Abstract
Normal aging impairs olfactory functioning both centrally and peripherally. The P3 peak of the event related potential (ERP), evoked by active response to a target stimulus, is considered a reflection of central cognitive processing. It can also be evoked in a passive task to both auditory and visual stimuli. Our goal was to investigate whether age influences amplitude and latency of the ERP differentially in active and passive tasks to olfactory stimuli. Olfactory and visual event-related potentials were elicited with a single-stimulus paradigm in separate active and passive task response conditions. Participants included 30 healthy individuals from three age groups, young, middle age, and older adults. Results indicated that P3 ERP latency increased with age in both sensory modalities. P3 latencies for active versus passive tasks were similar across age groups for visual ERPs, but in the olfactory modality, older adults demonstrated significantly longer latencies in the passive task compared to the active task. Future directions should include research on specific clinical populations utilizing active versus passive task conditions.
Keywords: Active attention, Age, Event-related potentials, P3, Visual, Olfactory, Smell
1. Introduction
The production of the P3 cognitive component of the event-related potential (ERP) typically necessitates a participant to respond actively to a target stimulus (button press, finger lift, silent counting, etc.). These types of “active” paradigms, however, can be problematic when working with cognitively impaired participants or those who may not fully understand the task requirements (e.g. dementing illnesses, mental retardation, difficult psychiatric patients, or children). Often these types of participants need to be regularly reminded of the task instructions during the recording session in order to adequately engage in the task. A “passive” task has been used in ERP research where the participants are told to not respond to the stimuli and are encouraged to daydream while stimuli are being presented. With this method intentional discrimination of stimuli is not requested or required of the participant. One advantage is that participants do not need to understand much about the task and do not need to respond to any stimuli, thus they do not need to focus attention on the task. Visual and auditory research studies have demonstrated that these passive paradigms can also elicit the P3 component (Bennington and Polich 1999; Ford et al., 1976; Polich 1986a,b, 1987; Roth, 1973). Bennington and Polich (1999) demonstrated that in young participants auditory passive paradigms elicit P3 waveforms similar in amplitude and latency to active paradigms. They found that visual ERPs, however, produce much smaller P3 amplitudes in passive conditions compared to active conditions, but similar latencies. Friedman et al. (1998) examined aging effects in an auditory active versus passive experiment. They found no latency effects but did find differences between old and young participants in amplitude. Their study demonstrated that young participants exhibited a relative reduction in P3 amplitude at the frontal electrode sites for the ignore condition compared with the attend condition, but older subjects did not show a difference between attend and ignore conditions.
Olfactory ERP (OERP) research has also examined the passive versus active task differences in young participants. Pause et al. (1997), utilized a between subjects design with young adult participants where some participants engaged in an active task and other participants in a passive task. Their single stimulus paradigm experiment, with a 50 second inter-stimulus interval (ISI), demonstrated larger P3 amplitudes in active compared to passive conditions, with no significant differences in latency between the two tasks. Krauel et al. (1998) also studied young adults, utilizing a within-subjects, two-stimulus (oddball) paradigm. Odors were presented 8 seconds apart with the infrequent odor occurring 20% of the time, and a 60 second ISI after every block of five odors, resulting in the target odors being spaced apart on average a minimum of 60 seconds. They demonstrated increased P3 amplitudes and decreased latencies for the active compared to passive conditions. Masago et al. (2001) demonstrated similar results when they studied young adults in an oddball paradigm with 15–25 second randomized ISIs, with the infrequent odor occurring 25% of the time, resulting in target odors spaced 50 seconds or greater apart. Geisler and Murphy (2000) utilized a within-subjects, single stimulus paradigm, with 60 second ISIs, also with young adult participants. They demonstrated larger P3 amplitudes for the active task but similar latencies between the two task conditions. To date we know of no published study that has examined active versus passive task condition OERP effects in groups other than young adults.
Normal aging impairs odor sensitivity, odor identification, odor recall and odor recognition memory (Doty et al., 1984, Murphy, 1983, 1985, 1986, 2008; Murphy and Cain, 1986; Murphy et al., 1991, 1997, 2002; Schiffman, 1977, 1986). Olfactory changes in aging stem from changes to the olfactory system including the olfactory receptor cells, the olfactory bulb, the olfactory tract, and the cortical processing areas of the brain such as the hippocampus and amygdala (Liss and Gomez, 1958; Price et al., 1991; Scheibel and Scheibel, 1975; Smith, 1942). Several ERP studies have demonstrated increased OERP peak latencies and decreased peak amplitudes with aging (Covington et al., 1999; Evans et al., 1995; Morgan et al., 1997, 1999; Murphy et al., 2000; Nordin et al., 1999; Stuck et al., 2006; Thesen and Murphy, 2001). While these early studies did not indicate effect sizes, typical correlation coefficients for amplitude with age were −0.39 and for latency 0.46 (Murphy et al., 2000). Morgan et al. (1997) demonstrated effects of ISI on aging and the OERP, specifically that older males produced significantly smaller P2 amplitudes at short ISIs (45 sec & 60 sec) compared to older females and younger participants. Shorter ISIs allow less time for the olfactory system to recover from adaptation and adaptation and recovery have been shown to be age-related, with older adults adapting more quickly and recovering more slowly from olfactory stimulation (Stevens et al., 1989).
While the main focus of the current study was on olfactory ERPs and attention, visual ERPs were also recorded as a comparison modality. Research in the visual modality has also demonstrated changes in the visual ERP with aging (Celesia, 1986; Pfefferbaum et al., 1985; Polich 1996, 1997). Consistent with other studies, Polich (1997) demonstrated that as age increases, target stimulus amplitude generally decreases and peak latency increases. While effect sizes were not documented in these studies, correlation coefficients between aging and visual amplitude ranged from −0.24 to −0.33 and for latency from 0.32 to 0.34 (compared to the −0.39 and 0.46 correlations for OERP amplitude and latency). Given the age-related changes that take place in both olfactory and visual ERPs and age-related changes in cognition, it is important that we study ERP-related attentional task demands across the lifespan in order to better understand the effects of aging on these systems. In the current study we expected greater effects of aging on the ERP in the olfactory modality than the visual modality. We hypothesized that older participants would demonstrate longer olfactory P3 latencies in general, and further investigated whether older adults would demonstrate greater difference in the active task than in the passive task, compared to the young. Additionally given the shorter, 30 second, ISI used in this study, and likely greater adaptation/habituation due to that short ISI, we hypothesized that older participants would exhibit less robust OERP waveforms than younger participants in both amplitude and latency.
