Abstract
The role of STAT3 in infectious diseases remains undetermined, in part because unphosphorylated STAT3 has been considered an inactive protein. Here, we report that unphosphorylated STAT3 contributes to cholinergic anti-inflammation, prevents systemic inflammation, and improves survival in sepsis. Bacterial endotoxin induced STAT3 tyrosine phosphorylation in macrophages. Both alpha7 nicotinic receptor (alpha7nAChR) activation and inhibition of JAK2 blunt STAT3 phosphorylation. Inhibition of STAT3 phosphorylation mimicked the alpha7nAChR signaling, inhibiting NF-κB and cytokine production in macrophages. Transfection of macrophages with the dominant-negative mutant STAT3F, to prevent its tyrosine phosphorylation, reduced TNF production but did not prevent the alpha7nAChR signaling. However, inhibition of STAT3 protein expression enhanced cytokine production and abrogated alpha7nAChR signaling. Alpha7nAChR controls TNF production in macrophages through a mechanism that requires STAT3 protein expression, but not its tyrosine phosphorylation. In vivo, inhibition of STAT3 tyrosine phosphorylation by stattic prevented systemic inflammation and improved survival in experimental sepsis. Stattic also prevented the production of late mediators of sepsis and improved survival in established sepsis. These results reveal the immunological implications of tyrosine-unphosphorylated STAT3 in infectious diseases.
Keywords: Alpha7 nicotinic receptor, Inflammation, Sepsis, STAT3, TNF
Introduction
Sepsis is the third leading cause of death in developed societies affecting 750 000 patients and accounting for 9.3% of the overall annual death toll in the United States [1, 2]. Even with modern antimicrobial therapies and new technologies, sepsis is still a major cause of death in actual medicine [1]. Originating from an infection, sepsis is characterized by systemic immune responses leading to multiple organ failure. During an infection, immune cells produce inflammatory cytokines and excessive cytokine release causes organ damage and dysfunction [3–6]. TNF is believed to be a major factor triggering septic shock as TNF inhibition prevents it [3]. Strategies to neutralize TNF have been successful in other inflammatory disorders including rheumatoid arthritis, Crohn's disease, ankylosing spondylitis, and psoriasis [6, 7]. However, these strategies produce modest effects in clinical trials for sepsis. Consistently, endotoxin is lethal in TNF-deficient mice and TNF neutralization fails to prevent mortality in polymicrobial peritonitis [8–10]. A potential explanation for the failure of anti-TNF strategies is that sepsis is not produced by a single cytokine and hence successful treatments may require inhibiting several, rather than a single cytokine. This notion is supported by recent studies suggesting that late cytokines may provide clinical advantages for sepsis. Extracellular high-mobility group B protein-1 (HMGB1) is a characteristic “late” factor contributing to organ injury in sepsis. However, the development of strategies to control HMGB1 has been hampered by the limited knowledge about its mechanism of secretion [11, 12]. Indeed, sepsis is a major problem in actual medicine with more than 30 unsuccessful clinical trials. The broad clinical definition of sepsis and the spectrum of pathological stages suggest that strategies targeting single cytokines may produce limited effects in clinical trials [13]. Thus, composite anti-inflammatory mechanisms modulating multiple cytokines may provide pharmacological advantages in sepsis [6].
We previously reported that nicotinic agonists attenuate the production of multiple inflammatory cytokines from macrophages and improve survival in sepsis [14, 15]. Nicotine prevents macrophage activity by the alpha7 nicotinic receptor (alpha7-nAChR) [14–16]. Nicotinic agonists prevent NF-κB activation and inhibit cytokine release in WT but not in alpha7nAChR-deficient macrophages [14]. In vivo, nicotinic agonists decrease inflammation and increase survival in severe sepsis [14]. Nicotine has already been used in clinical trials for inflammatory disorders such as ulcerative colitis [17], but its clinical use has been limited by its collateral toxicity. Despite the clinical interest of this mechanism, there are two scientific considerations limiting its translational potential [15]: (i) identification of specific alpha7-nAChR-agonists and (ii) characterization of their anti-inflammatory signaling pathway modulating cytokine production in sepsis. Here, we analyze the nicotinic signaling pathway that controls cytokine production in sepsis. Our findings indicate that alpha7nAChR prevents STAT3 tyrosine phosphorylation and that unphosphorylated STAT3 controls the NF-κB pathway to modulate the innate immune responses to infection. To our knowledge, this article is the first study to reveal the immunological implications of unphosphorylated STAT3 in infectious diseases.
