Abstract
Background
Cancer-related fatigue and insomnia are common distressing symptoms and may affect mood and performance status.
Objective
to describe fatigue, sleep, pain, mood and performance status and the relationships among these variables in 187 patients newly diagnosed with multiple myeloma (MM) and conduct an analysis using the correlates of fatigue.
Interventions/Methods
Data were from baseline measures from the study, using the Profile of Mood States (POMS) and the Functional Assessment of Cancer Therapy - Fatigue to assess fatigue, the Actigraph to measure sleep, the Wong/Baker Faces Pain Rating Scale to assess pain, the POMS to assess mood, and the 6-minute walk test along with a back/leg/chest dynamometer to test muscle strength to assess performance status. Data analysis consisted of descriptive statistics, Pearson and Spearman rho correlations and multiple regression using fatigue as the dependent variable. All p values were two-sided, and those with < .05 considered significant.
Results
Patients newly diagnosed with MM presented with fatigue, pain, sleep and mood disturbances, and diminished functional performance. The regression model, which included all of these variables along with age, gender and stage of disease, was statistically significant with a large measure of effect. Mood was a significant individual contributor to the model.
Conclusions
Among patients with MM, fatigue, pain, sleep, mood and functional performance are interrelated.
Implications for Practice
Interventions are needed to decrease fatigue and pain and to improve sleep, mood and functional performance.
Multiple myeloma (MM), a cancer of the plasma cell, is an incurable but treatable disease. Symptoms include fatigue, bone pain, recurrent infections and renal failure that result from plasma cells crowding the bone marrow, direct tumor mass effects, activation of osteoclasts and inactivation of osteoblasts leading to osteolytic bone destruction, high levels of monoclonal immunoglobulins not effective in immune function, and renal insufficiency from hypercalcemia and deposits of immunoglobulins or amyloids. In the U.S., approximately 20,580 individuals were diagnosed with and 10,580 people died of MM in 2009.1 More men than women and more African-Americans than Caucasians are affected and the median age at diagnosis is approximately 71 years.2 Several promising, new therapies are helping patients live longer, healthier lives. Patients with MM often receive intensive multidisciplinary treatment and experience multi-organ complications as a result of their disease and treatment. At least 60% of patients with MM are anemic [hemoglobin (Hb) < 12 g/dL] at diagnosis, and nearly all become anemic during treatment.3 Therefore, fatigue continues to be a distressing symptom. Fatigue can lead patients to abandon treatment, and can be so overwhelming that some patients say they would rather die.4
Sleep-wake disturbances are common among people with cancer5 and insomnia is frequently related to fatigue in patients with cancer.6–12 Inadequate sleep may contribute to the development of mood disorders and depressive symptoms.13 Increased daytime sleep and fatigue may decrease daytime physical activity which may lead to physiologic deconditioning and diminished activity tolerance.14
Bone pain is the most common presenting symptom of multiple myeloma with approximately 70% of patients presenting with pain from lytic bone lesions and pathologic fractures which limits physical activity.15 Increased pain is associated with increased mood disturbance in patients with multiple myeloma.16 Among patients with lung cancer, the symptom cluster of pain, fatigue and insomnia showed significant interaction among the three symptoms.17 The purpose of this paper is to describe fatigue, sleep, pain, mood and performance status and the relationships among these variables in patients newly diagnosed with MM and to conduct an analysis using the correlates of fatigue. Data were baseline measures from our exercise intervention study.
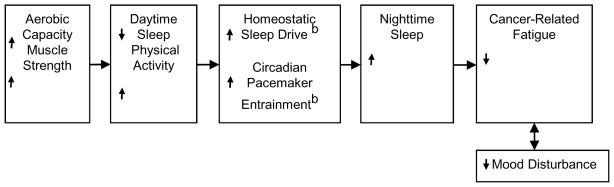
Conceptual Model
Figure 1 displays the conceptual model depicting the main study variables and covariates for the cross sectional analysis of the baseline data from our exercise intervention study. Our model proposes that sleep is regulated by two processes. First is a homeostatic process in which either curtailed sleep augments sleep propensity or excess sleep reduces sleep propensity. A circadian process is the second of the two processes and involves a clock-like mechanism with alternating periods of high and low sleep propensity.18 The homeostatic process rises during waking, declines during sleep, and interacts with the circadian process with excessive daytime sleep adversely affecting the homeostatic process.18 The primary synchronizer for the circadian pacemaker is light, but physical activity also exerts an effect.19–21 Thus, increased daytime sleep and physical inactivity may harm both the homeostatic sleep drive and entrainment of the circadian rhythm in patients with cancer, leading to decreased nighttime sleep. Increased aerobic capacity and muscle strength lead to increased physical activity, thereby strengthening circadian pacemaker entrainment. In addition, the increased physical activity will decrease daytime sleep, thereby increasing the homeostatic sleep drive. Increased nighttime sleep leads to improved mood and decreased cancer related fatigue. Pain can decrease physical activity thus negatively affecting performance status, mood and sleep, resulting in increased fatigue.
