Abstract
Background
Risk stratifying heart failure patients for primary prevention implantable cardioverter-defibrillators (ICDs) remains a challenge, especially for African Americans, who have an increased incidence of sudden cardiac death but have been underrepresented in clinical trials. We hypothesized that the S1103Y cardiac sodium channel SCN5A variant influences the propensity for ventricular arrhythmias in African American patients with heart failure and reduced ejection fraction.
Methods and Results
112 African Americans with ejection fractions (EF) <35% receiving primary prevention ICDs were identified from the Duke Electrophysiology Genetic and Genomic Studies (EPGEN) biorepository and followed for appropriate ICD therapy (either antitachycardia pacing or shock) for documented sustained ventricular tachycardia or fibrillation. The S1103Y variant was over-represented in patients receiving appropriate ICD therapy compared to subjects who did not (35% vs 13%, p=0.03). Controlling for baseline characteristics, the adjusted hazard ratio using a Cox Proportional Hazard Model for ICD therapy in Y1103 allele carriers was 4.33 (95% CI 1.60–11.73, p=<0.01). There was no difference in mortality between carriers and non-carriers.
Conclusion
This is the first report that the S1103Y variant is associated with a higher incidence of ventricular arrhythmias in African Americans with heart failure and reduced ejection fraction.
Keywords: heart failure, death, sudden, genetics, ion channels, race/ethnicity
Introduction
African Americans disproportionately suffer from heart failure, ventricular arrhythmias, and sudden cardiac death (SCD) when compared to Caucasians.1–3 While implantable cardioverter-defibrillators (ICDs) effectively reduce mortality from SCD in heart failure patients,4, 5 the ability to identify prospectively patients who will derive benefit from these therapies is lacking. Current guidelines, based upon clinical trials, fail to identify the majority of patients who will suffer SCD.6 New risk stratification algorithms are urgently needed, especially for African Americans who have been underrepresented in clinical trials. Genetic based risk stratification offers the potential for both identifying high-risk patients who do not qualify for primary prevention strategies and excluding low-risk individuals who do. While a diverse array of genetic loci have been associated with SCD, ion channel genes are particularly intriguing candidates as they underlie the vast majority of inherited arrhythmias.7 Moreover, a sizable percentage of ion channel mutations only elicit a pro-arrhythmic phenotype in the setting of environmental stressors such as exposure to a QT-prolonging medication.8 Thus, we hypothesize that mutations in ion channels could contribute to the acquired arrhythmias in heart failure.
For African Americans, the common variant S1103Y in the cardiac sodium channel gene SCN5A has been associated with a predisposition to acquired arrhythmias and was a candidate for testing our hypothesis. The S1103Y variant, found almost exclusively in persons of African descent and present in 13% of African Americans, has been associated in multiple studies with life-threatening arrhythmias in the setting of provocative environmental stimuli, such as QT-prolonging medications.9 In addition, this mutation has been associated with both sudden infant death syndrome10, 11 and SCD in the presence of mild to moderate cardiomegaly.12 In this report we tested whether the S1103Y variant was associated with the occurrence of arrhythmias in African Americans with heart failure and reduced ejection fraction.
Methods
Study population
The Duke Electrophysiology Genetic and Genomic Studies (EPGEN) biorepository is a prospective single-center repository that archives DNA, RNA, and protein samples obtained at the time of an electrophysiologic evaluation or intervention.13 Demographic, laboratory and cardiovascular risk factor data are collected at the time of ICD implantation. Device type was determined by the implanting operator and was not restricted by manufacturer. ICD programming and follow-up intervals were left to the discretion of the treating electrophysiologist. Between May 2005 and April 2008, 137 consecutive self-identified African American patients aged 18 or older undergoing ICD placement for purposes of primary prevention with a left ventricular ejection fraction (EF) <35%, were identified. Twenty-three subjects were excluded because of insufficient clinical follow-up variables or insufficient DNA. Two were excluded because of suspected or confirmed inherited arrhythmia syndromes.
