Abstract
INTRODUCTION
Concern exists over the subsequent development of hypertension after use of shock wave lithotripsy (SWL) for the treatment of symptomatic urolithiasis. Referral bias and lack of long-term follow-up has been a limitation of prior studies.
METHODS
We identified all Olmsted County, Minnesota residents with a diagnosis of urolithiasis from 1985 to 2008. The charts were electronically queried for hypertension and obesity by diagnostic codes and use of SWL by surgical codes. All patients first diagnosed with hypertension before or up to 90 days after their first documented kidney stone were considered to have prevalent hypertension and excluded. Cox proportional hazards models were used to assess the association of SWL with a subsequent diagnosis of hypertension.
RESULTS
We identified 6,077 incident urolithiasis patients with greater than 90 days follow-up. We excluded 1,295 (21.3%) of the population for prevalent hypertension leaving 4,782 incident urolithiasis patients for analysis. During an average follow-up of 8.7 years, new-onset hypertension was diagnosed in 983 (20.6%) of the cohort at a mean of 6.0 years from index stone date. Only 400 (8.4%) of the cohort received SWL therapy. There was no significant association between SWL treatment and the development of hypertension in univariate (P=0.33) and multivariate modeling controlling for age, gender, and obesity (Hazard ratio [95% CI] =1.03[0.84, 1.27], P=0.77).
CONCLUSION
In a large population based cohort of kidney stone formers, we failed to identify an association between SWL and the subsequent long-term risk of hypertension.
Keywords: lithotripsy, urolithiasis, hypertension
Introduction
The initial successful treatment results achieved with shock wave lithotripsy (SWL) for symptomatic renal and ureteral calculi led to widespread dissemination of this minimally invasive technology in 19851. Since its introduction, SWL has become one of the most widely utilized surgical treatments for urolithiasis. It has been generally perceived that SWL is non-invasive and safe, and that multiple treatments can be applied without risk. In fact, an early misconception of SWL was that shock waves did not produce injury and passed harmlessly through the body.2 However, although SWL is indeed well-tolerated clinically, multiple studies have demonstrated shock waves do not merely pass through the body without consequence, but rather can produce acute tissue damage to the kidney and surrounding organs.3–5
Since the kidney can potentially experience significant damage secondary to SWL, the development of new-onset hypertension after SWL has been a significant concern. However, this is a controversial topic since several studies have reported a link between SWL and hypertension6–12 with one noting a dose dependent relationship,11 while other studies have not demonstrated this association even at long-term follow-up.13–18 Past studies were generally small cohorts, based on stone patients seen in urology referral practices, and in some cases relied on patient self-report of hypertension. The objective of this study was to evaluate the long-term risk of clinician-diagnosed hypertension in a large population based cohort of symptomatic kidney stone formers.
Materials and Methods
After institutional review board approval, baseline data on the diagnosis of stone disease was obtained through the Rochester Epidemiology Project (REP). This unique resource contains the linked medical records of all health care providers for all residents of Olmsted County. Diagnostic codes (manually or automatically coded from the final diagnoses in clinical notes) dating back to 1935 are indexed and linked among virtually all Olmsted County providers through the Rochester Epidemiology Project.19 We have successfully used this resource to identify associations of nephrolithiasis with other chronic conditions such as chronic kidney disease.20 Residents with urolithiasis events between 1985 and 2008 were identified using International Classification of Diseases (ICD)-9 codes 592, 594, and 274.11. The first stone event documented in Olmsted County in the 1985 to 2008 period was defined as the “index stone.”
Olmsted County residents who did not have Minnesota Research Authorization, those with documented urolithiasis episodes prior to 1985, and those without 90 days or more of follow-up were excluded. The remaining individuals were used to study the risk of hypertension after SWL. Dates of SWL (first available for use in 1985) were identified by querying surgical codes available in the REP. Dates of the first diagnosis of hypertension were identified by ICD-9 codes 401.0, 401.1, and 401.9. These codes have been used previously to successfully identify hypertensive patients in the REP.21 Patients with prevalent hypertension before the “index stone” were excluded. Since there is the possibility that more medical care is received at the time of diagnosis and follow-up of a symptomatic kidney stone we excluded residents with hypertension diagnosed in the first 90 days after the index stone as likely having prevalent hypertension. Adjustments were made for possible confounding factors including age, gender, and presence of obesity based on ICD-9 codes 259.9, 278.0, 278.01, and 783.1. Follow-up was censored at last health care contact, death, or hypertension.
