Abstract
Lesions of the rat medial prefrontal cortex (mPFC) produce behavioral impairments in the 5-choice serial reaction time (5CSRT) task, a widely-used measure of sustained and selective visual attention. This experiment compared the effects of “dorsal” (centered on prelimbic and infralimbic cortices) and “ventral” (centered on dorsal peduncular cortex and tenia tecta) mPFC lesions on performance in a variant of the 5CSRT task. Because in some associative learning theories, the predictive validity of events determines the allocation of attention to them, we also examined the effects of cue validity in this task. Operant nosepoke responses to some briefly-illuminated ports were consistently (100%) reinforced (CRF) with food, whereas for other ports, responding was reinforced on only 50% of the trials (partial reinforcement, PRF). Different patterns of impairment emerged depending on lesion location within the mPFC. Dorsal- and sham-lesioned rats responded more to CRF than to PRF cues, but ventral-lesioned rats responded similarly to CRF and PRF cues. Additionally, under some conditions of increased attentional demands, dorsal-lesioned rats failed to respond on many trials, whereas the impairment in ventral-lesioned rats was manifested as an increase in response errors. These results demonstrate separable roles for dorsal and ventral mPFC subregions in controlling attention.
Keywords: attention, medial prefrontal cortex, partial reinforcement, tenia tecta, 5CSRT task
1. Introduction
Much animal research implicates the medial prefrontal cortex (mPFC) in attentional function. A variety of test paradigms has been employed in this research, including the 5-choice serial reaction time (5CSRT) task [1–4], signal discrimination tasks [5–7], and attentional set-shifting procedures [8,9]. Effects of mPFC manipulations have been demonstrated in each of these tasks, suggesting a relatively broad role for the mPFC in attentional processes. Here we examined further the role of mPFC in attention by examining the effects of cue validity and other parameters on performance in the 5CSRT task after lesions of mPFC subregions.
The 5CSRT task has been extensively used to assess sustained and selective visual attention in rats (for reviews of this task, see [10,11]). This task requires rats to poke their noses into a briefly illuminated stimulus-response port out of an array of five ports to earn food reward. After baseline acquisition of the task, challenges (such as a shortened duration port light) can be introduced to make the task more demanding of attentional resources. A number of studies have used the 5CSRT task to explore the involvement of the mPFC in attention. For example, Muir, Everitt and Robbins [1] found that mPFC-lesioned rats showed reduced accuracy, increased correct response latencies, and increased perseverative responding, even under their baseline version of the task, prior to any attentional challenges. Although much of the work subsequent to this observation concentrated on the role of the cholinergic input to the mPFC [3,4,12,13], another line of research focused on subregional specialization within the mPFC [2,14,15]. Consistent with modern views of anatomical and functional specialization within the mPFC [16], Chudasama and colleagues [2, 17] found different effects of dorsal vs. ventral neurotoxic mPFC lesions in the 5CSRT task. Specifically, dorsal mPFC lesions, which targeted anterior cingulate cortex (Zilles’s Cg1), resulted in reduced accuracy, but did not cause an increase in perseverative responding, whereas ventral mPFC lesions, which targeted prelimbic (PL) and infralimbic (IL) cortex, increased perseverative responding but caused only a transient impairment in accuracy.
A subsequent study by Chudasama and colleagues [14] found differential effects of anterior cingulate cortex (ACC) and IL cortex lesions in the 5CSRT task. ACC lesions impaired task accuracy, whereas IL cortex lesions resulted in increased premature (anticipatory) responding, greater omissions, and faster response latencies. Interestingly, the IL-lesioned rats in that study did not show increased perseverative responding, suggesting that the observed increase in perseverative responding in the previous (Passetti et al. [2]) study, which utilized a combined PL+IL lesion, may have been due primarily to damage to the PL cortex. In order to further dissociate PL and IL function, Murphy and colleagues [15] examined the effects of NMDA receptor antagonism within each subregion prior to the rats being assessed in the 5CSRT task. Infusions of a NMDA receptor antagonist into either the PL or IL cortex resulted in decreased accuracy and increased omissions. However, premature (anticipatory) responding was increased only by infusions into IL, but not PL, cortex. These authors [15] did not find any effect of infusions into either mPFC subregion on perseverative responding, but this apparent discrepancy with the results of Passetti et al. [2] could be due to the different surgical manipulations (permanent lesion vs. transient pharmacological effect) or slightly different training contingencies used between the two studies. Regardless of the exact specifics of these earlier studies, it is clear that different regions of the rat mPFC subserve different but possibly interrelated functions in attentional processes.
In contrast to areas of the mPFC examined in previous studies (anterior cingulate, prelimbic, and infralimbic cortices), there is relatively little existing research into the function of the ventral-most mPFC (specifically, dorsal peduncular cortex and tenia tecta). There is reason to suggest that these ventral-most mPFC regions could be involved in different aspects of attentional function than the regions just dorsal to them. For example, Ottersen [18] reported a projection from the tenia tecta to the central nucleus (CEA) of the amygdala. Cassell and Wright [19] replicated this finding, and moreover, made it clear that this projection from tenia tecta, unlike projections from other mPFC regions, was specific to CEA, and did not innervate the basolateral amygdala (BLA). This is an important distinction, because a substantial body of evidence has accumulated indicating dissociable roles for the CEA and the BLA in a variety of paradigms, including those that assess attentional function (for reviews of amygdala circuitry in cognitive processes, see [20,21]). These studies suggest that CEA is broadly involved in attentional processes in associative learning, whereas BLA is more involved in other processes, such as reward learning and outcome representational processes. Most relevant to the present study, lesions of CEA impair performance in the multiple-choice reaction time paradigm [13,22]. As such, it is possible that the tenia tecta are part of a circuit, including CEA and other structures, which regulates attentional function. In the present experiment, we compared the effects of “ventral” (centered on dorsal peduncular cortex and tenia tecta) and “dorsal” (centered on prelimbic and infralimbic cortices) lesions of the mPFC on the allocation of visual attention in the multiple-choice reaction time task.
A key feature of the experiment reported here was the inclusion of assessments of the effects of cue validity on performance in the 5CSRT task after these lesions. Many theorists [23–25] have stressed the importance of the predictive validity of a conditioned stimulus (CS) in determining the allocation of attention to that CS in the framework of associative learning. We examined the effects of the predictive validity of the port cues in the 5CSRT task by manipulating reinforcement probability. After initial training in which all correct responses were reinforced, a partial reinforcement contingency was imposed on responding to two of the ports. We expected that consistently reinforced (CRF) cues would command more attention in the performance of this task, relative to partially reinforced (PRF) cues, in intact rats. If mPFC subregions regulate the deployment of attention, then lesions of these regions might interfere with the normal pattern of responding to CRF vs. PRF cues. Given the anatomical relation between ventral mPFC and CEA, and the results of prior studies that implicated CEA in mediating effects of cue validity on attention in other test paradigms (e.g., [26,27]), we especially anticipated impairments after lesions of ventral mPFC. Behavioral impairments may be evident even under baseline training conditions, or may only become evident under conditions of increased attentional load. To address this issue, these experiments included a set of attentional challenge tests designed to tax the rats’ attentional resources.
2. Materials and Methods
2.1 Subjects
Twenty-four male Long-Evans rats (Charles River Laboratories, Raleigh, NC), weighing between 300–325 grams when they arrived in the laboratory vivarium, were used in this experiment. The rats were housed individually, and were given a 1 week acclimatization period to adjust to their new surroundings and to being handled by the experimenter on a daily basis. During this period, the rats had free access to food and water. Thereafter, they were food deprived to 85% of their ad libitum weights, and remained in this food deprived state for the entire course of the experiment. The laboratory viviarium was illuminated from 7 A.M. to 7 P.M.
