Abstract
High-density lipoprotein (HDL) cholesterol and its apolipoproteins each capture unique lipid and cardiometabolic information important to risk quantification. It was hypothesized that metabolic factors, including insulin resistance and type 2 diabetes, would confound the association of HDL cholesterol with coronary artery calcification (CAC) and that apolipoprotein A-I (apoA-I) and/or apolipoprotein A-II (apoA-II) would add to HDL cholesterol in predicting CAC. Two community-based cross-sectional studies of white subjects were analyzed: the Penn Diabetes Heart Study (PDHS; n = 611 subjects with type 2 diabetes, 71.4% men) and the Study of Inherited Risk of Coronary Atherosclerosis (SIRCA; n = 803 subjects without diabetes, 52.8% men) using multivariable analysis of apoA-I, apoA-II, and HDL cholesterol stratified by diabetes status. HDL cholesterol was inversely associated with CAC after adjusting for age and gender in whites with type 2 diabetes (tobit ratio for a 1-SD increase in HDL cholesterol 0.58, 95% confidence interval [CI] 0.44 to 0.77, p <0.001) as well as those without diabetes (tobit ratio 0.72, 95% CI 0.59 to 0.88, p = 0.001). In contrast, apoA-I was a weaker predictor in subjects with (tobit ratio 0.64, 95% CI 0.45 to 0.90, p = 0.010) and without (tobit ratio 0.79, 95% CI 0.66 to 0.94, p = 0.010) diabetes, while apoA-II had no association with CAC. Control for metabolic variables, including triglycerides, waist circumference, and homeostasis model assessment of insulin resistance, attenuated these relations, particularly in subjects without diabetes. In likelihood ratio test analyses, HDL cholesterol added to apoA-I, apoA-II, and atherogenic apolipoprotein B lipoproteins but improved CAC prediction over metabolic factors only in subjects with diabetes. In conclusion, HDL cholesterol outperformed apoA-I and apoA-II in CAC prediction, but its association with CAC was attenuated by measures of insulin resistance.
There is growing interest in alternatives to high-density lipoprotein (HDL) cholesterol measurement,1 and data indicate that metabolic markers confound the inverse link of HDL parameters to atherosclerotic coronary heart disease (CHD).2 In studies of subjects with and without type 2 diabetes mellitus (T2DM) and free of clinical CHD, we examined the associations of HDL parameters with coronary artery calcification (CAC), a quantitative index of atherosclerotic burden that strongly correlates with risk for CHD.3 We addressed the hypotheses that apolipoprotein A-I (apoA-I) and/or apolipoprotein A-II (apoA-II) add to HDL cholesterol in CAC prediction and that metabolic factors, particularly measures of insulin resistance, confound the association of HDL parameters with CAC.
Methods
Details of the Penn Diabetes Heart Study (PDHS) and the Study of Inherited Risk of Coronary Atherosclerosis (SIRCA) have been described previously, including in our report that apolipoprotein B is superior to low-density lipoprotein cholesterol in predicting CAC in T2DM.3,4 These community-based cross-sectional studies were coordinated at the University of Pennsylvania using the same research center, staff, computed tomographic scanner, and lipid laboratory. Inclusion criteria included age fourth to eighth decades and a family history of premature CHD in SIRCA or the presence of T2DM in PDHS. Exclusion criteria included a clinical history of CHD (as evidenced by myocardial infarction, coronary revascularization, angiographic disease, or positive stress test results), elevated creatinine in both studies and, in SIRCA only, the presence of T2DM. This report focuses on unrelated white subjects (n = 611 with and n = 803 without T2DM). The studies were approved by the University of Pennsylvania institutional review board, and informed consent was obtained from all participants.
Participants were evaluated at the General Clinical Research Center of the University of Pennsylvania Medical Center after a 12-hour overnight fast. In the University of Pennsylvania's Centers for Disease Control and Prevention–certified lipid laboratory, standard lipid panels and apolipoproteins were measured enzymatically (Cobas Fara II; Roche Diagnostic Systems, Somerville, New Jersey) in lipoprotein fractions after ultracentrifugation (β-quantification technique) in PDHS and in whole serum in SIRCA. ApoA-I, apoA-II, and high-sensitivity C-reactive protein were assayed by immunoturbidimetric assays. Plasma levels of leptin, adiponectin, and insulin were measured by enzyme-linked immunosorbent assays (Linco Research, St. Charles, Missouri). Laboratory test results were generated by personnel blinded to the clinical characteristics and CAC scores of research subjects.