Results
Alpha7nAChR prevents STAT3 activation
First, we analyzed whether nicotine modulates TNF responses to bacterial endotoxin via the alpha7nAChR by using the alpha7-nAChR-knockout mice. Nicotine inhibited serum TNF levels in both WT and alpha7nAChR-knockout mice (Fig. 1A), indicating that nicotine is not specific for the alpha7nAChR, and the study of this receptor will require specific agonists. Among a diversity of tested agonists, choline functioned as a specific alpha7nAChR-agonist by inhibiting serum TNF levels in WT but not in the alpha7nAChR-knockout mice (Fig. 1B). Because nicotine controls systemic inflammation in sepsis by inhibiting TNF production in the spleen [18], we analyzed the effects of choline in splenocytes. Choline inhibited TNF production in WT but not in alpha7-nAChR-deficient splenocytes (Fig. 1C). Choline also inhibited TNF in different cell lines including murine and human macrophages. Since nicotine can activate JAK2 [19], we analyzed whether choline requires JAK2 to inhibit TNF production. JAK2 inhibition with AG490 (a well-known inhibitor for JAK2 [20]) did not resume TNF levels in macrophages treated with choline (Fig. 1D). Indeed, AG490 further decreased TNF levels suggesting that JAK2 inhibition may mimic alpha7nAChR signaling. Since STAT3 is phosphorylated by JAK2, we analyzed whether choline inhibits STAT3 tyrosine phosphorylation. Choline (3h) inhibited the tyrosine phosphorylation of STAT3 and did not change STAT3 protein concentration or STAT3 serine phosphorylation (Fig. 1E). Together, these results suggested that alpha7nAChR may control TNF production by preventing STAT3 tyrosine phosphorylation. Furthermore, endotoxin induced STAT3 phosphorylation in tyrosine, which is proportional to endotoxin concentration (Fig. 2A). In order to mimic the inhibition of STAT3 tyrosine phosphorylation induced by the alpha7nAChR signaling, we used AG490. Similar to alpha7nAChR, JAK2 inhibition with AG490 inhibited the tyrosine phosphorylation of STAT3 without changing its serine phosphorylation (Fig. 2B), and blunted TNF responses (Fig. 2C and D). Viability analysis with Trypan blue and MTT showed that AG490 was not toxic at the used concentrations. These results suggested that AG490 inhibited TNF production by preventing STAT3 tyrosine phosphorylation.
Figure 1.
Alpha7nAChR inhibits STAT3 tyrosine phosphorylation. WT or alpha7nAChR-knockout (α7KO) mice were treated with (A) nicotine (0.5 mg/Kg) or (B) choline (10 mg/kg; i.p.) 30 min prior to LPS (6 mg/kg; i.p.). TNF levels in serum were quantified by ELISA at 90 min after LPS. *p<0.01 versus LPS(n = 4/group; One-way ANOVA with Bonferroni's corrections). (C) Splenocytes from WT and alpha7nAChR-knockout mice were treated with choline 30 min before treatment with LPS (100 ng/mL). (D) RAW264.7 cells were treated with choline alone or with AG490 for half an hour before LPS, and TNF levels were quantified 3 h later. Upper panel represents cell survival determined by MTT assay. *p<0.01 versus LPS (n = 3/group; One-way ANOVA with Bonferroni's corrections).(E) Human THP1 cells were pretreated with choline (3h) and STAT3 phosphorylation in tyrosine (705) and serine (727) was determined by Western blot. The left panel shows a representative Western blot of three different experiments. Total STAT3 was used as a loading control. The films were scanned and the densitometric data were represented as mean±SD (n = 3) in the right panel.
Figure 2.