Follow-up, and Classification of Events
Patients were classified as having received appropriate ICD therapy if they experienced antitachycardia pacing or shock for documented sustained ventricular tachycardia or ventricular fibrillation. Patients that only received inappropriate ICD therapy or did not receive any antitachycardia pacing or shock were classified as having no appropriate ICD therapy. Events were adjudicated at the time of device interrogation by review of the treating electrophysiologist, who was blinded to the genotype. The research protocol was approved by the Duke University Institutional Review Board and all patients consented to use of their samples for the EPGEN biorepository.
Statistical Analysis
Standard descriptive statistics were used, including percentages for discrete variables and means for continuous variables. Baseline characteristics were tested for significance with the Chi-square statistic for categorical variables and with the Student’s t-test for continuous variables. Hardy-Weinberg Equilibrium for the single nucleotide polymorphism (SNP) was tested using Chi-square. Allele frequencies were compared between patients receiving appropriate ICD therapy and patients that did not receive appropriate ICD therapy using Fisher’s Exact Test. Our primary analysis was a time-to-event analysis to assess the relationship between time to appropriate ICD therapy and genotype, and thus a life-table Kaplan-Meier survival analysis was performed using the log-rank statistic. Risk relationships were characterized as hazard ratios and 95% confidence intervals, generated with the use of a multivariable Cox Proportional Hazard model with covariates known to be associated with mortality and sudden cardiac death: age, gender, non-ischemic cardiomyopathy, hyperlipidemia, hypertension, history of tobacco use, diabetes, and genotype. The assumption of proportional hazards for the regression model was not violated, as evidenced by the parallel nature of the plots of the cumulative hazard functions for the allele strata (data not shown). Further evidence of the validity of this assumption was provided by the lack of association of a time-varying covariate in the regression model (p=0.92). Two sided P-values of <0.05 were considered as statistically significant. All statistical analyses were performed with SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Genotyping
DNA was extracted using PureGene (Gentra Systems, Minneapolis, MN) following manufacturers standard protocol. Genotypes for rs7626962 (S1103Y) were determined using the 7900HT Taqman genotyping system (Applied Biosystems, Foster City, CA), which incorporates a standard PCR-based, dual fluorophore, allelic discrimination assay. Assays were purchased from Applied Biosystems. QC samples, composed of 12 reference controls, were included in each quadrant of the plate. SNPs showing mismatches on QC samples were reviewed by an independent supervisor. The SNP was successfully genotyped for all of the individuals in the study. Error rate estimates for SNPs meeting QC benchmarks were <0.2%.
Results
Patient Characteristics
A total of 112 African Americans who received ICDs for primary prevention were included. Mean follow-up was 865 days (interquartile range 585 – 1131 days). The mean age was 62.8 and 34% of patients were women. Twenty-three patients (21%) received appropriate ICD therapy and 89 patients (79%) did not receive appropriate ICD therapy. Twelve of the 23 patients (52%) that received appropriate ICD therapy experienced ATP only as their classifying event. Baseline characteristics with respectto diabetes, tobacco use, hypertension, hyperlipidemia, atrial fibrillation, serum potassium level, New York Heart Association (NYHA) class, ejection fraction, maximum LV wall thickness, or medications at time of enrollment did not differ significantly between patients that received appropriate ICD therapy and those that did not (Table 1). The corrected QT duration did not differ between the two groups (463 vs 465 ms, p=0.41). There was a higher percentage of patients classified as having non-ischemic cardiomyopathy among those receiving appropriate ICD therapy (74% vs 48%, p=0.02).
Table 1.