Clinical parameters, including blood pressure, body mass index, and waist circumference were assessed as previously reported.3,4 Framingham risk scores were calculated using low-density lipoprotein cholesterol (similar results were obtained using total cholesterol). Homeostasis model assessment of insulin resistance (HOMA-IR; fasting glucose [mmol/L] × fasting insulin [mU/ml]/22.5) was used as a measure of insulin resistance. Subjects with T2DM taking insulin were excluded for the calculation of HOMA-IR, yielding a sample size of 513. Subjects were classified as having metabolic syndrome using the revised National Cholesterol Education Program definition (glucose cut point 100 mg/dl).3 All subjects with T2DM were assumed to meet the metabolic syndrome criterion for glycemia. Global Agatston CAC scores were quantified by electron-beam computed tomography, as described.3,4
Data are reported as median (interquartile range) or as mean ± SD for continuous variables and as proportions for categorical variables. Chi-square and Mann-Whitney tests were used to compare variables by T2DM status. The crude association of apolipoproteins and HDL cholesterol with lipid, metabolic, and inflammatory parameters was examined by Spearman's correlation.
Multivariable analysis of CAC scores was performed using tobit conditional regression to accommodate the unusual distribution of CAC data (many zero scores but also a marked right skew).3,4 Tobit regression combines a logistic regression of the presence of CAC (any CAC present vs CAC zero score) with a linear regression (of log-transformed CAC) when CAC is present to produce a single estimate for the relation of risk factors with CAC data. A tobit ratio of 0.80 indicates a 20% reduction in the CAC score for every SD increase in a lipid parameter. Multivariable associations of apoA-I, apoA-II, and HDL cholesterol levels with CAC were assessed in incremental tobit models: model 1 was adjusted for age and gender; model 2 was additionally adjusted for medications (statins, niacin, fibrates, insulin, metformin, thiazolidinediones, sulfonylureas, and, in women, hormone replacement therapy; each medication was adjusted for individually), systolic and diastolic blood pressure, low-density lipoprotein cholesterol, tobacco use, alcohol use, exercise, family history of premature CHD, body mass index, waist circumference, triglycerides, adiponectin, leptin, and high-sensitivity C-reactive protein; model 3 added HOMA-IR (subjects taking insulin were excluded from T2DM analyses). Given significant interaction by T2DM status for HDL cholesterol association with CAC (interaction p = 0.007 in age- and gender-adjusted model, p = 0.035 in fully adjusted model), results are stratified by T2DM status. Non-normal variables were log transformed (triglycerides, leptin, high-sensitivity C-reactive protein, and HOMA-IR).
Interactions of apolipoproteins and HDL cholesterol with T2DM, gender, statin use, and triglycerides in association with CAC were assessed by likelihood ratio testing. We applied likelihood ratio testing in nested models to assess the incremental value of apoA-I, apoA-II, and HDL cholesterol over other lipoprotein parameters as well as measures of insulin resistance and adiposity in predicting CAC scores. Statistical analyses were performed using Stata version 10.0 (StataCorp LP, College Station, Texas).
Results
Table 15,6 lists study sample characteristics by T2DM status. Subjects with T2DM were older, were more likely male, were more obese, had a greater a prevalence of metabolic syndrome, and had lower insulin sensitivity as assessed by HOMA-IR. Total and low-density lipoprotein cholesterol were lower in those with T2DM, likely because of greater statin use. ApoA-I, apoA-II, and HDL cholesterol levels were significantly lower in patients with T2DM versus subjects without T2DM. In those with T2DM, median glycosylated hemoglobin was 6.8%. A difference in the presence of CAC by T2DM status disappeared after age and gender adjustment in logistic regression (p = 0.79). Referencing subjects aged ≥45 years to Multi-Ethnic Study of Atherosclerosis (MESA) percentiles,7 our median woman with T2DM was at the 69th percentile, median man with T2DM at the 72nd percentile, median woman without T2DM at the 76th percentile, and median man without T2DM at the 78th percentile, suggesting that family history of premature CAD and T2DM are associated with higher CAC burden.
Table 1.
