parasite. In any case, the finding that a single 3′ UTR can give regulated expression of numerous transgene constructs (Figure 1b – g) has allowed us to develop a collection or ‘toolkit’ of modular vectors for S. stercoralis (39) and make this available to the research community <www.addgene.org>.
Table 1.
An endogenous 3′ UTR is required for regulated transgene expression in Strongyloides stercoralis. Expression of constructs with various combinations of regulatory elements from C. elegans and S. stercoralis
| Expression in |
|||||
|---|---|---|---|---|---|
| Plasmid | Promoter | Coding gene | 3′ end | Embryoa | Larvae |
| pPV102.7 | Ss-era-1 | gfpS65C | Ce-unc-54 | + | − |
| pPV230.3 | Ss-era-1 | gfpS65C | Ss-era-1 | + | + |
| pAJ08 | Ss-act-2 | gfpS65C | Ss-era-1 | + | + |
| pAJ09 | Ss-gpa-3 | gfpS65C | Ss-era-1 | + | + |
| pAJ20 | Ss-rps-21 | gfpS65C | Ss-era-1 | + | + |
| pPV101.1 | Ss-actin-2 | gfpS65C | Ce-unc-54 | + | − |
| pTG96_2 | Ce-sur-5 | gfpS65C + NLS | Ce-unc-54 | + | − |
| pPD118.33 | Ce-myo-2 | gfpS65C | Ce-let-858 | + | − |
| pPD117.01 | Ce-mec-7 | gfpS65C | Ce-let-858 | + | − |
Denotes expression in degenerating embryos only. Expression of the Ss era-1 reporter in larvae is tissue specific and comparable to its orthologue cdc-48.1 in C. elegans.
Transformation markers and transformant selection
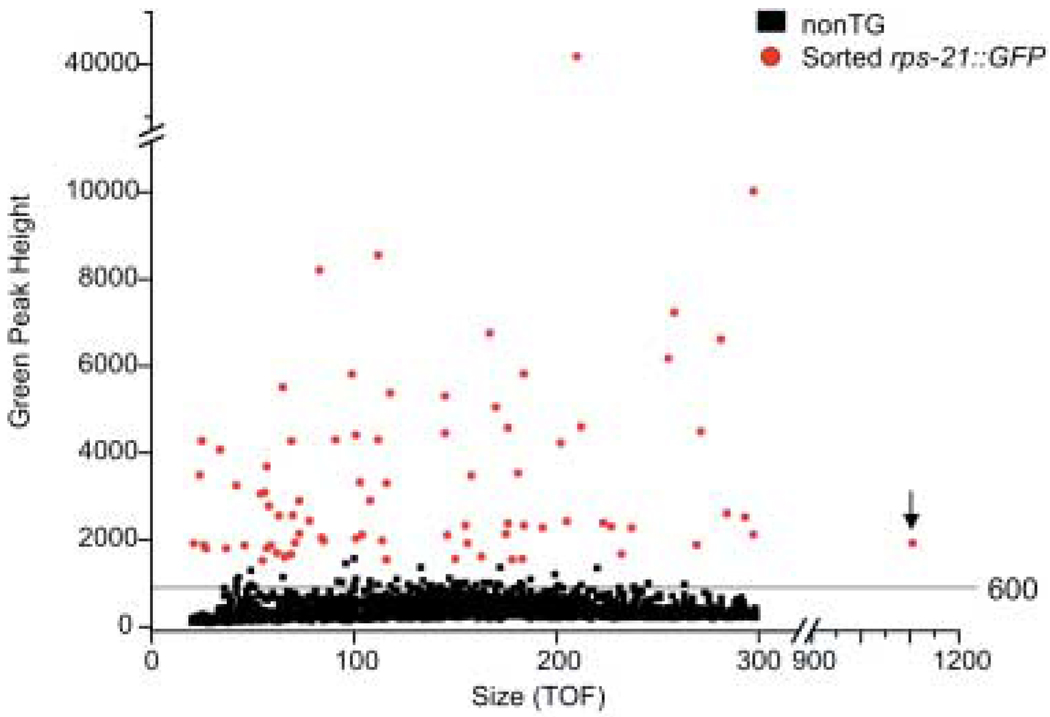
Efficient marking and/or selection of transgenic worms will be an important component of any system in which these organisms are used in immunological experiments. The sensitivity of protozoan parasites to a range of antibiotics and other drugs has allowed selectable marker genes conferring drug resistance to be used routinely for efficient selection of transgenic individuals (53–61). By contrast, rapid, large-scale selection of transgenic C. elegans, particularly those resulting from biolistic gene transfer, has usually been based upon rescue of conditional lethal or behavioural mutations by co-transformation with rescuing gene sequences along with the transgene of interest (37,48,62,63). Small-scale manual selection of transformed C. elegans may be accomplished using dominant mutant sequences such as rol-6 (64), which confer behavioural phenotypes that can be easily recognized and picked by hand from among a mixed population on agar plates. None of the mutant genes described above as selectable markers for C. elegans have been characterized in Strongyloides sp., and it is doubtful that dominant markers that affect motility such as rol-6 would be practical for immunological experiments with parasitic nematodes where ability to invade and migrate normally in the host would be a prerequisite. Similarly, with the exception of an apparently low sensitivity to hygromycin (65), none of the antibiotics commonly used in the selectable genetic marker systems described above are known to be effective against Strongyloides sp. However, the availability of GFP-encoding transgenes for S. stercoralis allows us to select transformed parasites based on fluorescence. Where relatively small numbers (≤ 102) of transformants are involved, this may be done manually using a stereomicroscope equipped with coaxial epifluorescence. Recently, we have been able to automatically isolate larger numbers (102–103) of gfp-expressing transformed infective third-stage larvae from a mixed population using a COPAS Biosorter (Figure 2) (39).