2.2 Surgical procedures
Surgery was performed after the rats had undergone an initial period of CRF training on the 5CSRT task. Bilateral dorsal and ventral neurotoxic lesions of the mPFC were made under aseptic conditions, using isoflurane anesthesia. Note that we use the terms “dorsal” and “ventral” comparatively; thus they do not necessarily correspond to the usage of other authors. Lesions were made using NMDA at a concentration of 12.5 mg/ml in PBS (Sigma, St. Louis, MO). Dorsal lesions were made using the following coordinates: AP: +3.0, ML: +/− 0.7, and DV: −4.8 and −3.8 from the skull surface. Ventral lesions were made using the following coordinates: AP: +3.0, ML: +/− 0.6, and DV: −5.0 and −4.0 from the dura. A volume of 0.15 μl (for the ventral coordinates) or 0.20 μl (for the dorsal coordinates) was infused, using a 2.0 μl Hamilton syringe, at each injection site over a period of 90 s. The needle was left in place at each injection site for 1 min before infusing and 4 min after infusing the toxin. Sham-lesioned rats received an equal amount of vehicle (PBS) infused with the same coordinates and paramaters. All rats were given a 14-day recovery period after surgery before behavioral training and testing began again.
2.3 Apparatus
The behavioral training apparatus consisted of four individual 5CSRT chambers (25 cm × 25 cm × 25 cm; Cambridge Cognition, Cambridge, UK). Each chamber had aluminum front and side walls and a clear acrylic back wall and top. Five 2.5 cm × 2.5 cm × 4 cm (deep) stimulus-response ports were spaced 2.5 cm apart, and centered on the front, curved wall of the chamber, 2 cm above a grid floor. A 3 W lamp at the back of each port provided illumination as the port cues; responding in the ports was detected with infrared phototransistors. A recessed food cup was located in the center of the back wall of the chamber. This food cup was fitted with a lamp that was illuminated when food pellets were delivered and a transparent acrylic flap to detect food cup entries. A 3 W overhead lamp was mounted at the center of the chamber ceiling that could be illuminated to serve as a ready signal to indicate the upcoming port cue trial. Each chamber was enclosed in a sound-attenuating box where ventilation fans provided masking noise (70 dB). A bank of infrared LEDS provided background illumination for video monitoring and recording, but this illumination was invisible to the rats. A television camera was mounted within each box to allow for video recording during behavioral training and testing.
2.4 Behavioral training procedures
The rats were first familiarized with the apparatus in four sessions. In the first 15-min session, ten 45-mg grain food pellets (Research Diets, New Brunswick, NJ) were present in the illuminated food cup, and the acrylic flap to the food cup was propped open. In the next 15-min session, five food pellets were again placed in the illuminated food cup, but the flap door was not propped open (such that the rats had to push it open to retrieve the food pellets). In addition, all five port lights were continuously illuminated, and two food pellets were placed in each response port (in addition to the five pellets placed in the food cup). In each of the next two 32-min sessions, there were 16 deliveries of a food pellet, accompanied by a 1-s illumination of the food cup light, to train the rats to collect food from the food cup. After successful magazine training, the rats began baseline training of the 5CSRT task.
In the baseline task used here, the beginning of a trial was signaled at random intervals by the illumination of the overhead lamp ready signal. After a constant 5-s ready period, one of the five target ports was illuminated for 1 s. Each port was equally likely to be illuminated on any trial. The first response to the correct port during port illumination was reinforced with the delivery of a food pellet to the food cup (accompanied by a 1-s illumination of the food cup) and the darkening of both the port light and the ready signal. If no correct response was made before the end of the 1-s response window, the ready signal and port light were terminated and the trial ended. Responses to the ports that were not illuminated on a trial were recorded as errors but had no scheduled consequences. Sixty trials were presented in random order at predetermined intervals within each 30-min session; trial delivery was not affected by the rats’ behavior. Each rat received two sessions daily.
Rats were shaped to this procedure gradually, but all rats received the same treatment (the shaping was not individualized). Between sessions, the duration of port illumination was reduced, and the number of trials were increased, from 30 s (6 of each port cue per session) in the first two to three training sessions to 1 s (12 of each port cue per session) over the course of 10–12 sessions. After the 1-s cue duration level was initially reached, rats required an additional 30 training sessions at the 1-s level to be able to perform this task quickly enough for their responses to register as correct. Once all rats reached a criterion of approximately 80% accuracy, the rats underwent surgery. Following a 14-day post-operative recovery period, the rats were given six reminder training sessions identical to those received immediately prior to surgery.
Thereafter, the final reinforcement contingencies were introduced. For each rat, two of the ports were designated for CRF, two for PRF (50%), and one for no reinforcement (extinction, EXT). Each session included 12 trials with each port cue, as before. CRF trials were identical to those presented previously. Responding on half of the trials with the PRF port cues was reinforced as before, but on the other half of those trials, responses had no effect. On trials with the EXT port cues, responses had no consequences. The EXT port was always the center port in the 5-port array, but the critical types of ports, the CRF and PRF ports, were spatially counterbalanced within the array.
After 20 CRF/PRF training sessions, all rats received a series of attentional challenge sessions, to determine the effects of the PRF contingencies on the allocation of attention. In the first two challenge sessions (port cue challenge), the port cue duration was reduced to 250 ms; otherwise these sessions were identical to the training sessions. Although the port cue was illuminated for only 250 ms, the rats had the full 1-s period to make a response. Next, the rats were returned to the original 1-s target condition (referred to as baseline) for two sessions that were identical to the final training sessions. In the second two challenge sessions (ready signal challenge), the duration of the house light ready signal before the 1-s port cues were illuminated was made variable, with equally probable values of 1, 5, or 9 s. To avoid changes in the total numbers of trials or session duration, each of these challenge test sessions included trials with each of the three ready signal durations, but with only one CRF port and one PRF port, as well as the EXT port. Thus, the only difference between the two challenge test sessions was the specific CRF and the specific PRF port (spatial location) used in each session. Following these sessions, the rats received another two baseline sessions.
2.5 Response measures and data analysis
Behavioral performance was assessed with several measures. The primary measures of performance were the percentage of trials on which at least one correct response (a response to an illuminated port) occurred, the percentage of trials on which at least one error (a response to a non-illuminated port) was made, and the percentage of trials on which no responses occurred (“response omissions”). For each of these 3 measures, the eligible period was the 1-s interval of port light illumination in baseline sessions and in the ready cue challenge test, or the 1-s interval comprised of the 250-ms port light illumination and 750-ms limited hold period in the port cue challenge test. Note that because on each trial, there was one correct and 4 incorrect ports, chance performance (given a completed trial) was 20% for a correct response and 80% for an error. In addition, measures of correct port entry response latency (time after onset of the port light when the first correct response was made), premature responses (port entries that occurred during the ready signal but before the onset of a port light stimulus), and perseverative responses (port entries that occurred after food delivery) were recorded.
Separate analyses of variance (ANOVAs) were conducted for each of the six response measures. Lesion effects were assessed in comparison with the appropriate sham-lesioned rats; thus, separate ANOVAs were conducted for the dorsal and ventral groups in all analyses. Initial inspection of responding after the PRF and EXT ports were added showed lower responding to the EXT port than to CRF or PRF ports; furthermore, unlike with CRF and PRF ports, rats were no more likely to respond to the EXT port when it was illuminated (correct) than when it was dark (error). Thus, we focused on analysis of responding to the CRF and PRF ports. Once PRF contingencies were introduced, ANOVAs for all training, baseline and test phases included the between-subjects lesion (lesion vs. sham) variable and the within-subject repeated measure of contingency (CRF vs. PRF ports). Finally, the effects of the challenge manipulations were assessed within subjects by comparing average performance in the two test sessions with averaged performance in the corresponding two baseline sessions. Thus, most of the analyses were 2 × 2 ×2, lesion (lesion vs. sham) × contingency (CRF vs. PRF) × test (baseline vs. test) ANOVAs. The level of statistical significance adopted was p < .05; in addition, differences with ps > .05 but less than 0.08 were described as of marginal significance.