Figure 1. Conceptual Model Depicting Main Study Variables and Covariatesa.
a Pain will most likely disrupt the entire cycle. It can decrease physical activity thus negatively affecting performance status, mood and sleep, resulting in increased fatigue.
b Not measured in this study
METHODS
Setting and Sample
The study site was an international referral center for patients with MM. The study population included patients who were newly diagnosed with MM and eligible for treatment with an intensive chemotherapy treatment protocol for MM that includes tandem peripheral stem cell transplants. As patients decided to enroll in the treatment protocol for MM, they were assessed for inclusion in the exercise intervention trial. Those who were not at high risk for impending pathologic fracture or cord compression, as determined by magnetic resonance imaging and other radiology reports and physician assessments, were invited to participate in the exercise study. Patients were excluded if they showed any of the following attributes/conditions: inability to understand the intent of the study, current diagnosis with a major psychiatric illness, presence of microcytic or macrocytic anemia, uncontrolled hypertension, red cell transfusions within two weeks, and recombinant epoetin alfa within eight weeks. The last four criteria were necessary because patients received prophylactic recombinant epoetin alfa as part of the research study. We collected baseline (before beginning chemotherapy) assessments on hemoglobin, fatigue, sleep, pain, mood and performance status. Performance status as defined for this study consisted of aerobic capacity and muscle strength.
Instruments/Measurements
Profile of Mood States
We used the Profile of Mood States (POMS – EdITS/Educational and Industrial Testing Service, San Diego, CA) to assess fatigue and mood. The POMS fatigue-inertia adjective rating scale assesses fatigue. Five other rating scales assess depression-dejection, tension-anxiety, anger-hostility, vigor-activity and confusion-bewilderment. Subtracting the vigor-activity score from the sum of the other scores yields a total mood disturbance score (TMDS). The possible range of scores for the TMDS is −40 through 192 with higher scores indicating greater mood disturbance. Examination of the individual items defining each mood scale supports content validity, and four areas of research have provided evidence of the predictive and construct validity of the POMS.22 All the internal consistency reliabilities are highly satisfactory, ranging from 0.87–0.95.22
Several researchers have established Cronbach’s alpha for the POMS total mood disturbance in patients with cancer at 0.90–0.96.23–25 Test-retest coefficients range from 0.65–0.74.22 We asked patients to complete the 65-item version of the form with the following instructions: “Below is a list of words that describe feelings people have. Please read each one carefully, then fill in one circle under the answer to the right which best describes how you have been feeling during the past week, including today.” Most subjects completed the POMS in 3–5 minutes.
Functional Assessment of Cancer Therapy - Fatigue (FACT-F)
We used the Functional Assessment of Cancer Therapy - Fatigue (FACT-F) as an additional measure of fatigue. The FACT-F questionnaire identifies patients with chronic disease whose quality of life is affected by fatigue. The FACT-F has demonstrated good stability (r = .87) and strong internal consistency (alpha = .95). Test-retest reliability coefficients showed good stability (r = .84 to .90).26, 27 The 13-item form asks patients to respond using a Likert-type scale to describe their status during the past seven days. Scores range from 0–52 with higher scores, indicating less fatigue. A raw score of 43 was 92% sensitive and 69% specific for distinguishing anemic cancer patients from the general population with 84% overall accuracy.28 FACT-F scores correlate well with physical function scores and researchers found when the scores are 45–52, fewer than 10% of subjects report they were “very limited” in climbing several flights of stairs compared to more than 80% being impaired in stair climbing when their FACT-F scores were less than 15.29
Actigraphy
Nighttime sleep and daytime sleep were measured using the Actigraph (Ambulatory Monitoring, Ardsley, NY), a device worn on the wrist, similar in size and shape to a large wristwatch, that senses and stores physical motion. The Actigraph signals were processed on-line, and stored data were transferred to a computer for display, interpretation and conversion into sleep parameters. The Actigraph has minute-by-minute agreement of 85–95% between activity-based sleep-wake scoring and traditional polysomnography-based scoring in healthy individuals.30 Additional sleep variables were collected for descriptive purposes. They included frequency of awakenings and percent time asleep while in bed at night (sleep efficiency). Patients wore the Actigraph for 72 hours to decrease the effect of random variance, and they recorded their bedtimes and rise-times on a log during the 3-day measurement periods. Down intervals (nighttime sleep) were determined by using the Custom Interval Boundary to select peak epochs for downtime and rise-time.
A 3-day measurement period allowed us to control for any unusual daily variations resulting from interpersonal or environmental factors. The Actigraph translates body movement into an electrical signal which a microprocessor records and stores in solid-state memory. We downloaded the activity data into a personal computer for display and analysis and analyzed the activity data using Action 3.31 software (Ambulatory Monitoring, Inc).
Wong/Baker Faces Rating Scale
The Wong/Baker Faces Rating Scale is an established valid and sensitive measure of pain intensity. Using the 0–10 version of the Wong/Baker Pain Rating Scale with six faces and, with 0 = “no hurt” and 10 = “hurts worse” we asked patients to rate their pain before they took the six-minute walk test and we measured their muscle strength.