JAK2 inhibition halts STAT3 phosphorylation and cytokine production. (A) RAW264.7 cells were treated with LPS for 3 hs, and STAT3 phosphorylation was analyzed by Western blot using total STAT3 protein as a loading control. The upper panel shows a representative, whereas the lower panel shows the densitometric data of three different experiments (mean±SD, normalized as percent of the control treatment). (B) AG490 inhibited LPS-induced STAT3 phosphorylation in tyrosine (705). RAW264.7 cells were pretreated with JAK2 inhibitor (AG490) 30 min prior to LPS stimulation. The upper panel shows a representative Western blot, whereas the lower panel represents the densitometric data of three different experiments (mean±SD, normalized as percent of the LPS treatment). Cells were treated with AG490 half an hour before LPS on (C) RAW264.7 or (D) THP1 cells. TNF levels were determined by ELISA at 3 h posttreatment. Upper panel represents cell survival as determined by MTT assay. *p<0.01 versus LPS (n = 3; One-way ANOVA with Bonferroni's corrections).
Inhibition of STAT3 phosphorylation blunts TNF responses, but not STAT3 protein depletion
To confirm the implications of STAT3 phosphorylation, we used stattic, a well-characterized inhibitor of STAT3 phosphorylation [21]. Inhibition of STAT3 phosphorylation with stattic blunted TNF production in human THP1 cells (Fig. 3A). Similarly to that described for choline, stattic was specific for the STAT3 tyrosine phosphorylation and no statistically significant effect was observed in the STAT3 serine phosphorylation. Viability analysis with MTT showed that stattic is not toxic at the indicated concentrations. To analyze whether the anti-inflammatory mechanism of alpha7-nAChR or stattic requires STAT3 tyrosine phosphorylation, macrophages were transfected with STAT3F vector. STAT3F is a dominant-negative form that has the tyrosine 705 mutated by phenylalanine to prevent its phosphorylation and has been previously used in similar studies [22]. Both choline and stattic inhibited TNF production in control peritoneal macrophages and those transfected with STAT3F (Fig. 3B). These results show that the anti-inflammatory potential of choline and stattic did not require STAT3 tyrosine phosphorylation. To confirm that the anti-inflammatory potential of the alpha7nAChR requires STAT3 protein expression, we used small interference siRNA to inhibit STAT3 protein expression. Inhibition of STAT3 protein expression enhanced TNF responses proportionally to concentration, suggesting that STAT3 protein can inhibit TNF production (Fig. 3C). Furthermore, choline inhibited TNF production in control macrophages but not when STAT3 protein expression was inhibited by using siRNA (Fig. 3D). Thus, alpha7nAChR can control TNF production in macrophages through a mechanism that requires STAT3 protein expression.
Figure 3.
Inhibition of STAT3 tyrosine phosphorylation halts cytokine production. (A) THP1 cells were pretreated with stattic, and TNF levels were quantified by ELISA at 3 h after LPS stimulation. *p<0.01 versus LPS (n = 3; One-way ANOVA with Bonferroni's modifications). (B) STAT3 phosphorylation was determined in peritoneal macrophages by Western blot. The upper panel shows a representative Western blot, whereas the lower panel represents the densitometric data of three different experiments (mean±SD, normalized as percent of the LPS treatment). (C) Control peritoneal macrophages or cells transfected with the dominant-negative form of STAT3F were pretreated with choline or stattic and LPS, and the TNF levels were quantified by ELISA and represented in mean±SD. *p<0.01 versus LPS (n = 3; One-way ANOVA with Bonferroni's corrections). (D) STAT3 depletion increased TNF production. THP1 cells were pretreated with control RNA or the STAT3 siRNA at the indicated concentrations for 24 h prior to LPS stimulation. STAT3 expression in control and treated cells were analyzed by Western blot using β-actin as a loading control (upper panel). Graph shows the data of three different experiments in mean±SD. (E) THP1 cells pretreated with control RNA or STAT3 siRNA were treated with choline (50 mM) 30 min prior to LPS stimulation. Graph shows data of three experiments in mean±SD. *p<0.01 versus LPS (n = 3; One-way ANOVA with Bonferroni's corrections).