2. Methods
2.1. Participants
Participants totaled 30 adults consisting of 10 in each of three age groups, Young Adults (5M/5F, Mean age = 23.9 years, SD = 2.8), Middle Age Adults (5M/5F, Mean Age = 50.6 years, SD = 2.4), and Older Adults (5M/5F, Mean age = 70.2, SD = 4.3). Participants were recruited from an ongoing participant pool at the Lifespan Human Senses Laboratory, from San Diego State University, and from the community in general and were paid for their time. All participants were screened for cognitive impairment using the Dementia Rating Scale (DRS; Mattis, 1976) and for nasal health using an odor threshold test (Cain, 1989; Murphy et al., 1990). All participants with DRS scores lower than 124 out of 144 possible points or odor threshold scores lower than 5 (out of 9 steps) were excluded from the experiment. Smoking history was obtained from each participant and compared between groups. All participants signed informed consent forms approved by the human subject committees at both San Diego State University and the University of California, San Diego.
2.2 ERP stimulus presentation
Olfactory stimuli were presented in a single stimulus paradigm via a computer controlled olfactometer incorporating aspects of previously designed olfactometers (Kobal and Hummel, 1988; Lorig et al., 1999, 2000; Morgan et al., 1999; Murphy et al., 1994). The olfactory stimulus was amyl acetate presented for 200 msec at an ISI of 30 sec for 20 trials. Velopharyngeal closure was employed to restrict breathing to the mouth and thereby maintain a constant odorant flow rate (Kobal and Hummel, 1988; Lorig, 1996, 2000; Thesen and Murphy, 2001). As a comparison modality, in a separate experimental task, visual ERPs were also recorded in response to visual stimuli presented on a computer monitor consisting of a 76 × 76 mm blue square on a light gray background for 200 msec with an ISI of 30 sec for 20 trials. Every participant was tested in separate active and passive condition experiments and in separate olfactory and visual experiments, such that every participant was tested in each of 4 different experimental conditions (olfactory passive [OP], olfactory active [OA], visual passive [VP], visual active [VA]. Order of presentation of the 4 different experiments was randomized so that some participants were given the following sequence: OP-VP-OA-VA, and others: VP-OP-VA-OA. The passive condition was always presented before the corresponding active condition. The two olfactory experiments were never presented sequentially in order to reduce adaptation/habituation effects. In the passive conditions participants were instructed to ignore any stimuli presented. In the active conditions participants were asked to press a button with their dominant hand once they detected the stimulus. Only trials where the participant pressed the button within five seconds of stimulus presentation were analyzed. Missed targets and late responses after five seconds were excluded. The button press response served not only to verify target detection but also reaction time data were collected with this button press. STIM2 (Compumedics, Charlotte, NC) software was utilized to present and trigger stimuli for all conditions and modalities and to record subject responses in the active conditions.
2.3 Electrophysiolgical recording
Olfactory and visual ERPs were obtained via Compumedics 64-electrode AG/AG/CL sintered Quick-Cap and Quick-Cell system, amplified via Synamps 2 amplifiers, and recorded on computer hard disk via the Compumedics Neuroscan software package. Electrode impedances were kept below 10 kΩ. EEG recordings from central electrodes Fz, Cz, and Pz as well as lateral electrodes F7, T7, P7, F8, T8, P8, electrodes sites were used for analysis, referenced monopolarly to linked A1 and A2. Ongoing EEG activity was recorded throughout the experiment and then trials were epoched offline to 500 msec pre-stimulus and 1500 msec post-stimulus. Artifactual eyeblink activity was recorded and corrected offline via the Neuroscan software utilizing the Occular Artifact Reduction (OAR) method within the software. Neuroscan’s OAR method includes several steps: 1. A scan is made of the continuous pre-epoched EEG for maximum eye movement potentials. 2. An average electrooculogram (EOG) artifact response is constructed. From this average, transmission coefficients (b) are computed by estimating the covariance (cov) of the averaged potentials of the ocular channel with the EEG channels. The transmission coefficients (b) are computed according to the following equation: b=cov(EOG,EEG)/var(EOG). The EOG is subtracted from the EEG channels on a sweep-by-sweep, point-by-point basis in the following manner: corrected EEG = original EEG-b*EOG. Trials were then epoched from the continuous EEG data and individual trials were analyzed for other artifacts, rejecting any trials where voltage exceeded ±75 μV. Individual trials were filtered offline .01 to 9 Hz, baseline corrected, and averaged for each participant and condition. Peak amplitude was measured from pre-stimulus baseline (averaged over 500 ms) to maximum peak amplitude. Latency windows from previous OERP studies (e.g. Morgan et. al., 1997, 1999) were used as guides to identify peak components. OERP latencies for N1 were between 400 and 600, P2 500 and 800, N2 600 and 1000, P3 700 and 1100. For Visual ERPS the P3 component was defined as the largest positive-going peak occurring within 300–800 msec of stimulus onset at each electrode. While not discernable in the visual grand averages due to filtering and averaging over many subjects, the majority of the individual subject averages exhibited distinguishable P2 peaks preceding the P3 peak. In the few individual averages were the P2 was not discernable from the P3 a point half way between N1 and P3 was used as an estimated P2. Latencies were measured from the time of stimulus release/onset to the maximal peak. As in previous OERP studies, amplitudes and latencies were measured for electrode sites Fz, Cz, and Pz. In order to better capture differences between groups in the relatively slow OERP and extend the research on OERPs we also analyzed area measures around peak components at electrode sites closer to the fronto-temporal olfactory processing areas of the brain (F7, F8, T7, T8) and as comparison posterior sites P7 and P8. Area measures were computed for lateral electrodes F7, T7, P7, F8, T8, and P8 utilizing 200 msec windows around each mean component latency taken from the Cz component as measured above for each age group. 200 msec (100 msec pre- and post-peak amplitude) windows were chosen in order to sufficiently capture the slower OERP brain activity. The same sites were analyzed for visual ERPs in order to demonstrate differing effects at those exact sites from the OERP, the main focus of this study.