Six-minute Walk Test
Aerobic capacity was assessed with the 6-minute walk test which is a simple procedure that measures the distance covered by walking on a level surface in 6 minutes. Since it is a submaximal exercise test, it is associated with much smaller increments in heart rate, blood pressure and plasma catecholamines than cardiopulmonary exercise testing. Further, this test is characterized by very small intra-individual variance, and the results are closely related to peak VO2.31–34 The test is well accepted by patients and reflects their daily activity better than more strenuous classic tests.35 Its noninvasive nature, zero cost, and lack of need for special equipment or place to perform, make the 6-minute walk test a theoretically satisfactory method of evaluating the exercise capacity of patients with MM. Hamilton and Haennel tested the 6-minute walk test in a cardiac rehabilitation population. They found that the test was linearly related to maximum metabolic equivalents (METs) (r = 0.687, p < .001), supporting its validity; and the 6-minute walk test had strong test-retest reliability (intraclass correlation = .97).36 In a nonrandomized pilot study of older individuals with mobility impairment, the intraclass correlation coefficient for test-retest reliability was .93 for the 6-minute walk test.37 The 6-minute walk test was performed in a level hallway in the treatment facility and administered by a research assistant blinded to the patients’ group assignment. The patients were instructed to cover the greatest distance possible during the allotted time, at a self-determined walking speed, pausing to rest as needed. We told patients to stop if they developed pain or became short of breath or dizzy when walking. We used a stopwatch to time the test and a manual counter to record the number of lengths in the hall the patient completes. The distance covered in feet was the number of hall lengths the patient walks, multiplied by the length in feet (110 ft.) of the hall. At the end of the test, we recorded the distance covered and the patient’s heart rate and rate of perceived exertion (RPE), using the BORG scale. The correlation between perceived exertion and target heart rate is helpful in evaluating the patient’s aerobic capacity.
Dynamometer Measurement of Muscle Strength
Since muscle strength affects performance status, a research assistant blinded to the patient’s group assignment conducted muscle strength tests. Patients without lifting restrictions were tested for muscle strength using a back/leg/chest dynamometer, which is highly correlated (r = 0.77, p <.001) with manual muscle tests.38 Designed to measure the isometric force produced by the musculature of the legs, arms, shoulders and upper back, the device is widely used in physical therapy settings. The dynamometer measures static strength. An external force applied to the dynamometer compresses a steel spring and moves a pointer. Knowing the force required to move the pointer a particular distance, one can determine exactly how much external force has been applied to the dynamometer.38 The dynamometer thus provides a measure of the force the patient generates while performing a standing extension of the torso. The range of measurement is 0 to 660 pounds in 10-pound increments, and the accuracy at 220 pounds is reported to be + 1.1 pounds.
The patient was asked to assume approximately a 30-degree flexion of the knees and flexion of the hips while maintaining an appropriate lordotic curve, and then told to pull up on the dynamometer using the major muscle groups of the legs and arms.39 The average of two strength measurements at each testing period separated learning effects from training effects. Patient safety during testing was assured because the strength measurement involves only gradual application of force. The patient was in complete control of the amount of force applied, and the instructions included a caution to the patient to pull only as hard as he or she could without causing discomfort. Patients wore low heeled shoes for the test.
Data Analysis
Data analysis consisted of descriptive statistics, Pearson and Spearman rho correlations as indicated, and multiple regression using fatigue as the dependent variable. All p-values were two-sided, and those with < .05 considered significant.
RESULTS
The sample (n = 187) was primarily Caucasian (n = 169, 90%) with an average age of 56 years (SD 10) and a higher percentage of males (n = 109, 58%). Using the International Staging System, the majority of the sample was stage I (n = 127, 68%) with 38 (20%) stage II and 22 (12%) stage III.40 Table 1 gives the study outcomes by gender.
Table 1.
Study Variables by Gender
| Variable | Males | Females | p values |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age | 56.0 (10.1) | 56.4 (9.6) | .82 |
| Hemoglobin | 11.9 (1.8) | 11.2 (1.7) | .009a |
| Fatigue (FACT) | 38.7 (9.6) | 32.1 (12.8) | .000b |
| Pain rating | 1.4 (1.9) | 1.8 (2.1) | .15 |
| POMS Total Mood Disturbance score | 18.4 (31) | 31.3 (34) | .008a |
| Pounds lifted | 204 (99.3) | 73.2 (39.5) | .000b |
| Feet walked in six minutes | 1710 (428) | 1452 (369) | .000b |
| Hours of nighttime sleep | 6.6 (1.7) | 7.3 (1.5) | .007a |
| % (SD%) | % (SD%) | ||
| Sleep efficiencyc (%) | 78 (16) | 84 (10) | .007a |
| % (n) | % (n) | X2p =.46 | |
| ISS Disease Stage | |||
| I | 67 (73) | 69 (54) | |
| II | 23 (25) | 17 (13) | |
| III | 10 (11) | 14 (11) | |
Abbreviations: SD, Standard Deviation.
p< .01
p< .005
Sleep efficiency=Percent of time asleep while in bed at night
N = 178 to 187 for all variables except for “pounds lifted” as only 88 patients were without lifting restrictions
Baseline Hb levels were similar for males and females. However, a greater percentage of females were anemic before starting chemotherapy than males in that 70% of females had an Hb level below 12 g/dL compared to 45% of males having a Hb level below 12 g/dL.
Patients presented with fatigue with mean FACT-F scores of 32.1 for females and 38.7 for males. Severe fatigue was present at baseline with 36/187 (19.3%) patients having a FACT-F score < 25. Fatigue was more of a problem for the females with 30.8% (24/78) of them having scores < 25. Fatigue scores from the FACT-F and the POMS-F were highly correlated (Pearson r = −0.84,), only FACT-F scores were used in the regression model for fatigue.