Since our studies suggest that unphosphorylated STAT3 can restrain cytokine production, we analyzed whether the inhibition of STAT3 phosphorylation can modulate NF-κB, a critical factor modulating immune responses [14]. STAT3 inhibition with stattic prevented the LPS-induced activation of p65RelA in peritoneal macrophages transfected with the NF-κB luciferase reporter (Fig. 4A). The specificity of this inhibition was evaluated by analyzing the different NF-κB proteins using specific DNA probes for each pathway. LPS enhanced the NF-κB canonical pathway by activating both p65 and p50 (Fig. 4B and C), and stattic prevented this activation. This inhibition of p65/p50 was specific as stattic failed to affect p52, c-Rel, and RelB.
Figure 4.
STAT3 modulates the classical p65RelA/p50NF-κB1 pathway. (A) Peritoneal macrophages were transfected with the NF-κB gene reporter, and the cells were treated with the indicated concentration of the STAT3 inhibitor stattic. NF-κB activity was analyzed by luminescence. (B–F) THP1 cells were pretreated with stattic, and the specific NF-κB pathways were analyzed in nuclear extracts by using the TransAM DNA-Binding. Graphs show the luciferase arbitrary units of three different experiments in mean±SD (*p<0.01 versus LPS; n = 3 experiments; One-way ANOVA with Bonferroni's corrections).
Inhibition of STAT3 attenuates inflammation and mortality in sepsis
Pretreatment with stattic 30 min prior to the LPS challenge inhibited serum TNF levels (Fig. 5A). Stattic inhibited 50% TNF levels at 25 mg/kg (i.p.). The anti-inflammatory effects of stattic appear to be selective to particular organs, inducing major effects in the spleen without affecting TNF levels in the thymus or heart (Fig. 5A). Since the spleen modulates systemic inflammation in sepsis, we analyzed whether stattic modulates serum TNF responses through a mechanism dependent on the spleen. Stattic attenuated serum TNF levels in sham but not in splenectomized animals (Fig. 5B). These results suggested that stattic restrains systemic inflammation by modulating cytokine production in the spleen, a mechanism similar to that described for the alpha7-nAChR [18]. The anti-inflammatory potential of stattic appeared to be clinically significant by improving survival in endotoxemic mice by over 50% (Fig. 5C). This effect was dose dependent with the lowest concentration of stattic failing to provide survival benefits. Survival was analyzed for 15 days and no deaths were found after 7 days, meaning that stattic confers a lasting protection. In addition to TNF, we also analyzed “late” inflammatory mediators, because of their clinical potential to treat established sepsis. Given that LPS induces HMGB1 secretion at ~20 h after stimulation [10, 23, 24], we analyzed whether stattic inhibits HMGB1 secretion. Stattic prevented HMGB1 extracellular secretion in culture of macrophages (Fig. 5D). Similar results were found in vivo, as stattic also prevented systemic HMGB1 responses in endotoxemic animals (Fig. 5E). The anti-inflammatory potential of stattic was also studied in experimental sepsis using a cecal ligation and puncture procedure. A single dose of stattic administered 1 day after the CLP procedure increased survival over 70% (Fig. 5F). STAT3 inhibition with stattic decreased the clinical signs of sepsis including piloerection, diarrhea, and lethargy. Animals were observed for 15 days to confirm that stattic confers a lasting protection.
Figure 5.
STAT3 inhibition controls inflammation and rescues animals in sepsis. (A) Endotoxemic animals were treated with vehicle or the STAT3 inhibitor stattic (25 mg/kg), 30 min before LPS challenge. TNF levels in the samples were normalized to total protein (*p<0.01 versus LPS; n = 6/group). (B) BALB/c mice underwent sham surgery or splenectomy 5 days before LPS challenge. Animals were treated with stattic, and serum TNF levels were analyzed by ELISA. (C) Animals were treated with vehicle, 10 or 25 mg/kg stattic, 30 min prior to endotoxin. Animals were followed for 2wk (*p<0.01 versus control, n = 10/group Logrank Test). (D) RAW264.7 macrophages were treated with stattic and HMGB1 levels were analyzed by Western blot in the extracellular media (*p<0.01 versus LPS; n = 3 experiments; ANOVA with Bonferroni's modifications). (E) Animals were treated with vehicle or stattic and systemic HMGB1 levels were determined at 24 h. (*p<0.01 versus LPS; n = 6 mice/group; One-way ANOVA with Bonferroni's corrections). (F) BALB/c mice underwent CLP and the stattic treatment (25 mg/kg; i.p.) was started 24 h later. Animals were observed for 2 wk. (*p<0.01 versus control, n = 10 mice/group; Logrank Test).