2.6 Histological procedures
After completion of behavioral testing, the rats were deeply anesthesized with isoflurane, and perfused transcardially with 0.9% saline followed by 10% (v/v) formalin. The brains were removed and stored in 0.1 M PBS with 20% (w/v) sucrose, 2.5% (w/v) formaldehyde, and 1.25% (w/v) dimethyl sulfoxide (DMSO) for 48–72 hours. Sections (40 μm) from each brain were collected on a freezing microtome. Every third section was mounted on glass microscope slides and Nissl-stained to verify lesion placement.
3. Results
3.1 Histology
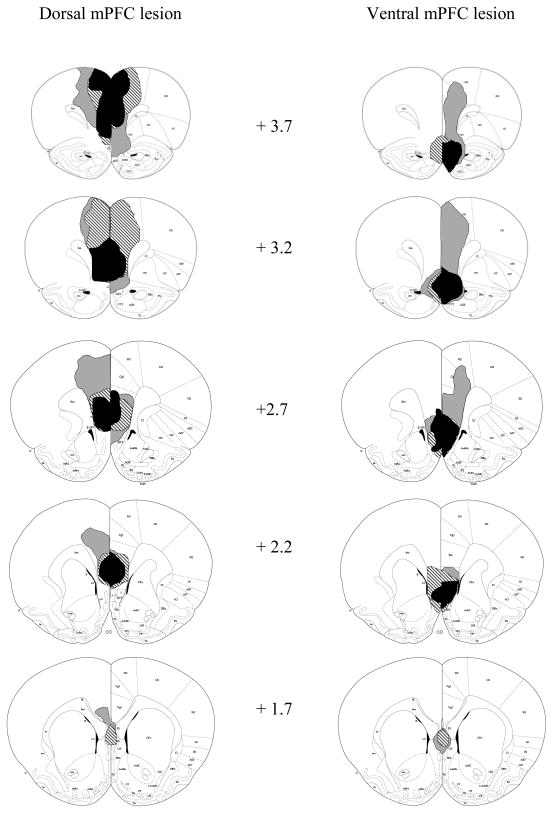
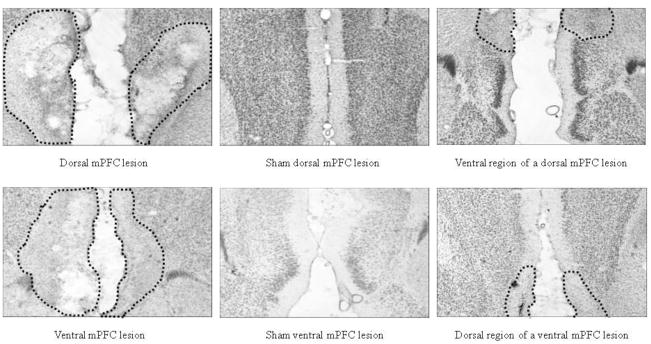
Of the 7 rats that received dorsal mPFC lesions, 1 had only minor damage to the targeted area, leaving a total of 6 acceptable dorsal mPFC-lesioned rats. These 6 dorsal mPFC-lesioned rats had damage centered on PL and IL, although some ACC damage (mostly of a mechanical nature) also occurred. Of the 8 rats that received ventral mPFC lesions, 1 died during surgery and 1 had damage that extended too far dorsally (to PL), leaving a total of 6 acceptable ventral mPFC-lesioned rats. These 6 ventral mPFC- lesioned rats had damage centered on DP and TT, although partial IL damage was present. For both types of lesions, rats had to sustain bilateral damage from at least +3.20 to +2.20 mm anterior to bregma, according to the atlas of Paxinos and Watson [28], to qualify as acceptable for inclusion in the behavioral analysis. Of the 5 rats that received dorsal sham surgery, 1 was excluded from analysis due to an infection that damaged parts of the mPFC and the corpus callosum, leaving 4 acceptable dorsal sham-lesioned rats. The 4 rats that received ventral sham surgery were all acceptable as shams. Figure 1 shows drawings of the largest, smallest, and representative dorsal and ventral mPFC lesions. Figure 2 shows photomicrographs of dorsal, ventral, and sham mPFC lesions.
Figure 1.
Extents of minimum (black), maximum (gray), and representative (stripes) dorsal (left) and ventral (right) mPFC lesions at various distances anterior to bregma. Coronal sections from Paxinos and Watson [28].
Figure 2.
Photomicrographs of dorsal (top panels) and ventral (bottom panels) neurotoxic (far left panels) and sham (middle panels) mPFC lesions. The far right panels show the non-lesioned subregion for each neurotoxic lesion type (i.e., ventral region of a dorsal-lesioned rat; dorsal region of a ventral-lesioned rat). The dotted lines signify the boundaries of the lesion.
3.2 Behavior
3.2.1 Presurgical training
All rats learned to perform the basic requirement of the task, that is, to nosepoke in response to an illuminated port light. Over the course of initial training, all rats adapted to the decreasing duration port cues. By the end of training, all rats exhibited similar levels of correct responding, with correct responses occurring on 75% – 80% of the trials.
3.2.2 Postsurgical reminder training
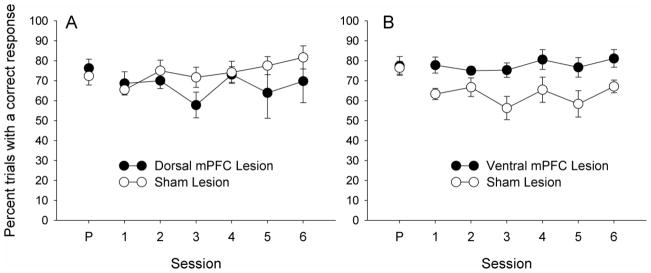
All rats continued to successfully perform the task after surgery. Figure 3 shows postsurgical reminder session performance, as measured by percent trials with a correct response. Dorsal-lesioned and their corresponding sham-lesioned rats performed similarly in the six reminder training sessions; a lesion by session ANOVA showed no significant main effects or interaction, Fs < 1.08, ps > .390. Ventral-lesioned rats performed better than their corresponding sham-lesioned rats in these sessions, F(1,8) = 8.04, p = .020. However, additional ANOVAs for each lesion group, comparing the last two sessions of training before surgery with the first two sessions of training after surgery, showed no significant effects or interactions for either the dorsal, Fs < 1.95, ps > .200, or ventral, Fs ≤ 3.10, ps ≥ .120, groups. Thus, neither surgery nor the passage of postsurgical recovery time affected correct responding for either group.
Figure 3.
Correct nosepoke responding in the six post-surgical reminder sessions for the dorsal (panel A) and ventral (panel B) mPFC-lesioned rats. The point labeled P on the abscissa shows pre-surgical performance (correct response measure averaged for the last two sessions of training before surgery).
3.2.3 Postsurgical CRF/PRF training
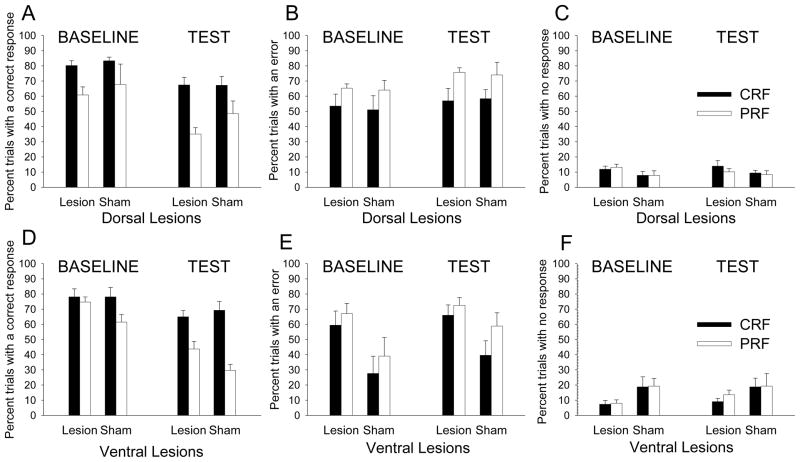
After the six reminder training sessions of the baseline 5CSRT task, partial reinforcement contingencies were introduced. This CRF/PRF training continued for 20 sessions. All groups were sensitive to the reinforcement contingency manipulation; there were more correct responses to the CRF than to the PRF port lights regardless of lesion condition.