Characteristics of study sample
| Variable | Subjects Without T2DM (n = 803) | Subjects With T2DM (n = 611) | p Value |
|---|---|---|---|
| Age (years) | 48 (42–54) | 60 (54–68) | <0.001 |
| Men | 52.8% | 71.4% | <0.001 |
| Alcohol use | 67.8% | 58.4% | <0.001 |
| Current smoker | 11.3% | 8.4% | 0.07 |
| HDL cholesterol (mg/dl) | 48 (39–59) | 45 (37–53) | <0.001 |
| ApoA-I (mg/dl) | 128 (110–150) | 124 (113–137) | <0.001 |
| ApoA-II (mg/dl) | 34 (31–37) | 32 (29–35) | <0.001 |
| Total cholesterol (mg/dl) | 205 (177–228) | 174 (152–198) | <0.001 |
| Triglycerides (mg/dl) | 117 (87–159) | 134 (92–197) | <0.001 |
| Low-density lipoprotein cholesterol (mg/dl) | 126 (103–148) | 97 (79–119) | <0.001 |
| ApoB (mg/dl) | 98 (84–114) | 82 (71–94) | <0.001 |
| ApoB/apoA-I ratio | 0.76 (0.61–0.94) | 0.66 (0.55–0.77) | <0.001 |
| Blood pressure (mm Hg) | |||
| Systolic | 126 (117–136) | 131 (122–140) | <0.001 |
| Diastolic | 77 (72–84) | 75 (71–81) | <0.001 |
| Body mass index (kg/m2) | 27 (24–30) | 32 (28–36) | <0.001 |
| Waist circumference (cm) | 89 (81–99) | 107 (98–117) | <0.001 |
| Metabolic syndrome | 25.8% | 76.6% | <0.001 |
| Leptin (ng/ml) | 8.4 (4.5–16.4) | 11.7 (6.5–20.9) | <0.001 |
| Adiponectin (μg/ml) | 16.4 (11.6–24.6) | 9.1 (6.1–14.9) | <0.001 |
| HOMA-IR | 1.4 (0.9–2.1) | 4.2 (2.8–6.2) | <0.001 |
| High-sensitivity | 1.2 (0.5–2.6) | 1.6 (0.8–3.4) | <0.001 |
| C-reactive protein (mg/dl) | |||
| Interleukin-6 (pg/ml) | 1.3 (0.8–1.9) | 1.3 (0.8–2.1) | 0.34 |
| 10-year Framingham risk | 5% (3%–8%) | 13% (8%–20%) | <0.001 |
| Medications | |||
| Statin | 14.0% | 57.5% | <0.001 |
| Niacin | 3.0% | 5.6% | 0.02 |
| Fibrate | 1.1% | 10.0% | <0.001 |
| Insulin | 14.9% | ||
| Metformin | 63.8% | ||
| Thiazolidinedione | 27.3% | ||
| Sulfonylurea | 40.3% | ||
| Hormone replacement therapy (women) | 28.2% | 45.1% | <0.001 |
| CAC | 3 (0–45) | 89 (1–456) | <0.001 |
| >0 | 68.9% | 75.3% | 0.008 |
| >100 | 16.4% | 49.1% | <0.001 |
| ln(CAC) if CAC >0 | 2.8 ± 2.1 | 5.2 ± 1.8 | <0.001 |
Data are expressed as median (interquartile range), percentages, or mean ± SD. As a reference, the National Cholesterol Education Program Adult Treatment Panel III5 classifies HDL cholesterol levels <40 mg/dl as “low” and ≥60 mg/dl as “high”; low-density lipoprotein cholesterol levels <100 mg/dl are classified as “optimal,” 100 to 129 mg/dl as “near or above optimal,” 130 to 159 mg/dl as “borderline high,” 160 to 189 mg/dl as “high,” and ≥190 mg/dl as “very high.” Regarding apolipoprotein reference values, the 2009 Canadian guidelines6 identify an apolipoprotein B level <80 mg/dl and an apolipoprotein B/apo A-I ratio <0.8 as therapeutic targets.
apo = apolipoprotein; CAC = coronary artery calcium; HOMA-IR = homeostasis model assessment of insulin resistance.
Supplementary Appendix: Table A1 lists Spearman's correlations for the interrelation of HDL cholesterol, apoA-I, and apoA-II and their relations with other CHD risk factors by T2DM status. Correlation of HDL cholesterol was higher with apoA-I compared to apoA-II (p <0.001 in subjects with and without T2DM). Triglycerides, adiposity, and HOMA-IR were inversely correlated with HDL cholesterol and apoA-I and less so with apoA-II in subjects with and without T2DM. HDL cholesterol and its apolipoproteins were correlated modestly, or not at all, with high-sensitivity C-reactive protein.
