Abstract
To assess the relationship between serum C3 or C4 levels and lupus renal flare, C3 and C4 levels were measured bimonthly in 71 lupus nephritis patients for a mean of 35 months, during which time 70 renal flares were identified. Comparing baseline, pre-flare, and at-flare values indicated that neither C3 nor C4 levels decreased pre-flare, but both decreased on average significantly at flare. However, sensitivity/specificity for C3 (75%/71%) and C4 (48%/71%) were low. To account for other influencing factors, multiple regression was performed that included bimonthly values of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), and genotype data on C3 (S/F), CRP (1846G > A), and the complement regulator factor H (Y402H). This analysis revealed that reduced levels of C4, but not C3, were independently associated with the two-month pre-flare period. Conversely, reduced levels of C3, but not C4, were independently associated with the flare visit. Significant pro-flare interactions included low C3 levels with the factor H 402HH-encoding genotype, and low CRP levels with the C3 F allele. Together these data suggest that C4 activation is critical for initiating renal flare while C3 activation is involved in the actual tissue damage, and that these effects are influenced by genetic variability in complement activation and regulation.
Keywords: C3, C4, lupus nephritis, renal flare
Introduction
Clinical renal disease is manifested in about 40–50% of all systemic lupus erythematosus (SLE) patients (lupus nephritis), and is associated with significant morbidity and a poor prognosis. Patients experiencing spikes in their renal disease activity (renal flares) risk permanent tissue damage, and generally have a worse prognosis.1 Although therapies exist to effectively treat renal flares, these therapies are themselves toxic. Unfortunately, despite the negative impact that renal flares have on SLE management, the pathogenesis of renal flare remains unclear, and there are no known forecasters of an impending renal flare.
Complement activation is thought to be involved in the tissue damage associated with SLE flare.2 Studies to determine whether changes in plasma levels of complement C3 and C4 can serve as biomarkers of SLE flare have reported conflicting results.3–9 Why these results conflict is unclear. One possible explanation may relate to study design, as they have been either cross-sectional or based on a few irregular longitudinal measurements biased around flare, with data analyzed against indices of disease activity. No study has assessed complement measurements at regular (unbiased) and frequent intervals leading up to flare to rigorously test the true role of C3 or C4 as biomarkers of flare.
Another explanation may concern individual differences in the complement system, where genetic variation in complement activation proteins and complement regulators, affecting both expression levels and function, could have an influence on the role of complement in the pathogenesis of flare. In support of this, there is a growing recognition for a role for genetic variation in complement regulators in a number of pathologies involving the complement system.10
The goal of the present study was to critically evaluate whether decreases in serum C3 or C4 protein levels could either forecast or mark a renal flare, or be informative about the pathogenesis of renal flare.Over 1000 serial measurements of serum C3 and C4 levels were made at regular bimonthly intervals in 71 lupus nephritis patients over an average of 35 months, during which time 70 renal flares were identified. These measurements were analyzed in a univariate fashion as well as through multiple regression analysis incorporating other variables of inflammation and complement activation/regulation. The variables included a polymorphism in the complement control protein, factor H (FH), that influences its ability to interact with C-reactive protein (CRP) in controlling complement activation at tissue sites,11,12 a CRP polymorphism that affects constitutive CRP plasma levels,13 and a common C3 polymorphism with currently no consensus physiological relevance.14
Materials and methods
Patient population
This study, conducted in accordance with the Declaration of Helsinki, involved 71 lupus nephritis patients (biopsy-verified) enrolled in the Ohio SLE Study (OSS) after informed consent was obtained. Each patient was followed every 2 months for a minimum of 4 consecutive months and an average of 34.6 months (median = 38), with >90% visit compliance. At each study visit, an extensive clinical evaluation and laboratory clinical tests were performed, including serum C3, C4, and CRP levels, and erythrocyte sedimentation rate (ESR).
Clinical measurements
Bimonthly measurements of C3, C4, CRP, and ESR were performed at the University Reference Laboratories at The Ohio State University Hospital Systems. Nephelometry was used for C3, C4, and CRP, while an automated assessment of sedimentation rate was used for ESR. The University Reference Laboratories normal range for C3 is 80–178 mg/dl, for C4 is 12–42 mg/dl, for CRP is 0.0–3.0 mg/l, and for ESR is 0–14 mm/h for males under 50 years old, 0–19 mm/h for females under 50 years old, 0–19 for males between 50 and 80 years old, and 0–29 mm/h for females between 50 and 80 years old.
Adjudication and classification of SLE renal flare
After all clinical results were compiled from each study visit, SLE renal flares were identified using pre-specified criteria as we have previously reported in detail.15–19 These criteria are based on changes in serum creatinine or proteinuria that were attributable to SLE. At least 4 months had to elapse after the previous flare before another flare could be declared, unless there was an increase in flare severity. If an SLE flare occurred during the time between two consecutive visits, for accounting purposes, the flare was assigned to the more recent study visit. A total of 70 renal flares were identified.