2.4 Statistical analysis
For purposes of analysis the active versus passive comparison will be called the “Task” comparison hereafter. Amplitude and latency measures for each peak component at central electrode sites, were analyzed utilizing separate repeated-measures multivariate analyses of variance for visual ERPs and OERPs (MANOVA, Age group x Electrode site x Task). Area measures for lateral electrodes F7, T7, P7, F8, T8, P8, were also analyzed utilizing similar statistical analysis grouping electrodes by left or right hemisphere (MANOVA, Age group x Hemisphere x Electrode site x Task). Greenhouse-Geisser corrections were applied to all MANOVAs. Only the probability values from corrected d.f. are reported below. Significant main effects and interactions were further analyzed with post hoc Newman Keuls Multiple Range Tests (alpha 0.05)
3. Results
3.1 Screening & demographic measures
Table 1 summarizes screening and demographic measures for each age group. Dementia Rating Scale scores did not differ significantly between age groups (p >.05). The middle age group had more current tobacco smokers than the young or older groups. Number of people who had ever smoked was similar between the three age groups. Any smokers who had developed anosmia or severe hyposmia were screened out of the study due to low odor detection thresholds. Analysis of odor threshold scores indicated better odor threshold performance in young participants compared to middle age and older participants (p<.05), with all participants scoring in the normosmic range of performance.
Table 1.
Means & Standard Deviations for demographic, screening, and behavioral data for each age group.
| Young | Middle | Older | |
|---|---|---|---|
| Age (years) | 23.9 (2.8) | 50.6 (2.4) | 70.1 (4.4) |
| Education (years) | 16.6 (2.6) | 14.5 (2.6) | 17.1 (3.0) |
| Number of current smokers | 0/10 | 4/10 | 0/10 |
| Number who ever smoked during lifetime | 5/10 | 6/10 | 6/10 |
| Dementia Rating Scale Score (144 max) | 142.2 (2.0) | 138.5 (5.4) | 140.9 (2.7) |
| Odor Threshold (dilution steps, 9 max) | 7.7 (1.2) | 6.0 (1.5) | 5.8 (1.7) |
| Olfactory ERP Active Reaction Time (msec) | 1824 (852) | 1752 (862) | 2105 (514) |
| Olfactory ERP Active % Correct Trials | 79.5% | 77.5% | 82.5% |
| Olfactory ERP Active # Trials in Average (20 max) | 11.0 (3.4) | 10.9 (4.5) | 11.3 (2.0) |
| Olfactory ERP Passive # Trials in Average (20 max) | 15.1 (3.9) | 16.5 (2.7) | 13.8 (3.1) |
| Visual ERP Active Reaction Time (msec) | 316 (33) | 413 (177) | 338 (81) |
| Visual ERP Active % Correct Trials | 89.0% | 89.5% | 96.5% |
| Visual ERP Active # Trials in Average (20 max) | 12.1 (4.5) | 10.5 (4.3) | 11.5 (2.0) |
| Visual ERP Passive # Trials in Average (20 max) | 14.9 (4.0) | 16.3 (2.2) | 12.3 (2.9) |
3.2 ERP behavioral assessment
Table 1 summarizes behavioral performance. In the active olfactory and visual conditions there were no significant differences between age groups for reaction time, percent correct trials, or number of trials included in each average (p>.05). In the passive conditions there were no significant differences between age groups for number of trials included in each average (p>.05).
3.3 ERP waveform analysis
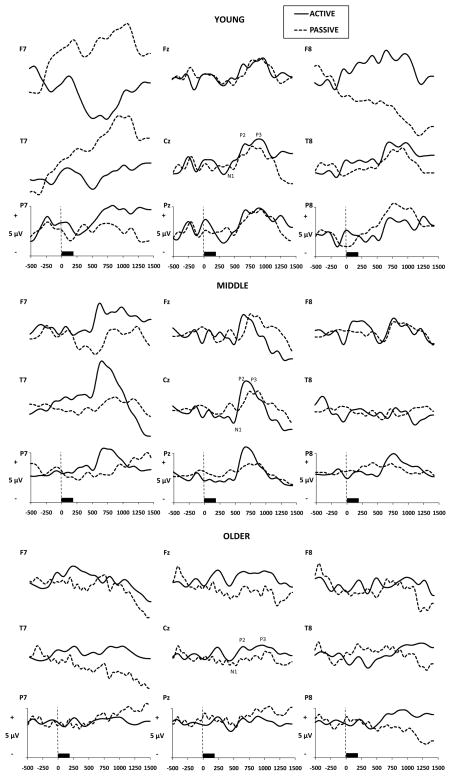
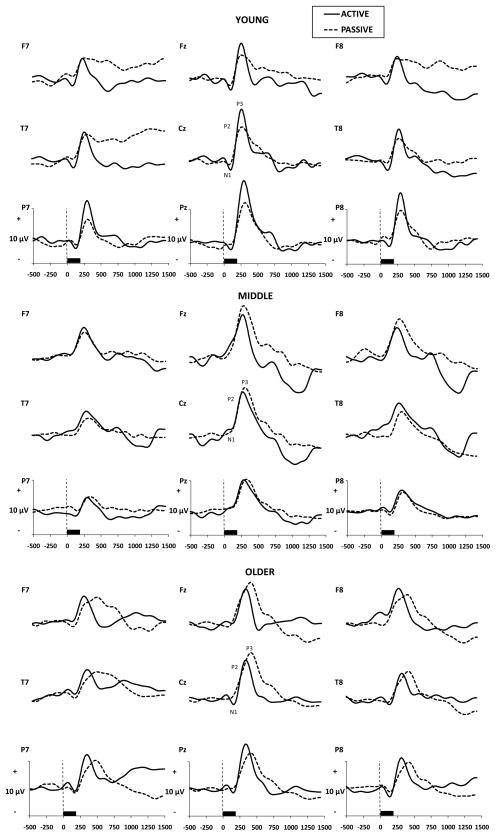
Grand averaged OERP and visual ERP waveforms are presented in Figs. 1 and 2 by Age group, Electrode Site, and Task. Significant results and effect sizes are summarized in Table 2.
Fig. 1.
Grand averaged olfactory ERPs by age group, electrode site, and passive vs. active task (n=10 per age group).
Fig. 2.
Grand averaged visual ERPs by age group, electrode site, and passive vs. active task (n=10 per age group).