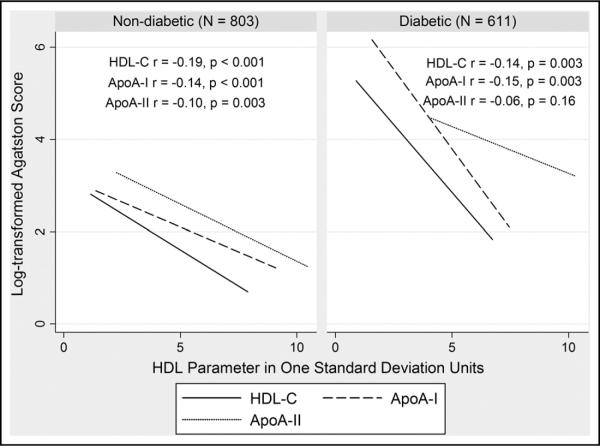
Unadjusted inverse correlations of HDL parameters with CAC are shown in Figure 1 by diabetes status. HDL cholesterol was inversely associated with CAC after adjusting for age and gender in whites with and without T2DM (Table 2, model 1). In contrast, apoA-I displayed a weaker inverse relation in subjects with and without T2DM, while apoA-II had no association with CAC. After adjustment for risk factors, the associations of HDL cholesterol and apoA-I with CAC were attenuated (Table 2, model 2) with a trend for HDL cholesterol prediction of CAC remaining in those with T2DM but not those without T2DM. This difference was not explained by the higher risk factor milieu in T2DM, as the association of HDL cholesterol with CAC was stronger in subjects with T2DM below the median level of Framingham risk (tobit ratio 0.53, 95% confidence interval 0.29 to 0.95, p = 0.03 in model 2).
Figure 1.
Unadjusted association of HDL cholesterol (HDL-C), apoA-I, and apoA-II, with Agatston score by diabetes status. One standard deviation for HDL cholesterol is 14.70 mg/dl, for apoA-I is 26.16 mg/dl, and for apoA-II is 4.86 mg/dl.
Table 2.
Association of plasma levels of high-density lipoprotein cholesterol and its apolipoproteins with coronary calcium in tobit multivariable models
| Variables Adjusted for | Ratio in Subjects Without T2DM (95% CI) |
Ratio in Subjects With T2DM (95% CI) |
||
|---|---|---|---|---|
| (n = 803) | p Value | (n = 611) | p Value | |
| HDL cholesterol | ||||
| Age, gender | 0.72 (0.59–0.88) | 0.001 | 0.58 (0.44–0.77) | <0.001 |
| Age, gender, medications, BP, LDL-C, tob, EtOH, exercise, FH, RF | 0.89 (0.70–1.11) | 0.30 | 0.72 (0.51–1.02) | 0.07 |
| Age, gender, medications, BP, LDL-C, tob, EtOH, exercise, FH, RF, HOMA-IR | 0.90 (0.72–1.14) | 0.40 | 0.84 (0.58–1.22) | 0.36 |
| ApoA-I | ||||
| Age, gender | 0.79 (0.66–0.94) | 0.01 | 0.64 (0.45–0.90) | 0.01 |
| Age, gender, medications, BP, LDL-C, tob, EtOH, exercise, FH, RF | 0.86 (0.71–1.04) | 0.11 | 0.82 (0.57–1.16) | 0.26 |
| Age, gender, medications, BP, LDL-C, tob, EtOH, exercise, FH, RF, HOMA-IR | 0.88 (0.73–1.07) | 0.19 | 0.92 (0.63–1.32) | 0.64 |
| ApoA-II | ||||
| Age, gender | 0.87 (0.72–1.05) | 0.16 | 0.96 (0.76–1.21) | 0.75 |
| Age, gender, medications, BP, LDL-C, tob, EtOH, exercise, FH, RF | 0.83 (0.68–1.01) | 0.06 | 0.88 (0.68–1.13) | 0.32 |
| Age, gender, medications, BP, LDL-C, tob, EtOH, exercise, FH, RF, HOMA-IR | 0.83 (0.69–1.01) | 0.06 | 0.91 (0.70–1.18) | 0.49 |
Results of tobit regression are presented as the ratio of increase in CAC score for 1-SD increases in HDL cholesterol (14.70 mg/dl), apoA-I (26.16 mg/dl), or apoA-II (4.86 mg/dl); SD for pooled cohort standardizes comparison across T2DM status. Medications included statins, niacin, fibrates, insulin, metformin, thiazolidinediones, sulfonylureas, and hormone replacement therapy.
apo = apolipoprotein; BP = systolic and diastolic blood pressures; EtOH = alcohol use; FH = family history of premature CHD; HOMA-IR = homeostasis model assessment of insulin resistance; LDL-C = low-density lipoprotein cholesterol; RF = other risk factors (triglycerides, body mass index, waist circumference, adiponectin, leptin, and high-sensitivity C-reactive protein); tob = tobacco use.