Determination of C3, CRP, and FH genotypes
The genotypes for the C3 S/F polymorphism14 and the CRP nucleotide 1846G > A polymorphism13 were determined by standard restriction length fragment polymorphism (RFLP) assays, using genomic DNA isolated from peripheral blood leukocytes. The FH amino acid Y402H polymorphism11,20 was genotyped using the polymerase chain reaction (PCR) pyrosequencing in a PSQ™ 96MA System (Pyrosequencing Uppsala, Sweden)21 using a similar to method to that described previously22 (primer sequences are available upon request). The results of the C3 and CRP genotyping were analyzed as homozygous wildtype or minor allele carrier due to low minor allele frequency. The FH polymorphism was analyzed as genotypes.
Performance of C3 and C4 alone in the diagnosis or forecast of renal flare
To test the relationship between serum levels of C3 or C4 and renal flare, two approaches were taken. First, an initial screen was done using only moderate-to-severe flares that had no confounding flares occurring in the months before and after flare (n = 33). For these, mean baseline values were compared to 2 months prior to and at flare, using a repeated measures analysis of variance (ANOVA). This was followed by a more extensive analysis using all 70 renal flares where the bimonthly complement values were used to calculate false positive and negative rates for C3 and C4 at or 2 months before renal flare. The false negative rate was determined as: (number of normal values at or 2 months before flare)/(total number of renal flares). The false positive rate was defined as (number of abnormal values at non-flare)/(total number of non-flare measurements). To avoid confounding the false positive data, C3 and C4 values were not used from any visits within 4 months before or after any flare (renal or non-renal) of any severity. The sensitivity, specificity, and positive and negative predictive values of C3 and C4 were calculated from the false positive and false negative rates for concurrent and future renal flare. In addition, receiver-operating characteristic (ROC) curves were generated to determine whether diagnostic utility could be improved using different definitions of the lower limit of normal (LLN).
Performance of C3 and C4 in combination with other variables in the diagnosis or forecast of renal flare
To clarify the relationship of C3 and C4 consumption with SLE renal flare, a multivariate analysis was performed. This allowed us to take into account other variables (predictors) that might strengthen C3 and C4 as clinical biomarkers. Two general categories were selected for inclusion as predictors. First, because C3 and C4 are both acute phase proteins, two other measurements of acute phase reactivity, namely CRP and ESR, were included. Second, genotypic variation in the C3 gene and in genes for proteins involved in C3 regulation (CRP and factor H) were measured and included in the analysis. Finally, age was included because we previously showed this to be a predictor of SLE renal flare.19
The multivariate analysis was done using stepwise multiple logistic regression in the generalized estimating equation (GEE) framework, as we have reported previously.19 In brief, to identify markers of concurrent renal flare, the predictors analyzed were every bimonthly level of C3, C4, ESR, and CRP, the age of the patient, the polymorphism data for C3 (SS or F carrier), CRP (1846GG or A carrier), and FH (402-YY, -YH, or -HH). The responses were ‘renal flare—yes’ (70 events) or ‘renal flare—no’ (>1000 events) at the time of the office visit. The same approach and predictors were used to identify forecasters of renal flare, except the bimonthly predictors were those measured the previous 2 months, and excluded the values measured at the time of flare.
To identify main effectors of renal flare, univariate analyses were performed for each predictor. Those surviving at a p-value < 0.25 were included as predictors in the multiple regression models. Predictors with a p-value > 0.1 were discarded, and the multivariate analysis was repeated until only predictors with p < 0.05 remained. Those remaining were included in a second round of stepwise regression in which interactions between C3 or CRP and the three genotypes were tested as predictors of renal flare. The levels of significance for those variables identified as main effectors or as interaction effectors were taken from the final step of the interaction analysis. Prediction equations derived from these analyses were used to construct risk curves to quantify the effects of changes in the significant effectors on risk for flare.