Mean sleep onset latency was 29 minutes (SD = 38.9), mean nocturnal sleep time was 414 minutes (6.9 hours) (SD = 98.5), mean percent of nighttime sleep while in bed was 80% (SD = 14.2), and the mean number of wake episodes was 12 times (SD = 5.7). The mean number of daytime sleep episodes was 10 (SD = 10), the mean total daytime sleep time was 98 minutes (1.6 hours) (SD = 98.6), and the mean total sleep time (per 24 hours) was 512 minutes (8.5 hours) (SD = 157.1). Although the average total sleep time appears adequate, nocturnal sleep time comprises only 81% while daytime sleep time accounts for 19% of the 24-hour total. Further, there was much individual variability in sleep among the sample as indicated by the large standard deviations. Some patients were sleeping only one to four hours at night. Five patients (2.8%) were sleeping less than four hours at night. Average sleep efficiency (mean per cent of nighttime sleep while in bed) differed for males and females with the males having less than the desired 85% sleep efficiency.41
Since pain was not normally distributed, the Spearman rho statistic was used for all correlations between pain and the other study variables. Pain was moderately correlated with fatigue as measured by the FACT-F (rs = −.33), with the total mood disturbance score (rs =.25) and with the performance status measurements of the six-minute walk test (rs = −.36) and the dynamometer measurement of muscle strength (rs = −.37). Pain was minimally correlated with sleep efficiency (rs= −.12).
Mean POMS TMDS was 31.3 (SD = 34) for females and 18.4 (SD = 31) for males indicating mood disturbance. There was high variability among the individual patients as 26 (13.9%) patients with very high mood disturbance scores (> 50.7 TMDS) needed immediate psychological intervention.42, 43 Fatigue from the FACT-F and mood disturbance from the POMS TMDS were highly correlated (Pearson r = −.70,).
The performance status measurement using the 6-minute walk test and strength tests scores showed great individual variability with some patients not able to walk or lift any weight while others showed high performance on both tests. The 6-minute walk test and strength test scores were correlated (Pearson r = .57,). Table 2 shows the correlation matrix with all the study variables.
Table 2.
Correlations of Study Variables (Pearson r for all variables except Spearman Rho r2 or pain rating and ISS Stage of Disease)
| Age | Hemoglobin | Fatigue | Pain | Mood Disturbance | Pounds Lifted | Distance walked | Night Time Sleep | Sleep Efficiency | ISS Stage of Disease | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age r | 1 | −.112 | −.088 | .021 | .013 | −.078 | −.159a | −.154a | −.255b | .251 b |
| Sig. (2-tailed) | .125 | .230 | .772 | .862 | .470 | .030 | .040 | .001 | .001 | |
| N | 187 | 187 | 187 | 187 | 187 | 88 | 186 | 178 | 178 | 187 |
| Hemoglobin r | −.112 | 1 | .193b | .069 | −.120 | .023 | .096 | −.032 | −.014 | −.462b |
| Sig. (2-tailed) | .125 | .008 | .349 | .103 | .835 | .194 | .668 | .856 | .000 | |
| N | 187 | 187 | 187 | 187 | 187 | 88 | 186 | 178 | 178 | 187 |
| Fatigue (FACT) r | −.088 | .193b | 1 | −.320b | −.703b | .355b | .411b | −.025 | .148a | −.219b |
| Sig. (2-tailed) | .230 | .008 | .000 | .000 | .001 | .000 | .742 | .048 | .003 | |
| N | 187 | 187 | 187 | 187 | 187 | 88 | 186 | 178 | 178 | 187 |
| Pain r2 | .006 | .035 | −.326b | 1.000 | .273b | −.329b | −.385b | −.091 | −.174a | −.029 |
| Sig. (2-tailed) | .936 | .630 | .000 | . | .000 | .002 | .000 | .229 | .020 | .691 |
| N | 187 | 187 | 187 | 187 | 187 | 88 | 186 | 178 | 178 | 187 |
| Mood Disturbance r | .013 | −.120 | −.703b | .250b | 1 | −.319b | −.238b | −.034 | −.071 | .166a |
| Sig. (2-tailed) | .862 | .103 | .000 | .001 | .002 | .001 | .651 | .344 | .023 | |
| N | 187 | 187 | 187 | 187 | 187 | 88 | 186 | 178 | 178 | 187 |
| Pounds Lifted r | −.078 | .023 | .355b | −.372b | −.319b | 1 | .565b | −.021 | −.027 | −.025 |
| Sig. (2-tailed) | .470 | .835 | .001 | .000 | .002 | .000 | .847 | .806 | .820 | |
| N | 88 | 88 | 88 | 88 | 88 | 88 | 88 | 86 | 86 | 88 |
| Distance Walked | −.159 a | .096 | .411b | −.363b | −.238b | .565b | 1 | .081 | .239b | −.144 |
| Sig. (2-tailed) | .030 | .194 | .000 | .000 | .001 | .000 | .282 | .001 | .051 | |
| N | 186 | 186 | 186 | 186 | 186 | 88 | 186 | 178 | 178 | 186 |
| Nighttime Sleep r | −.154a | −.032 | −.025 | −.075 | −.034 | −.021 | .081 | 1 | .740b | −.189a |
| Sig. (2-tailed) | .040 | .668 | .742 | .322 | .651 | .847 | .282 | .000 | .012 | |
| N | 178 | 178 | 178 | 178 | 178 | 86 | 178 | 178 | 178 | 178 |
| Sleep Efficiencyc | −.255b | −.014 | .148a | −.123 | −.071 | −.027 | .239b | .740b | 1 | −.200b |
| Sig. (2-tailed) | .001 | .856 | .048 | .101 | .344 | .806 | .001 | .000 | .007 | |
| N | 178 | 178 | 178 | 178 | 178 | 86 | 178 | 178 | 178 | 178 |
| ISS Stage of Disease r2 | .256b | −.441b | −.226b | −.029 | .153a | −.033 | −.162a | −.185a | −.223b | 1.000 |
| Sig. (2-tailed) | .000 | .000 | .002 | .691 | .036 | .760 | .027 | .014 | .003 | . |
| N | 187 | 187 | 187 | 187 | 187 | 88 | 186 | 178 | 178 | 187 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Sleep efficiency=Percent of time asleep while in bed at night.