The associations of HDL parameters with CAC in T2DM was attenuated further after adjusting for HOMA-IR (analysis excluded subjects taking insulin; Table 2, model 3). When confounders from Table 2 beyond age and gender were examined individually, metabolic variables, namely, triglycerides, body mass index, waist circumference, and HOMA-IR, most strongly attenuated HDL parameter associations with CAC (Supplementary Appendix Table A2). In fact, with baseline control for age and gender, further adjustment for any 1 of these 4 metabolic factors led to complete loss of the association of HDL cholesterol with CAC in subjects without T2DM. In contrast, relative to an age- and gender-controlled model of CAC prediction by HDL cholesterol (Table 2, model 1), further adjustment for high-sensitivity C-reactive protein did not substantially alter estimates: 0.56 (95% confidence interval 0.42 to 0.74, p <0.001) in subjects with T2DM and 0.75 (95% confidence interval 0.62 to 0.92, p = 0.005) in those without T2DM (similarly, there was no significant attenuation by high-sensitivity C-reactive protein on the association of apoA-I with CAC in model 1).
Table 3 lists findings, stratified by diabetes status, for the incremental value of adding HDL cholesterol, apoA-I, or apoA-II to one another or additional lipid and metabolic parameters. To a greater extent in T2DM, HDL cholesterol improved CAC prediction when added to apoA-I, apoA-II, total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein B. In patients with T2DM, but not in those without, HDL cholesterol added to triglycerides, waist circumference, and HOMA-IR in predicting CAC. In contrast, neither apoA-I nor apoA-II added value to HDL cholesterol, waist circumference, or HOMA-IR. In a CAC model adjusted for age, gender, medications, and diabetes, adding the apolipoprotein B/apoA-I ratio provided essentially the same predictive value as adding apolipoprotein B alone (likelihood ratio test chi-squares 31.7 and 32.9, respectively, p <0.001 for both), whereas when apoA-I was added as an individual variable to apolipoprotein B in this same model, it provided incremental value (chi-square 6.3, p <0.001).
Table 3.
Incremental value of high-density lipoprotein cholesterol and its apolipoproteins over lipoprotein and metabolic parameters in coronary calcium prediction
| Added to | HDL Cholesterol |
ApoA-I |
ApoA-II |
|||
|---|---|---|---|---|---|---|
| No T2DM (n = 803) | T2DM (n = 611) | No T2DM (n = 803) | T2DM (n = 611) | No T2DM (n = 803) | T2DM (n = 611) | |
| HDL cholesterol | — | — | 0.01 | 0.74 | 0.03 | 0.49 |
| ApoA-I | 4.09* | 8.59† | — | — | 0.11 | 0.00 |
| ApoA-II | 8.24† | 11.20‡ | 4.38* | 2.86 | — | — |
| Total cholesterol | 17.10‡ | 13.49‡ | 13.93‡ | 5.12* | 8.21† | 2.15 |
| Low-density lipoprotein cholesterol | 9.35† | 12.13‡ | 6.78* | 4.21* | 4.07* | 1.54 |
| ApoB | 4.15* | 9.43† | 6.37* | 3.97* | 4.38* | 1.91 |
| Triglycerides | 3.06 | 4.46* | 4.56* | 2.37 | 3.85* | 1.88 |
| Waist circumference | 2.39 | 8.10† | 2.98 | 3.12 | 1.60 | 0.89 |
| HOMA-IR | 2.64 | 4.47* | 2.12 | 1.38 | 2.26 | 0.30 |
Likelihood ratio testing was applied in nested tobit models primarily to assess the incremental value of HDL cholesterol over its apolipoproteins, and vice versa, in predicting CAC scores. All models presented in the table included age, gender, and medications. Chi-square values are presented.
p <0.05
p <0.01
p <0.001.