Discussion
Our study indicates that STAT3 mediates the anti-inflammatory pathway of the alpha7nAChR to control innate immune responses to infection. Inhibition of STAT3 tyrosine phosphorylation restrained the inflammatory responses and increased survival in sepsis. These findings provide an explanation of the detrimental consequences of STAT3 genetic deletion in sepsis [25, 26]. Genetic strategies to study STAT3 protein require conditional tissue-specific gene deletion because STAT3-deficient mice died during early embryogenesis [27]. LysMcre/Stat3flox/– mice were designed for cell-specific STAT3 disruption in macrophages and neutrophils. These mice are born normal but they have overwhelming TNF responses to bacterial endotoxin, which make them very susceptible to endotoxemia [25] and sepsis [26]. Similar results were confirmed in two different STAT3-conditional knockout animals, indicating that STAT3-deficient macrophages have higher TNF production in response to endotoxin [25, 28]. Similar to STAT3 knockout mice, our results with siRNA confirm that inhibition of STAT3 protein expression enhances inflammatory responses. The molecular mechanism for these overzealous responses to sepsis in STAT3-deficient mice was unknown. Since unphosphorylated STAT3 was considered inactive, it was suggested that phosphorylated STAT3 could restrain cytokine production. However, our current results provide a new perspective, indicating that these knockout animals have an overwhelming susceptibility to sepsis because they lack the anti-inflammatory potential of unphosphorylated STAT3. To our knowledge, this article is the first study to reveal the biological implications of unphosphorylated STAT3 in infectious diseases and provide a molecular mechanism for those results. Transfection of macrophages with STAT3F (a dominant-negative mutant form that prevents its tyrosine phosphorylation) blunted TNF production but it did not prevent the anti-inflammatory potential of neither choline nor stattic. Despite earlier reported acute phosphorylation of Stat3 via nAChR activation [19], the current results suggest that the alpha7nAChR signaling requires STAT3 protein rather than its tyrosine phosphorylation. Unlike STAT3 genetic deletion, pharmacological inhibition of STAT3 tyrosine phosphorylation prevents systemic inflammation in sepsis. Thus, STAT3 genetic deletion enhances deleterious inflammatory responses, whereas inhibition of STAT3 tyrosine phosphorylation restrains inflammatory responses to bacterial endotoxin.
STAT3 and NF-κB are central transcription factors in both innate and adaptive immunity. Yet, the clinical implications of the crosstalk of these factors in infectious diseases are unknown. This signaling crosstalk has particular interest because it can account for the anti-inflammatory potential of unphosphorylated STAT3. Previous studies indicate that inhibition of STAT3 protein expression enhanced NF-κB activation in bone marrow-derived dendritic cells from the STAT3-deficient mice [28]. We further studied this mechanism by showing that inhibition of STAT3 protein expression with siRNA increases LPS-induced TNF production in macrophages. These results can be explained by the recent study indicating that activated STAT3 from tumor cells can inhibit the phosphorylation of IκBα by IKKβ [29]. However, our results indicate that neither choline nor stattic requires STAT3 phosphorylation. Similar to our studies, a recent study indicates that unphosphorylated STAT3 can bind to NF-κB in competition with IκBα [30]. Although the role of unphosphorylated STAT3 has not been yet established [31], our results suggest that unphosphorylated STAT3 can mediate the anti-inflammatory pathway of alpha7nAChR in infectious diseases (Fig. 6). In normal conditions, JAK2 phosphorylates STAT3, and phosphorylated STAT3 dimerizes and translocates into the nucleus. In these conditions, some STAT3 can prevent NF-κB activation by inhibiting IKKβ as described previously [29]. Inhibition of STAT3 protein expression prevents this mechanism and enhances NF-κB in STAT3-deficient mice contributing to overzealous inflammatory responses in these knockout animals. However, inhibition of STAT3 tyrosine phosphorylation results in more unphosphorylated STAT3 available to bind to NF-κB. Our results now propose that the binding of unphosphorylated STAT3 to NF-κB inhibits NF-κB-mediated