Results
Performance of C3 and C4 individually in the diagnosis or forecast of renal flare
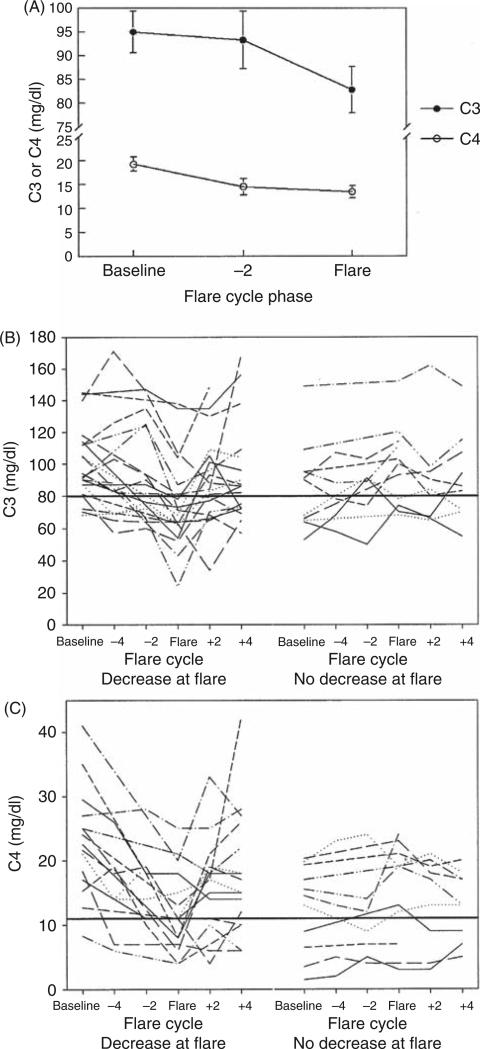
In the initial assessment of 33 moderate-to-severe renal flares, both C3 and C4 fell significantly at flare, compared with baseline (Figure 1A). C4 also showed a tendency to decline below baseline 2 months before flare, but this did not reach significance (p = 0.06). However, when C3 and C4 levels were examined over time for individual patients, the response was less predictable (Figure 1B and C). While approximately two-thirds of the patients showed a fall in C3 and C4 at flare compared with baseline, the flare values did not dip below the LLN established by the hospital laboratory in several cases. In addition, in some cases, the baseline values were already below the LLN, and at flare declined further. Finally, about one-third of the patients did not show a fall in C3 or C4, and some of these showed an increase at flare.
Figure 1.
Complement values over time in a cohort of renal systemic lupus erythematosus (SLE) patients. (A) The average C3 and C4 values at baseline, pre-flare, and flare are shown for a subset of 33 well-characterized moderate and severe renal flares. Baseline values were taken as the average of two or three measurements obtained during non-flare periods and 6 or more months removed from any flare activity. C3 and C4 are significantly lower than baseline at flare (*p = 0.01 and 0.002, respectively, based on a repeated measures analysis of variance (ANOVA)). (B) and (C) Complement values over time for individual patients, showing changes in C3 or C4 levels from baseline, 4 and 2 months pre-flare, at flare, and 2 and 4 months postflare. The data are stratified in each panel according to whether or not C3 or C4 levels fell at flare, compared with baseline.
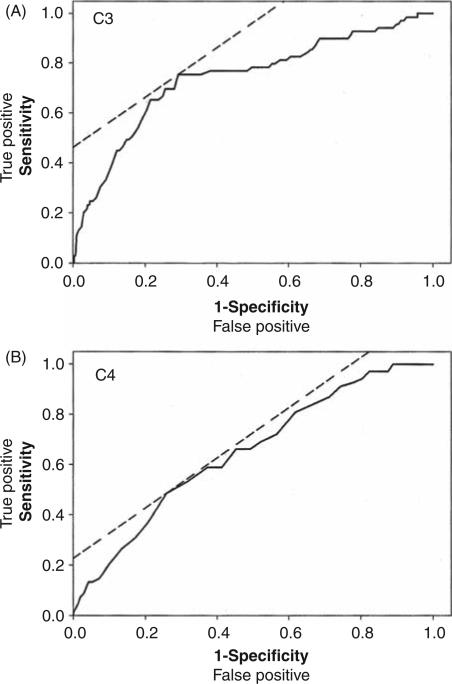
Given this individual variability, the false positive and negative rates for C3 and C4 decline at flare were calculated for all 70 renal flares. As shown in Table 1, both complement components had high false positive and negative rates. Sensitivity, specificity, and positive predictive values were correspondingly low. ROC curves were generated for C3 and C4, and showed area under the curve values of 0.74 and 0.65, respectively (Figure 2). The optimal LLN of C3 (81 mg/dl) and C4 (11 mg/dl) determined from these ROC curves were not markedly different than the established values of the clinical laboratory (C3 = 80 mg/dl; C4 = 12 mg/dl), and did little to improve sensitivity or specificity of C3 (sensitivity/specificity 75%/71%) or C4 (sensitivity/specificity 48%/71%).
Table 1.
Univariate performance of serum levels of C3 or C4 as diagnostic biomarkers for systemic lupus erythematosus renal flare. All data expressed as percentages
| False Negative | False Positive | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| C3 | 30 | 27 | 70 | 73 | 22 | 97 |
| C4 | 51 | 26 | 49 | 74 | 17 | 95 |
PPV, positive predictive value; NPV, Negative predictive value.
Figure 2.
Receiver-operating characteristic (ROC) curves of (A) C3 and (B) C4 sensitivity and specificity to mark concurrent renal flare. The dashed line is the 45° angle tangent to each ROC curve. The area under the curve (AUC) is 0.74 for the C3 curve, and 0.65 for C4 curve.