Statistical Analysis
Standard survival analysis methods (Kaplan-Meier, log-rank test, proportional hazards models) were used to analyze time from incident nephrolithiasis diagnosis to development of hypertension. As SWL may be an initial treatment or given later in response to recurrence, it was analyzed as a time-dependent covariate in the Cox proportional hazards model. Hazard ratios and 95% confidence interval estimates were reported for the effect of SWL treatment on subsequent hypertension. Potential confounders such as age, gender, and obesity were controlled for in multivariate modeling. The landmark method22 was used to display the effect of prior SWL (time-dependent covariate) on subsequent hypertension. This was done by stratifying on the use of SWL in the first two-years after stone diagnosis, and then plotting (K-M plot) rates of hypertension after year two. All tests were two-sided with significance level 0.05, and performed using SAS 9.1 (SAS, NC).
Results
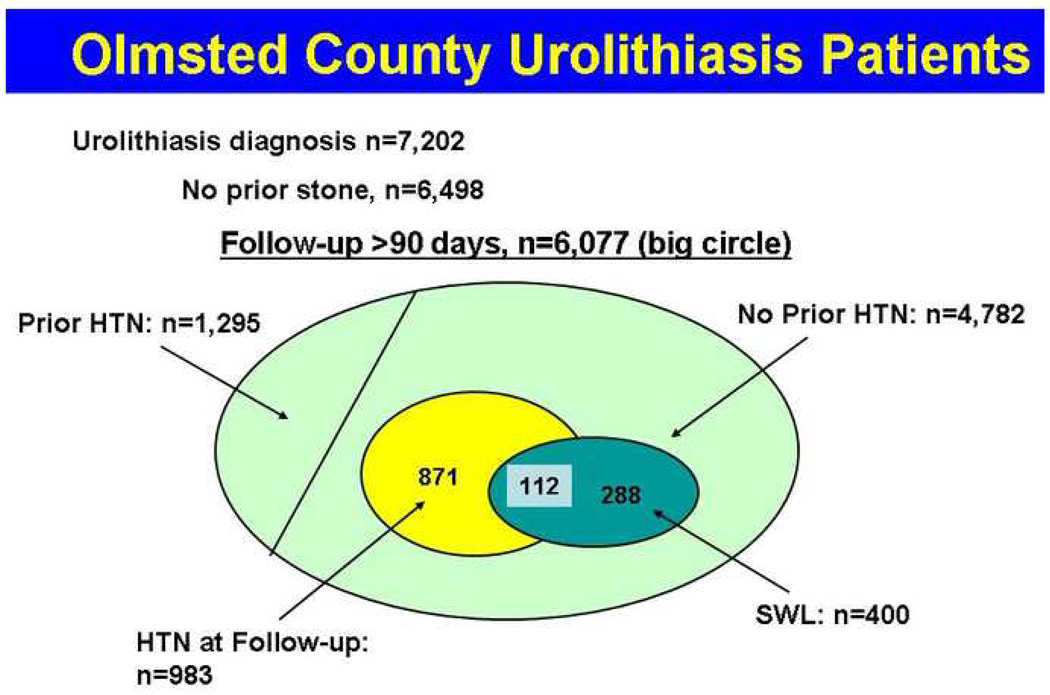
From January 1, 1985 through December 31, 2008 we identified 7,202 Olmsted County residents with a diagnosis code for nephrolithiasis. A stone diagnosis prior to the 1985 was identified in 704 (9.8%) patients and these patients were omitted leaving 6,498 incident nephrolithiasis patients. Four hundred and twenty-one patients had less than 90 days follow-up after index stone date and they were also omitted leaving a total of 6,077 incident nephrolithiasis patients for the analysis (Figure 1).
Figure 1. Diagram of stone former selection based on diagnoses codes.
Only those patients without prior stone episodes and greater than 90 days follow-up were analyzed. All patients with history of hypertension (HTN) were excluded. Of the patients without hypertension, 264 were treated with shock wave lithotripsy (SWL), and 126 went on to develop hypertension.