Figure 2.
Selecting transgenic S. stercoralis L3i using the COPAS Biosorter. Sorting Ss act-2::gfp transformants based on GFP fluorescence from a mixed population of GFP-expressing and non-GFP-expressing parasites. Intensity of GFP fluorescence in units of green peak height is plotted against time of flight (TOF), a measure of worm size. Typically, setting the sorting gate at a green peak height of 600 as shown yields a population of worms highly enriched for transformants while retaining parasites with lower levels of fluorescence, possibly indicating a lower transgene copy number, more optimal for heritable transgenesis. The outlier in time of flight (arrow) may indicate two or more worms passing the detector in tandem.
HERITABLE VS. TRANSIENT TRANSGENESIS
In addition to employing transiently transfected nematode parasites in studies of the host immune response (see below), in vivo analysis of the development and progression of anti-nematode immune responses would be greatly facilitated by the availability of stable transgenic lines of Strongyloides sp. or other parasitic nematodes. We have detected transgene DNA as late as the F5 generation following gonadal microinjection in S. stercoralis, indicating that heritable transformation is possible, even frequent, using this approach (16). However, to date none of these constructs has been expressed in the F2 transgenic generation and beyond, and, consequently, we have not yet succeeded in deriving a stable transgene expressing line. Existing stable lines of P. trichosuri were derived by in vitro passage of free-living stages and subsequently carried forward by passage in the mammalian host (14). This approach is unavailable for all but a few parasitic nematodes. It appears likely that transgenes in strongyloidoid nematodes are actively silenced during host passage.
We envisage two potential mechanisms that could act alone or in concert to account for this apparent transgene silencing. The first hinges on the likelihood that, as in C. elegans, transgene constructs microinjected into the gonads of these parasites are assembled into tandem multicopy extrachromosomal arrays or episomes in F1 progeny. Such genetic elements, by virtue of their repetitive nature, their extrachromosomal location or both, may be particularly subject to epigenetic silencing (66,67). A practical remedy for such silencing would be to adopt gene transfer methods that favour low-copy-number, chromosomal integration over episomal array formation. One such method involves introduction of transgenes in the presence of a phage-encoded integrase. Such an approach, involving the bacteriophage ϕC31 integrase and plasmid constructs encoding the bacterial attachment site attB, succeeded in driving transgene sequences into the genome of Xenopus laevis (66). Another approach that should also be attempted in parasitic nematodes is transposon-mediated transgene integration. Tc1 and related mariner elements have proven capable of mediating chromosomal integration of plasmid-encoded sequences in C. elegans (68), and another insect transposon, piggyBac, was recently used to mediate integrative transformation of the blood fluke Schistosoma mansoni (43,69).
The possibility that parasitic nematodes may simply silence transgene sequences more efficiently than C. elegans regardless of their episomal or chromosomal location should also be considered. This contingency was also explored in the Xenopus study (66), where it was found that constructs in which transgenes were flanked by the HS4 insulator sequence from the chicken β-globin gene were less subject to epigenetic silencing than constructs lacking this element. Such vertebrate insulator sequences or related elements from constitutively expressed helminth genes might serve to prevent or forestall silencing of transgenes in parasitic nematodes.
Although heritable transgenesis should be the ultimate goal of work in this area, the utility of transient transformation should not be discounted, especially where it occurs in a significant number of individuals and transgene expression appears to be regulated appropriately. Transient transformation systems have already found application in studies of mRNA processing and translation in A. suum (70,71) and in an analysis of promoter structure and function, and of message transplicing in B. malayi (72,73). Our own work with transiently transformed S. stercoralis generally underscores the conservation of anatomical gene expression patterns between this parasite and the distantly related free-living nematode C. elegans. The facts that F1 transgenic S. stercoralis can be sorted efficiently from a mixed population having an excess of nontransformed individuals (Figure 2) and that these transformants can establish as parasitic females in a susceptible host (Figure 1h,i) (39), mean that experimental infections with worms transiently transformed to express model antigens, though somewhat laborious, are already feasible with methodology currently in hand. The same considerations would hold for worms that were genetically attenuated by expression of altered transgenes.
APPLICATIONS OF TRANSGENIC NEMATODE PARASITES
Molecular cloning techniques have greatly facilitated studies of the dynamics of CD8+ and CD4+ T cell responses to viral and prokaryotic pathogens. These methods have made it feasible to identify and clone dominant epitopes from these organisms and associate them with T cell populations having cognate antigen receptors using approaches such as MHC tetramer staining. Where knowledge of specific native antigens is lacking for these pathogens, and for more complex single-celled eukaryotic pathogens, transgenic organisms engineered to express various model antigens such as avian ovalbumin, for which specific TCR-transgenic T cells are available, have been used extensively to characterize pathogen-specific cellular responses (5,28–31). By contrast, similar ‘tracking’ of nematode-specific T cell responses has been hampered by the difficulty in associating specific T cell populations with endogenous helminth antigens and by the virtual lack of methods for expressing model antigens in these worms (32).