Baseline Characteristics Stratified by ICD Therapy
| Characteristic * | Entire Cohort (n=112) | Appropriate ICD Therapy (n=23) | No Appropriate ICD Therapy (n=89) |
|---|---|---|---|
| Age, Mean (SD), y | 63 (12) | 64 (9) | 63 (13) |
| Male, No. (%) | 74 (66) | 18 (78) | 56 (63) |
| Medical History | |||
| History of Non-Ischemic Cardiomyopathy, No. (%) + | 60 (54) | 17 (74) | 43 (48) |
| History of Diabetes, No. (%) | 57 (51) | 11 (46) | 46 (52) |
| Tobacco Use, No. (%) | 59 (53) | 12 (52) | 47 (53) |
| History of Hypertension, No. (%) | 101 (90) | 22 (96) | 79 (89) |
| History of Hyperlipidemia, No. (%) | 81 (72) | 18 (78) | 63 (71) |
| History of Atrial Fibrillation, No. (%) | 51 (46) | 9 (39) | 42 (47) |
| NYHA Class, Mean (SD) | 2.4 (0.6) | 2.3 (0.7) | 2.4 (0.6) |
| Echo Parameters | |||
| Ejection Fraction, Mean (SD), % | 25 (6) | 24 (7) | 25 (6) |
| Maximal LV Wall Thickness, Mean (SD), cm | 1.4 (0.3) | 1.4 (0.3) | 1.4 (0.3) |
| ECG Parameters | |||
| QTc, Mean (SD), ms | 464 (41) | 463 (45) | 465 (40) |
| Lab Values | |||
| Serum Potassium at Enrollment, Mean (SD), mmol/L | 4.2 (0.5) | 4.1 (0.4) | 4.2 (0.5) |
| Medication | |||
| Beta Blockers, No. (%) | 104 (93) | 22 (96) | 82 (92) |
| ACE Inhibitors, No. (%) | 79 (70) | 19 (82) | 60 (67) |
| Diuretics, No. (%) | 91 (81) | 19 (83) | 72 (81) |
| Aspirin, No. (%) | 98 (88) | 19 (83) | 79 (89) |
| Digoxin, No. (%) | 28 (25) | 6 (26) | 22 (25) |
QTc, QT Interval corrected using Bazett’s Formula (QTc=QT/√RR).
P>0.05 for all variables except Non-Ischemic Cardiomyopathy
P=0.03
Association of S1103Y and ICD therapy
Genotyping revealed a Y1103 allele frequency of 0.09 with 92 patients homozygous for the S1103 allele (SS), 19 heterozygous (SY) and 1 homozygous for the Y1103 allele (YY) (supplemental Table 1). There was no departure from Hardy-Weinberg equilibrium. Chi-Square analyses using a dominant model (at least one copy of the Y1103 allele) revealed a significant over representation of the Y1103 allele in patients that received appropriate ICD therapy versus no appropriate ICD therapy, (8 of 23 [35%] vs. 12 of 89 [13%], p=0.03) (Figure 1). The presence of the Y1103 allele correlated to an unadjusted odds ratio for appropriate ICD therapy of 3.42 (95% CI 1.20–9.80, p=0.02). Although study-independent, genotype-blinded electrophysiologists adjudicated all ICD events, a sensitivity analysis excluding the 24 subjects that received only inappropriate ICD therapy was performed to account for possible errors in adjudication. Consistent with the primary analysis, this revealed an unadjusted odds ratio of 3.32 (95% CI 1.09–10.07, p=0.03) for appropriate ICD therapy in Y1103 carriers. When analyzed by allele status, there was no significant difference in baseline characteristics (Table 2). To assess the relationship between genotype and time to appropriate ICD therapy, a time-to-event survival analysis was performed. Kaplan-Meier curves diverged early and continued to diverge throughout the follow-up period showing a higher incidence of appropriate ICD therapy in Y1103 carriers (Figure 2, log-rank p=0.01). The mean time to appropriate ICD therapy for carriers was 609 ± 59 days compared to 1057± 36 days for non-carriers. Utilizing a Cox Proportional Hazard Model, carriers of the Y1103 allele had an age and gender adjusted hazard ratio of 3.50 (95% CI 1.38 – 8.85, p=<0.01) for ICD therapy compared to non-carriers (Table 3). An additional multivariable Cox Proportional Hazard Model was performed adjusting for age and gender as well as non-ischemic cardiomyopathy, hyperlipidemia, hypertension, history of tobacco use, and diabetes and resulted in a similar hazard ratio of 4.33 (95% CI 1.60–11.73, p=<0.01) for appropriate ICD therapy in carriers of the Y1103 allele (Table 3). There were four deaths among patients that received appropriate ICD therapy (one carrier of the Y1103 allele) and 15 deaths in those patients that did not receive appropriate ICD therapy (three were carriers of the Y1103 allele). There was no association between all-cause mortality and Y1103 carrier status, p=0.69.