Of the 6,077, mean (±SD) age at index stone diagnosis was 45.4 ± 17.5 years (median 44, interquartile range (IQR) 32) and 56.4% were male. During the follow-up (mean 8.4 years, range 0.33 to 25 years), SWL was performed on 478 (7.9%) of the nephrolithiasis patients. Of the 6,077 patients, pre-existing hypertension was present in 1,295 (21.3%) (Table 1). Among the 4,782 patients without pre-existing hypertension at time of stone diagnosis, new-onset hypertension diagnosed greater than 90 days after the index stone was found in 983 (20.6%), at a median of 6.0 years after stone diagnosis (Table 2). Mean follow-up for the subgroup of urolithiasis patient without hypertension was also 8.7 years (range 0.3 to 25 years).
Table 1.
Timing of the diagnosis of new onset hypertension related to index stone date in 6,077 index nephrolithiasis stone patients in Olmsted County.
| Timing of Hypertension Diagnosis Related to Index Stone | N=6,077 | % |
|---|---|---|
| Prior to index stone | 1,163 | 19.1 |
| 0 to 30 days after index stone | 112 | 1.8 |
| 30 to 60 days after index stone | 14 | 0.2 |
| 60 to 90 days after index stone | 6 | 0.1 |
| Total pre-existing hypertension | 1,295 | 21.3 |
Table 2.
Timing of shock wave lithotripsy in relation to hypertension diagnosis for 4,782 index nephrolithiasis patients without pre-existing hypertension.
| Diagnosis | N=4,782 | % |
|---|---|---|
| Never hypertension or SWL | 3,511 | 73.4 |
| SWL only | 288 | 6.0 |
| SWL then hypertension | 99 | 2.1 |
| Hypertension only | 871 | 18.2 |