activation of TNF transcription and restrains systemic inflammation in sepsis. Unlike the inhibition of STAT3 tyrosine phosphorylation, STAT3 protein depletion prevents the physiological implications of unphosphorylated U-STAT3. It is worth noting that a distinction should be made between STAT3 inhibitors and unspecific inhibitors affecting STAT3. The former specifically inhibits STAT3 phosphorylation by direct binding to the protein, whereas the latter refers to compounds that may affect STAT3 by indirect, and often undetermined, mechanisms. The vast majority of the reported STAT3 inhibitors do not really target STAT3, and thus they are more likely to result in collateral inhibition of additional signaling pathways than the targeting of downstream molecules [32]. Indeed, stattic is a selective inhibitor that prevents STAT3 phosphorylation without affecting other related proteins such as Stat1, Stat5, or Lck [21]. These results challenge the current perspective of unphosphorylated STAT3 as an inactive protein. Our results suggest that inhibition of STAT3 tyrosine phosphorylation does not necessarily indicate an inactivation of all STAT3 functions but rather a modulation of its pathway. Indeed, the binding of unphosphorylated STAT3 to NF-κB may prevent NF-κB-activation of TNF transcription, but the resulting STAT3–NF-κB complex may enhance the production of other factors [30].
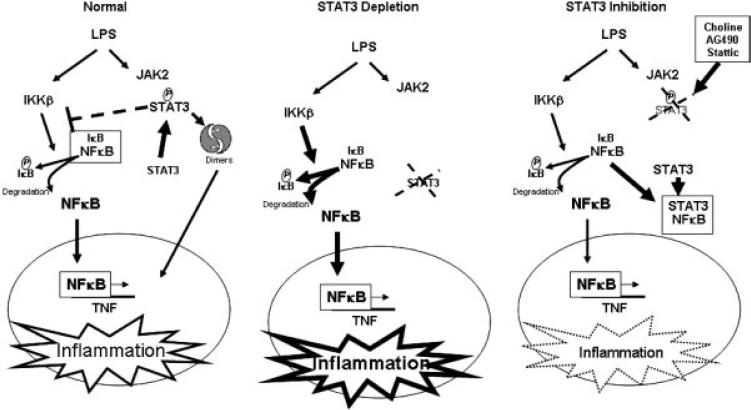
Figure 6.
Regulation of NF-κB by targeting STAT3. Activation of STAT3 by inflammatory signals induces STAT3 dimers that move into the nucleus, whereas a proportion of activated STAT3 restrains NF-κB activation by inhibiting IKKβ-mediated activation of IκBα [29]. STAT3 silencing by genetic depletion enhances LPS-induced inflammatory responses by preventing the inhibition of IKKβ by activated STAT3. Inhibition of STAT3 activation attenuates cytokine production in macrophages by preventing the SH2 domain-mediated dimerization and interaction with proteins such as a JAK2. STAT3 binds to NF-κB in competition with IκBα and prevents NF-κB-induced TNF activation [30].
From a physiological perspective, inhibition of STAT3 phosphorylation modulates serum TNF responses through a mechanism mediated by the spleen. This is a mechanism similar to that described for the vagus nerve and the alpha7nAChR [18]. The contribution of the spleen to organ injury in severe trauma or sepsis was recently analyzed in clinical studies, indicating that patients with severe trauma who underwent splenectomy had significantly shorter hospital length of stay, and better secondary outcomes than patients who were managed nonoperatively or with splenorrhaphy [33]. Our study indicates that STAT3 can control systemic inflammation by restraining TNF production in the spleen. This mechanism can provide clinical advantages to prevent overzealous splenic responses without affecting beneficial responses produced in other organs. In addition to “early” TNF response, inhibition of STAT3 tyrosine phosphorylation prevents extracellular HMGB1 secretion. Our results concur with the recent studies suggesting that JAK2 can modulate HMGB1 release in RAW264.7 cells [34, 35]. Our results can have clinical implications because serum HMGB1 is a late cytokine released 18–24 h after the onset of the pathogenesis and mimic septic death kinetic [10]. Thus, pharmacological strategies inhibiting HMGB1 responses may be clinically advantageous for treating established sepsis. Our results imply that inhibition of STAT3 phosphorylation does not modulate serum HMGB1 by restraining serum TNF responses, because the treatment with stattic was started after the production of serum TNF, which peaks at 90 min after the induction of sepsis. This pharmacological strategy has the advantage to rescue animals from established sepsis and improve survival even if the treatment is delayed a day after the induction of the sepsis. Other strategies like anti-TNF antibodies are not efficient if used after CLP [36]. Anti-MIF-strategy is ineffective when started at 8 h after sepsis [37]. Lysophosphatidylcholine is efficient if started within 10 h after sepsis [38]. These findings show that STAT3 may be useful for the treatment of sepsis. However, additional investigation is required to establish their clinical translation, because clinical sepsis usually involves elderly patients with additional complications.