Figure 1.
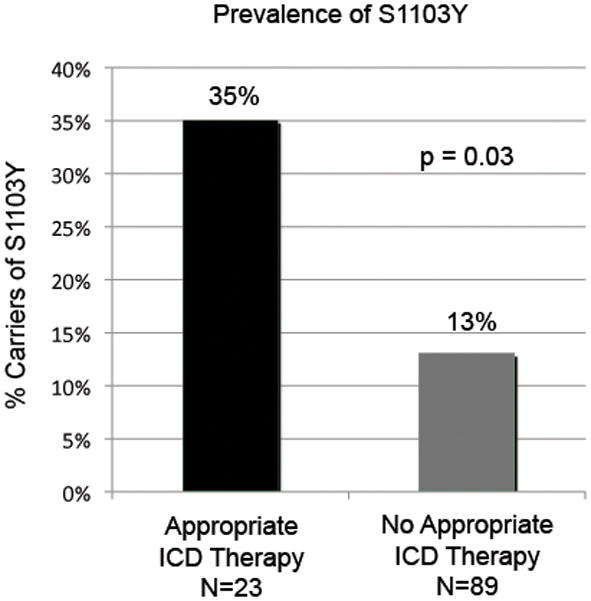
Prevalence of carriers for the minor alleles indicated, stratified by ICD therapy.
Table 2.
Clinical Characteristics and Events Stratified by Y1103 Allele Status
| Characteristic | Y1103 Carrier (n=20) | Y1103 Non Carrier (n=92) |
|---|---|---|
| Appropriate ICD Events, No. (%)* | 8 (40) | 15 (16) |
| Non-Ischemic Cardiomyopathy No. (%) | 11 (55) | 49 (53) |
| Ejection Fraction, Mean (SD) | 26 (7) | 24 (6) |
| QTc, Mean (SD), ms | 458 (47) | 466 (40) |
p=0.03
Figure 2.
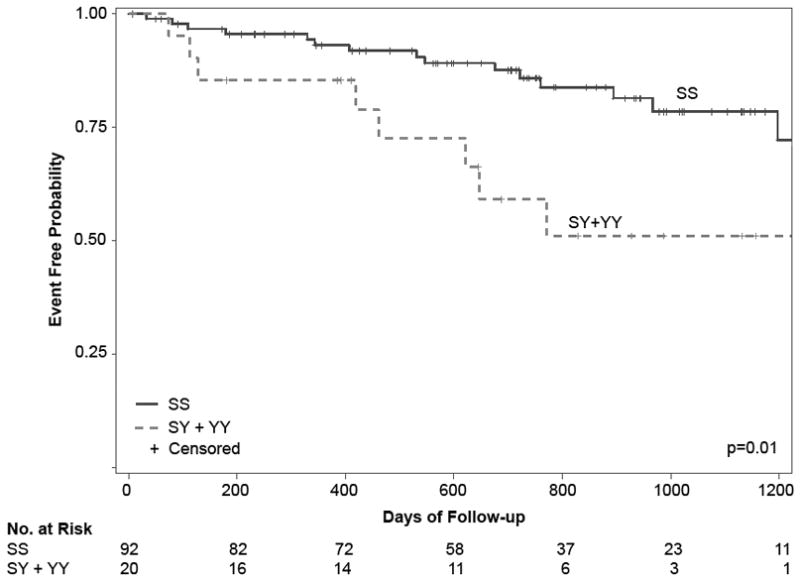
Kaplan-Meier curve for appropriate ICD therapy stratified by genotype, patients homozygous for the major allele (SS) and heterozygous or homozygous for the minor allele (SY + YY).