Generation of a multivariate model for the diagnosis or forecast of renal flare
Given the lack of sensitivity and specificity of C3 and C4 as individual clinical biomarkers, a multivariate approach incorporating C3, C4, and other relevant clinical and genetic variables was undertaken. These multiple regression analyses were run to identify both forecasters of flare (changes that occurred 2 months prior to flare), and markers of flare (changes that occurred at the time of flare). For the forecaster analysis (Table 2), the main pro-flare effectors were lower C4 levels (p = 0.002), higher ESR (p = 0.005), younger age (p = 0.012), and the CRP wildtype genotype (1846GG, 40% frequency) (p = 0.004). An interaction was identified between CRP levels and the C3 S/F genotype (p = 0.013), where lower CRP levels associated with renal flare in F allele carriers (18% frequency).
Table 2.
Results of multivariate analysis for forecasters of renal flare
| p-values |
||||
|---|---|---|---|---|
|
Main effector analysis |
Interaction analysis |
|||
| Predictors for identification of forecasters | Initial univariate analysis | Final multivariate analysis | Final multivariate analysis | Pro-flare effect of individual RF forecaster |
| C3 | 0.016 | 0.691 | 0.619 | |
| C4 | 0.002 | <0.001 | 0.002 | lower C4 levels |
| ESR | 0.013 | 0.029 | 0.005 | higher ESR levels |
| CRP | 0.895 | – | 0.014 * | |
| Age | 0.022 | 0.009 | 0.012 | younger age |
| C3 carrier | 0.637 | – | <0.001 * | |
| FH genotype | 0.198 | 0.665 | 0.840 | |
| CRP4 carrier | 0.015 | 0.004 | 0.004 | 1846GG (WT) |
| C3 × C3 carrier | 0.410 | |||
| C3 × FH genotype | 0.879 | |||
| C3 × CRP4 carrier | 0.133 | |||
| CRP × C3 carrier | 0.013 | |||
| CRP × FH genotype | 0.399 | |||
| CRP × CRP4 carrier | 0.125 | |||
CRP and C3 carrier are significant only through their interactions. Specifically, lower CRP levels are associated with flare only in C3 F carriers.
RF, renal flare; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FH, factor H; WT, wildtype.
For the marker analysis (Table 3), the main proflare effectors also included higher ESR (p = 0.002), younger age (p = 0.028), and the CRP wildtype genotype (1846GG, p = 0.023). However, in contrast to the forecaster analysis where lower C4 was a significant effector renal flare, the marker analysis identified lower C3 as a significant effector of renal flare (p < 0.001). In addition, an interaction was identified between C3 levels and FH genotype (p = 0.016).
Table 3.
Results of multivariate analysis for markers of renal flare
| p-values |
||||
|---|---|---|---|---|
|
Main effector analysis |
Interaction analysis |
|||
| Predictors for identification of markers | Initial univariate analysis | Final multivariate analysis | Final multivariate analysis | Pro-flare effect of individual RF marker |
| C3 | <0.001 | <0.001 | <0.001 | lower C3 levels |
| C4 | <0.001 | 0.787 | ||
| ESR | <0.001 | 0.003 | 0.002 | higher ESR levels |
| CRP | 0.561 | 0.765 | ||
| Age | 0.022 | 0.015 | 0.028 | younger age |
| C3 carrier | 0.637 | 0.139 | ||
| FH genotype | 0.198 | 0.193 | 0.030 * | |
| CRP4 carrier | 0.015 | 0.017 | 0.023 | 1846GG (WT) |
| C3 × C3 carrier | 0.062 | |||
| C3 × FH genotype | 0.016 | |||
| C3 × CRP4 carrier | 0.067 | |||
| CRP × C3 carrier | 0.307 | |||
| CRP × FH genotype | 0.421 | |||
| CRP × CRP4 carrier | 0.100 | |||
FH genotype is significant only through its interactions with C3.
RF, renal flare; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FH, factor H; WT, wildtype.