Table 2.
Summary of analyses performed and effect sizes for peak component amplitudes, latencies and area measures.
| Peak Measures | Amplitude | Latency | ||||||
|---|---|---|---|---|---|---|---|---|
| N1 | P2 | N2 | P3 | N1 | P2 | N2 | P3 | |
| Visual Stimuli | ||||||||
| Age (A) | * (η=.32) | - | - | - | * (η=.36) | *** (η=.54) | ***(η=.50) | *** (η=.46) |
| Electrode (E) | - | - | * (η=.11) | - | - | * (η=.14) | - | - |
| Task (T) | * (η=.22) | - | - | - | - | - | - | *** (η=.34) |
| A x E | - | - | - | - | - | - | - | - |
| A x T | - | - | - | - | - | - | - | - |
| E x T | - | - | - | ** (η=.18) | - | - | - | - |
| A x E X T | - | - | - | - | - | - | - | - |
| Olfactory Stimuli | ||||||||
| Age (A) | - | - | * (η=.15) | - | * (η=.24) | ***(η=.52) | ***(η=.63) | ***(η=.81) |
| Electrode (E) | - | - | - | - | - | - | - | - |
| Task (T) | - | - | - | - | ** (η=.32) | ** (η=.34) | ** (η=.26) | ***(η=.38) |
| A x E | - | - | - | - | - | - | - | - |
| A x T | - | - | - | - | - | ** (η=.42) | ***(η=.49) | ***(η=.57) |
| E x T | - | - | - | - | - | - | - | - |
| A x E X T | - | - | - | - | - | - | - | - |
| Area Measures | Visual | Olfactory | ||||||
|
| ||||||||
| N1 Area | P2 Area | N2 Area | P3 Area | N1 Area | P2 Area | N2 Area | P3 Area | |
|
| ||||||||
| Age (A) | - | - | - | - | - | - | - | - |
| Hemisphere (H) | - | - | - | - | - | - | - | - |
| Electrode (E) | * (η=.17) | - | - | - | - | - | - | - |
| Task (T) | - | - | - | - | - | - | - | - |
| All two-way interactions | - | - | - | - | - | - | - | - |
| H x E x T | - | - | - | - | ** (η=.19) | * (η=.16) | * (η=.15) | - |
| All other three-way interactions | - | - | - | - | - | - | - | - |
| A x H x E x T | - | - | - | - | * (η=.21) | * (η=.23) | * (η=.23) | * (η=.24) |
P<0.05,
P<0.01,
P<0.001
3.4 P3 amplitude and latency
Visual P3 amplitude demonstrated a significant interaction effect of task x electrode (F(2, 48) = 5.91, p<.01, η2=.18). In the active condition amplitude increased from the frontal to parietal electrode sites, whereas in the passive condition amplitudes were similar across the three electrode sites. Visual amplitudes did not differ significantly between active and passive tasks. Olfactory P3 amplitudes did not significantly differ across tasks, electrode sites, or age groups and there were no significant interaction effects (p>.05).
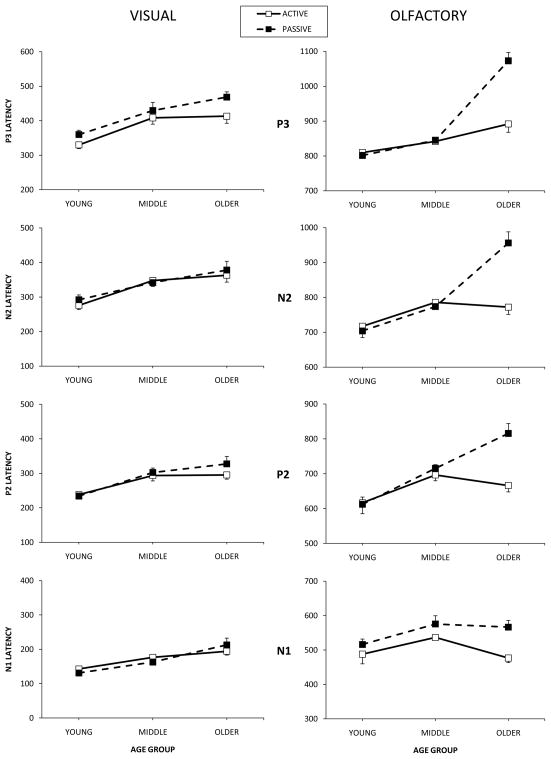
Fig. 3 presents visual and olfactory peak latencies by peak component, age group, and task for the central electrode sites. Visual P3 latency demonstrated significant main effects of age group with middle age and older participants producing significantly longer P3 peak latencies than young participants (F(2, 27) = 11.39, p<.001, η2=.46). There was also a significant main effect of task on visual P3 latency demonstrating significantly longer visual P3 latencies for the passive condition compared to the active condition (F(1, 27) = 13.79, p<.001, η2=.34). In order to better understand the effects of aging on ERPs in each task condition, separate analyses were completed for the active and passive conditions. Analysis of the visual active task condition by itself demonstrated a significant effect of age group (F(2, 27)=20.03, p<.001, η2=.42) with young participants producing significantly shorter latencies than both middle age and older participants. Analysis of the visual passive task condition by itself demonstrated the same results with young participants producing significantly shorter latencies than the other two age groups (F(2, 27)=9.07, p<.01, η2=.40)
Fig. 3.
Visual and olfactory average latencies for active versus passive tasks in young, middle age, and older adults for each ERP peak. Error bars represent the standard error of the mean (SEM).
Olfactory P3 latency demonstrated a significant interaction effect of task x age group (F(2, 27) = 17.67, p<.001, η2=.57). The older participant group demonstrated significantly longer P3 latencies in the passive condition compared to the active condition, while in the other two age groups latencies were similar between passive and active conditions. Further analysis of simple main effects revealed significant main effects for task (F(1, 27)=16.34, p<.001, η2=.38) with the passive task producing longer latencies, and for age (F(2, 27)=59.07, p<.001, η2=.81) with older participants producing longer latencies than middle age and young participants. Separate analyses for active and passive task conditions were also completed for OERP P3 latency. Analysis of the olfactory active task condition by itself demonstrated a significant effect of age group (F(2, 27)=4.35, p<.05, η2=.24) with young participants producing significantly shorter P3 latencies than older participants. Middle age participants did not differ significantly from either young or older participants for active P3 latency (p>.05). The olfactory passive condition also demonstrated a significant effect of age group (F(2, 27)=64.60, p<.001, η2=.83) with the older group producing significantly longer P3 latency than both the young and middle age groups.