Studies carried out over the last 20 years employing in vivo deletion or depletion of distinct cell types, cytokines or their receptors have demonstrated that immunity to gastrointestinal nematode parasites in murine model systems is critically dependent on CD4+ T cells that express T helper type 2 cytokines. Th2 cells are characterized by expression of an array of cytokines including IL-3, IL-4, IL-5, IL-9, IL-13, IL-25 and IL-31 (74–79). In multiple nematode parasite systems, including Heligmosomoides polygyrus, Nippostrongylus brasiliensis, Trichinella spiralis, Trichuris muris and Strongyloides spp., Th2 cytokines provoke physiologic changes in host tissue such as mastocytosis, altered smooth muscle contractility, changes in intestinal epithelial cell turnover, and differentiation and development of alternatively activated macrophages, all of which have been implicated in worm expulsion and/or resistance to re-infection (80–85). While Th2 cytokines confer protective immunity against infection, in some circumstances, Th1 cell-derived IFNγ can promote parasite persistence and chronic infection and development of intestinal inflammation (86–88).
Despite growing insights into cytokine regulation of resistance and susceptibility to infection, there is limited understanding of where and when nematode-specific Th2 cytokine responses are initiated and what immunoregulatory pathways govern these processes. For example, although most studies to date have studied anti-nematode responses in the mesenteric LN that drain the intestine, the anatomical locations in which nematode-specific CD4 T or CD8 T cells are primed and the temporal kinetics of these responses remain unknown. Recent studies have highlighted that robust anti-nematode responses are detectable in components of the gut-associated lymphoid tissues such as lymphoid follicles and the lamina propria (85,89), raising the prospect that anti-nematode responses may be initiated within the intestinal environment. Access to transgenic nematode parasites such as OVA-expressing Strongyloides, coupled with OVA-specific T cells, will enable direct analysis of where parasite-specific T cell responses are first activated and where programmed T cell division and differentiation occur. As discussed in other articles in this volume, this approach has been extremely valuable in understanding protozoan-specific immune responses in vivo (1–4).
Recent studies have also highlighted that naïve CD4 T cells can differentiate into multiple T helper cell subsets including Th1, Th2, Th3, Treg, Th17 and Tr1 cells (90,91), and many of these distinct cell lineages have been identified following exposure to nematode parasites. In addition to enabling analysis of nematode-specific T cell activation and proliferation, the availability of transgenic nematode parasites will enable detailed in vivo analysis of the cell types, cytokines, chemokines and co-stimulatory molecules that influence the differentiation and expansion of distinct nematode-specific T helper cell subsets. Employed in association with cytokine reporter mice, infection with transgenic nematode parasites will also facilitate the isolation of live nematode-specific T helper cell subsets for subsequent functional analysis of their plasticity and cell fate, including determination of where and when nematode-specific effector vs. memory T cells develop.
Lastly, as outlined above, we and others have now developed approaches to allow generation of transgenic nematode parasites in which model antigens can be expressed in specific cells and tissues within parasitic nematodes including the cuticle, intestine and pharynx (see above). The development of techniques to enable inducible expression, coupled with our pre-existing nematode tissue-specific promoters, will allow in vivo analysis of how tissue-specific nematode antigens, expressed at different points during the infection, can influence the type of T helper cell response that develops. The technology to generate transiently and stably transformed nematode parasites will allow, for the first time, the development of model antigen-expressing transgenic nematode parasites that will enable investigators to address critical questions regarding how nematode-specific CD4 T cell responses are regulated in vivo.
TARGETED CELL ABLATION
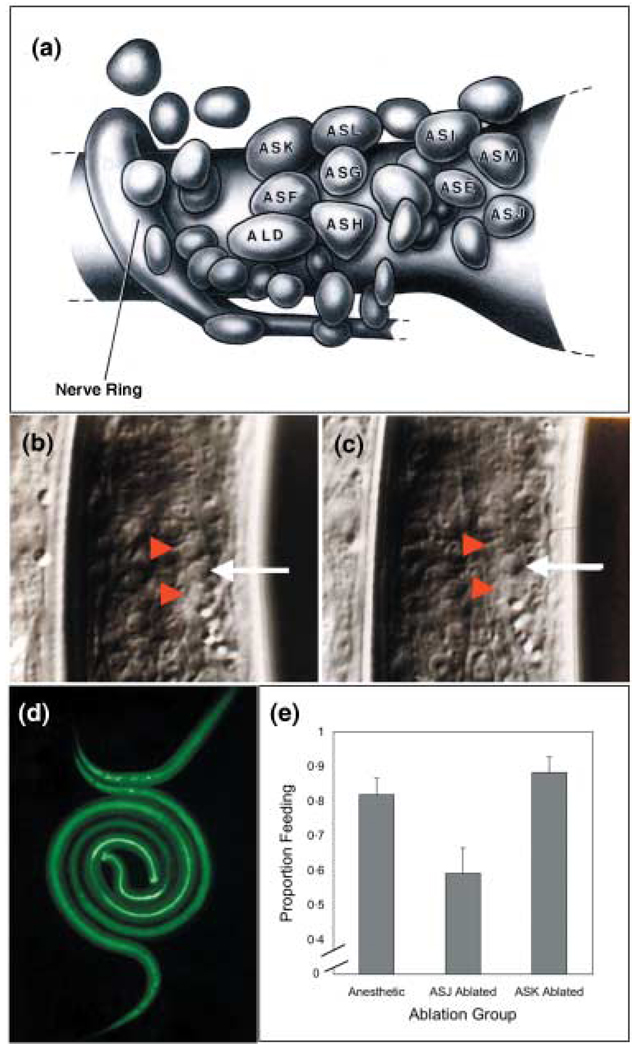
Precise, targeted ablation of cells by laser microbeam in C. elegans followed by phenotypic analysis has provided a means of studying the functions of mature cells such as chemo- and mechanosensory neurones (92,93) and the fates and interactions of cells in developing embryos and larvae (94,95). Many aspects of cell morphology and position in S. stercoralis are similar to C. elegans, and, like C. elegans, larval S. stercoralis are optically transparent. These similarities have facilitated adaptation of laser microsurgical technique developed for C. elegans to the neurobiology and behaviour of this parasite (27). Careful morphological study of anterior chemosensory neurones (96,97), involving complete three-dimensional reconstruction (Figure 3a), coupled with laser microsurgical techniques adapted from C. elegans methodology (Figure 3b,c), has allowed the neuronal basis of many behaviours associated with the infective process in S. stercoralis to be determined (98–102).