The regression analysis with FACT-F as the dependent variable and age, gender, stage of disease, hemoglobin at baseline, nocturnal sleep time, sleep efficiency, pain rating, POMS TMDS, 6-minute walk test, and strength test as the independent variables in the model showed the POMS total mood disturbances score as an individual significant contributor to the model. The model, which includes all of the variables, is statistically significant (p<.0005). The measure of effect is adjusted R2 =.58. According to Cohen, this is a large effect (0.01 is the minimum for a small effect, 0.09 for a moderate effect, and 0.25 for a large effect).44 Table 3 shows the regression results.
Table 3.
Summary of Multiple Regression Analysis for Variables Predicting Total FACT Scores
| Variable | Total FACT Scores |
|
|---|---|---|
| B | 95% CI | |
| Constant | 27.84c | [11.97, 43.71] |
| Gender | 2.54 | [−0.98, 6.06] |
| Age | −0.01 | [−0.13, 0.12] |
| Hemoglobin | 0.31 | [−0.40, 1.02] |
| Pain Rating | −0.36 | [−1.00, 0.27] |
| POMS Total Mood Disturbance | −0.22d | [−0.25, −0.18] |
| Pounds lifted (n=88) | −0.002 | [−0.02, 0.02] |
| Feet walked in six minutes | 0.004a | [0.001, 0.01] |
| Nighttime Sleep Hours | −1.51b | [−2.54, −0.47] |
| Daytime Sleep in Minutes | −0.004 | [−0.16, 0.01] |
| Sleep Efficiencye | 0.19b | [0.059, 0.31] |
| ISS Stage of Disease | −1.06 | [−3.02, 0.89] |
| R2 (adj.)f | 0.61 (0.58) | |
| F | 23.37d | |
Note. N=178. CI=confidence interval.
p ≤.05
p ≤.01
p ≤.001
p ≤.0005
Sleep efficiency=Percent of time asleep while in bed at night
Adjusted R2 adjusting for the number of variables in the model
CONCLUSIONS
Patients newly diagnosed with MM presented with fatigue, sleep and mood disturbances, and diminished functional performance. Over half the patients were anemic before starting chemotherapy and this is known to contribute to fatigue, affecting their quality of life. Severe fatigue and anemia were more prevalent among females than males. Sleep disturbances included frequent awakenings at night and increased daytime sleep with almost 1/5th of total sleep time occurring during the day. Increased sleep efficiency was associated with less fatigue. Increased mood disturbance was associated with more fatigue. Decreased aerobic capacity as indicated by the 6-minute walk test was associated with more fatigue. Increased muscle strength may improve aerobic capacity. Interventions are needed to decrease fatigue, improve sleep and functional performance. Pain needs to be alleviated as pain can decrease physical activity thus negatively affecting performance status, mood and sleep, resulting in increased fatigue.
DISCUSSION
Cancer-related fatigue remains poorly defined, but there is general agreement that it is multidimensional, subjective, perceived as abnormal and distressing, and inadequately relieved by rest.4, 7, 12 The National Comprehensive Cancer Network (NCCN) definition further states that cancer-related fatigue impairs usual functioning.45 The incidence of cancer-related fatigue varies with diagnosis and treatment regimens, with estimates for some disease/treatment combinations as high as 100%.46 Fatigue levels may vary within the individual patient and between individuals.47 Among the female participants in our study, almost 31% of them reported fatigue at baseline. Cancer-related fatigue leads to decreased activity which results in physiologic deconditioning, which, in turn, diminishes activity tolerance.48 The result of this downward spiral can be loss of physical function and chronic fatigue that may persist for months or even years after successful cancer treatment.11, 12, 49–51 Our findings support this association between fatigue and physical activity and between physiologic deconditioning and activity tolerance.
Adults with cancer may have disturbed sleep. In a study of 2,646 patients with cancer, 39% reported insomnia.52 Patients with cancer not only may experience decreased nighttime sleep and increased daytime sleep but may experience rapid eye movement (REM) sleep during afternoon sleep, which is considered abnormal.53 Although participants in our study had an adequate average total sleep time in 24 hours; some only had one to four hours of nighttime sleep and 19% of the 24-hour sleep total was daytime sleep.
Accurate assessment of sleep disturbance is a critical starting point for clinicians.
Polysomnography is the preferred sleep assessment modality because simply seeking a verbal report of whether patients have problems sleeping is inadequate.54 Actigraphy, a commonly used research tool based on measurement of movement and/or activity, is an objective, less costly, and less cumbersome sleep measurement device compared with polysomnography, but it cannot provide information about REM or other sleep stages and wave patterns. Neither polysomnography nor Actigraphy may be practical in everyday use by clinicians for patient care; thus, most will continue to rely on patient report via interview or questionnaires.