3.5 N1, P2, N2 amplitudes and latencies
Visual N1 amplitude demonstrated significant main effects of task (F(1, 27)=7.38, p<.05, η2=.22) and age group (F(2, 27)=6.35, P<.05, η2=.32). Across age groups the active condition produced larger N1 amplitudes than the passive condition. Across task conditions the young age group produced significantly larger N1 amplitudes than the middle age group. Visual P2 amplitudes demonstrated no significant main or interaction effects. Visual N2 amplitude demonstrated a significant main effect of electrode site (F(2, 54)=3.43, p<.05, η2=.11) with significantly larger peak amplitudes at the Cz and Pz sites compared to Fz.
There was a significant main effect of age group on visual N1 latency (F(2,27)=7.54, p<.05, η2=.36) with older participants producing significant longer N1 latencies than younger participants for both tasks. Visual P2 latency demonstrated main effects of age group (F(2, 27)=15.93, p<.001, η2=.54) and electrode site (F(2,54)=4.21, p<.05, η2=.14). Middle age and older participants produced significantly longer P2 latencies than young participants and across age groups latencies increased from the Fz to the Pz electrode sites. Visual N2 latency demonstrated a main effect of age group (F(2,27)=13.20, p<.001, η2=.50) with middle and older participants producing longer latencies than young participants.
Olfactory N1 and P2 amplitudes did not demonstrate any significant main or interaction effects. Olfactory N2 amplitude demonstrated a significant main effect of electrode site (F(2, 54)=4.75, p<.05, η2=.15) with amplitudes gradually increasing from the Fz to the Pz electrode site.
Olfactory N1 latency demonstrated significant main effects of task (F(2, 27)=12.96, p<.01), η2=.32) with longer latencies in the passive condition. N1 latency also showed a significant main effect of age group (F(2, 27)=4.33, p<.05, η2=.24) with young participants producing significantly shorter latencies than middle and older participants. Olfactory P2 latency demonstrated a significant interaction effect of task x age group (F(2,27)=9.59, p<.01, η2=.42). In the young and middle age groups P2 peak latencies were similar, however the older age group produced significantly longer latencies in the passive task compared to the active task. N2 latency also demonstrated a significant interaction effect of task x age group (F(2,27)=13.20, p<.001, η2=.49) with older participants producing significantly longer latencies in the passive condition compared to active condition, and no significant task latency difference for young and middle age subjects.
3.6 Area measures of lateral electrodes
Analysis of lateral electrode visual area measures F7, T7, P7, F8, T8, P8 revealed a significant effect of electrode site for the N1 component only (F(2, 54)=5.43, p<.05, η2=.17) with peak area increasing from the parietal electrodes to the frontal electrodes. All other interactions and main effects of visual areas did not reach statistical significance.
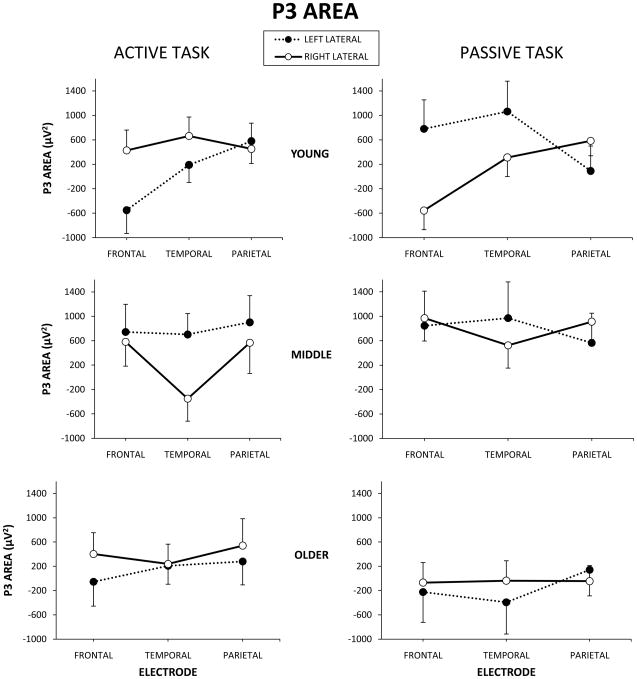
Analyses of lateral electrode olfactory area measures F7, T7, P7, F8, T8, P8 revealed significant 4-way interactions of age group x hemisphere x electrode site x task for all ERP components (N1: F(3, 54)=3.18, p<.05, η2=.21; P2: F(3, 54)=3.39, p<.05, η2=.23; N2: F(3, 54)=3.16, p<.05, η2=.23; P3: F(3, 54)=3.57, p<.05, η2=.24). Fig. 4 demonstrates this interaction effect for P3 area measures. N1, P2, & N2 components demonstrated similar patterns to P3. Further analysis by age group demonstrated that in young participants larger peak areas were present for the active task compared to the passive task at the F8 electrode site, whereas the opposite was true at F7 with larger areas for the passive task versus active task (p<.01). Middle age and older participants did not demonstrate statistically significant lateral area differences by hemisphere, electrode site, or task (p>.05).
Fig. 4.
P3 lateral area measures for young, middle age, and older participants by electrode site for active and passive task conditions. Error bars represent the standard error of the mean (SEM).
4. Discussion
The P3 component of ERPs, including the OERP, represents endogenous processing of a stimulus, involving stimulus classification speed and the ability to attend to and evaluate a stimulus (Donchin et al., 1984; Polich 1998). The amplitude of P3 has been interpreted theoretically as reflecting representational changes of working memory (Donchin and Coles, 1988; Johnson, 1988), while the latency of P3 is regarded as the speed of stimulus evaluation (Magliero et al., 1984). P3 latency negatively correlates with cognitive capability in normal subjects, such that shorter latencies are related to faster processing speed that reflects superior cognitive performance (Emmerson et al., 1989; Mertens and Polich, 1997; O’Donnell et al., 1992; Polich and Martin, 1992). OERP P3 latency correlates with neuropsychological tests that measure memory and cognitive processing speed (Geisler et al., 1999). As in the current study, previous studies have demonstrated that OERPs tend to be quite noisy, especially to readers who are most familiar with ERPs from other modalities (for OERP examples see: Hawkes et al., 1997; Krauel et al., 1998; Masago et al., 2001).