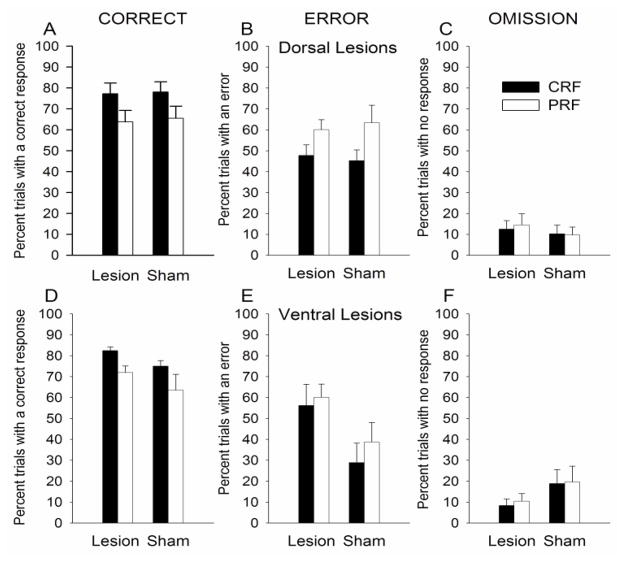
Figure 4 shows correct, error responses, and response omissions for this training phase. For the dorsal-lesioned groups, there were no effects of lesion on correct (Figure 4A), error (Figure 4B), or omission (Figure 4C) response measures. ANOVA showed that correct responding varied over sessions, F(19,152) = 15.88, p < .001, and that rats made more correct responses to the CRF port lights than to the PRF port lights, F(1,8) = 7.85, p = .020. No other main effect or interaction was significant for correct responses. In the ANOVA for errors, only the main effect of contingency was significant: rats made more errors to the PRF port lights than the CRF port lights, F(1,8) = 10.27, p = .010. In the ANOVA for omissions, only the main effect of session was significant, F(19,152) = 1.90, p = 0.018.
Figure 4.
Correct (left panels A and D), error (middle panels B and E), and omission (right panels C and F) nosepoke responding in the post-surgical CRF/PRF port light training sessions for the dorsal (top panels) and ventral (bottom panels) mPFC-lesioned rats. The port light duration was the baseline training level of 1 s. CRF = consistently reinforced; PRF = partially reinforced. The graph in panel A appears with a missing entry because the session 10 correct response data for the dorsal and their corresponding sham mPFC-lesioned rats was lost due to an equipment malfunction.
Similarly, for the ventral-lesioned groups, there were no effects of lesion on correct (Figure 4D) or error (Figure 4E) responses. ANOVA showed that correct responding varied over sessions, F(19,152) = 3.94, p < .001, and rats made more correct responses to the CRF port lights than to the PRF port lights, F(1,8) = 8.77, p = .018. No other effects or interactions were significant for correct responses. In the ANOVA for errors, the number of errors varied across training sessions, F(19,152) = 2.96, p < .001. This effect of session interacted with reinforcement contingency: the difference in number of errors for the CRF vs. PRF port lights varied across training sessions, F(19,152) = 1.79, p = .029. No other effects or interactions were significant for errors. Finally, in the ANOVA for omissions, there was a significant main effect of session, F(19,152) = 4.43, p<0.001, and a significant session X reinforcement contingency interaction, F(19,152) = 2.26, p = 0.003. The lesion variable and its interaction with sessions were marginally significant, F(1,8) = 4.62, p = 0.064, and F(19,152) = 1.64, p = 0.054, respectively, with ventral-lesioned rats making marginally fewer omissions than their sham-lesioned controls.
3.2.4 Reduced port light duration challenge
The primary data of this experiment are the results of the two attentional challenge tests. Each challenge test comprised two identical baseline training sessions followed by a pair of challenge sessions. For each of the two challenge tests, average performance in the two challenge sessions was compared to average performance in the two preceding baseline sessions.
For both sets of rats, the port light duration challenge impaired performance, and the reinforcement contingency manipulation resulted in a superiority of CRF port lights over PRF port lights in the control of behavior. Critically, the effects of dorsal and ventral lesions differed. There were no effects of the dorsal mPFC lesion, but the ventral mPFC lesion resulted in a less powerful influence of reinforcement contingency over behavior: ventral-lesioned rats showed a smaller difference in correct responding between CRF and PRF port lights, relative to their sham-operated controls. In addition, ventral-lesioned rats made more errors than their sham-lesioned counterparts.
The primary measures of performance were the percentages of trials with correct responses, errors, or no responses (omissions). These measures are presented in Figure 5, and the three other measures (correct response latency, premature responses, and perseverative responses) are presented in Table 1. Detailed descriptions of the ANOVAs for the primary measures are provided in the following paragraphs, and summaries of those ANOVAs for the remaining measures are given in Table 1.
Figure 5.
Correct (left panels A and D), error (middle panels B and E), and omission (right panels C and F) nosepoke responding in the port cue duration challenge test for dorsal (top panels) and ventral (bottom panels) mPFC-lesioned rats. The duration of the port light stimulus was reduced from the baseline training level of 1 s to the test level of 250 ms. CRF = consistently reinforced; PRF = partially reinforced.
Table 1.
Means and standard errors for the three auxiliary measures in each of the two attentional challenge tests, for dorsal and ventral mPFC-lesioned rats and their sham-lesioned controls. The results of the ANOVAs for these measures are summarized in this table, according to the key provided.