Table 3.
Multivariable Cox Proportional Hazard Model
| Variant | HR (95% CI) for ICD Therapy | P-value |
|---|---|---|
| S1103Y | ||
| Age and gender adjusted | 3.50 (1.38 – 8.85) | < 0.01 |
| Multivariable model | 4.33 (1.60 – 11.73) | < 0.01 |
Multivariable model controlled for age, gender, Non-Ischemic Cardiomyopathy, HTN, HL, TOB, and Diabetes.
Discussion
To the best of our knowledge this is the first report that the S1103Y variant in SCN5A is associated with a higher incidence of ventricular arrhythmias in African Americans with heart failure and reduced ejection fraction. Consistent with prior studies that have implicated this common genetic variant with drug-induced Long QT Syndrome (LQTS),9 SCD with cardiomegaly,12 and SIDS,10, 11 this finding strengthens the evidence that this variant is pro-arrhythmic. The specific association between the S1103Y genotype and an increased risk of arrhythmia in heart failure may have particular significance as a novel risk factor for African Americans.
While the exact mechanism by which the S1103Y variant contributes to SCD in heart failure is unclear, we propose that heart failure is an environmental stressor for Y1103 carriers. Previously, Splawski et al. demonstrated that the S1103Y variant causes a small, negative shift in sodium channel activation and increases peak transient current.9 An in silico modeled ventricular action potential was not affected by these changes at baseline, but the addition of hERG blockade (e.g., by a QT-prolonging medication) or acidosis significantly prolonged the action potential and promoted arrhythmia susceptibility.9, 10 In our study population we propose that the metabolic abnormalities and cellular derangements accompanying heart failure may serve as pro-arrhythmic stressors for Y1103 carriers. Splawski et al. also found that decreased extracellular potassium prolonged action potentials generated with the Y1103 allele (compared to S1103 allele) and promoted arrhythmias.9 Although the serum potassium at the time of event was not universally available in our data set, we did not observe a significant difference in serum potassium level at the time of implant between patients receiving appropriate ICD therapy and those patients that did not.
We found that non-ischemic cardiomyopathy was more prevalent in patients that received appropriate ICD therapy. The association of the Y1103 allele was independent of this finding. As previous studies have not shown significant differences in the rates of ICD therapy or ventricular arrhythmias between ischemic or non-ischemic origins of cardiomyopathy14 the difference may be secondary to sample size and should be investigated further during validation. This association of S1103Y with arrhythmias in heart failure highlights increasing evidence for the general concept that certain acquired arrhythmias, such as drug-induced LQTS, manifest more commonly in individuals with a genetic predisposition; recent data suggests that up to 40% of drug-induced LQTS patients harbor otherwise clinically silent ion channel mutations.8 Arrhythmogenesis because of ion channel mutations can therefore be considered a “two-hit” model, in which specific triggers exacerbate the abnormal function of a mutant channel. For patients with an inherited arrhythmia syndrome, such as LQTS, the effects of the mutation on channel functional are more severe and require only mild arrhythmogenic triggers, such as bradycardia during sleep in LQT3 patients with SCN5A mutations.15 In contrast, acquired arrhythmias due to mutations in the same ion channel loci occur with more severe environmental stressors (e.g., QT-prolonging drugs or heart failure) upon channels that are less functionally compromised.
The association of a genetic variant with life-threatening arrhythmias in heart failure may offer the possibility of genotype-specific risk-stratification strategies and targeted therapies. In the specific case of the S1103Y variant of SCN5A, which has been shown to increase the “late” or sustained Na+ current16, consideration of the use of agents such as ranolazine that diminish the sustained current to reduce arrhythmic events is worthy of speculation.