| Hypertension then SWL | 13 | 0.3 |
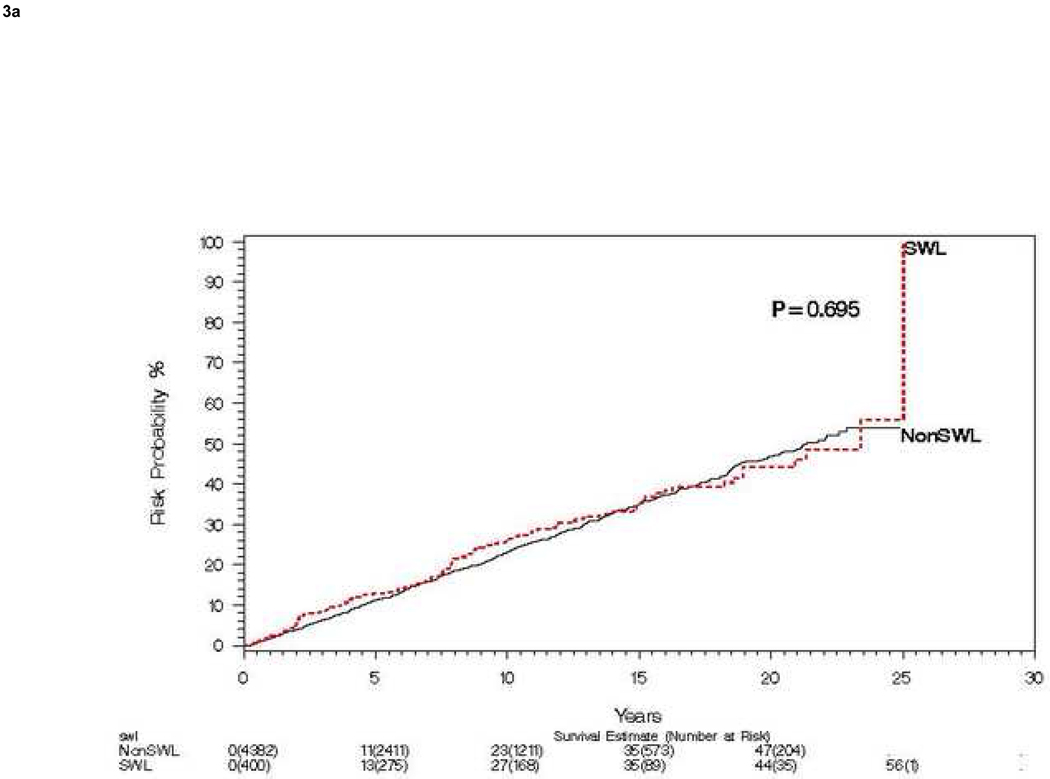
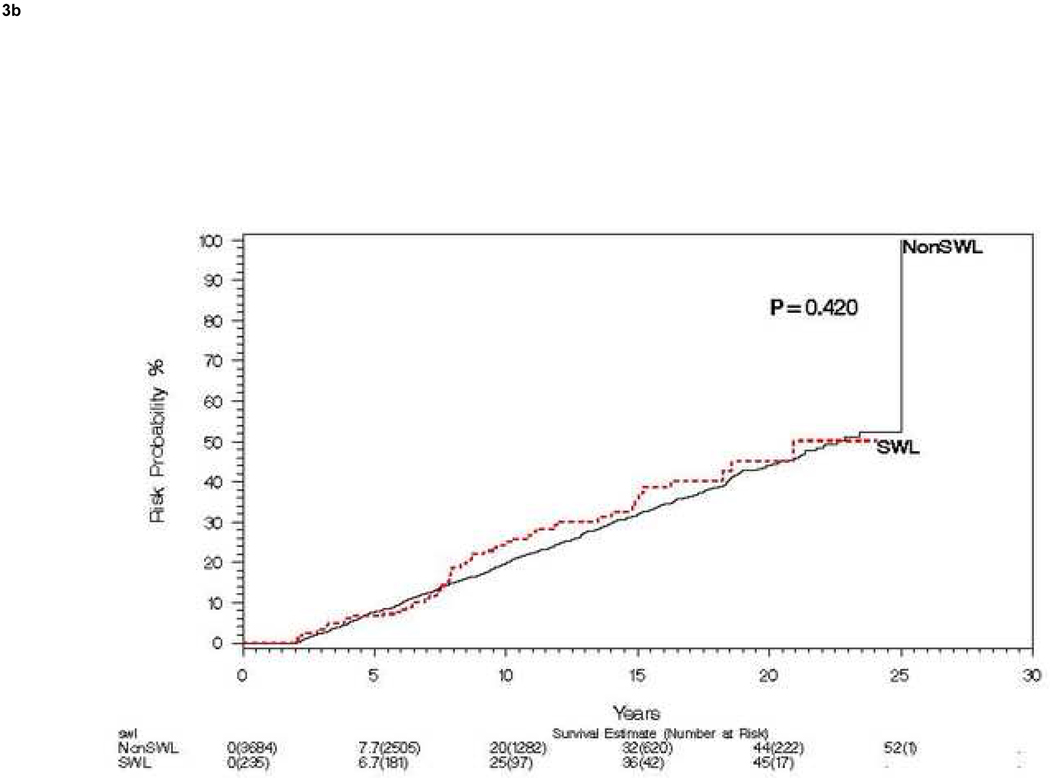
Of the 4,782 patients without pre-existing hypertension, 400 (8.4%) underwent SWL (78 patients treated with SWL had pre-existing hypertension and were thus excluded), 48 receiving SWL for the index stone event (Figure 2). One SWL was performed in 339 patients, two SWLs in 44 patients, three SWLs in 14 patients, four SWLs in 1 patient, and five SWLs in 2 patients. The timing of SWL and the diagnosis of hypertension is summarized in Table 2. Ignoring the timing of SWL, risk of hypertension by 15 years was 35% for both the SWL and non SWL groups (Figure 3a). For those without hypertension at 2 years, risk of hypertension (landmark method) at 15 years was 36% and 32%, for SWL and no SWL respectively (Figure 3b). When comparing those urolithiasis patients who never received SWL to those that ever received SWL we found no significant differences in age (P=0.12), sex (P=0.26), or baseline obesity (P=0.16). Specifically comparing those patients receiving SWL for the index stone (defined as SWL within 1 year of index stone), to all other index stone patients we found no significant difference in gender (P=0.50) or baseline obesity (P=0.77); however, patients who received SWL within 1 year of the index stone were significantly older than those who did not receive SWL within one year of index stone, mean (standard deviation) 44.1 (14.7) vs. 41.2 (16.0) years, respectively (P=0.002).