| Challenge | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lesion group | Dorsal lesions | Dorsal-Sham lesions | |||||||
| Contingency | ANOVA | CRF | PRF | CRF | PRF | CRF | PRF | CRF | PRF |
| 1. Port light duration | 1000 | 1000 | 250 | 250 | 1000 | 1000 | 250 | 250 | |
| correct latency | R,t | 0.548 ± 0.034 | 0.749 ±0.016 | 0.566 ± 0.039 | 0.779 ± 0.029 | 0.539 ±0.023 | 0.654 ± 0.086 | 0.581 ± 0.044 | 0.681 ± 0.067 |
| premature | R | 16.71 ± 3.39 | 4.21 ± 1.17 | 17.83 ± 2.82 | 4.38 ± 1.41 | 21.19±6.74 | 8.75 ± 3.24 | 22.00 ± 4.93 | 12.31 ± 3.75 |
| perseverative | R | 3.33 ± 1.08 | 0.25 ± 0.11 | 3.75 ± 1.24 | 0.1.7 ±0.10 | 3.00 ±0.41 | 0.50 ± 0.20 | 3.37 ± 0.88 | 0.63 ± 0.31 |
| 2. Ready signal duration | 5000 | 5000 | 1000 | 1000 | 5000 | 5000 | 1000 | 1000 | |
| correct latency | L,R,T,RT | 0.499 ± 0.038 | 0.683 ± 0.034 | 0.865 ± 0.025 | 0.837 ±0.025 | 0.500 ± 0.027 | 0.652 ± 0.039 | 0.707 ±0.099 | 0.764 ± 0.047 |
| premature | l,R,T | 19.58 ±4.34 | 5.63± 1.39 | 2.50 ± 1.12 | 1.67 ± 1.67 | 25.13 ± 7.22 | 11.25 ±3.19 | 15.00 ±8.90 | 1.25± 1.25 |
| perseverative | R,T,RT | 3.66 ± 0.96 | 0.58 ± 0.33 | 0.25 ± 0.25 | 0.17 ± 0.11 | 4.12 ± 0.96 | 1.00±0.54 | 0.63 ± 0.37 | 0.38 ± 0.13 |
| Lesion group | Ventral lesions | Ventral-Sham lesions | |||||||
| Contingency | ANOVA | CRF | PRF | CRF | PRF | CRF | PRF | CRF | PRF |
| 1. Port light duration | 1000 | 1000 | 250 | 250 | 1000 | 1000 | 250 | 250 | |
| correct latency | R,T,RT,lr | 0.554 ± 0.030 | 0.615 ± 0.034 | 0.594 ±0.031 | 0.725 ± 0.032 | 0.541 ± 0.032 | 0.736± 0.032 | 0.577 ±0.042 | 0.818 ±0.031 |
| premature | R,rt | 14.83 ± 3.66 | 8.50± 1.61 | 17.50 ±4.88 | 7.50 ±2.02 | 11.38±2.22 | 1.69±0.94 | 16.38 ± 2.70 | 2.13 ±0.75 |
| perseverative | R,t,lrt | 0.71 ±0.41 | 0.37 ± 0.24 | 1.67 ± 0.42 | 0.67 ± 0.40 | 2.75 ± 1.23 | 0.00 ± 0.00 | 3.00± 1.04 | 0.62 ± 0.47 |
| 2. Ready signal duration | 5000 | 5000 | 1000 | 1000 | 5000 | 5000 | 1000 | 1000 | |
| correct latency | R,T,RT,lrt | 0.567 ± 0.028 | 0.669 ± 0.063 | 0.770 ± 0.049 | 0.849 ± 0.018 | 0.492 ± 0.020 | 0.751 ±0.037 | 0.851 ±0.027 | 0.863 ± 0.037 |
| premature | r,T,RT | 14.67 ± 4.26 | 8.92 ± 2.02 | 3.33 ± 2.11 | 2.50 ±2.50 | 15.94 ±2.39 | 2.25 ± 0.51 | 1.25± 1.25 | 0.00±0.00 |
| perseverative | L,R,T,LR,LT,RT,LRT | 1.63 ± 0.55 | 0.62 ± 0.46 | 0.58 ± 0.30 | 0.08 ± 0.08 | 5.50 ± 1.06 | 0.12 ± 0.13 | 0.25 ± 0.14 | 0.13 ± 0.13 |
L = lesion; R = reinforcement contingency; T = test (challenge). Upper case letters (L, R, T) in the column labelled ANOVA represent a statistically significant effect or interaction (p < 0.05) for the listed variable(s). Lower case letters (l, r, t) in the same column represent a marginally significant effect or interaction (0.05 < p < 0.10) for the listed variable(s).
Dorsal-lesioned rats performed similarly to sham-lesioned rats on all measures of performance. There was no effect of lesion on either correct responding (Figure 5A) or errors (Figure 5B). Rats made fewer correct responses, F(1,8) = 44.90, p < .001, and more errors, F(1,8) = 10.03, p = .013, in the test sessions as compared to the baseline sessions, thus demonstrating the effectiveness of the reduced port light duration as a challenge to performance. In addition, correct responding was greater, F(1,8) = 15.47, p = .004, and errors were fewer, F(1,8) = 9.40, p = .015, to the CRF port lights relative to the PRF port lights, thus showing the rats’ sensitivity to reinforcement contingency in this paradigm. No other factors or interactions were significant in either of these ANOVAs. Response omissions (Figure 5C) were negligible and the ANOVA for that measure showed no significant effects.
Although in many respects the performance of ventral-lesioned rats was similar to that of the dorsal-lesioned rats, some evidence suggested that the ventral lesions affected the rats’ sensitivity to reinforcement contingencies or their ability to use contingency information to appropriately guide behavior. Consider first the aspects of performance that were similar to that of dorsal-lesioned rats. As with the dorsal groups, there was a main effect of test on both correct responses (Figure 5D) and errors (Figure 5E): rats made fewer correct responses, F(1,8) = 36.76, p < .001, and a greater number of errors, F(1,8) = 15.17, p = .005, in the challenge test compared to the baseline sessions, again validating the reduction in port light duration as an effective way to challenge performance. Also as with dorsal groups, there was a main effect of reinforcement contingency on both correct responses and errors: rats made more correct responses, F(1,8) = 37.82, p < .001, and fewer errors, F(1,8) = 8.22, p = .020, to the CRF port lights than to the PRF port lights, demonstrating the control of the reinforcement contingency manipulation over performance in this task. Moreover, the difference in correct responding to CRF vs. PRF port lights was greater in the test sessions than it was in the baseline sessions, F(1,8) = 9.13, p = .017, suggesting that the increased attentional demands of the challenge sessions especially impacted responding to PRF cues. Finally, as with the dorsal groups, there were relatively few response omissions (Figure 5F), and the ANOVA showed no significant effects or interactions.
However, in contrast to the dorsal groups, the performance of ventral-lesioned and sham-lesioned rats differed in some respects. First, the ventral-lesioned rats displayed more errors overall, F(1,8) = 5.33, p < .050, than the ventral-sham rats. Perhaps more important, there was an interaction of lesion with reinforcement contingency: the difference in correct responding to CRF vs. PRF port lights was smaller for the ventral-lesioned rats than it was for the sham-lesioned rats, F(1,8) = 5.77, p = .040. Similar, but only marginally significant, interactions were found for correct response latencies and perseverative responses. This result suggests that ventral-lesioned rats were either insensitive to reinforcement contingency or impaired in using reinforcement contingency information to appropriately guide behavior.
Some caution concerning the interpretation of our observation of lesion effects on errors after ventral lesions but not after dorsal lesions may be in order. Comparison of Figures 5B and 5E suggests that this difference was more the result of abnormally high error rates in the dorsal-sham control rats, compared to the error rates observed in the ventral-sham rats. However, it is notable that our observation of reduced sensitivity to reinforcement contingency after ventral lesions but not after dorsal lesions is not compromised in that fashion. First, the differences between both correct and error responses to CRF and PRF cues (Figures 5A, 5B, 5D, and 5E) were very similar in the two sham groups for all but correct responses in test responding. Second, regardless of any apparent differences in the responding of sham controls, direct comparisons between the two lesion groups showed greater differences between CRF and PRF responding in the dorsal-lesioned rats than in the ventral-lesioned rats. These issues are considered further in section 3.2.5.
3.2.5 Variable duration ready signal challenge
Although performance on 5-s and 9-s trials showed less evidence of disruption, reduction in ready-signal duration to 1s was an effective challenge to performance in both dorsal- and ventral-lesioned rats. The nature of performance deficits on 1-s trials differed by lesion group. Dorsal-lesioned rats made fewer correct responses (Figure 6A) and more omissions (Figure 6C) than their sham-lesioned controls, whereas ventral-lesioned rats made marginally more errors (Figure 6E) compared to their sham-lesioned controls, but did not show increased omissions (Figure 6F). This observation suggests that the two lesion groups may have adopted different strategies when attempting to perform under this challenge. The dorsal-lesioned rats seemed to “give up”, as evidenced by increased omissions, whereas the ventral-lesioned rats seemed to “guess”, as evidenced by (marginally) increased errors. In addition, and in keeping with the findings of the port light duration challenge test, the ventral lesion, but not the dorsal lesion, altered the rats’ responses to variations in reinforcement contingency.
Figure 6.
Correct (left panels A and D), error (middle panels B and E), and omission (right panels C and F) nosepoke responding in the ready signal challenge test for dorsal (top panels) and ventral (bottom panels) mPFC-lesioned rats. These graphs represent the trials on which the duration of the ready signal house light was reduced from the baseline training level of 5 s to the test level of 1 s within a test session of intermixed trial types (1, 5, or 9 s duration ready signal). CRF = consistently reinforced; PRF = partially reinforced.