There are several limitations to our study. Often used as a surrogate for SCD in heart failure, ICD therapy had previously been shown to occur more frequently than SCD in a heart failure population.17 Secondly, some patients were classified as having appropriate ICD therapy if the only ICD event recorded was appropriate ATP therapy. Although a well validated method for termination of ventricular arrhythmias, individualized programming parameters could bias detection; a number of sustained ventricular tachycardia events could have been missed with conservative device programming.18, 19 Most importantly, given the limited sample size, these findings must be validated in an independent cohort. Our attempts to identify one were unsuccessful, due to the lack of well-phenotyped cohorts with sufficient African American enrollment. This fact furthers strengthens the importance of minority recruitment and enrollment in clinical and genetic cardiovascular trials. Our finding is consistent with previous reports implicating the S1103Y variant with Sudden Cardiac Death, Sudden Infant Death Syndrome, and Drug induced Long QT Syndrome and thus enhances the growing body of literature demonstrating the inherent susceptibility to potentially lethal arrhythmias in Y1103 allele carriers
In combination with traditional risk stratification tools such as EF, ECG analysis, and medical history,20 the addition of a genetic component could further strengthen the ability to identify heart failure patients at highest risk for sudden cardiac death and to identify mechanisms to prevent it. Given the relatively high prevalence of the S1103Y variant, this finding has a potentially large impact for the African American community. The possible identification of a genetic contribution to sudden cardiac death in heart failure could establish a new paradigm and is an area fertile for further investigation.
Supplementary Material
Acknowledgments
Patient enrollment and data collection was assisted by Amy Hughes, Valerie Cumbea and Anthony Waldron; and genotyping was assisted by Karen Abramson and Elizabeth Grass, all of Duke University.
Funding Sources: This study was supported in part by a grant from Medtronic, Inc. to Duke University as part of the Medtronic-Duke University Strategic Alliance (MEDUSA), a National Institutes of Health T32 training grant T32HL007101-35 (Dr Sun) and National Institutes of Health National Heart, Lung, and Blood Institute grants R01 HL71165 and R01 HL088089 (Dr Pitt) and American Heart Association Established Investigator award (Dr Pitt).
Footnotes
Conflict of Interest Disclosures. Albert Y. Sun: Research Grant - Medtronic Inc., Amount: >= $10,000, Jason I. Koontz: Research Grant - Medtronic Inc., Amount: >= $10,000, Svati H. Shah: Research Grant - Medtronic Inc., Amount: >= $10,000 Other Research Support - Medtronic Inc., Amount: >= $10,000, Jonathan P. Piccini: Research Grant - Medtronic Inc., Amount: >= $10,000, Kent R. Nilsson: Research Grant - Medtronic Inc., Amount: >= $10,000, Damian Craig: Research Grant - Medtronic Inc., Amount: >= $10,000, Carol Haynes: Research Grant -Medtronic Inc., Amount: >= $10,000, Simon G. Gregory: Research Grant - Medtronic Inc., Amount: >= $10,000, Patrick M. Hranitzky: Research Grant - Medtronic Inc., Amount: >= $10,000 Other Research Support - Medtronic Inc., Amount: >= $10,000, Geoffrey S. Pitt: Research Grant - Medtronic Inc., Amount: >= $10,000
References
- 1.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 2.Leyris J-P, Gondeau C, Charnet A, Delattre C, Rousset M, Cens T, Charnet P. RGK GTPase-dependent CaV2.1 Ca2+ channel inhibition is independent of CaV{beta}-subunit-induced current potentiation. FASEB J. 2009;23:2627–2638. doi: 10.1096/fj.08-122135. [DOI] [PubMed] [Google Scholar]