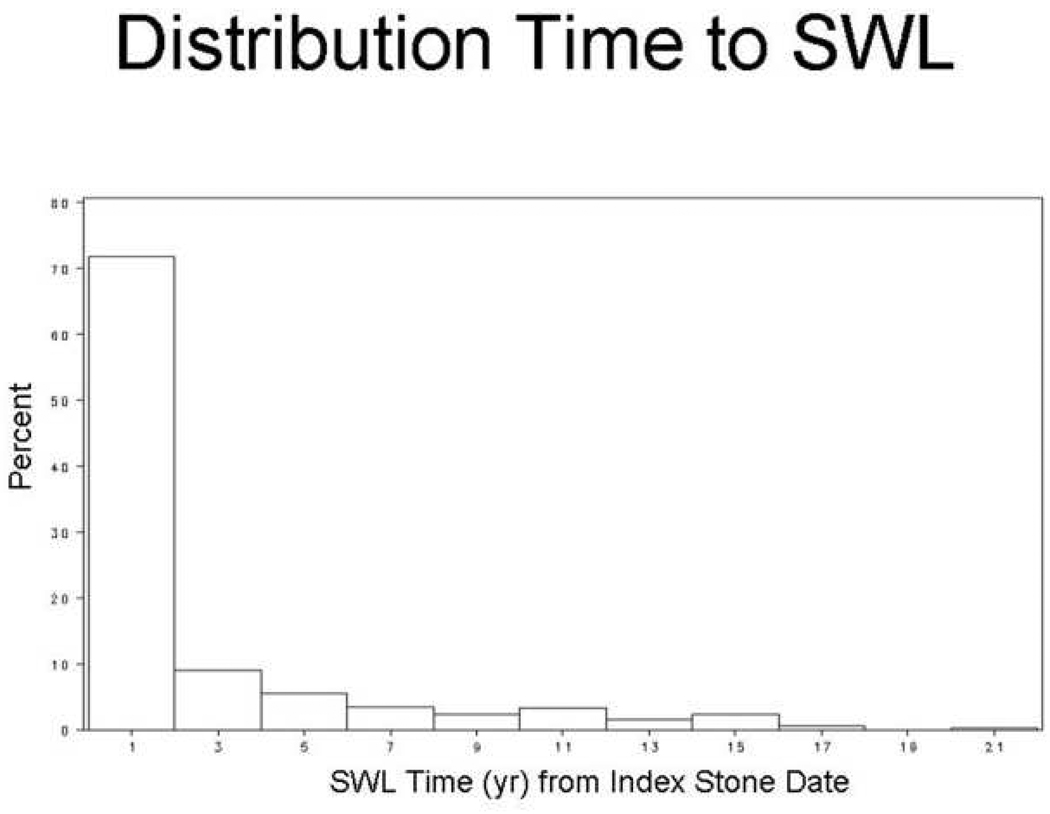
Figure 2. Time from index stone to Shock wave lithotripsy (SWL).
Of the 400 patients treated with SWL, 72% had SWL performed within 2 years of the index stone diagnosis.
Figure 3. Risk of subsequent hypertension events among patients treated with and without SWL.
A) Compares risk of hypertension over 15 years among patients treated with and without SWL starting at time of incident stone. B) Compares the same risk starting at 2 years post incident stone, stratified on SWL in first 2 years among those still at risk (landmark method).
After considering the timing of SWL in the analysis, we still found no significant increased risk of new-onset hypertension after SWL among these incident stone formers (Hazard ratio [95% CI] 1.11 [0.90, 1.37], P=0.33). Furthermore, multivariate analysis controlling for age, gender and baseline obesity again found no significant association (P=0.77) between SWL and subsequent development of hypertension (Table 3). Analysis in a subgroup who were possibly at higher risk for hypertension (age older than median: men >43 years and women >35 years) also did not demonstrate an association between SWL and hypertension (p=0.71 and p=0.40, respectively) (Table 3). Furthermore, analysis limited to patients greater than age 60 at index stone again did not demonstrate an increased risk of hypertension after SWL therapy (Hazard ratio [95% CI] 1.10 [0.74, 1.65], P=0.64).
Table 3.