Previous OERP studies have demonstrated larger P3 amplitudes for active tasks compared to passive tasks, but comparisons of amplitude differences did not reach statistical significance in the current study. These previous studies utilized longer ISIs ranging on average at least 45 seconds, while the current study utilized a relatively short ISI of 30 seconds. Morgan et al. (1997) demonstrated a reduction in peak amplitudes as ISIs decreased from 90 to 45 seconds, especially in older participants and most significantly in older males. This likely is a result of greater habituation and adaptation taking place at the shorter ISIs. Therefore it is not surprising, given the even shorter ISI in the present study, that amplitudes were reduced and amplitude differences between groups did not reach significance.
In the present study, greater age-related P3 latency differences were demonstrated in the olfactory modality (η2=.81) compared to the visual modality (η2=.46) along the central electrode sites. Latencies in both stimulus modalities were longer for older participants compared to young participants. In the visual modality active condition P3 latencies were shorter than in the passive condition with no interactions of age group. In the olfactory modality, however, young and middle age participants produced similar latencies in the conditions, but older participants produced much longer latencies in the passive condition compared to the active condition. These results demonstrate that in the olfactory modality the P3 is relatively similar regardless of active or passive presentation in young and middle aged individuals, however older adults are sensitive to task condition.
Regarding the early sensory OERP components, N1, P2, and N2, previous studies on active versus passive conditions have been mixed and have only been researched in young participants. Geisler & Murphy (2000) and Pause et al. (1997) both demonstrated amplitude effects for the early components with larger amplitudes in the active condition, but no latency effects. Masago et al. (2001) showed increased amplitude in the ignore (passive) condition only for N2, and decreased early component latencies under the attend (active) condition compared to the ignore condition. Krauel et al.(1998) also demonstrated larger N2 amplitudes in the ignore condition and decreased latencies for early components in the attend condition.
One of the primary functions of the attentional system is to enable cognitive processing to focus on stimulus attributes and ignore or inhibit irrelevant aspects (Chao and Knight, 1997). Karayanidis et al. (1995) suggested that older adults have a reduced gating of irrelevant input, which may lead to processing overload and consequently contribute to impairments in attentional processing and habituation. Hasher and Zacks (1988) suggested that older subjects are more susceptible to distractibility due to a decline in the efficiency of inhibitory mechanisms. Other researchers have discussed the “default network” that is active when individuals are not actively attending to a task (Buckner et al., 2008; Raichle et al., 2007). Research has shown that over the life-span, deactivation within this default network is reduced in older adults relative to middle aged and younger adults, suggesting an age-dependent inability to filter out information that is irrelevant to the task (Grady et al., 2006; Stevens et al., 2008).
Krauel et al. (1998) discuss that in the visuo-spatial domain allocation of attention is considered to provide a sensory gain in the attended channel and the effect of attention is manifest as an amplitude modulation or sensory gain. They go on to state that in the olfactory system it is not the amount of sensory gain (amplitude) but the speed of sensory processing (latency) that appears to be enhanced by attention. They further state that this implies a high share of temporal coding within olfactory stimulus processing. Krauel et al. (1998) indicate that the first, more exogenous components of OERPs seem to vary with attentional investment, suggesting that attentional modulation takes place at relay stations before the cortical representation.
In the present study young participants demonstrated an interesting OERP interaction with the right lateral electrode site F8 producing differing activation between the active and passive tasks than the left lateral F7 site. While Masago et al. (2001) did not use laterality as a statistical variable, and only compared one electrode site to others, they demonstrated larger OERP P3 amplitudes at right frontal electrodes including F8 and F4 compared to left electrode sites such as F7 and F3. They did not indicate if these results were combined across task conditions or presented just for the active condition, but they report no interaction effects, as demonstrated in the present study. While not easily generalized across types of studies, brain imaging studies have also demonstrated preferential activation in the right hemisphere when processing higher order olfactory information (Cerf-Ducastel and Murphy, 2006; Gottfried and Zald, 2005; Royet et al., 2003). Zatorre and Jones-Gotman (2000) suggested the primary sensory olfactory response appears to be bilateral, while higher processing preferentially involves the right orbitofrontal cortex. This may suggest that, at least in younger participants, higher processing of an actively attended stimulus may be processed by different areas of the brain than processing of an ignored stimulus. Petit et al. (2007) showed not only greater right than left frontal activity in an fMRI auditory attend task in young participants, but an enhancement of this rightward asymmetry in response to attended tones versus unattended tones. Friedman et al. (1998) demonstrated auditory ERPs recorded from frontal electrode sites differed between attend and ignore conditions for young participants but did not differ for older participants. They surmised that, for the elderly, the frontal generator(s) are as selectively engaged in the ignore condition as they are in the attend condition.
In conclusion, in the clinical arena passive ERP tasks may be more easily implemented, especially with cognitively impaired patients, however the current findings demonstrate that it is essential to take into consideration task demands when interpreting olfactory ERP performance. In persons being evaluated for dementia for example, if a passive task is used, one must be careful to not interpret increased OERP latencies necessarily as a sign of dementia, when in reality the observed latency increases may be related to the specific task parameters. Future research in this area needs to focus on performance differences in these specific clinical populations in order to develop normative data for each population given specific task demands.