Materials and methods
Experimental animals
Experiments were performed in accordance with the National Institutes of Health Guidelines under protocols approved by the Institutional Animal Care and Use Committee of the UMDNJ-New Jersey Medical School. Male mice WT 6- to 8-wk-old C57BL/6J (Jackson Labs) were randomly assigned to a group and blindly treated by the investigators [14, 39]. Antibiotic was administered (Enrofloxacine 0.9 mg/kg, s.c.) in saline solution following the surgery and twice a day for 3 days [39]. Organs were collected and homogenized (Homogenizer; Omni International, GA) in lysis buffer. The suspension was centrifuged at 10 000 × g for 25 min at 41C and the supernatant was collected and quantified by Bradford protein assay (Bio-Rad, Hercules, CA).
Cellular cultures
THP1 and RAW264.7 cells are cultured as we reported earlier [14]. Primary culture of splenocytes and peritoneal macrophages was done according with our previous report [18]. Cells were plated at 3 × 105 cells/well total 1 mL volume per well. The medium was replaced, after overnight incubation, with OPTIMEM I serum-free medium for the experimental procedure [39].
Cytokines
Organ samples were homogenized at 4°C PBS and standardized by protein concentration. TNF in cells supernatant was quantified by ELISA (eBioscience San Diego, CA) 3 h after stimulation. Serum and organ's TNF levels were measured 90 min after the endotoxic shock. Western blot analyses were used for HMGB1 detection (Abcam, Cambridge, MA) in serum or supernatant resolved on SDS-PAGE gels 4–20%.
STAT3 and NF-κB analyses
Phosphorylated and total STAT3 protein were analyzed with antibodies from Cell Signaling. TransAM ELISA kits (Active Motif, Cambridge, MA) were used to analyze NF-κB proteins binding to DNA. RAW264.7 were stably transfected with a NF-κB luciferase reporter construct (Clontech, Mountain View, CA) in which a PDNA3.1(+) derived neomycin resistance TK cassette was inserted (referred to as pNF-κBneo-luc). Resistant cells were subcloned and the clones were cultured up to 20 passages with RPMI-containing neomycin. For assay, cells were pretreated with stattic at the concentration indicated for 20 min, washed, and subsequently stimulated with LPS (100 ng/mL; Sigma) for 3 h. After treatment, the medium was removed, the cells were washed three times with ice-cold PBS, and lysed with Passive Lysis Buffer supplied in the LuciferaseTM Reporter Assay Kit (Promega, Madison, WI) and the lysate was assayed for luciferase activity according to the manufacturer's instructions.
Transfections
RAW264.7 cells were transfected with STAT3F reporter constructs (kindly provided by Dr. Touw, I., Erasmus University Rotterdam and Prof. T. Hirano ) using Jet PEI transfection reagent (PolyTransfection), according to the manufacturer's instructions. After 16 h, medium was refreshed and cells were pretreated with choline or stattic for 20 min, followed by LPS (100 ng/mL) stimulation for 3 h.
STAT3 silencing by siRNA
THP1 cells were nucleofected using Amaxa Cell Line Nucleofector Kit V (Lonza) with 0.25–2 nmol/mL Non-Targeting DHARMACON siCONTROL (CAT# D-001206-13) or siGENOME SMART pool STAT3 (AMAXA Biosystem Nucleofector; Cat# M-003544-00). Twenty four hours after nucleofection, cells were treated with choline 50 mM for 30 min prior to LPS stimulation (400 ng/mL) for 3 h. Supernatant was collected and analyzed for ELISA.