People with cancer may experience pain from their disease process. Fatigue, pain, and decreased functional status may be present concurrently and levels differ according to disease or treatment.55 Cancer pain may interrupt or delay sleep and increase fatigue, however pain may be mediated by sleep problems and both pain and sleep problems may mediate fatigue and lead to daytime sleepiness.56 In contrast, we found no significant correlations between sleep variables and pain. Both consistent pain and breakthrough pain may be associated with decreased function; and breakthrough pain is associated with depression.57 Our findings are similar in that fatigue, mood disturbance and performance status were moderately correlated with pain. Results using pain in our study need to be interpreted with the knowledge that our sample probably is not representative of all patients with multiple myeloma. We could not include patients with, or at high risk of, pathologic fracture and bone pain is the main source of pain with multiple myleoma. Psychosocial factors such as mood may also influence sleep and fatigue in people with cancer. In our study, baseline POMS scores indicated mood disturbance, including some very high scores. Our findings and those of others54 indicate that disturbed mood is strongly associated with fatigue severity. Depression and anxiety are associated with cancer-related fatigue, but may not predict it.58, 59 It is important for clinicians to recognize baseline mood disturbance because cancer treatment may compound mood problems. Mood changes and fatigue may result from medications used to treat MM such as thalidomide and steroids.60, 61 Patients have reported stress from steroid-related mood alterations, feeling depressed while needing to stay busy, and a desire to have less interaction with others.62
Good performance status is often one of the eligibility requirements for treatment of MM and other cancers. Patients with MM are at risk for decreased performance status at the time of diagnosis as well as during treatment. Those with low performance status may go untreated and those who are treated may face increasing performance status problems during treatment related to altered hematopoietic function and effects of medications. We found great variability in performance among participants in this study, including some individuals who were unable to walk or to lift any weight at baseline. Our findings as well as those of others indicate that performance status may be inversely associated with fatigue. 59–63
Fatigue and muscle wasting occur in 50% of patients with MM.64 Close to 100% of patients receiving intensive treatment experience fatigue. Thus, patients with MM are susceptible to virtually all treatment-associated complications that other patients with cancer experience, including cancer-related insomnia and cancer-related fatigue. Patients undergoing stem cell transplant treatment for MM are especially at high risk for developing both acute (during treatment) and chronic (post treatment) fatigue and functional deficits. 65
Research has shown that exercise can decrease patients’ fatigue and improve physical performance and psychological outlook.66–69 Individualized, moderate prescriptive exercise may lead to decreased fatigue which may then improve quality of life.70 Fatigue, mood, and functional performance may improve with a comprehensive exercise program incorporating low-to-moderate intensity aerobic and resistance exercise, education classes, and support from peers and cancer exercise program specialists.71 An individualized comprehensive exercise program may then address the holistic care needs of the patient with cancer-related fatigue, including fatigue, sleep and mood disturbances, and decreased performance status. Our analysis of baseline data from our exercise intervention study showed that fatigue, pain, sleep, mood and functional performance are interrelated. Our randomized trial of an individualized exercise program is designed to show how these variables are interrelated.
Acknowledgments
National Institutes of Nursing Research (NIH) [grant #R01 NR008937-01A1]
Ortho Biotech Clinical Affairs, LLC, Bridgewater, NJ, USA
References
- 1.American Cancer Society. [Accessed July 16, 2010];What are the Key Statistics About Multiple Myeloma. 2010 http://www.cancer.org/Cancer/MultipleMyeloma/DetailedGuide/multiple-myeloma-key-statistics.
- 2.Zaidi AA, Vesole DH. Multiple myeloma: an old disease with new hope for the future. CA Cancer J Clin. 2001;51(5):273–285. doi: 10.3322/canjclin.51.5.273. quiz 286–279. [DOI] [PubMed] [Google Scholar]
- 3.Coleman EA, Coon SK, Kennedy RL, et al. Effects of exercise in combination with epoetin alfa during high-dose chemotherapy and autologous peripheral blood stem cell transplantation for multiple myeloma. Oncol Nurs Forum. 2008;35(3):E53–61. doi: 10.1188/08.ONF.E53-E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 5.Vena C, Parker K, Cunningham M, Clark J, McMillan S. Sleep-wake disturbances in people with cancer part I: an overview of sleep, sleep regulation, and effects of disease and treatment. Oncol Nurs Forum. 2004;31(4):735–746. doi: 10.1188/04.ONF.735-746. [DOI] [PubMed] [Google Scholar]
- 6.Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 1999;26(10):1663–1671. [PubMed] [Google Scholar]
- 7.Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: a pilot study. Oncol Nurs Forum. 2000;27(9):1443–1448. [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 9.Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998;16(5):1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage. 1999;18(4):233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 11.Okuyama T, Akechi T, Kugaya A, et al. Factors correlated with fatigue in disease-free breast cancer patients: application of the Cancer Fatigue Scale. Support Care Cancer. 2000;8(3):215–222. doi: 10.1007/s005200050288. [DOI] [PubMed] [Google Scholar]
- 12.Portenoy RK. Cancer-related fatigue: An immense problem. Oncologist. 2000;5(5):350–352. doi: 10.1634/theoncologist.5-5-350. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl) 2001;10(4):245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum. 1994;21(1):23–36. [PubMed] [Google Scholar]
- 15.Grethlein SJ, Thomas LM. [Accessed July 16, 2010];Multiple Myeloma. emedicine from WebMD; http://emedicine.medscape.com/article/204369-overview.