An initial ANOVA, which included all three ready signal duration types as well as baseline trials, indicated that only performance on 1-s trials differed substantially from that on baseline trials. Subsequently, we conducted ANOVAs that contrasted baseline performance with each of the three different ready signal duration trial types (1, 5, and 9 s) separately, to allow for individual analysis on those trials that had a ready signal that was shorter (1 s), the same (5 s), or longer (9 s) than the rats were accustomed to experiencing in baseline training. For 5-s and 9-s trials, there were few significant effects or interactions. Each of these ANOVAs revealed significant main effects of reinforcement contingency, Fs(1,8) > 6.83, ps < .031, with more robust responding on CRF trials than on PRF trials for both dorsal and ventral groups. In addition, there was a significant three-way interaction of lesion, reinforcement contingency and test for the dorsal-lesioned groups on 5-s trials, F(1,8) = 10.30, p = 0.012, and for the ventral-lesioned groups on 9-s trials, F(1,8) = 5.87, p = 0.042. No other main effects or interactions were significant, ps > .10, in these analyses. Thus, we focused on the rats’ performance on 1-s trials, on which the ready signal was shorter than on baseline trials.
Correct, error and response omissions for the 1-s trials are displayed in Figure 6, and the three auxiliary measures on those trials are presented in Table 1, together with summaries of the results of ANOVAs for those measures. On the 1-s trials, there was a main effect of lesion on correct responding in the dorsal-lesioned rats: these rats made fewer correct responses (Figure 6A) than their sham-lesioned counterparts, F(1,8) = 14.11, p = .006, and omitted responding altogether (Figure 6C) on more trials, F(1,8)=10.13, p = .013, an effect mirrored in several other measures (Table 1). There was also a main effect of test on all three primary measures: rats made fewer correct responses, F(1,8) = 15.50, p < .005, fewer errors, F(1,8) = 78.36, p < .001, and more response omissions, F(1, 8) = 32.40, p < .001, in the test compared to the baseline sessions. Note that this attentional challenge test resulted in fewer errors (Figure 6B) relative to baseline performance in dorsal-lesioned rats, which at first may seem odd. However, this finding can be explained by the observation of more omissions in the test sessions: there were fewer errors because there were fewer trials with responses (more omissions). Thus, the challenge test was clearly an effective way of taxing performance; indeed, performance on each of the auxiliary measures was also significantly degraded in test.
Although the effects of reinforcement contingency did not meet conventional levels of statistical significance for correct responses or errors in the dorsal groups, Fs(1,8) < 4.69, .050 < ps ≤ .100, it is notable that those effects were significant for several other measures of performance (Table 1). The rats showed quicker correct response latencies, more premature responses, and more perseverative responses to the CRF compared to the PRF ports, Fs(1,8) ≥ 7.92, ps ≤ .023, but omissions were not affected significantly by reinforcement contingency. As in challenge test 1, the dorsal lesion did not significantly affect rats’ sensitivity to reinforcement contingency; that variable did not interact significantly with lesion, ps >.10 Finally, there was a marginally significant interaction of lesion and test on the number of errors: the difference in the number of errors made between the baseline and the test sessions was marginally larger for the dorsal-lesioned than for the sham-lesioned rats, F(1,8) = 4.21, p = .074, suggesting that the test placed a greater strain on attention for the dorsal-lesioned rats.
For the ventral-lesioned groups, there was a main effect of test on all three primary measures: rats made fewer correct responses (Figure 6D), F(1,8) = 33.00, p < .001, fewer errors (Figure 6E), F(1,8) = 23.38, p = .001, and more omissions (Figure 6F), F(1,8) = 52.82, p < .001, in the challenge test relative to the baseline sessions. As with the dorsal-lesioned rats, this overall reduction in the number of errors in test was likely attributable to greater omissions. However, unlike the dorsal-lesioned rats, which tended to make fewer errors but more omissions than their sham-lesioned controls, ventral-lesioned rats made marginally more errors than sham-lesioned rats, F(1,8) = 4.17, p = .075, overall, and the number of omissions was numerically (but not significantly, p > .10) lower in the ventral-lesioned rats than in their sham controls. For correct responses, there was a significant main effect of reinforcement contingency and a significant interaction of test with reinforcement contingency: rats made more correct responses to CRF than PRF ports, F(1,8) = 5.38, p = .049, and this CRF vs. PRF difference was larger in the baseline than in the test sessions, F(1,8) = 9.56, p = .015. In addition, there was also a significant three-way interaction of lesion, test, and reinforcement contingency, F(1,8) = 5.60, p = .046, consistent with a pattern of a greater baseline CRF vs. PRF difference in correct responding for the sham-lesioned rats than for the ventral-lesioned rats in the baseline, but a general disruption of performance in both groups in test. Thus, as in the port cue duration challenge, correct responding of ventral- (but not dorsal-) lesioned rats was less sensitive to reinforcement contingency than that of shams. Finally, omissions were unaffected by reinforcement contingency.
As in the port cue challenge test, there were apparent (but nonsignificant) differences between the performances of the two sham-lesioned control groups. It is possible that the lack of an effect of the dorsal lesion on the sensitivity of correct responding to reinforcement contingency in this challenge test was at least partially due to an abnormally small effect of contingency in the dorsal-shams, compared to the effect noted in ventral-shams. However, it is important to note that our observations of differential effects of the lesions on errors and omissions in this test are not compromised by any such differences in responding between the sham controls: direct comparisons of responding in the two lesion groups showed more omissions and fewer errors in test in the dorsal-lesioned rats than in the ventral-lesioned rats. Furthermore, across both this challenge test and the preceding one, it is notable that the apparent differences between the two sham-lesioned groups were neither systematic (that is, sometimes the performance of the dorsal-shams was superior and other times inferior to that of the ventral-shams), nor typically statistically significant (although because of the relatively small number of subjects such comparisons lack power). Thus, we believe our comparisons of the performance of each lesion group to its own sham control provides the most appropriate comparison throughout for evaluating the lesion effects.
4. Discussion
The performance of sham-lesioned rats was degraded by the two attentional challenges and by partial reinforcement (PRF). Lesions of medial prefrontal cortex further disrupted performance. Notably, the patterns of impairment differed depending on the loci of those lesions. First, whereas rats with lesions that targeted PL and IL (“dorsal” lesions) showed more response omissions but no higher rates of error responses than their sham-lesioned controls, rats with lesions that targeted DP and TT (“ventral” lesions) showed more error responses than their sham controls, but comparable rates of response omissions. Casually speaking, the dorsal-lesioned rats “gave up” on performing the tasks, whereas the ventral-lesioned rats “guessed”. Second, whereas the dorsal lesions tended to impair responding under conditions of attentional challenge (that is, when physical/temporal characteristics of the various visual stimuli were altered), but had less effect on the distribution of responding to CRF and PRF cues, the ventral lesion more frequently yielded aberrant responding to PRF cues, but was less likely to impair performance attributable solely to the challenge manipulations. This distinction suggests that the dorsal lesion may have more directly affected visual processing, albeit modulated by top-down influences, than the ventral lesion, which may have altered, for example, aspects of reward processing. Third, the lesions produced different patterns of impairment across the two attentional challenge tests, suggesting that different aspects of performance were challenged in each test. The dorsal lesion had no effects on performance in the port light duration challenge, but altered performance substantially in the ready signal duration challenge. By contrast, the ventral lesion had substantial effects on performance in both the port light and ready signal duration challenges.