Multivariate and subgroup analysis assessing the association between SWL and the development of a new diagnosis of hypertension in index stone formers.
| Adjustment | SWL HR* | 95% CI | P-value |
|---|---|---|---|
| No adjustment | 1.11 | 0.90, 1.37 | 0.33 |
| Age, gender | 1.03 | 0.84, 1.27 | 0.76 |
| Age, gender, baseline obesity | 1.03 | 0.84, 1.27 | 0.77 |
| Subgroup | SWL HR* | 95% CI | P-value |
| Male, Age < Median of 43 (N=1,351) | 1.13 | 0.69, 1.84 | 0.63 |
| Male, Age >=Median of 43 (N=1,307) | 0.94 | 0.69, 1.29 | 0.71 |
| Female, Age < Median of 35 (N=1,031) | 1.10 | 0.57, 2.14 | 0.78 |
| Female, Age >=Median of 35 (N=1,093) | 1.19 | 0.79, 1.78 | 0.40 |
Estimate SWL hazard ratio for development of hypertension from Cox model with SWL analyzed as a time dependent covariate.
Discussion
More than 20 years have passed since the introduction of SWL for the treatment of human urolithiasis.23,24 Its initial high success rates and minimally invasive nature have made SWL one of the most widely used treatments for symptomatic urolithiasis, and 80–90% of calculi are currently considered to be amenable to SWL.15 Although clinical and experimental studies have demonstrated the safety of SWL,25–27 clear evidence also suggests SWL can cause acute tissue damage and long-term renal scar formation.4 In fact, almost every abdominal organ has been reported to be injured during clinical SWL treatment.3–5 Therefore, since the kidney can potentially experience significant damage secondary to SWL the potential for development of long-term renal dysfunction and new-onset hypertension after SWL has been a significant concern. No studies to date have provided strong evidence that the risk of chronic kidney disease is increased after SWL treatment.5 However, there are multiple conflicting studies on the role of SWL in the development of new onset hypertension in urolithiasis patients.1–2, 6–18
Our study attempted to identify an association between SWL and new-onset hypertension in a large community based cohort of urolithiasis patients. By focusing on all stone patients in a geographically defined community setting, we attempted to limit referral bias, which can occur in a high volume referral center. Over a 23 year period, we identified 6,077 incident stone formers living in Olmsted County with greater than 3 months follow-up. New-onset hypertension after the index stone was diagnosed in 21% of the cohort. We found no statistically significant associations between SWL and the risk of hypertension in the community setting, even after adjusting for age, gender, and obesity or limiting the analysis to older men and women.
The first study to note an increase in systemic blood pressure after SWL was by Peterson and Finlayson in 1986.12 Since this initial report multiple investigators have evaluated the association between SWL and hypertension.1–3, 6–18, 23 Janetschek and colleagues found that patients over 60 years of age were at risk for SWL induced elevated renal resistive indices measured by ultrasound. In the 26 month follow-up study elevated resistive indices (45% of patients) were noted almost exclusively in patients over the age of 60 years with a rate of new onset hypertension of 17.5%.9 Our study failed to demonstrate such an association in the older patient population, even when focusing only on those patients greater than 60 years of age at time of stone diagnosis.
In 2006 we studied all patients (not just Olmsted County residents) treated with SWL at our institution in the year 1985 for long-term sequelae via mail survey. At 19 years of follow-up this study identified an increased risk of developing hypertension in stone patients treated with SWL compared to patients treated with conservative management. The risk for hypertension was also associated with bilateral SWL. Sato and colleagues have subsequently evaluated their long-term SWL results.13 They compared patients treated with SWL for renal calculi to those undergoing SWL for ureteral calculi and noted no increase in HTN in the renal SWL treatment group at 17 years follow-up.
There are several possible reasons why an increased risk of hypertension with SWL is not evident in the community setting, but is evident in studies of urology referral patients at a tertiary care centers. Evaluating stone formers in the community setting should theoretically capture the “typical” stone former, whereas focusing on patients treated at a tertiary care facility is likely to capture more severe stone disease or more complex patients with a higher number of comorbidities who might be at increased risk for hypertension. There may also be a detection bias between patients with and without past SWL treatments regarding self-reporting of medical conditions such as hypertension. It is also possible that patients with large stone burden who underwent multiple treatments with SWL at referral centers were more likely to incur the amount of parenchymal damage necessary to induce hypertension. Another possible explanation is differences in study design. The prior study focused on chart review and patient self reporting, while the current study relied on diagnosis codes to assess outcomes. Both designs are subject to error: patients may over or under self report conditions and diagnosis codes may also over or under represent conditions. Of note the current study included over 6,000 urolithiasis patients, and although only a minority of patients received shock wave therapy, 400 without prior hypertension, it is still one of the larger SWL cohorts analyzed to date with long-term follow-up. With a larger cohort we would expect to more easily observe associations between treatment and different outcomes such as hypertension. However, the follow-up for the current community based study is shorter than our prior study (mean of 8.7 years vs. 19 years), thus it is possible that post-SWL hypertension requires longer follow-up to be detected.