Acknowledgments
Supported by NIH Grant DC02064 to Claire Murphy. The authors would like to thank John Polich, Krystin Corby, Joel Kowalewski, Jessica Bartholow, Barbara Cerf-Ducastel, Roberto Zamora, Richard Vail and Lori Haase for research assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennington JY, Polich J. Comparison of P300 from passive and active tasks for auditory and visual stimuli. Int J Psychophysiol. 1999;34:171–177. doi: 10.1016/s0167-8760(99)00070-7. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cain WS. Testing olfaction in a clinical setting. Ear Nose Throat J. 1989;68:316, 322–318. [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Celesia GG. EEG and event-related potentials in aging and dementia. J Clin Neurophysiol. 1986;3:99–111. doi: 10.1097/00004691-198604000-00001. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. Neural substrates of cross-modal olfactory recognition memory: an fMRI study. Neuroimage. 2006;31:386–396. doi: 10.1016/j.neuroimage.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Covington JW, Geisler MW, Polich J, Murphy C. Normal aging and odor intensity effects on the olfactory event-related potential. Int J Psychophysiol. 1999;32:205–214. doi: 10.1016/s0167-8760(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles M. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–427. [Google Scholar]
- Donchin E, Heffley E, Hillyard SA, Loveless N, Maltzman I, Ohman A, Rosler F, Ruchkin D, Siddle D. Cognition and event-related potentials. II. The orienting reflex and P300. Ann N Y Acad Sci. 1984;425:39–57. doi: 10.1111/j.1749-6632.1984.tb23522.x. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Emmerson RY, Dustman RE, Shearer DE, Turner CW. P3 latency and symbol digit performance correlations in aging. Exp Aging Res. 1989;15:151–159. doi: 10.1080/03610738908259769. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Cui L, Starr A. Olfactory event-related potentials in normal human subjects: effects of age and gender. Electroencephalogr Clin Neurophysiol. 1995;95:293–301. doi: 10.1016/0013-4694(95)00055-4. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roth WT, Kopell BS. Auditory evoked potentials to unpredictable shifts in pitch. Psychophysiology. 1976;13:32–39. doi: 10.1111/j.1469-8986.1976.tb03333.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski VA, Cycowicz YM. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology. 1998;35:508–520. doi: 10.1017/s0048577298970664. [DOI] [PubMed] [Google Scholar]
- Geisler MW, Morgan CD, Covington JW, Murphy C. Neuropsychological performance and cognitive olfactory event-related brain potentials in young and elderly adults. J Clin Exp Neuropsychol. 1999;21:108–126. doi: 10.1076/jcen.21.1.108.935. [DOI] [PubMed] [Google Scholar]
- Geisler MW, Murphy C. Event-related brain potentials to attended and ignored olfactory and trigeminal stimuli. Int J Psychophysiol. 2000;37:309–315. doi: 10.1016/s0167-8760(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Res Rev. 2005;50:287–304. doi: 10.1016/j.brainresrev.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks R. Working memory, comprehension and aging: a review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Academic Press; New York: 1988. pp. 192–225. [Google Scholar]
- Hawkes CH, Shephard BC, Kobal G. Assessment of olfaction in multiple sclerosis: evidence of dysfunction by olfactory evoked response and identification tests. J Neurol Neurosurg Psychiatry. 1997;63:145–151. doi: 10.1136/jnnp.63.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ. The amplitude of the P300 component of the event-related potential: review and synthesis. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in Psychophysiology. Jai Press; Greenwich, CT: 1988. pp. 69–137. [Google Scholar]
- Karayanidis F, Andrews S, Ward PB, Michie PT. ERP indices of auditory selective attention in aging and Parkinson’s disease. Psychophysiology. 1995;32:335–350. doi: 10.1111/j.1469-8986.1995.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel C. Cerebral chemosensory evoked potentials elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroencephalogr Clin Neurophysiol. 1988;71:241–250. doi: 10.1016/0168-5597(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Krauel K, Pause BM, Sojka B, Schott P, Ferstl R. Attentional modulation of central odor processing. Chem Senses. 1998;23:423–432. doi: 10.1093/chemse/23.4.423. [DOI] [PubMed] [Google Scholar]
- Liss L, Gomez F. The nature of senile changes of the human olfactory bulb and tract. AMA Arch Otolaryngol. 1958;67:167–171. doi: 10.1001/archotol.1958.00730010173006. [DOI] [PubMed] [Google Scholar]
- Lorig TS. The effects of active and passive stimulation on chemosensory event-related potentials. Int J Psychophysiol. 1996;23:199–205. doi: 10.1016/s0167-8760(96)00061-x. [DOI] [PubMed] [Google Scholar]
- Lorig TS. On the similarity of odor and language perception. Neurosci Biobehav Rev. 1999;23:391–398. doi: 10.1016/s0149-7634(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Lorig TS. The application of electroencephalographic techniques to the study of human olfaction: a review and tutorial. Int J Psychophysiol. 2000;36:91–104. doi: 10.1016/s0167-8760(99)00104-x. [DOI] [PubMed] [Google Scholar]
- Magliero A, Bashore TR, Coles MG, Donchin E. On the dependence of P300 latency on stimulus evaluation processes. Psychophysiology. 1984;21:171–186. doi: 10.1111/j.1469-8986.1984.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Masago R, Shimomura Y, Iwanaga K, Katsuura T. The effects of hedonic properties of odors and attentional modulation on the olfactory event-related potentials. J Physiol Anthropol Appl Human Sci. 2001;20:7–13. doi: 10.2114/jpa.20.7. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Katasu TB, editors. Geriatric psychiatry: A handbook for psychiatrists and primary care physicians. Grune and Statton; New York: 1976. pp. 77–121. [Google Scholar]
- Mertens R, Polich J. P300 from a single-stimulus paradigm: passive versus active tasks and stimulus modality. Electroencephalogr Clin Neurophysiol. 1997;104:488–497. doi: 10.1016/s0168-5597(97)00041-5. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Covington JW, Geisler MW, Polich J, Murphy C. Olfactory event-related potentials: older males demonstrate the greatest deficits. Electroencephalogr Clin Neurophysiol. 1997;104:351–358. doi: 10.1016/s0168-5597(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Geisler MW, Covington JW, Polich J, Murphy C. Olfactory P3 in young and older adults. Psychophysiology. 1999;36:281–287. doi: 10.1017/s0048577299980265. [DOI] [PubMed] [Google Scholar]
- Murphy C. Age-related effects on the threshold, psychophysical function, and pleasantness of menthol. J Gerontol. 1983;38:217–222. doi: 10.1093/geronj/38.2.217. [DOI] [PubMed] [Google Scholar]