Figure 3.
Microlaser ablation of chemosensory neurones in S. stercoralis: a possible means of attenuating or restricting parasite development to early events that are key to initiation of the immune response. (a) Diagrammatic representation of amphidial cell bodies in the head of an L1 of S. stercoralis based on computer-generated three-dimensional reconstruction of electron micrographic sections From Ashton et al. (98), reproduced with permission. (b,c) Cell bodies of ampidial neurones before and after, respectively, microlaser ablation of ASI and ASF (upper and lower red arrow heads, respectively). Un-operated neurone ASG is highlighted (white arrow) for a point of reference. Images courtesy of G. A. Schad. (d) Ingestion of FITC from medium by S. stercoralis L3i following transfer to culture under host-like conditions, an indicator of resumption of development. (e) Effect of laser ablation of ASJ chemosensory neurones on resumption of feeding. Controls consisted of un-operated worms and worms in which an unrelated neurone pair, ASK was ablated. Images in panels b and c are from Ashton et al. (102); reproduced with permission.
Microlaser surgery remains the preferred method of cell ablation in C. elegans and in S. stercoralis. However, genetic methods of targeting and killing specific cells or groups of cells have been demonstrated in C. elegans and may prove useful in parasites such as Strongyloides for generating the larger numbers of attenuated individuals that would be required for immunological experiments. Driscoll, Chalfie and colleagues devised a method of ‘genetically targeted cell disruption’ for C. elegans based upon the expression of transgenes containing the coding sequence for a cytotoxic gene under the control of promoters specific for the cells of interest (103). The cytotoxic gene used in this case was the mutant mechanosensory channel forming protein, MEC-4d, which causes degeneration of target cells expressing it ectopically (104). Theoretically, timing of such genetically targeted cell ablation could be achieved through the use of conditional promoters. Through a process called chromophore assisted light inactivation (CALI), genetically encoded photosensitizing molecules, such as KillerRed, generate reactive oxygen species upon irradiation, resulting in the strategically timed degeneration of cells expressing the marker but not surrounding nonexpressing cells (105). These genetic targeting methods would allow ablation of single cells or groups of cells depending on promoter specificity. Known promoters in C. elegans could be used to achieve this type of cell specificity. For example, the dauer regulatory gene daf-7, is expressed in a single pair of amphidial neurones, ASI, in first stage larvae (106). The promoter for this gene could be used to target expression of a toxic or photosensitizing molecule to specifically ablate this pair of neurones. As the expression patterns of more genes in parasitic nematodes are uncovered, and those of a relatively cell-specific nature identified (see Figure 1d,e), genetically targeted cell ablation may become practical in strongyloidoid and other parasitic nematodes.
APPLICATIONS OF NEMATODE PARASITES WITH TARGETED CELL ABLATIONS
In addition to transgenesis, targeted ablation of specific neurones in parasitic nematodes offers a new approach to interrogate the host–parasite interaction following infection. For example, a number of studies have focused on neuronal control of host seeking and other positioning behaviours that are prerequisites for infection but occur outside the host. Recent studies demonstrated that worms in which the two ASJ-class amphidial neurones had been ablated were unable to completely resume pharyngeal pumping and food ingestion following transfer to host-like in vitro culture conditions (Figure 3d,e) (102). Resumption of feeding is one of the first events to occur after L3i initiate the host phase of development following skin penetration and, as such, could be subject to regulation by the host immune system. Employing laser ablated parasites in in vivo infection studies could facilitate a systematic analysis of which if any parasite neurones are subject to immunologic attack. Given that many successful anti-nematode drugs actively target nematode chemo- and neurosensory functions, such information could provide a rationale approach in the design of a new generation of chemotherapeutic agents to target nematode infection. In addition, worms rendered deficient in this critical behaviour by neuronal ablation could be considered developmentally attenuated and therefore useful for immunologic studies to address where and when anti-parasite effector and memory T cell responses develop.