Certain limitations of the study should be mentioned. First, the population of Olmsted County in 2000 comprised approximately 124,000 persons (90% white). Socio-economically, the community resembles the U.S. white population19 and findings in other race groups may differ. The National Health and Nutrition Examination Survey (NHANES II) demonstrated that stone disease is more prevalent in whites compared to other racial groups28; thus, the Olmsted County cohort should be a reasonable representative of the majority of stone disease experienced in the U.S. population. Second, the study relied on diagnostic codes to identify nephrolithiasis, hypertension, and comorbidities, which is subject to misclassification. A random subset of 1097 charts has been reviewed as part of a separate study. This chart review showed that 89% of the patients with a diagnosis code for urolithiasis had evidence in the medical record supporting the diagnosis, and in 11% clear evidence of stones was not present. Finally, patients were not randomized to SWL vs. other treatment options, thus there may be unmeasured factors contributing to the results. Despite these limitations, this is one of the largest population based studies to assess the impact of a common stone treatment on the potential long-term complication of hypertension.
Conclusion
This study in a large population based cohort of incident stone formers failed to demonstrate an increased risk of hypertension after SWL treatment for urolithiasis. These findings are reassuring that the use of at least one SWL treatment does not subject most urolithiasis patients to an increased risk of hypertension. However, it is still possible that multiple SWL treatments may increase the subsequent risk of hypertension, or that certain subgroups (e.g. those with chronic kidney disease) might be more susceptible.
Acknowledgment
We would like to acknowledge the contributions of Timothy Roth, M.D. to the study design. Funding for the study was through National Institute of Health O’Brien Center Grant number DK83007.
Key of Definitions
- SWL
Shock Wave Lithotripsy
- HTN
hypertension
- ICD
International Classification of Diseases
- REP
Rochester Epidemiology Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lingeman JE, Newman D, Mertz JH, et al. Extracorporeal shock wave lithotripsy: the Methodist Hospital of Indiana experience. J Urol. 1986;135:1134. doi: 10.1016/s0022-5347(17)46016-2. [DOI] [PubMed] [Google Scholar]
- 2.Chaussy C, Fuchs G. Extracorporeal lithotripsy in the treatment of renal lithiasis. 5 years’ experience. J Urol (Paris) 1986;92:339. [PubMed] [Google Scholar]
- 3.Lingeman JE, Matlaga BR, Evan AP, et al. Surgical management of upper urinary tract calculi. In: Kavoussi LR, Novick AC, Partin AW, et al., editors. Campbell-Walsh Urology. 9th ed. Philadelphia: Saunders-Elsevier; 2007. pp. 1431–1507. [Google Scholar]
- 4.Lingemann JE, McAteer JA, Assimos DG, et al. Current perspectives on adverse effects in shock wave lithotripsy. American Urological Education Series, White Paper. 2010 [Google Scholar]
- 5.Krambeck AE, Lingeman JE. Clinical and Bioeffects of Shock Wave Lithotripsy. American Urologic Association Update series Lesson 25. 2009 [Google Scholar]
- 6.Krambeck AE, Gettman MT, Rohlinger AL, et al. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of follow-up. J Urol. 2006;175:1742. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 7.Williams CM, Kaude JV, Newman RC, et al. Extracorporeal shock-wave lithotripsy: long-term complications. Am J Roentgenol. 1988;150:311. doi: 10.2214/ajr.150.2.311. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery BS, Cole RS, Plafrey EL, Shuttleworth KE. Does extracorporeal shockwave lithotripsy cause hypertension? B J Urol. 1989;64:567. doi: 10.1111/j.1464-410x.1989.tb05309.x. [DOI] [PubMed] [Google Scholar]