Overall, the results of this study suggest separable roles for dorsal and ventral mPFC in processing variations in reinforcement contingency and in adapting to effects of various attentional challenges. Consider first the effects of reinforcement contingency. Both groups of sham-lesioned rats showed more and faster correct responses and fewer errors when CRF cues were presented than when PRF cues were presented. This greater value of CRF port cues was also evident in premature and perseverative responding: Rats were more likely to make responses to the ports that were associated with CRF even before they were illuminated, and after they were darkened. On the whole, this favoring of CRF cues was not affected by the attentional challenges themselves, except that reduction of the ready signal duration to 1 s in the ready signal duration challenge substantially suppressed many measures of responding to the CRF cues. Thus, it is uncertain whether the reduced performance to PRF cues relative to CRF cues in that test reflected a reduced allocation of attention to the PRF cues for purposes of controlling action [13,14,24,29] or simply lower associative strength. Regardless, it is notable that whereas the effects of the dorsal mPFC lesion on the distribution of responding to CRF and PRF cues were negligible (e.g., there were no significant lesion X reinforcement contingency interactions for any of the measures in either of the challenges), rats with ventral mPFC lesions failed to show differential responding to CRF and PRF cues in both of the attentional challenges. This latter deficit was evident in both baseline and challenge sessions, and thus appears to be related to the detection or use of reinforcement contingencies themselves (whether in the allocation of attention or otherwise), perhaps independent of any reallocation of resources under demanding conditions. Indeed, the use of the word “deficit” to describe these rats’ pattern of responding to the cues based on their reinforcement contingencies may be somewhat misleading: although they certainly distributed their responding in an aberrant fashion relative to sham-lesioned rats, they actually made more correct responses to PRF cues (but also more errors) than sham-lesioned rats.
Next, consider the nature of our two attentional challenges, and their differential effects on rats with the two mPFC lesions. Each challenge involved the manipulation of the duration of a visual cue. However, whereas the port light duration challenge manipulated the duration of the port cue to which rats were required to direct their responding, the ready signal challenge test manipulated the duration and interval of a signal that initiated the trial and provided information about the time when the port cues would be presented. Thus, the two challenges might tap different processes. For example, the port light duration challenge likely involved increased load on spatial attention, whereas the ready signal challenge may have placed a greater emphasis on attention to aspects of timing or task routine. Interestingly, although shortening the port light duration substantially impaired performance, the dorsal lesions had no additional effect on that performance, and the ventral lesions’ effects in that challenge (on the distribution of responding to CRF and PRF cues) was not specific to the short-duration test cue. Furthermore, unlike with the ready signal challenge, the probability of response omissions was not affected by reducing the port light duration, nor by either of the lesions.
Although the port light duration manipulation did not provide a major change to the routine of the task (that is, its structure, organization or timing), the ready signal challenge did. With that alteration, the rats’ ability to discern the start, and thus subsequent timing, of a trial might be affected, such that the target port light occurred at unexpected times. Effects of the dorsal mPFC lesion in the ready signal challenge but not in the port light duration challenge are consistent with a view of PL/IL function that emphasizes behavioral flexibility in response to changes in task demands (e.g., [30]). The results of this study thus join those of several other studies in which PL/IL lesion deficits have been reported in tasks that require substantial adaptations in behavior to correctly perform a task, such as strategy set-shifting or attentional set-shifting [8,31–34].
An apparent discrepancy between the results of this experiment and earlier studies deserves comment. Muir and colleagues [1] found that excitotoxic lesions of the rat mPFC caused a reduction in accuracy, an increase in response latency, and increased perseverative responding, in baseline performance of the 5CSRT task. These impairments were evident across ten training sessions that took place two weeks after the rats had received surgery to induce the lesions. In contrast to those findings, we did not find any detrimental effect of our mPFC lesions in the postsurgical baseline training sessions. In fact, ventral mPFC-lesioned rats displayed greater correct responding than their sham-lesioned controls in the postsurgical baseline training sessions (although overall, surgery or postsurgical recovery time had no effect on correct responding). Thus, it is clear that neither our dorsal nor ventral mPFC lesions impaired correct responding in the baseline version of our task. Interestingly, the mPFC-lesioned rats in the Muir et al. [1] study were eventually able to perform the task at levels comparable to that of sham-lesioned control rats, after receiving additional baseline training sessions after surgery. This result shows that any lesion-induced impairment on their baseline version of the task was transient, and could be overcome with further training.
A notable difference between our version of the 5CSRT task and that used in the Muir et al. [1] study (and indeed most 5CSRT studies, e.g., [2,14]) is the degree of control over trial events ceded to the rat. In our task, trials (which were signalled by a ready cue) and all trial events were scheduled without regard to the rats’ behavior. By contrast, in more typical 5CSRT procedures, trial onset is initiated by the rat and time-out periods are imposed such that any incorrect or extraneous responses (e.g., anticipatory and perseverative responses), or even omissions, resulted in a 5-s period of darkness. Any further responding in the ports during time-out periods restarted the time-out period. Thus, any “mistakes” made by the rat in effect delayed its opportunity to earn food reward. As such, the rats in the Muir et al. [1] study were much more stringently trained than the rats in our study, which did not involve time-out procedures. Thus it is hardly surprising that Muir et al.’s [1] procedures were more sensitive to effects of lesions, which may have affected their rats’ abilities to master the various operant contingencies they imposed. Although most users of the 5CSRT task assert that this complexity in the task allows it to be used for the assessment of a range of psychological characteristics [11], we [22] have argued that these additional operant contingencies might generate substantial between-subject variability in the sequencing and spacing of trials. Furthermore, this variability could potentially be confounded with lesion treatment if lesions affected the subjects’ ability to master these contingencies. Minimizing the subjects’ control over event scheduling, as in this study, makes such confounds less likely. Similarly, our simplification of the task made it possible for each rat to learn without individualized shaping. In most studies that used standard 5CSRT procedures, the training parameters were adjusted as necessary for each rat during acquisition, perhaps resulting in their exposure to very different initial learning contingencies. Thus, our simplified procedure may provide an assessment of visuospatial attention less confounded by other factors. On the other hand, it could also be argued that our simplifications may also have substantially reduced the attentional demands of the task. Nonetheless, the training conditions used here were sufficiently sensitive to detect the effects of lesions when attentional demands were increased.
There is evidence to suggest that the time-out parameter also influences other aspects of behavior in this task. Passetti et al. [2] punished perseverative responding with a time-out period, whereas Murphy et al. [15] did not (although they did impose a time-out period for other kinds of inappropriate responding). Interestingly, Passetti et al.[2] found a mPFC manipulation to increase perseverative responding, whereas Murphy et al. [15] did not find any effect of mPFC manipulation on that measure. Taken together with our results, all of these findings suggest that mPFC-lesioned rats may be more susceptible to conditions that make the task more difficult, such as the time-out procedure and attentional challenges.
It is interesting to note that in a previous study using 5CSRT procedures similar to those used in the present study, Maddux et al. [13] found that lesions that destroyed the cholinergic innervation of the mPFC resulted in a pattern of baseline responding similar to that observed here in the rats with lesions of ventral mPFC. Although as in the present study, sham-lesioned control rats showed more correct responding to CRF than to PRF cues, mPFC-lesioned rats showed comparably high levels of responding to both CRF and PRF cues. Cholinergic depletion in the mPFC in that study was most evident in PL and IL, but did extend dorsally to the ACC and ventrally to the DP in some cases. The neurotoxic dorsal mPFC lesions in this study primarily damaged PL and IL, with some ACC mechanical damage, whereas the neurotoxic ventral mPFC lesions primarily damaged DP and TT, but with some IL damage. Thus, IL was a region of overlap for the dorsal and ventral lesions in the present study. However, only ventral mPFC-lesioned rats displayed the reinforcement contingency impairment here. From this observation, it follows that the impairment observed in the ventral-lesioned rats is likely a result of damage to DP and/or TT, and that the reinforcement contingency impairment observed in Maddux et al.’s [13] study might have been primarily due to the cholinergic depletion in DP, despite the fact that depletion was more obvious in PL and IL. Furthermore, it is notable that in that study [13] we found a similar reinforcement contingency impairment pattern after neurotoxic lesions of CEA, which as mentioned in the introduction, has a different pattern of connectivity with ventral than with dorsal mPFC [18,19].