Discussion
We report that plasma levels of HDL cholesterol were superior to those of its apolipoproteins, apoA-I and apoA-II, in predicting the extent of CAC in asymptomatic white subjects with and without T2DM. Nonetheless, no HDL parameter remained independently predictive of CAC after fully accounting for cardiometabolic variables, with particular attenuation by triglycerides, adiposity, and the HOMA-IR measure of insulin resistance. Overall, these results challenge the hypothesis that apoA-I or apoA-II add value to HDL cholesterol levels as markers of atherosclerotic CHD and question whether HDL cholesterol is a truly independent CHD marker.
Over the decades since the original prospective epidemiologic studies linking HDL cholesterol levels inversely to CHD,8–10 it has been debated whether this relation represents causality or an epiphenomenon. Supportive of the latter, mouse11 and human12 HDL cholesterol deficiency do not result in an obvious premature atherosclerotic phenotype, and when the cholesteryl ester transfer protein inhibitor torcetrapib was used to increase HDL cholesterol in humans,13 there was no evidence for a protective effect, although off-target adverse events confounded results for this specific cholesteryl ester transfer protein inhibitor. While data from the small Framingham Offspring Study (n = 454) demonstrated an association between the change in HDL cholesterol and CHD outcomes in patients taking lipid-modifying agents,14 a meta-analysis of 108 randomized trials involving 299,310 participants did not.15
Our results, and others,2 indicate significant confounding of CHD's association with HDL parameters by other metabolic factors. As in El Harchaoui et al's2 nested case-control analysis, triglyceride levels were an operative confounder in our study, as were waist circumference and HOMA-IR, such that HDL parameters lost association with CAC. It is possible that, with larger samples, increased power would identify modest significant associations independent of metabolic confounders. The degree of attenuation, however, by metabolic parameters is striking. Although prospective epidemiologic studies10 tended to include triglycerides and measures of adiposity (in some cases, simply weight), they did not include measures of insulin resistance. If HDL cholesterol's relation to CHD is partially driven by insulin resistance, then one would expect HDL cholesterol to more strongly confer CHD risk in patients with T2DM relative to subjects without T2DM, as we observed in our less adjusted CAC models. Such differences between subjects with and without T2DM should be reduced with adjustment for measures of insulin resistance, as we also saw in our data.
Whether or not HDL cholesterol is truly independently related to CHD, given its strong association with other cardiometabolic factors, HDL cholesterol integrates a large amount of risk information into a single measurable phenotype. It helps mark patients with atherogenic dyslipidemia who remain at high residual risk for vascular events despite meeting low-density lipoprotein cholesterol goals.16 In this role as a marker of residual risk, an important question is the utility of HDL parameters beyond HDL cholesterol.1 Clinically available alternatives to cholesterol-based testing include the quantification of HDL-associated apoA-I and apoA-II, which have independent functions and track roughly with total (apoA-I) and dense (apoA-II) HDL particle number.17,18 They are standardized3 and may be less influenced than HDL cholesterol by hypertriglyceridemia and cholesteryl ester transfer protein activation in insulin-resistant states.
Our study is novel in examining the CAC relations for apoA-I and A-II while extending previous smaller studies of HDL cholesterol. Previous studies of the association of HDL cholesterol with CAC were inconclusive.19–22 In subjects with T2DM, the Patients With Renal Impairment and Diabetes Undergoing Computed Tomography (PREDICT) study19 of 495 subjects with T2DM showed no relation of HDL cholesterol, or any measured lipid parameter, with the extent of CAC in a multivariable model. When Wagenknecht et al20 examined 97 subjects with T2DM, they found an inverse relation of HDL cholesterol with the extent of CAC in a model controlled for age. In subjects without T2DM, Arad et al21 reported a univariate inverse relation of HDL cholesterol with the extent of CAC in 1,160 asymptomatic men and women, lost in a multivariable model including central adiposity and other CHD risk factors. Finally, Hecht et al22 found no correlation of HDL cholesterol levels with CAC in 930 asymptomatic subjects without T2DM. Our findings, synthesized with these previous studies, highlight confounding in HDL cholesterol's relation with CAC (particularly by metabolic factors) and are novel in demonstrating a lack of added value in measuring apoA-I and A-II. We note that apoA-II trended toward significant CAC association with more adjusted models in subjects without T2DM, which may be due to model fitting or to less influence of confounders. Given limited and conflicting data for apoA-II in CHD risk prediction, greater follow-up in larger studies with clinical events is warranted.