CONCLUSIONS
Transgenesis, a method that has strongly benefited immunological studies with the parasitic protozoa, has been unavailable for parasitic nematodes until recently. Recent efforts by multiple groups have successfully pioneered these methodologies in various helminths. Because of their similarities to C. elegans, parasites in the superfamily Strongyloidoidea (including Strongyloides spp. and Parastrongyloides spp.) have lent themselves most readily to the adaptation of a well-established method for genetic transformation involving gonadal microinjection of DNA. Other methods for gene transfer such as particle bombardment may be used in the near future to achieve heritable transformation of other nematode parasites. Along with the growing genomic resources for many of the medically important nematodes, these methods will constitute a resource for immunological study in the future. The applications of transgenesis in such studies would include the tracking of cellular immune responses to infection using parasites engineered to express model antigens coupled with immune cells bearing receptors specific for that model antigen. Genetic attenuation of parasitic nematodes, that is introducing genetic alterations (e.g. ones resulting in ablation of key neurones) that restrict development to key processes in eliciting the immune response, might also be an important application of this methodology. Microsurgical or genetically targeted cell ablation could also be used to generate parasites that are altered strategically in their abilities to undertake developmental or migratory steps that are key to invoking various aspects of the cellular immune response.
ACKNOWLEDGEMENTS
Supported by grants from the Ellison Medical Foundation (Grant No. ID-IA-0037–02 to J. B. Lok), the Crohn’s and Colitis Foundation of America William and Shelby Modell Family Foundation (to D. Artis), the National Institutes of Health (AI-50688 and AI-22662 to J. B. Lok and AI-61570 to D. Artis) and from the Center for Infectious Diseases and the Research Foundation of the University of Pennsylvania. We thank E. J. Pearce, M. J. Sundaram, G. A. Schad, H. J. Massey, Jr. A. J. Junio and S. T. Lamitina for helpful discussion.
REFERENCES
- 1.Beattie L, Evans KJ, Kaye PM, Smith DF. Transgenic Leishmania and the immune response to infection. Parasite Immunol. 2008;30:255–266. doi: 10.1111/j.1365-3024.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzierszinski FS, Hunter CA. Advances in the use of genetically engineered parasites to study immunity to Toxoplasma gondii. Parasite Immunol. 2008;30:235–244. doi: 10.1111/j.1365-3024.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 3.Mansfield JM, Paulnock DM. Genetic manipulation of African trypanosomes as a tool to dissect the immunobiology of infection. Parasite Immunol. 2008;30:245–253. doi: 10.1111/j.1365-3024.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 4.Thompson J, Millington OR, Garside P, Brewer JM. What can transgenic parasites tell us about the development of Plasmodium-specific immune responses? Parasite Immunol. 2008;30:223–233. doi: 10.1111/j.1365-3024.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- 5.Pepper M, Dzierszinski F, Crawford A, et al. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infect Immun. 2004;72:7240–7246. doi: 10.1128/IAI.72.12.7240-7246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubbels MJ, Striepen B, Shastri N, et al. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect Immun. 2005;73:703–711. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertholet S, Debrabant A, Afrin F, et al. Antigen requirements for efficient priming of CD8+ T cells by Leishmania major-infected dendritic cells. Infect Immun. 2005;73:6620–6628. doi: 10.1128/IAI.73.10.6620-6628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertholet S, Goldszmid R, Morrot A, et al. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177:3525–3533. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 9.Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J Immunol. 2004;172:3793–3797. doi: 10.4049/jimmunol.172.6.3793. [DOI] [PubMed] [Google Scholar]
- 10.Kebaier C, Uzonna JE, Beverley SM, Scott P. Immunization with persistent attenuated Δ lpg2 Leishmania major parasites requires adjuvant to provide protective immunity in C57BL/6 mice. Infect Immun. 2006;74:777–780. doi: 10.1128/IAI.74.1.777-780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 12.De Ley P, Blaxter M. Systematic position and phylogeny. In: Lee DL, editor. Biology of Nematodes. London: Harwood Academic Publishers; 2001. [Google Scholar]
- 13.Dorris M, Viney ME, Blaxter ML. Molecular phylogenetic analysis of the genus Strongyloides and related nematodes. Int J Parasitol. 2002;32:1507–1517. doi: 10.1016/s0020-7519(02)00156-x. [DOI] [PubMed] [Google Scholar]
- 14.Grant WN, Skinner SJM, Howes JN, et al. Heritable transgenesis of Parastrongyloides trichosuri: a nematode parasite of mammals. Int J Parasitol. 2006;36:475–483. doi: 10.1016/j.ijpara.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Lok JB, Massey HC., Jr Transgene expression in Strongyloides stercoralis following gonadal microinjection of DNA constructs. Mol Biochem Parasitol. 2002;119:279–284. doi: 10.1016/s0166-6851(01)00414-5. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Massey HC, Nolan TJ, et al. Successful transgenesis of the parasitic nematode Strongyloides stercoralis requires endogenous non-coding control elements. Int J Parasitol. 2006;36:671–679. doi: 10.1016/j.ijpara.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Jackstadt P, Wilm TP, Zahner H, Hobom G. Transformation of nematodes via ballistic DNA transfer. Mol Biochem Parasitol. 1999;103:261–266. doi: 10.1016/s0166-6851(99)00089-4. [DOI] [PubMed] [Google Scholar]