- 16.Poulos AR, Gertz MA, Pankratz VS, Post-White J. Pain, mood disturbance, and quality of life in patients with multiple myeloma. Oncol Nurs Forum. 2001;28(7):1163–71. [PubMed] [Google Scholar]
- 17.Hoffman AJ, Given BA, von Eye A, et al. Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum. 2007;34(4):785–92. doi: 10.1188/07.ONF.785-792. [DOI] [PubMed] [Google Scholar]
- 18.Borbely A. Sleep homeostasis and models of sleep regulation. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. Philadelphia: WB Saunders; 2000. pp. 377–390. [Google Scholar]
- 19.Bliwise D. Sleep and circadian rhythm disorders in aging and dementia. In: Turek F, Zee P, editors. Regulation of sleep and circadian rhythms. 1999. pp. 487–525. [Google Scholar]
- 20.Czeisler CA, Khalsa S. The human circadian timing system and sleep-wake regulation. In: King M, Roth T, Dement W, editors. Principles and practice of sleep machine. Philadelphia: WB Saunders; 2000. pp. 353–375. [Google Scholar]
- 21.Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF, 3rd, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;274(4 Pt 2):R991–996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- 22.McNair D, Lorr M, Droppleman L. EITS Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 23.Cella DF, Jacobsen PB, Orav EJ, Holland JC, Silberfarb PM, Rafla S. A brief POMS measure of distress for cancer patients. J Chronic Dis. 1987;40(10):939–942. doi: 10.1016/0021-9681(87)90143-3. [DOI] [PubMed] [Google Scholar]
- 24.Classen C, Koopman C, Angell K, Spiegel D. Coping styles associated with psychological adjustment to advanced breast cancer. Health Psychol. 1996;15(6):434–437. doi: 10.1037//0278-6133.15.6.434. [DOI] [PubMed] [Google Scholar]
- 25.Richardson MA, Post-White J, Grimm EA, Moye LA, Singletary SE, Justice B. Coping, life attitudes, and immune responses to imagery and group support after breast cancer treatment. Altern Ther Health Med. 1997;3(5):62–70. [PubMed] [Google Scholar]
- 26.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13–19. [PubMed] [Google Scholar]
- 27.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 28.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 29.Patient-reported fatigue scores reflect physical function impairment. J Support Oncol. 2004;2(5):416. [PubMed] [Google Scholar]
- 30.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 31.Chuang ML, Lin IF, Wasserman K. The body weight-walking distance product as related to lung function, anaerobic threshold and peak VO2 in COPD patients. Respir Med. 2001;95(7):618–626. doi: 10.1053/rmed.2001.1115. [DOI] [PubMed] [Google Scholar]
- 32.Faggiano P, D’Aloia A, Gualeni A, Lavatelli A, Giordano A. Assessment of oxygen uptake during the 6-minute walking test in patients with heart failure: preliminary experience with a portable device. Am Heart J. 1997;134(2 Pt 1):203–206. doi: 10.1016/s0002-8703(97)70125-x. [DOI] [PubMed] [Google Scholar]
- 33.Riley M, McParland J, Stanford CF, Nicholls DP. Oxygen consumption during corridor walk testing in chronic cardiac failure. Eur Heart J. 1992;13(6):789–793. doi: 10.1093/oxfordjournals.eurheartj.a060258. [DOI] [PubMed] [Google Scholar]
- 34.Zugck C, Kruger C, Durr S, et al. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur Heart J. 2000;21(7):540–549. doi: 10.1053/euhj.1999.1861. [DOI] [PubMed] [Google Scholar]
- 35.Nail LM, Winningham ML. Fatigue and weakness in cancer patients: the symptoms experience. Semin Oncol Nurs. 1995;11(4):272–278. doi: 10.1016/s0749-2081(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton DM, Haennel RG. Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. J Cardiopulm Rehabil. 2000;20(3):156–164. doi: 10.1097/00008483-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 37.King MB, Judge JO, Whipple R, Wolfson L. Reliability and responsiveness of two physical performance measures examined in the context of a functional training intervention. Phys Ther. 2000;80(1):8–16. [PubMed] [Google Scholar]
- 38.McArdle W, Katch F, Katch V. Exercise Physicology: Energy, Nutrition, and Human Performance. 4. Baltimore: Williams and Wilkins; 1996. [Google Scholar]
- 39.Mazzeo K. Aerobics: the Way to Fitness. Englewood, CO: Morton publishing Co; 1992. [Google Scholar]
- 40.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 41. [Accessed March 5, 2010];Sleep Study Report. http://sleepapneafaq.wikispaces.com/Sleep+Study+report.