Moving beyond subclinical to clinical CHD, numerous retrospective studies report substantially lower apoA-I levels in patients with CHD.23 Those that attempted to assess the relative associations of apoA-I and HDL cholesterol with CHD yielded conflicting results.23 ApoA-II is less extensively studied but is also inversely associated with CHD in subjects without24 and with25 T2DM, although 1 study suggested a positive association with angiographic CAD.26 Data also compare HDL cholesterol versus its apolipoproteins in predicting CHD outcomes. The Casale Monferrato Study27 looked at 11-year CHD mortality in 1,565 Mediterranean subjects with T2DM and showed that HDL cholesterol, but not apoA-I, predicted outcomes after controlling for age, gender, and CHD risk factors. In populations without T2DM, Rader et al23 reviewed 6 prospective, pre-mid-1990s studies of the correlation of apoA-I with primary CHD events. All demonstrated inverse correlation, and 3 of the 6 directly compared apoA-I and HDL cholesterol, each concluding that apoA-I was no better than HDL cholesterol. More recently, the Atherosclerosis Risk in Communities (ARIC) study28 but not the Prospective Epidemiological Study of Myocardial Infarction (PRIME)29 reached similar conclusions, and thus the balance of evidence is against added value for apoA-I over HDL cholesterol in discriminating primary CHD risk. Similarly, although with fewer data, apoA-II did not predict future CHD risk30 or did not improve on traditional risk factors.18 Our results for subclinical atherosclerosis in asymptomatic subjects agree with these outcomes studies.
Our study had several limitations. Analyses were cross-sectional, thus causal and longitudinal relations were not addressed. Our modeling was based on the assumption that a cross-sectional representation of risk factors reflects previous levels and exposures that contributed to atherosclerosis over time. Given lipid and CAC variability by race,3 our findings cannot be generalized beyond whites. Also, CAC is an estimate and not a direct measure of coronary atherosclerosis and thus may fail to detect some coronary atherosclerotic plaques. In our study, there was differential statin use between participants with and without T2DM, although we adjusted for statins in our analyses. Finally, HOMA-IR is a surrogate index of insulin sensitivity.
Supplementary Material
Footnotes
Supplementary Data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.amjcard.2010.09.033.
References
- 1.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 3.Martin SS, Qasim AN, Mehta NN, Wolfe M, Terembula K, Schwartz S, Iqbal N, Schutta M, Bagheri R, Reilly MP. Apolipoprotein B but not LDL cholesterol is associated with coronary artery calcification in type 2 diabetic whites. Diabetes. 2009;58:1887–1892. doi: 10.2337/db08-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly MP, Wolfe ML, Localio AR, Rader DJ. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis. 2004;173:69–78. doi: 10.1016/j.atherosclerosis.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, Couture P, Dufour R, Fodor G, Francis GA, Grover S, Gupta M, Hegele RA, Lau DC, Leiter L, Lewis GF, Lonn E, Mancini GB, Ng D, Pearson GJ, Sniderman A, Stone JA, Ur E. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can J Cardiol. 2009;25:567–579. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 8.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 9.Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 10.Barter PJ, Rye KA. High density lipoproteins and coronary heart disease. Atherosclerosis. 1996;121:1–12. doi: 10.1016/0021-9150(95)05675-0. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Reddick RL, Maeda N. Lack of apoA-I is not associated with increased susceptibility to atherosclerosis in mice. Arterioscler Thromb. 1993;13:1814–1821. doi: 10.1161/01.atv.13.12.1814. [DOI] [PubMed] [Google Scholar]
- 12.Frikke-Schmidt R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis. 2010;208:305–316. doi: 10.1016/j.atherosclerosis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 14.Grover SA, Kaouache M, Joseph L, Barter P, Davignon J. Evaluating the incremental benefits of raising high-density lipoprotein cholesterol levels during lipid therapy after adjustment for the reductions in other blood lipid levels. Arch Intern Med. 2009;169:1775–1780. doi: 10.1001/archinternmed.2009.328. [DOI] [PubMed] [Google Scholar]