- 18.Higazi TB, Merriweather A, Shu L, et al. Brugia malayi: transient transfection by microinjection and particle bombardment. Exp Parasitol. 2002;100:95–102. doi: 10.1016/S0014-4894(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 19.Davis RE, Parra A, LoVerde PT, et al. Transient expression of DNA and RNA in parasitic helminths by using particle bombardment. Proc Natl Acad Sci USA. 1999;96:8687–8692. doi: 10.1073/pnas.96.15.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitreva M, McCarter JP, Martin J, et al. Comparative genomics of gene expression in the parasitic and free-living nematodes Strongyloides stercoralis and Caenorhabditis elegans. Genome Res. 2004;14:209–220. doi: 10.1101/gr.1524804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson FJ, Mitreva M, Barker GL, et al. An expressed sequence tag analysis of the life-cycle of the parasitic nematode Strongyloides ratti. Mol Biochem Parasitol. 2005;142:32–46. doi: 10.1016/j.molbiopara.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Kalinna BH, Brindley PJ. Manipulating the manipulators: advances in parasitic helminth transgenesis and RNAi. Trends Parasitol. 2007;23:197–204. doi: 10.1016/j.pt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Vol. 48. San Diego, CA: Academic Press, Inc; 1995. pp. 225–250. [Google Scholar]
- 24.Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang CF, Vanhoven MK, Fetter RD, et al. An innexindependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell. 2007;129:787–799. doi: 10.1016/j.cell.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 26.Bargmann CI. Genetic and cellular analysis of behavior in C. elegans. Annu Rev Neurosci. 1993;16:47–71. doi: 10.1146/annurev.ne.16.030193.000403. [DOI] [PubMed] [Google Scholar]
- 27.Ashton FT, Li J, Schad GA. Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes. Vet Parasitol. 1999;84:297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 28.Pope C, Kim SK, Marzo A, et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Tarleton RL. Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. J Immunol. 2001;166:4596–4603. doi: 10.4049/jimmunol.166.7.4596. [DOI] [PubMed] [Google Scholar]
- 30.Pearce EL, Shedlock DJ, Shen H. Functional characterization of MHC class II-restricted CD8+CD4 and CD8CD4 T cell responses to infection in CD4−/− mice. J Immunol. 2004;173:2494–2499. doi: 10.4049/jimmunol.173.4.2494. [DOI] [PubMed] [Google Scholar]
- 31.Yrlid U, Svensson M, Hakansson A, et al. In vivo activation of dendritic cells and T cells during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2001;69:5726–5735. doi: 10.1128/IAI.69.9.5726-5735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce EJ. An increased understanding of T-cell responses during helminth infections. Crystal Ball: Parasite Immunol. 2006;28:235–269. [Google Scholar]
- 33.Kimble J, Hodgkin J, Smith T, Smith J. Suppression of an amber mutation by microinjection of suppressor tRNA in C. elegans. Nature. 1982;299:456–458. doi: 10.1038/299456a0. [DOI] [PubMed] [Google Scholar]
- 34.Stinchcomb DT, Shaw JE, Carr SH, Hirsh D. Extrachromo-somal DNA transformation of Caenorhabditis elegans. Mol Cell Biol. 1985;5:3484–3496. doi: 10.1128/mcb.5.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fire A. Integrative transformation of Caenorhabditis elegans. EMBO J. 1986;5:2673–2680. doi: 10.1002/j.1460-2075.1986.tb04550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mello C, Kramer JM, Stinchcomb DT, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- 38.Evans TC. Transformation and microinjection (April 6, 2006) WormBook. ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.108.1, < http://www.wormbook.org>.
- 39.Junio AB, Li X, Massey HC, et al. Strongyloides stercoralis: cell-and tissue-specific transgene expression and co-transformation with vector constructs incorporating a common multifunctional 3′ UTR. Exp Parasitol. 2007 doi: 10.1016/j.exppara.2007.08.018. doi: 10.1016/j.exppara.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biewenga JE, Destree OH, Schrama LH. Plasmid-mediated gene transfer in neurons using the biolistics technique. J Neurosci Methods. 1997;71:67–75. doi: 10.1016/s0165-0270(96)00127-6. [DOI] [PubMed] [Google Scholar]
- 41.Beckmann S, Wippersteg V, El-Bahay A, et al. Schistosoma mansoni: germ-line transformation approaches and actin-promoter analysis. Exp Parasitol. 2007;117:292–303. doi: 10.1016/j.exppara.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Wippersteg V, Sajid M, Walshe D, et al. Biolistic transformation of Schistosoma mansoni with 5′ flanking regions of two peptidase genes promotes tissue-specific expression. Int J Parasitol. 2005;35:583–589. doi: 10.1016/j.ijpara.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Pearce EJ, Freitas TC. Reverse genetics and the study of the immune response to schistosomes. Parasite Immunol. 2008;30:215–221. doi: 10.1111/j.1365-3024.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 44.Praitis V. Creation of transgenic lines using microparticle bombardment methods. Methods Mol Biol. 2006;351:93–107. doi: 10.1385/1-59745-151-7:93. [DOI] [PubMed] [Google Scholar]