Prediction equations and risk curves for C4 and C3
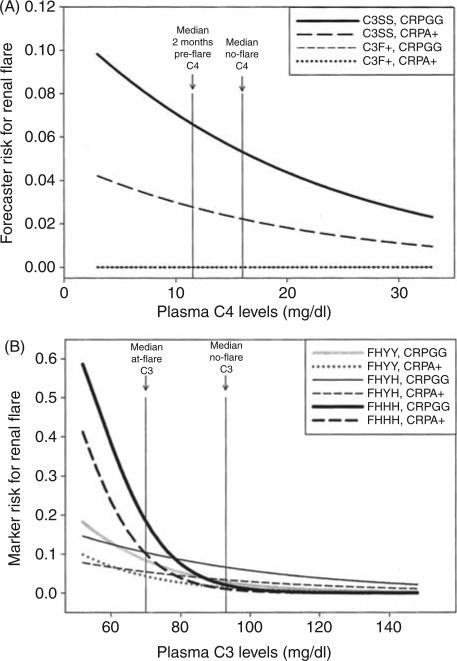
Risk curves were generated for C4 and C3 from prediction equations that resulted from the multivariate analyses. For C4 as a forecaster of flare, four curves were constructed, one for each geno-type combination (C3SS wildtype or F allele carrier, CRP 1846GG wildtype or 1846A allele carrier) (Figure 3A). These curves assumed constant values for the other main effectors, set as the average no-flare values for the OSS cohort (ESR = 27.7, CRP level = 7.98, age = 36). The scale of the X-axis was arbitrarily based on the middle 90% values of C4 levels in the OSS cohort during no-flare visits (3–33 mg/dl). As shown, the overall risk for flare in wildtype C3SS patients increased with decreasing C4 levels 2 months prior to flare. Risk estimates were higher in patients with the wildtype genotypes (C3SS, CRP 1846GG), and were greatest in those who were both C3SS and 1846GG. The impact of C4 as a forecaster of renal flare was reduced by more than half in CRP 1846A allele carriers, and was negligible in C3F allele carriers. The risk ratio for the median C4 value measured 2 months prior to flare (11.5 mg/dl) and the median no-flare value (16.0 mg/dl) in all C3SS patients was 1.2.
Figure 3.
Risk curves derived from prediction equations, using (A) C4 levels as forecasters of flare or (B) C3 levels as markers of renal flare. Each risk curve is generated for the significant genotype combinations identified in the generalized estimating equation (GEE) analysis. Genotypes that are analyzed as carriers include C3 F carriers (C3F+ versus homozygous wildtype C3SS) and C-reactive protein (CRP) 1846A carriers (CRPA+ versus homozygous wildtype CRPGG). The factor H (FH) polymorphism is noted as homozygous wildtype 402YY (FHYY), heterozygous 402YH (FHYH), or homozygous variant 402HH (FHHH). The X-axis ranges represent the middle 90% of the Ohio SLE Study (OSS) cohort during no-flare visits. The vertical lines identify the median no-flare, pre-flare, or at-flare values for C4 or C3 in the OSS cohort. Risks are calculated from the estimates of the log odds obtained from the respective prediction equation. Note in (A) that the two risk curves for patients who were C3F+ are negligible, and appear superimposed.
For C3 as a marker of renal flare, six risk curves were drawn based on the FH and CRP genotypes identified as significant, and the same constant values of ESR and age as above (Figure 3B). The scale of the X-axis was based on the middle 90% values of C3 levels in the OSS cohort during noflare visits (54–146 mg/dl). As can be seen, patients with the wildtype CRP 1846GG genotype experienced higher overall risk for renal flare as C3 decreased, similar to what was seen in Figure 3A. The effect of decreasing C3 levels on risk for renal flare was also influenced by the FH genotype. In particular, the risk for flare increased the most for patients with the FH 402HH genotype (7% frequency), with a risk ratio for the median C3 value measured at flare (70 mg/dl) and the median no-flare value (93.0 mg/dl) of 8.0.
Discussion
The current investigation utilized the resources of the Ohio SLE Study, which is a longitudinal study designed to identify forecasters, markers, and causal factors in the pathogenesis of SLE flare through regular bimonthly follow-up of a large cohort of recurrently active SLE patients. Thus, this study provided an opportunity to rigorously assess the temporal relationship between changes in circulating C3 and C4 levels and the onset of lupus nephritis flare. The results of this study indicate that isolated single measurements of C3 and C4 are weak clinical tools in terms of marking a concurrent flare, and cannot forecast an impending flare. Combining serial C3 and C4 measurements with other variables related to inflammation and complement regulation in a multivariate analysis did not provide a much better clinical tool. However, this analysis did offer novel insights into the role of complement and the pathogenesis of renal flare. First and foremost was the finding that lower C4 levels, but not C3 levels, significantly forecasted a renal flare. These observations suggest that the events that trigger renal flare in this immune complex disease involve classical pathway activation at least 2 months before clinical manifestation of the renal flare. In contrast, the finding that lower C3 levels significantly marked a renal flare in the absence of lower C4 levels suggests that the actual tissue damage that is manifested clinically as a renal flare involves amplified C3 activation, and thus predominantly the alternative pathway. Although this postulate remains to be confirmed through serial measurements of other complement proteins (e.g. factor B or Ba plasma levels), this is not unexpected in light of studies showing that deleting either the factor B gene23 or the factor D gene24 of the alternative pathway significantly reduced the degree of renal injury in the MRL/lpr murine lupus model.
The inclusion of other variables in the multivariate analysis provides additional insights into renal flare pathogenesis. The finding that an increased ESR is both an independent forecaster and marker of renal flare suggests that inflammatory processes, possibly driven by classical pathway (C4) activation, are also set in motion at least 2 months prior to clinical manifestations of the renal flare. In contrast to ESR, CRP levels did not increase prior to or at flare. This finding is in agreement with other reports showing a lack of correlation between changes in CRP and SLE disease activity.25,26 The relationship between CRP levels and flare was not influenced by the CRP genotype that affects constitutive CRP expression (1846G > A).