- 15.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, Whitt I, Alves da Silva S, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O'Regan C, Mills EJ, Bucher HC, Montori VM, Guyatt GH. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche JM, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A, Zimmet P. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102(suppl):1K–34K. doi: 10.1016/S0002-9149(08)01833-X. [DOI] [PubMed] [Google Scholar]
- 17.Albers JJ, Aladjem F. Precipitation of 125 I-labeled lipoproteins with specific polypeptide antisera. Evidence for two populations with differing polypeptide compositions in human high density lipoproteins. Biochemistry. 1971;10:3436–3442. doi: 10.1021/bi00794a019. [DOI] [PubMed] [Google Scholar]
- 18.Birjmohun RS, Dallinga-Thie GM, Kuivenhoven JA, Stroes ES, Otvos JD, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation. 2007;116:2029–2035. doi: 10.1161/CIRCULATIONAHA.107.704031. [DOI] [PubMed] [Google Scholar]
- 19.Elkeles RS, Feher MD, Flather MD, Godsland IF, Nugara F, Richmond W, Rubens MB, Wang D. The association of coronary calcium score and conventional cardiovascular risk factors in type 2 diabetic subjects asymptomatic for coronary heart disease (the PREDICT study) Diabet Med. 2004;21:1129–1134. doi: 10.1111/j.1464-5491.2004.01409.x. [DOI] [PubMed] [Google Scholar]
- 20.Wagenknecht LE, Bowden DW, Carr JJ, Langefeld CD, Freedman BI, Rich SS. Familial aggregation of coronary artery calcium in families with type 2 diabetes. Diabetes. 2001;50:861–866. doi: 10.2337/diabetes.50.4.861. [DOI] [PubMed] [Google Scholar]
- 21.Arad Y, Newstein D, Cadet F, Roth M, Guerci AD. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron-beam computed tomographic study. Arterioscler Thromb Vasc Biol. 2001;21:2051–2058. doi: 10.1161/hq1201.100257. [DOI] [PubMed] [Google Scholar]
- 22.Hecht HS, Superko HR, Smith LK, McColgan BP. Relation of coronary artery calcium identified by electron beam tomography to serum lipoprotein levels and implications for treatment. Am J Cardiol. 2001;87:406–412. doi: 10.1016/s0002-9149(00)01392-8. [DOI] [PubMed] [Google Scholar]
- 23.Rader DJ, Hoeg JM, Brewer HB., Jr. Quantitation of plasma apolipoproteins in the primary and secondary prevention of coronary artery disease. Ann Intern Med. 1994;120:1012–1025. doi: 10.7326/0003-4819-120-12-199406150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Buring JE, O'Connor GT, Goldhaber SZ, Rosner B, Herbert PN, Blum CB, Breslow JL, Hennekens CH. Decreased HDL2 and HDL3 cholesterol, apo A-I and apo A-II, and increased risk of myocardial infarction. Circulation. 1992;85:22–29. doi: 10.1161/01.cir.85.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Syvanne M, Kahri J, Virtanen KS, Taskinen MR. HDLs containing apolipoproteins A-I and A-II (LpA-I:A-II) as markers of coronary artery disease in men with non-insulin-dependent diabetes mellitus. Circulation. 1995;92:364–370. doi: 10.1161/01.cir.92.3.364. [DOI] [PubMed] [Google Scholar]
- 26.Alaupovic P, Mack WJ, Knight-Gibson C, Hodis HN. The role of triglyceride-rich lipoprotein families in the progression of athero-sclerotic lesions as determined by sequential coronary angiography from a controlled clinical trial. Arterioscler Thromb Vasc Biol. 1997;17:715–722. doi: 10.1161/01.atv.17.4.715. [DOI] [PubMed] [Google Scholar]
- 27.Bruno G, Merletti F, Biggeri A, Bargero G, Prina-Cerai S, Pagano G, Cavallo-Perin P. Effect of age on the association of non-high-density-lipoprotein cholesterol and apolipoprotein B with cardiovascular mortality in a Mediterranean population with type 2 diabetes: the Casale Monferrato study. Diabetologia. 2006;49:937–944. doi: 10.1007/s00125-006-0195-6. [DOI] [PubMed] [Google Scholar]
- 28.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 29.Luc G, Bard JM, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P. Value of HDL cholesterol, apolipoprotein A-I, lipoprotein A-I, and lipoprotein A-I/A-II in prediction of coronary heart disease: the PRIME study. Arterioscler Thromb Vasc Biol. 2002;22:1155–1161. doi: 10.1161/01.atv.0000022850.59845.e0. [DOI] [PubMed] [Google Scholar]
- 30.Sweetnam PM, Bolton CH, Downs LG, Durrington PN, MacKness MI, Elwood PC, Yarnell JW. Apolipoproteins A-I, A-II and B, lipoprotein(a) and the risk of ischaemic heart disease: the Caerphilly study. Eur J Clin Invest. 2000;30:947–956. doi: 10.1046/j.1365-2362.2000.00725.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.