- 45.Wilm T, Demel P, Koop HU, et al. Ballistic transformation of Caenorhabditis elegans. Gene. 1999;229:31–35. doi: 10.1016/s0378-1119(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 46.Berezikov E, Bargmann CI, Plasterk RH. Homologous gene targeting in Caenorhabditis elegans by biolistic transformation. Nucleic Acids Res. 2004;32:e40. doi: 10.1093/nar/gnh033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarov M, Schneider S, Pozniakovski A, et al. A recombineering pipeline for functional genomics applied to Caenorhabditis elegans. Nat Methods. 2006;3:839–844. doi: 10.1038/nmeth933. [DOI] [PubMed] [Google Scholar]
- 48.Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lok JB. Strongyloides stercoralis: a model for translational research on parasitic nematode biology (February 17, 2007) WormBook. doi: 10.1895/wormbook.1.134.1. ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.134.1, < http://www.wormbook.org>. [DOI] [PMC free article] [PubMed]
- 50.Fire A, Kondo K, Waterston R. Vectors for low copy transformation of C. elegans. Nucl Acids Res. 1990;18:4269–4270. doi: 10.1093/nar/18.14.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fire A, Harrison SW, Dixon D. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene. 1990;93:189–198. doi: 10.1016/0378-1119(90)90224-f. [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka K, Okubo Y, Suzaki T, Ogura T. Analysis of the two p97/VCP/Cdc48p proteins of Caenorhabditis elegans and their suppression of polyglutamine-induced protein aggregation. J Struct Biol. 2004;146:242–250. doi: 10.1016/j.jsb.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Brooks DR, McCulloch R, Coombs GH, Mottram JC. Stable transformation of trypanosomatids through targeted chromosomal integration of the selectable marker gene encoding blasticidin S deaminase. FEMS Microbiol Lett. 2000;186:287–291. doi: 10.1111/j.1574-6968.2000.tb09119.x. [DOI] [PubMed] [Google Scholar]
- 54.Buckner FS, Wilson AJ, Van Voorhis WC. Trypanosoma cruzi: use of herpes simplex virus-thymidine kinase as a negative selectable marker. Exp Parasitol. 1997;86:171–180. doi: 10.1006/expr.1997.4163. [DOI] [PubMed] [Google Scholar]
- 55.Jefferies D, Tebabi P, Le Ray D, Pays E. The ble resistance gene as a new selectable marker for Trypanosoma brucei: fly transmission of stable procyclic transformants to produce antibiotic resistant bloodstream forms. Nucl Acids Res. 1993;21:191–195. doi: 10.1093/nar/21.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee MG, van der Ploeg LH. The hygromycin B-resistance-encoding gene as a selectable marker for stable transformation of Trypanosoma brucei. Gene. 1991;105:255–257. doi: 10.1016/0378-1119(91)90159-9. [DOI] [PubMed] [Google Scholar]
- 57.Donald RG, Roos DS. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc Natl Acad Sci USA. 1993;90:11703–11707. doi: 10.1073/pnas.90.24.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donald RG, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- 59.Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- 60.Mamoun CB, Gluzman IY, Goyard S, et al. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1999;96:8716–8720. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nature Protoc. 2006;1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 62.Miller DM3rd, Niemeyer CJ, Chitkara P. Dominant unc-37 mutations suppress the movement defect of a homeodomain mutation in unc-4, a neural specificity gene in Caenorhabditis elegans. Genetics. 1993;135:741–753. doi: 10.1093/genetics/135.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller DM3rd, Niemeyer CJ. Expression of the unc-4 in Caenorhabditis elegans motor neurons specifies presynaptic input. Development. 1995;121:2877–2886. doi: 10.1242/dev.121.9.2877. [DOI] [PubMed] [Google Scholar]
- 64.Kramer JM, French RP, Park EC, Johnson JJ. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990;10:2081–2089. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leland S., Jr Effect of hygromycin on migrating and adult Strongyloides ransomi in weanling pigs. J Am Vet Med Assoc. 1972;160:58–60. [PubMed] [Google Scholar]
- 66.Allen BG, Weeks DL. Transgenic Xenopus laevis embryos can be generated using phiC31 integrase. Nat Methods. 2005;2:975–979. doi: 10.1038/nmeth814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen ZY, He CY, Meuse L, Kay MA. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11:856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- 68.Plasterk RH, Izsvak Z, Ivics Z. Resident aliens: the Tc1/ mariner superfamily of transposable elements. Trends Genet. 1999;15:326–332. doi: 10.1016/s0168-9525(99)01777-1. [DOI] [PubMed] [Google Scholar]
- 69.Morales ME, Mann VH, Kines KJ, et al. piggyBac transposon mediated transgenesis of the human blood fluke, Schistosoma mansoni. FASEB J. 2007;21:3479–3489. doi: 10.1096/fj.07-8726com. [DOI] [PubMed] [Google Scholar]
- 70.Cohen LS, Mikhli C, Friedman C, et al. Nematode m7GpppG and m3 (2,2,7) GpppG decapping: activities in Ascaris embryos and characterization of C. elegans scavenger DcpS. RNA. 2004;10:1609–1624. doi: 10.1261/rna.7690504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lall S, Friedman CC, Jankowska-Anyszka M, et al. Contribution of trans-splicing, 5′-leader length, cap-poly (A) synergism, and initiation factors to nematode translation in an Ascaris suum embryo cell-free system. J Biol Chem. 2004;279:45573–45585. doi: 10.1074/jbc.M407475200. [DOI] [PubMed] [Google Scholar]