- Murphy C. Cognitive and chemosensory influences on age-related changes in the ability to identify blended foods. J Gerontol. 1985;40:47–52. doi: 10.1093/geronj/40.1.47. [DOI] [PubMed] [Google Scholar]
- Murphy C. Taste and smell in the elderly. In: Meiselman HL, Rivlin RS, editors. Clnical measurement of taste and smell. Macmillan; New York: 1986. pp. 343–371. [Google Scholar]
- Murphy C. Senescence and clinical changes in the olfactory system: Psychological considerations [NIDCD Monograph] Development, Growth and Senescence in the Chemical Senses. 1993;3:153–160. [Google Scholar]
- Murphy C. The chemical senses and nutrition in the elderly. J Nutri for the Elderly. 2008:247–265. doi: 10.1080/01639360802261862. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS. Odor identification: the blind are better. Physiol Behav. 1986;37:177–180. doi: 10.1016/0031-9384(86)90402-6. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Gilmore MM, Skinner RB. Sensory and semantic factors in recognition memory for odors and graphic stimuli: elderly versus young persons. Am J Psychol. 1991;104:161–192. [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiol Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Murphy C, Morgan CD, Geisler MW, Wetter S, Covington JW, Madowitz MD, Nordin S, Polich JM. Olfactory event-related potentials and aging: normative data. Int J Psychophysiol. 2000;36:133–145. doi: 10.1016/s0167-8760(99)00107-5. [DOI] [PubMed] [Google Scholar]
- Murphy C, Nordin S, Acosta L. Odor learning, recall, and recognition memory in young and elderly adults. Neuropsychology. 1997;11:126–137. doi: 10.1037//0894-4105.11.1.126. [DOI] [PubMed] [Google Scholar]
- Murphy C, Nordin S, de Wijk RA, Cain WS, Polich J. Olfactory-evoked potentials: assessment of young and elderly, and comparison to psychophysical threshold. Chem Senses. 1994;19:47–56. doi: 10.1093/chemse/19.1.47. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. Journal of the American Medical Association (JAMA) 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Nordin S, Quinonez C, Morgan CD, Geisler MW, Polich J, Murphy C. Olfactory event-related potentials in young and elderly adults: evaluation of tracking task versus eyes open/closed recording. Chem Senses. 1999;24:459–464. doi: 10.1093/chemse/24.4.459. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Friedman S, Swearer JM, Drachman DA. Active and passive P3 latency and psychometric performance: influence of age and individual differences. Int J Psychophysiol. 1992;12:187–195. doi: 10.1016/0167-8760(92)90010-9. [DOI] [PubMed] [Google Scholar]
- Pause BM, Sojka B, Ferstl R. Central processing of odor concentration is a temporal phenomenon as revealed by chemosensory event-related potentials (CSERP) Chem Senses. 1997;22:9–26. doi: 10.1093/chemse/22.1.9. [DOI] [PubMed] [Google Scholar]
- Petit L, Simon G, Joliot M, Andersson F, Bertin T, Zago L, Mellet E, Tzourio-Mazoyer N. Right hemisphere dominance for auditory attention and its modulation by eye position: an event related fMRI study. Restor Neurol Neurosci. 2007;25:211–225. [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Wenegrat BG, Roth WT, Kopell BS. Clinical-application of the P3 component of event-related potentials .1. Normal aging. Electroen Clin Neuro. 1984;59:85–103. doi: 10.1016/0168-5597(84)90026-1. [DOI] [PubMed] [Google Scholar]
- Polich J. Attention, probability, and task demands as determinants of P300 latency from auditory stimuli. Electroencephalogr Clin Neurophysiol. 1986;63:251–259. doi: 10.1016/0013-4694(86)90093-3. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 development from auditory stimuli. Psychophysiology. 1986;23:590–597. [Google Scholar]
- Polich J. Comparison of P300 from a passive tone sequence paradigm and an active discrimination task. Psychophysiology. 1987;24:41–46. doi: 10.1111/j.1469-8986.1987.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33:334–353. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Polich J. EEG and ERP assessment of normal aging. Evoked Potential. 1997;104:244–256. doi: 10.1016/s0168-5597(97)96139-6. [DOI] [PubMed] [Google Scholar]
- Polich J, Martin S. P300, cognitive capability, and personality: A correlational study of university undergraduates. Personality and individual differences. 1992;13:533–543. [Google Scholar]
- Polich J, Hoffman LD. P300 and handedness: on the possible contribution of corpus callosal size to ERPs. Psychophysiology. 1998;35:497–507. doi: 10.1017/s0048577298970792. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Roth WT. Auditory evoked responses to unpredictable stimuli. Psychophysiology. 1973;10:125–138. doi: 10.1111/j.1469-8986.1973.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20:713–728. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Dendrite bundles, central programs and the olfactory bulb. Brain Res. 1975;95:407–421. doi: 10.1016/0006-8993(75)90118-3. [DOI] [PubMed] [Google Scholar]
- Schiffman S, Robinson D, Erickson R. Multidimensional-scaling of odorants - examination of psychological and physicochemical dimensions. Chemical Senses & Flavour. 1977;2:375–390. [Google Scholar]
- Schiffman SS. Age-related changes in taste and smell and their possible causes. In: Meiselman HL, Rivlin RS, editors. Clinical Measurement of Taste and Smell. Macmillan; New York: 1986. pp. 326–342. [Google Scholar]
- Schiffman SS, Gatlin CA. Clinical physiology of taste and smell. Annu Rev Nutr. 1993;13:405–436. doi: 10.1146/annurev.nu.13.070193.002201. [DOI] [PubMed] [Google Scholar]
- Smith CG. Age incidence of atrophy of olfactory nerves in man. Journal of Comparative Neurology. 1942;77:589–595. [Google Scholar]
- Stevens JC, Cain WS, Schiet FT, Oatley MW. Olfactory adaptation and recovery in old age. Perception. 1989;18:265–276. doi: 10.1068/p180265. [DOI] [PubMed] [Google Scholar]
- Stevens WD, Hasher L, Chiew KS, Grady CL. A neural mechanism underlying memory failure in older adults. J Neurosci. 2008;28:12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck BA, Frey S, Freiburg C, Hormann K, Zahnert T, Hummel T. Chemosensory event-related potentials in relation to side of stimulation, age, sex, and stimulus concentration. Clin Neurophysiol. 2006;117:1367–1375. doi: 10.1016/j.clinph.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Thesen T, Murphy C. Age-related changes in olfactory processing detected with olfactory event-related brain potentials using velopharyngeal closure and natural breathing. Int J Psychophysiol. 2001;40:119–127. doi: 10.1016/s0167-8760(00)00157-4. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M. Functional imaging of the chemical senses. In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Applications. Academic Press; San Diego: 2000. pp. 403–424. [Google Scholar]