The lack of an increase in CRP at the time of increased ESR suggests that there are other mechanisms that are influencing CRP levels just before and at the time of flare. One mechanism could involve anti-CRP antibodies. These have been reported in 70% of a small SLE cohort, and the anti-CRP levels increase with increased disease activity,27 which could counter any concurrent increase in CRP expression at the time of renal flare. Other mechanisms could relate to the involvement of CRP in the pathogenesis of renal flare. This involvement might be as a clearance protein for apoptotic or cellular debris28,29 that could arise as a consequence of renal flare. CRP could also be involved in flare pathogenesis through a complicated interaction with the complement system, in which CRP can activate the classical pathway30,31 as well as facilitate complement regulation by binding and targeting the complement regulator FH to sites of tissue damage.32 Inclusion of the CRP polymorphism that affects constitutive CRP expression (1846G > A) in the analysis provides some insight into these relationships. The wildtype 1846G allele has been reported to be associated with higher CRP levels, and with protection against SLE onset presumably through enhance clearance of apoptotic debris.13 Our finding that the higher expressing genotype (1846GG) is an independent effector of renal flare, both as a forecaster and marker, suggests that the effect of constitutively high CRP expression on flare pathogenesis in established SLE is different from its effect on the onset of SLE. More specifically, this suggests that CRP may be driving tissue damage through activation of the classical pathway to an extent that supersedes the protective effects of CRP due to FH-mediated complement regulation or clearance of apoptotic debris. As such, higher CRP levels would likely contribute to C4 consumption as renal flare is being triggered. Interestingly in this regard, the CRP 1846GG genotype is more significant as a forecaster of renal flare (p = 0.004) than as a marker of renal flare (p = 0.023).
This study also measured the impact of variation in complement regulation by including in the multivariate analysis the FH Y402H polymorphism. The 402-H allotype is associated with age-related macular degeneration (AMD),20,33,34 presumably due in part to its reduced binding to CRP, leading to less-efficient regulation of C3 activation at damaged cell surfaces.12,35 Here we show that the role of low C3 as a marker of renal flare is influenced by the Y402H polymorphism. Specifically, compared with 402-YY and 402-YH, the genotype encoding 402-HH is associated with the highest increase in flare risk as C3 levels drop from the median no-flare level (Figure 3B). This suggests that as C3 activation accelerates during increased disease activity, inefficient regulation of C3 at renal tissue surfaces leads to significant tissue damage. Thus, lupus renal flare appears to be, in addition to AMD, another pathology involving complement activation that is influenced by the FH Y402H polymorphism. Of interest, many other FH polymorphisms have been found to be associated with AMD, including a haplotype involving deletion of the FH-related genes 1 and 3 that appear to protect against AMD,36 and at least 12 other newly discovered FH single nucleotide polymorphisms that associate with AMD.37 However, these occur in relatively low frequency in the general population, and their roles in lupus nephritis flare were not investigated in the present study.
The C3 gene has a common polymorphism that affects its charge, with the basic form (S allele) being more frequent than the acidic form (F allele). The physiological consequence of this polymorphism is unclear, though studies have reported the F allele being associated with diseases such as IgA nephropathy,38 systemic vasculitis,39 and recently AMD.40,41 The present study found that the C3 S/F polymorphism alone is not a main effector of renal flare. However, an interaction between the C3 S/F polymorphism and CRP levels were identified as a forecaster of flare, in which a lower CRP level is an effector of renal flare in the presence of at least one C3 F allele. This finding contrasts with our observation that constitutive high expression of CRP (the 1846GG genotype) alone is a main effector of renal flare. The fact that the pro-flare effect of lower CRP levels requires the C3 F allele suggests that, in the balance between CRP-mediated complement activation and CRP/FH-mediated C3 regulation, discussed above, regulation predominates if the C3 F allotype is present (18% of the study cohort). This scenario, where the combination of reduced tissue-localized complement regulation (lower CRP levels) and the presence of the C3 F allele increased the risk for renal flare, is similar to a report that the combination of the 402H allele with the C3 F allele (along with a third risk allele) increased the risk for AMD.42 Thus, this is another example where similarity exists between lupus renal flare and AMD with regards to variability in both complement activation and regulation. It should be noted that the present study did not assess the interaction between the FH Y402H and the C3 S/F polymorphisms, as this would have resulted in unacceptably small samples sizes.