- 72.Higazi TB, Unnasch TR. Intron encoded sequences necessary for trans splicing in transiently transfected Brugia malayi. Mol Biochem Parasitol. 2004;137:181–184. doi: 10.1016/j.molbiopara.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Shu L, Katholi CR, Higazi T, Unnasch TR. Analysis of the Brugia malayi HSP70 promoter using a homologous transient transfection system. Mol Biochem Parasitol. 2003;128:67–75. doi: 10.1016/s0166-6851(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 74.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 75.Farrar JD, Asnagli H, Murphy KM. T helper subset development: roles of instruction, selection, and transcription. J Clin Invest. 2002;109:431–435. doi: 10.1172/JCI15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 77.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunol Rev. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 78.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 79.Reinhardt RL, Kang SJ, Liang HE, Locksley RM. T helper cell effector fates - who, how and where? Curr Opin Immunol. 2006;18:271–277. doi: 10.1016/j.coi.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Finkelman FD, Shea-Donohue T, Goldhill J, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 81.Grencis RK, Bancroft AJ. Interleukin-13: a key mediator in resistance to gastrointestinal-dwelling nematode parasites. Clin Rev Allergy Immunol. 2004;26:51–60. doi: 10.1385/CRIAI:26:1:51. [DOI] [PubMed] [Google Scholar]
- 82.Pennock JL, Grencis RK. The mast cell and gut nematodes: damage and defence. Chem Immunol Allergy. 2006;90:128–140. doi: 10.1159/000088885. [DOI] [PubMed] [Google Scholar]
- 83.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maizels RM, Balic A, Gomez-Escobar N, et al. Helminth parasites - masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 85.Anthony RM, Urban JF, Jr, Alem F, et al. Memory T (H) 2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayes KS, Bancroft AJ, Grencis RK. Immune-mediated regulation of chronic intestinal nematode infection. Immunol Rev. 2004;201:75–88. doi: 10.1111/j.0105-2896.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 87.Bancroft AJ, Else KJ, Sypek JP, Grencis RK. Interleukin-12 promotes a chronic intestinal nematode infection. Eur J Immunol. 1997;27:866–870. doi: 10.1002/eji.1830270410. [DOI] [PubMed] [Google Scholar]
- 88.Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 89.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 90.Weaver CT, Harrington LE, Mangan PR, et al. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 91.Cua DJ, Kastelein RA. TGF-β, a ‘double agent’ in the immune pathology war. Nat Immunol. 2006;7:557–559. doi: 10.1038/ni0606-557. [DOI] [PubMed] [Google Scholar]
- 92.Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 93.Driscoll M, Tavernarakis N. Molecules that mediate touch transduction in the nematode Caenorhabditis elegans. Gravit Space Biol Bull. 1997;10:33–42. [PubMed] [Google Scholar]
- 94.Garriga G, Guenther C, Horvitz HR. Migrations of the Caenorhabditis elegans HSNs are regulated by egl-43, a gene encoding two zinc finger proteins. Genes Dev. 1993;7:2097–2109. doi: 10.1101/gad.7.11.2097. [DOI] [PubMed] [Google Scholar]
- 95.Thompson BE, Lamont LB, Kimble J. Germ-line induction of the Caenorhabditis elegans vulva. Proc Natl Acad Sci USA. 2006;103:620–625. doi: 10.1073/pnas.0510264103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ashton FT, Bhopale VM, Fine AE, Schad GA. Sensory neuroanatomy of a skin-penetrating nematode parasite: Strongyloides stercoralis. I. Amphidial neurons. J Comp Neurol. 1995;357:281–295. doi: 10.1002/cne.903570208. [DOI] [PubMed] [Google Scholar]
- 97.Fine AE, Ashton FT, Bhopale VM, Schad GA. Sensory neuroanatomy of a skin-penetrating nematode parasite Strongyloides stercoralis. II. Labial and cephalic neurons. J Comp Neurol. 1997;389:212–223. [PubMed] [Google Scholar]
- 98.Ashton FT, Bhopale VM, Holt D, et al. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. J Parasitol. 1998;84:691–695. [PubMed] [Google Scholar]
- 99.Lopez PM, Boston R, Ashton FT, Schad GA. The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis. Int J Parasitol. 2000;30:1115–1121. doi: 10.1016/s0020-7519(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 100.Nolan TJ, Brenes M, Ashton FT, et al. The amphidial neuron pair ALD controls the temperature-sensitive choice of alternative developmental pathways in the parasitic nematode, Strongyloides stercoralis. Parasitology. 2004;129:753–759. doi: 10.1017/s0031182004006092. [DOI] [PubMed] [Google Scholar]
- 101.Forbes WM, Ashton FT, Boston R, et al. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet Parasitol. 2004;120:189–198. doi: 10.1016/j.vetpar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Ashton FT, Zhu X, Boston R, et al. Strongyloides stercoralis: amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Exp Parasitol. 2007;115:92–97. doi: 10.1016/j.exppara.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harbinder S, Tavernarakis N, Herndon LA, et al. Genetically targeted cell disruption in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1997;94:13128–13133. doi: 10.1073/pnas.94.24.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science. 1989;243:1027–1033. doi: 10.1126/science.2646709. [DOI] [PubMed] [Google Scholar]
- 105.Bulina ME, Chudakov DM, Britanova OV, et al. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 106.Ren P, Lim CS, Johnsen R, et al. Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]