- 9.Janetschek G, Frauscher F, Knapp R, et al. New onset hypertension after extracorporeal shock wave lithotripsy: age related incidence and prediction by intrarenal resistive index. J Urol. 1997;158:346. doi: 10.1016/s0022-5347(01)64475-6. [DOI] [PubMed] [Google Scholar]
- 10.Strohmaier WL, Schmidt J, Lahme S, et al. Arterial blood pressure following different types of urinary stone therapy. Eur Urol; Presented at the 8th European Symposium on Urolithiasis; Parma, Italy; 2000. p. 753. [DOI] [PubMed] [Google Scholar]
- 11.Lingeman J, Delius M, Evan A, et al. Bioeffects and physical mechanisms of SW effects in SWL. In: Segura J, Conort P, Khoury S, et al., editors. Stone Disease: First International Consultation on Stone Disease. Paris: Heath Publications; 2003. pp. 251–286. [Google Scholar]
- 12.Peterson JC, Finlayson B. Effects of ESWL on blood pressure. In: Gravenstein JS, Peter K, editors. Extracorporeal shock wave lithotripsy for renal stone disease: Technical and clinical aspects. Boston: Butterworths; 1986. [Google Scholar]
- 13.Sato Y, Tanda H, Kato S, et al. Shock wave lithotripsy for renal stones is not associated with hypertension and diabetes mellitus. J Urol. 2008;71:586. doi: 10.1016/j.urology.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 14.Liedle B, Jocham D, Lunz C, et al. Five year follow-up of urinary stone treatment with extracorporeal shock wave lithotripsy. J Endourol. 1988;2:157. [Google Scholar]
- 15.Lingeman JE, Woods JR, Toth PD. Blood pressure changes following extracorporeal shock wave lithotripsy and other forms of treatment for nephrolithiasis. JAMA. 1990;263:1789. [PubMed] [Google Scholar]
- 16.Puppo P, Germinale F, Ricciotti G. Hypertension after extracorporeal shock wave lithotripsy: A false alarm. J Endourol. 1988;3:401. [Google Scholar]
- 17.Elves AW, Tilling K, Menezes P, Wills M, Rao PN, Feneley RC. Early observations of the effect of extracorporeal shockwave lithotripsy on blood pressure: a prospective randomized control clinical trial. BJU International. 2000;85:611. doi: 10.1046/j.1464-410x.2000.00571.x. [DOI] [PubMed] [Google Scholar]
- 18.Jewett MAS, Bombardier C, Logan AG et al. A randomized controlled trial to assess the incidence of new onset hypertension in patients after shock wave lithotripsy for asymptomatic renal calculi. J Urol. 1998;160:1241–1243. [PubMed] [Google Scholar]
- 19.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 20.Rule AD, Bergstralh EJ, Melton LJ, 3rd, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:804. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieske JC, de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis. 2006;48:897. doi: 10.1053/j.ajkd.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 23.Chaussy C, Schmiedt E, Jocham D, Brendel W, Forssmann B, Walther V. First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol. 1982;172:417. doi: 10.1016/s0022-5347(17)53841-0. [DOI] [PubMed] [Google Scholar]
- 24.Chaussy C, Schuller J, Schmiedt E, Brandl H, Jocham D, Liedl B. Extracorporeal shock wave lithotripsy (ESWL) for the treatment of urolithiasis. Urology. 1984;23:59. doi: 10.1016/0090-4295(84)90243-7. [DOI] [PubMed] [Google Scholar]
- 25.Kerbl K, Rehman J, Landman J, et al. Current management of urolithiasis: progress or regress? J Endourol. 2007;16:281. doi: 10.1089/089277902760102758. [DOI] [PubMed] [Google Scholar]
- 26.Galvin DJ, Pearle MS. The contemporary management of renal and ureteric calculi. BJU Int. 2006;98:1283. doi: 10.1111/j.1464-410X.2006.06514.x. [DOI] [PubMed] [Google Scholar]
- 27.Argyropoulos AN, Tolley DA. Optimizing shock wave lithotripsy in the 21st century. Eur Urol. 2007;52:344. doi: 10.1016/j.eururo.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 28.Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994;46:893. doi: 10.1038/ki.1994.347. [DOI] [PubMed] [Google Scholar]