- 42.Profile of Mood States Technical Update. Toronto: Multi Health Systems; Copyright 2003, 2005. [Google Scholar]
- 43.Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55(1):79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 45.National Comprehensive Cancer Network (NCCN) [Accessed July 16, 2010];NCCN guidelines V.1. 2009 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 46.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25(1):51–62. [PubMed] [Google Scholar]
- 47.Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W. Taking fatigue seriously, II: variability in fatigue levels in cancer patients. Psychosomatics. 2007;48(3):247–252. doi: 10.1176/appi.psy.48.3.247. [DOI] [PubMed] [Google Scholar]
- 48.Winningham ML. Fatigue. In: Groenwald S, Hansen FM, Goodman M, Yargro C, editors. Cancer Symptom Management. Boston: Jones and Bartlett; 1996. pp. 42–54.89. [Google Scholar]
- 49.Evans WJ. Physical function in men and women with cancer. Effects of anemia and conditioning. Oncology (Williston Park) 2002;16(9 Suppl 10):109–115. [PubMed] [Google Scholar]
- 50.Grant M. Fatigue and quality of life with cancer. In: Winningham ML, Barton-Burke M, editors. Fatigue in Cancer: A multidimensional Approach. Boston: Jones and Bartlett; 2000. pp. 353–364. [Google Scholar]
- 51.Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist. 1999;4(1):1–10. [PubMed] [Google Scholar]
- 52.Bardwell WA, Profant J, Casden DR, et al. The relative importance of specific risk factors for insomnia in women treated for early-stage breast cancer. Psychooncology. 2008;17(1):9–18. doi: 10.1002/pon.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker KP, Bliwise DL, Ribeiro M, et al. Sleep/Wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26(15):2464–2472. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes R, Stone P, Andrews P, morgan R, Sharma S. Is cancer-related fatigue more strongly correlated to haematological or to psychological factors in cancer patients? Journal of Pain and Symptom Management. 2006;32(3):245–254. doi: 10.1016/j.jpainsymman.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Siefert ML. Fatigue, pain, and functional status during outpatient chemotherapy. Oncology Nursing Foru. 2010;37(2):E114–E123. doi: 10.1188/10.ONF.114-123. [DOI] [PubMed] [Google Scholar]
- 56.Stepanski EJ, Walker MS, Schwartzberg LS, et al. The Relation of Trouble Sleeping, Depressed Mood, Pain, and Fatigue in Patients with Cancer. J Clin Sleep Med. 2009;5(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 57.Montague L, Green CR. Cancer and breakthrought pin’s impact on a diverse population. Pain Medicine. 2009;10(3):549–561. doi: 10.1111/j.1526-4637.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 58.Romito F, Montanaro R, Corvasce C, Di Bisceglie M, Mattioli V. Is cancer-related fatigue more strongly correlated to haematological or to psychological factors in cancer patients? Support Care Cancer. 2008;16(8):943–946. doi: 10.1007/s00520-007-0357-1. [DOI] [PubMed] [Google Scholar]
- 59.Yennurajalingam S, Palmer JL, Zhang T, Poulter V, Bruera E. Association between fatigue and other cancer-related symptoms in patients with advanced cancer. Support Care Cancer. 2008;16(10):1125–1130. doi: 10.1007/s00520-008-0466-5. [DOI] [PubMed] [Google Scholar]
- 60.Faiman B, Bilotti E, Mangan PA, Rogers K. Steroid-associated side effects in patients with multiple myeloma: consensus statement of the IMF Nurse Leadership Board. Clin J Oncol Nurs. 2008;12(3 Suppl):53–63. doi: 10.1188/08.CJON.S1.53-62. [DOI] [PubMed] [Google Scholar]
- 61.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27(11):1788–1793. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 62.McGrath P, Patton MA, Leahy M. And Tell yourself, “This is not Me, it’s the Drug” Coping with the Psychological Impact of Corticosteroid Treatments in Hematology -Further Results from a Pilot Study. Patient. 2009;2(1):19–31. doi: 10.2165/01312067-200902010-00003. [DOI] [PubMed] [Google Scholar]
- 63.Kirkova J, Walsh D, Rybicki L, et al. Symptom severity and distress in advanced cancer. Palliat Med. 2010;24(3):330–339. doi: 10.1177/0269216309356380. [DOI] [PubMed] [Google Scholar]
- 64.Lockhorst H. Clinical features and diagnostic criteria. In: Mehta HSS, editor. Myeloma. London: Martin Dunitz; 2002. pp. 151–168. [Google Scholar]
- 65.Andrykowski MA, Brady MJ, Greiner CB, et al. ‘Returning to normal’ following bone marrow transplantation: outcomes, expectations and informed consent. Bone Marrow Transplant. 1995;15(4):573–581. [PubMed] [Google Scholar]
- 66.Dimeo F, Stieglitz RD, Novelli-Fischer U, Fetscher S, Mertelsmann R, Keul J. Correlation between physical performance and fatigue in cancer patients. Ann Oncol. 1997;8(12):1251–1255. doi: 10.1023/a:1008234310474. [DOI] [PubMed] [Google Scholar]
- 67.Dimeo FC. Effects of exercise on cancer-related fatigue. Cancer. 2001;92(6 Suppl):1689–1693. doi: 10.1002/1097-0142(20010915)92:6+<1689::aid-cncr1498>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 68.MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989;38(6):348–351. [PubMed] [Google Scholar]
- 69.Mock V, Dow KH, Meares CJ, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24(6):991–1000. [PubMed] [Google Scholar]
- 70.Liu RD, Chinapaw MJ, Huijgens PC, van Mechelen W. Physical exercise interventions in haematological cancer patients, feasible to conduct but effectiveness to be established: a systematic literature review. Cancer Treat Rev. 2009;35(2):185–192. doi: 10.1016/j.ctrv.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Hanna LR, Avila PF, meteer JD, Nicholas DR, Kaminsky LA. The Effects of a Comprehensive Exercise Program on Physical Function, Fatigue, and Mood in Patients with Various Types of Cancer. Oncology Nursing Forum. 2008;35(3):461–469. [Google Scholar]