In conclusion, using unbiased sample collection through regular and frequent patient follow-up, the current study demonstrates the poor clinical utility of serial serum C3 or C4 measurements alone to forecast or identify an SLE renal flare. Analyzing these variables together along with other related covariates reveals both the complexity by which complement activation and its regulation contribute to the development of renal flare, and how this varies from patient to patient. A further understanding of the role of complement in lupus nephritis flare in individual patients will require an expansion of this multivariate approach, with additional complement-related variables. One such variable is the gene copy number variation (CNV) and associated polymorphisms of complement C4, where individual gene copy numbers range from 0 (null) to 7 in a diploid genome among different individuals.43 C4 CNV is one of the major factors in determining the plasma or serum levels of C4.44,45 Low copy number of C4A is a common genetic risk factor for SLE onset.46 We are currently investigating CNVs of complement C4A and C4B in modifying the disease progression and flare of SLE.
Funding
This work was supported by the National Institutes of Health (P01-DK-55546, R01-DK-074661, R01-AR054459 and UL1RR025755-01).
Footnotes
Disclosure
The authors have no competing financial interests.
References
- 1.Moroni G, Quaglini S, Maccario M, Banfi G, Ponticelli C. “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int. 1996;50:2047–2053. doi: 10.1038/ki.1996.528. [DOI] [PubMed] [Google Scholar]
- 2.Bao L, Quigg RJ. Complement in lupus nephritis: the good, the bad, and the unknown. Semin Nephrol. 2007;27:69–80. doi: 10.1016/j.semnephrol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Esdaile JM, Joseph L, Abrahamowicz M, Li Y, Danoff D, Clarke AE. Routine immunologic tests in systemic lupus erythematosus: is there a need for more studies? J Rheumatol. 1996;23:1891–1896. [PubMed] [Google Scholar]
- 4.Esdaile JM, Abrahamowicz M, Joseph L, MacKenzie T, Li Y, Danoff D. Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail. Arthritis Rheum. 1996;39:370–378. doi: 10.1002/art.1780390304. [DOI] [PubMed] [Google Scholar]
- 5.Ricker D, Hebert L, Rohde R, Sedmak D, Lewis E, Clough J. Serum C3 levels are diagnostically more sensitive and specific for SLE activity than are serum C4 levels. Am J Kidney Dis. 1991;18:678–685. doi: 10.1016/s0272-6386(12)80609-3. [DOI] [PubMed] [Google Scholar]
- 6.Ho A, Barr SG, Magder LS, Petri M. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:2350–2357. doi: 10.1002/1529-0131(200110)44:10<2350::aid-art398>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Tsokos GC. Exploring complement activation to develop bio-markers for systemic lupus erythematosus. Arthritis Rheum. 2004;50:3404–3407. doi: 10.1002/art.20602. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd W, Schur PH. Immune complexes, complement, and anti-DNA in exacerbations of systemic lupus erythematosus (SLE). Medicine (Baltimore) 1981;60:208–217. doi: 10.1097/00005792-198105000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Moroni G, Radice A, Giammarresi G, et al. Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis. 2009;68:234–237. doi: 10.1136/ard.2008.094508. [DOI] [PubMed] [Google Scholar]
- 10.Richards A, Kavanagh D, Atkinson JP. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory states. The examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration. Adv Immunol. 2007;96:141–177. doi: 10.1016/S0065-2776(07)96004-6. [DOI] [PubMed] [Google Scholar]
- 11.Caprioli J, Castelletti F, Bucchioni S, et al. Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet. 2003;12:3385–3395. doi: 10.1093/hmg/ddg363. [DOI] [PubMed] [Google Scholar]
- 12.Laine M, Jarva H, Seitsonen S, et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J Immunol. 2007;178:3831–3836. doi: 10.4049/jimmunol.178.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell AI, Cunninghame Graham DS, Shepherd C, et al. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–147. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botto M, Fong KY, So AK, Koch C, Walport MJ. Molecular basis of polymorphisms of human complement component C3. J Exp Med. 1990;172:1011–1017. doi: 10.1084/jem.172.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rovin BH, Tang Y, Sun J, et al. Clinical significance of fever in the SLE patient receiving steroid therapy. Kidney Int. 2005;68:747–759. doi: 10.1111/j.1523-1755.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 16.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol. 2005;16:467–473. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 17.Rovin BH, Song H, Hebert LA, et al. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–1833. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 18.Birmingham DJ, Gavit KF, McCarty SM, et al. Consumption of erythrocyte CR1 (CD35) is associated with protection against systemic lupus erythematosus renal flare. Clin Exp Immunol. 2006;143:274–280. doi: 10.1111/j.1365-2249.2005.02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birmingham DJ, Nagaraja HN, Rovin BH, et al. Fluctuation in self-perceived stress and increased risk of flare in patients with lupus nephritis carrying the serotonin receptor 1A-1019 G allele. Arthritis Rheum. 2006;54:3291–3299. doi: 10.1002/art.22135. [DOI] [PubMed] [Google Scholar]