- 3.Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: New windows on complex neurological diseases. J Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the american association for thoracic surgery and society of thoracic surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Monaghan MM, Menegola M, Vacher H, Rhodes KJ, Trimmer JS. Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience. 2008;156:550–562. doi: 10.1016/j.neuroscience.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh H, Sakaguchi T, Ding WG, Watanabe E, Watanabe I, Nishio Y, Makiyama T, Ohno S, Akao M, Higashi Y, Zenda N, Kubota T, Mori C, Okajima K, Haruna T, Miyamoto A, Kawamura M, Ishida K, Nagaoka I, Oka Y, Nakazawa Y, Yao T, Jo H, Sugimoto Y, Ashihara T, Hayashi H, Ito M, Imoto K, Matsuura H, Horie M. Latent genetic backgrounds and molecular pathogenesis in drug-induced Long-QT syndrome. Circ Arrhythm Electrophysiol. 2009;2:511–523. doi: 10.1161/CIRCEP.109.862649. [DOI] [PubMed] [Google Scholar]
- 9.Splawski I, Timothy KW, Tateyama M, Clancy CE, Malhotra A, Beggs AH, Cappuccio FP, Sagnella GA, Kass RS, Keating MT. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 10.Plant LD, Bowers PN, Liu Q, Morgan T, Zhang T, State MW, Chen W, Kittles RA, Goldstein SA. A common cardiac sodium channel variant associated with sudden infant death in african americans, SCN5A S1103Y. J Clin Invest. 2006;116:430–435. doi: 10.1172/JCI25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Norstrand DW, Tester DJ, Ackerman MJ. Overrepresentation of the proarrhythmic, sudden death predisposing sodium channel polymorphism S1103Y in a population-based cohort of african-american sudden infant death syndrome. Heart Rhythm. 2008;5:712–715. doi: 10.1016/j.hrthm.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke A, Creighton W, Mont E, Li L, Hogan S, Kutys R, Fowler D, Virmani R. Role of SCN5A Y1102 polymorphism in sudden cardiac death in blacks. Circulation. 2005;112:798–802. doi: 10.1161/CIRCULATIONAHA.104.482760. [DOI] [PubMed] [Google Scholar]
- 13.Koontz JI, Haithcock D, Cumbea V, Waldron A, Stricker K, Hughes A, Nilsson K, Sun A, Piccini JP, Kraus WE, Pitt GS, Shah SH, Hranitzky P. Rationale and design of the duke electrophysiology genetic and genomic studies (EPGEN) biorepository. Am Heart J. 2009;158:719–725. doi: 10.1016/j.ahj.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Ermis C, Zhu AX, Vanheel L, Lemke MJ, Sakaguchi S, Lurie KG, Lu F, Lin J, Benditt DG. Comparison of ventricular arrhythmia frequency in patients with ischemic cardiomyopathy versus nonischemic cardiomyopathy treated with implantable cardioverter defibrillators. Am J Cardiol. 2005;96:233–238. doi: 10.1016/j.amjcard.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AAM, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the Long-QT syndrome: Gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 16.Splawski I, Timothy KW, Tateyama M, Clancy CE, Malhotra A, Beggs AH, Cappuccio FP, Sagnella GA, Kass RS, Keating MT. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 17.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–782. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 18.Wathen MS, Sweeney MO, DeGroot PJ, Stark AJ, Koehler JL, Chisner MB, Machado C, Adkisson WO. Shock reduction using antitachycardia pacing for spontaneous rapid ventricular tachycardia in patients with coronary artery disease. Circulation. 2001;104:796–801. doi: 10.1161/hc3101.093906. [DOI] [PubMed] [Google Scholar]
- 19.Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, Canby RC, Khalighi K, Machado C, Rubenstein DS, Volosin KJ. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing fast ventricular tachycardia reduces shock therapies (PAINFREE RX II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 20.Atwater BD, Thompson VP, Vest RN, 3rd, Shaw LK, Mazzei WR, Jr, Al-Khatib SM, Hranitzky PM, Bahnson TD, Velazquez EJ, Califf RM, Lee KL, Roe MT. Usefulness of the duke sudden cardiac death risk score for predicting sudden cardiac death in patients with angiographic (>75% narrowing) coronary artery disease. Am J Cardiol. 2009;104:1624–1630. doi: 10.1016/j.amjcard.2009.07.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