Statistical analyses
All data in the figures and text were expressed as mean±SD. One-Way ANOVA with the Bonferroni adjustments was used for the statistical analyses. Logrank test and Kaplan–Meier estimator were used for survival analyses. p<0.05 was estimated statistically significant.
Acknowledgements
Dr. I. Touw (Erasmus MC Rotterdam, NL) and Professor T. Hirano (Osaka, University, Japan) are gratefully acknowledged for providing Stat3 dominant negative constructs. The authors thank L. Ramos and B. Barton for their suggestions and critical reading of the manuscript. E. Z. and W. J. are supported by Top Institute Pharma T1-215, the European Community Framework Program Marie Curie, and the Netherlands Organisation for Scientific Research. L. U. is funded by Department of Surgery UMDNJ, USAMRMC#05308004, AHA06352230N, and the NIH GM084125.
Abbreviations
- alpha7nAChR
alpha7 nicotinic receptor
- HMGB1
high-mobility group B protein-1
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr. Pharm. Des. 2009;15:1918–1935. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr. Opin. Pharmacol. 2004;4:378–385. doi: 10.1016/j.coph.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat. Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 6.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol. Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 8.Grau GE, Maennel DN. TNF inhibition and sepsis – sounding a cautionary note. Nat. Med. 1997;3:1193–1195. doi: 10.1038/nm1197-1193. [DOI] [PubMed] [Google Scholar]
- 9.Remick D, Manohar P, Bolgos G, Rodriguez J, Moldawer L, Wollenberg G. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4:89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 11.Mantell LL, Parrish WR, Ulloa L. HMGB1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Sama AE, Wang H. Role of HMGB1 in cardiovascular diseases. Curr. Opin. Pharmacol. 2006;6:130–135. doi: 10.1016/j.coph.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angus DC. Caring for the critically ill patient: challenges and opportunities. J. Am. Med. Assoc. 2007;298:456–458. doi: 10.1001/jama.298.4.456. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 15.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 17.Thomas GA, Rhodes J, Mani V, Williams GT, Newcombe RG, Russell MA, Feyerabend C. Transdermal nicotine as maintenance therapy for ulcerative colitis. N. Engl. J. Med. 1995;332:988–992. doi: 10.1056/NEJM199504133321503. [DOI] [PubMed] [Google Scholar]
- 18.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 20.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 21.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, et al. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15:3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 23.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 24.Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 26.Matsukawa A, Takeda K, Kudo S, Maeda T, Kagayama M, Akira S. Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J. Immunol. 2003;171:6198–6205. doi: 10.4049/jimmunol.171.11.6198. [DOI] [PubMed] [Google Scholar]
- 27.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc. Natl. Acad. Sci. USA. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Stark GR. Roles of unphosphorylated STATs in signaling. Cell Res. 2008;18:443–451. doi: 10.1038/cr.2008.41. [DOI] [PubMed] [Google Scholar]
- 32.McMurray JS. A new small-molecule Stat3 inhibitor. Chem. Biol. 2006;13:1123–1124. doi: 10.1016/j.chembiol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Crandall M, Shapiro MB, West MA. Does splenectomy protect against immune-mediated complications in blunt trauma patients? Mol. Med. 2009;15:263–267. doi: 10.2119/molmed.2009.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Yao YM, Yu Y, Dong N, Yin HN, Sheng ZY. Role of Janus kinase/signal transducer and activator of transcription pathway in regulation of expression and inflammation-promoting activity of high mobility group box protein 1 in rat peritoneal macrophages. Shock. 2007;27:55–60. doi: 10.1097/01.shk.0000233197.40989.31. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Kim SJ, Lee IS, Lee MS, Uematsu S, Akira S, Oh KI. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J. Immunol. 2009;182:2458–2466. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- 36.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 37.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 38.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 39.Pena G, Cai B, Deitch EA, Ulloa L. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J. Mol. Med. 2010;88:851–859. doi: 10.1007/s00109-010-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]