- 20.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronaghi M. Pyrosequencing sheds light on DNA sequencing. Genome Res. 2001;11:3–11. doi: 10.1101/gr.11.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Boackle SA, Hanvivadhanakul P, et al. Association of a common complement receptor 2 haplotype with increased risk of systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2007;104:3961–3966. doi: 10.1073/pnas.0609101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe H, Garnier G, Circolo A, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164(2):786–794. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
- 24.Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, Gilkeson GS. Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int. 2004;65:129–138. doi: 10.1111/j.1523-1755.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- 25.Wallace DJ, Hahn BH. Dubois’ Lupus Erythematosus. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. [Google Scholar]
- 26.Williams RC, Jr, Harmon ME, Burlingame R, Du Clos TW. Studies of serum C-reactive protein in systemic lupus erythematosus. J Rheumatol. 2005;32:454–461. [PubMed] [Google Scholar]
- 27.Sjowall C, Bengtsson AA, Sturfelt G, Skogh T. Serum levels of autoantibodies against monomeric C-reactive protein are correlated with disease activity in systemic lupus erythematosus. Arthritis Res Ther. 2004;6:R87–R94. doi: 10.1186/ar1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mold C, Baca R, Du Clos TW. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcgamma receptors. J Autoimmun. 2002;19:147–154. doi: 10.1006/jaut.2002.0615. [DOI] [PubMed] [Google Scholar]
- 29.Gaipl US, Sheriff A, Franz S, et al. Inefficient clearance of dying cells and autoreactivity. Curr Top Microbiol Immunol. 2006;305:161–176. doi: 10.1007/3-540-29714-6_8. [DOI] [PubMed] [Google Scholar]
- 30.Volanakis JE, Kaplan MH. Interaction of C-reactive protein complexes with the complement system. II. Consumption of guinea pig complement by CRP complexes: requirement for human C1q. J Immunol. 1974;113:9–17. [PubMed] [Google Scholar]
- 31.Claus DR, Siegel J, Petras K, Osmand AP, Gewurz H. Interactions of C-reactive protein with the first component of human complement. J Immunol. 1977;119:187–192. [PubMed] [Google Scholar]
- 32.Jarva H, Jokiranta TS, Hellwage J, Zipfel PF, Meri S. Regulation of complement activation by C-reactive protein: targeting the complement inhibitory activity of factor H by an interaction with short consensus repeat domains 7 and 8–11. J Immunol. 1999;163:3957–3962. [PubMed] [Google Scholar]
- 33.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 34.Thakkinstian A, Han P, McEvoy M, et al. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet. 2006;15:2784–2790. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- 35.Skerka C, Lauer N, Weinberger AA, et al. Defective complement control of factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol Immunol. 2007;44:3398–3406. doi: 10.1016/j.molimm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Hageman GS, Hancox LS, Taiber AJ, et al. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38:592–604. [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rambausek M, van den Wall Bake AW, Schumacher-Ach R, et al. Genetic polymorphism of C3 and Bf in IgA nephropathy. Nephrol Dial Transplant. 1987;2:208–211. [PubMed] [Google Scholar]
- 39.Finn JE, Zhang L, Agrawal S, Jayne DR, Oliveira DB, Mathieson PW. Molecular analysis of C3 allotypes in patients with systemic vasculitis. Nephrol Dial Transplant. 1994;9:1564–1567. [PubMed] [Google Scholar]
- 40.Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 41.Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39:1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- 42.Seitsonen SP, Onkamo P, Peng G, et al. Multifactor effects and evidence of potential interaction between complement factor H Y402H and LOC387715 A69S in age-related macular degeneration. PLoS One. 2008;3:e3833. doi: 10.1371/journal.pone.0003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu YL, Savelli SL, Yang Y, et al. Sensitive and specific real-time polymerase chain reaction assays to accurately determine copy number variations (CNVs) of human complement C4A, C4B, C4-long, C4-short, and RCCX modules: elucidation of C4 CNVs in 50 consanguineous subjects with defined HLA genotypes. J Immunol. 2007;179:3012–3025. doi: 10.4049/jimmunol.179.5.3012. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Chung EK, Zhou B, et al. Diversity in intrinsic strengths of the human complement system: serum C4 protein concentrations correlate with C4 gene size and polygenic variations, hemolytic activities, and body mass index. J Immunol. 2003;171:2734–2745. doi: 10.4049/jimmunol.171.5.2734. [DOI] [PubMed] [Google Scholar]
- 45.Wu YL, Higgins GC, Rennebohm RM, et al. Three distinct profiles of serum complement C4 proteins in pediatric systemic lupus erythematosus (SLE) patients: tight associations of complement C4 and C3 protein levels in SLE but not in healthy subjects. Adv Exp Med Biol. 2006;586:227–247. doi: 10.1007/0-387-34134-X_16. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Chung EK, Wu YL, et al. Gene copy-number variation and associated polymorphisms of complement component C4 in human systemic lupus erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against SLE susceptibility in European Americans. Am J Hum Genet. 2007;80:1037–1054. doi: 10.1086/518257. [DOI] [PMC free article] [PubMed] [Google Scholar]