All in all, the results of the present experiment extend a dorsal-ventral distinction within the mPFC in the guidance of a well-established operant behavior, when attention was challenged either by temporal or reinforcement contingency manipulations. Most discussions of the functions of dorsal and ventral portions of the prefrontal cortex (e.g., [16, 36]) have contrasted a dorsal region embracing approximately the ACC, medial agranular cortex, and the dorsal-most portions of PL on the one hand, with a ventral region including ventral portions of PL, IL, medial orbital cortex and perhaps DP, on the other. Here, we distinguished between dorsal (PL, IL) and ventral (DP and TT, and perhaps ventral IL) and ventral subregions of what within such schemes, would all be considered “ventral mPFC “. Notably, even within this more limited ventral region, several dorsal-ventral variations in connectivity have been described. For example, several studies [16, 36, 37] have shown a gradient of mPFC projections to the striatum such that projections to the caudate putamen and nucleus accumbens core were prevalent in PL but grew sparser more ventrally in mPFC, whereas projections to accumbens shell were more prevalent in DP and ventral IL, growing more sparse more dorsally (but see [38]). Similarly, IL and DP project heavily to regions in the extended amygdala, such as the CeA and the bed nucleus of the stria terminalis, whereas these projections become lighter more dorsally in mPFC [16, 36, 37]. In a comparable manner, Gaykema and colleagues [39] noted that projections to the medial portion of the lateral septum were heavy in DP and grew sparser more dorsally. Furthermore, although this whole region is rich in glutamate neurons that project to the ventral tegmental area, these neurons are especially dense in DP [40]. Finally, the tenia tecta (another focus of our ventral lesions) may present a special case. Despite their location, they are not generally considered part of mPFC functionally, but rather as a part of the hippocampal continuation (dorsal TT) and the olfactory cortex ([41], but see [42]). Our study does not permit inferences about roles of this region alone in our task. However, two aspects of its connectivity are worth mentioning in this regard. First, as noted earlier, TT has strong projections to CeA [18, 19], a region also implicated in performance of this task. Second, some evidence links TT to function of hypocretin (orexin) neurons in the lateral hypothalamus, which we have linked to some aspects of attentional function in associative learning [43]. Although most of our ventral region (ventral IL, DP and TT) is rich in orexin receptors [44], TT seems to be especially rich [45].
The more well-studied prelimbic and infralimbic regions of mPFC cortices may be conceived to be more important for performance in the 5CSRT task used here, as our “dorsal” lesions of those regions resulted in increased response omissions when the temporal manipulations of the attentional challenge tests were imposed. Completely failing to respond (as our dorsal mPFC-lesioned rats did) could reasonably be considered a more severe deficit than making errors when responding (as our ventral mPFC-lesioned rats did). The importance of prelimbic and/or infralimbic mPFC regions in 5CSRT task performance is in keeping with other reported studies [1,14,15]. However, the observed ventral mPFC impairment is a new finding, and provides evidence that these lesser-studied regions also contribute to performance in this task, albeit perhaps to a lesser degree or in a different way than the regions just dorsal to them. Perhaps what is most interesting about the ventral mPFC lesion effects reported here are not those observed following the temporal manipulations of the attentional challenge tests, but rather those seen as a result of the reinforcement contingency manipulation. Recall that the reinforcement contingency manipulation more directly relates to CS-processing theories of associative learning [23–25], and that the ventral, but not the dorsal, mPFC lesion resulted in an abnormal distribution of responding to CRF vs. PRF port lights. This finding suggests that these relatively unexplored ventral mPFC regions of dorsal peduncular cortex and tenia tecta are, in fact, important for some associative learning processes, and warrant further research in order to more clearly discern their contributions to behavior. For example, it is unclear from the present work whether the observed ventral impairment stems from an inability to detect the difference between contingencies, or from an inability to use reinforcement contingency information to appropriately guide responding. The latter possibility would be of particular interest, as it might surprisingly implicate these regions in “higher-order” associative and/or cognitive processes.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (grant number MH53367).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function, dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebral Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- 2.Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cerebral Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- 3.Passetti F, Dalley JW, O’Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- 4.Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cerebral Cortex. 2004;14:922–932. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- 5.McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- 6.Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cerebral Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- 8.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neurosci. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bushnell PJ. Behavioral approaches to the assessment of attention in animals. Psychopharmacol. 1998;138:231–259. doi: 10.1007/s002130050668. [DOI] [PubMed] [Google Scholar]
- 11.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacol. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 12.McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddux JM, Kerfoot EC, Chatterjee S, Holland PC. Dissociation of attention in learning and action: effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behav Neurosci. 2007;121:63–79. doi: 10.1037/0735-7044.121.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacol. 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- 16.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Chudasama Y, Muir J. Visual attention in the rat: A role for the prelimbic cortex and thalamic nuclei? Behav Neurosci. 2001;115:417–428. [PubMed] [Google Scholar]
- 18.Ottersen OP. Connections of the amygdala of the rat IV: corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- 19.Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull. 1986;17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher M, Schoenbaum G. Functions of the amygdala and related forebrain areas in attention and cognition. Annals NY Acad Sci. 1999;877:397–411. doi: 10.1111/j.1749-6632.1999.tb09279.x. [DOI] [PubMed] [Google Scholar]
- 21.Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. TICS. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 22.Holland PC, Han J-S, Gallagher M. Lesions of the amygdala central nucleus alter performance on a selective attention task. J Neurosci. 2000;20:6701–6706. doi: 10.1523/JNEUROSCI.20-17-06701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Pelley ME. The role of associative history in models of associative learning: A selective review and a hybrid model. Quar J Exp Psychol Comp Physiol Psychol. 2004;57(B):193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- 24.Mackintosh NJ. A theory of attention, variations in the associability of stimuli with reinforcement. Psychol Rev. 1975;8:276–298. [Google Scholar]
- 25.Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- 26.Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav Neurosci. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- 27.Holland PC, Thornton JA, Ciali L. The influence of associability changes in negative patterning and other discriminations. J Exp Psychol Anim Behav Proc. 2000;26:462–476. doi: 10.1037//0097-7403.26.4.462. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. San Diego, CA: Academic Press; 1997. [Google Scholar]
- 29.Holland PC. Disconnection of the amygdala central nucleus and the substantia innominata/nucleus basalis magnocellularis disrupts performance in a sustained attention task. Behav Neurosci. 2007;121:80–89. doi: 10.1037/0735-7044.121.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gisquet-Verrier P, Delatour B. The role of the rat prelimbic/infralimbic cortex in working memory: not involved in the short-term maintenance but in monitoring and processing functions. Neurosci. 2006;141:585–596. doi: 10.1016/j.neuroscience.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cerebral Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- 32.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosc. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- 35.Hoover WB, Vertes RP. Anatomical analyses of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 36.Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sesnory, limibic and autonomic function. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds SM, Zahm DS. Specificity in the projections of the prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosi. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 39.Gaykema RP, van Weeghel R, Hersh LB, Luiten PG. Prefrontal cortical projections to the cholinergic neurons in the basal forebrain. J Comp Neurol. 1991;303:563–583. doi: 10.1002/cne.903030405. [DOI] [PubMed] [Google Scholar]
- 40.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents o fthe ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyss JM, Sripanidkulchai K. The indusium griseum and anterior hippocampal continuation in the rat. J Comp Neurol. 1983;219:251–272. doi: 10.1002/cne.902190302. [DOI] [PubMed] [Google Scholar]
- 42.Crosby EC, Schnitzlein HN. Comparative correlative neuroanatomy of the vertebrate telencephalon. New York: Macmillan; 1982. [Google Scholar]
- 43.Holland PC, Angeli N, Lasseter H, Wheeler DS. Unilateral lesions of lateral hypothalamic orexin neurons impair surprise-induced enhancements of learning. Society for Neuroscience. 2008 poster 387.16. [Google Scholar]
- 44.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 45.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. Fed Euro Biochem Soc Let. 1988;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]