Abstract
Objectives
The mechanism of action of treatment with tumour necrosis factor (TNF) blockers in rheumatoid arthritis (RA) is still not completely understood. The aim of this study was to test if adalimumab treatment could affect the influx of monocytes into the synovium.
Methods
A novel technique was used to analyse the migration of labelled autologous monocytes before and 14 days after initiation of adalimumab treatment using scintigraphy. CD14 monocytes were isolated from patients with RA, using a positive selection procedure with magnetic-activated cell sorting, and labelled with technetium-99m-hexamethylpropylene-amino-oxime. Scintigraphic scans were made 1, 2 and 3 h after re-infusion.
Results
As early as 14 days after the start of treatment with adalimumab a significant decrease in disease activity score evaluated in 28 joints was shown. There was no significant decrease in the influx of monocytes into the joint at this time.
Conclusions
This study indicates that adalimumab treatment does not reduce the influx of monocytes into the synovium early after initiation of treatment. As previous studies showed a rapid decrease in macrophage infiltration after TNF-antibody therapy, which could not be explained by increased cell death, this points to an important role for enhanced efflux of inflammatory cells from the synovium.
Introduction
Disease control has been improved by the use of tumour necrosis factor (TNF) blockade in patients with rheumatoid arthritis (RA) and other immune-mediated inflammatory diseases. However, the mechanisms by which TNF antagonists exert their effect is still not completely understood.1 Anti-TNF antibody treatment has been shown to result in marked reduction of synovial inflammation in RA and psoriatic arthritis.2–4 This decrease in synovial cellularity could be observed as early as 24–48 h after initiation of treatment.5–7
This early reduction in synovial inflammation after TNF blockade could not be explained by apoptosis induction at the site of inflammation,6 7 leaving either reduced cell influx or enhanced cell efflux to explain this process. In one study infliximab treatment significantly decreased the influx of 111In-labelled granulocyte migration into affected joints of RA patients,4 and in another study adalimumab significantly reduced influx of 99Tc-labelled leucocytes, whereas no decrease in influx was seen in patients treated with placebo.8
Monocytes and macrophages are key players in the pathogenesis of RA.9 Furthermore, the decrease in macrophage numbers in the synovium is associated with clinical improvement after effective treatment.10 Therefore, the effect of adalimumab treatment on monocyte migration towards the synovial compartment was examined. The authors recently developed a procedure using a combination of immunomagnetic cell selection with CD14-coated beads, labelling with technetium-99m-hexamethylpropylene-amino-oxime and scintigraphy to visualise the migratory behaviour of autologous monocytes.11 Applying this method, a continuous migration of monocytes into the inflamed synovial tissue of RA patients at a slow macrophage-replacement rate was shown.12 The slow rate of monocyte influx into the synovial compartment suggests that the rapid effect of anti-TNF therapy on macrophage infiltration cannot merely be explained by blockade of cell influx, as previously thought. Hence, this novel imaging technique was used to directly test if adalimumab treatment could affect the influx of monocytes into the synovium.
Patients and methods
Patients
Eight patients with established RA according to the revised American College of Rheumatology criteria for the diagnosis of RA13 were included. All patients had an indication for the use of anti-TNF therapy according to the guidelines of the Dutch Society for Rheumatology, which is active disease status (disease activity score evaluated in 28 joints (DAS28) ≥3.2) despite treatment with two conventional disease-modifying antirheumatic drugs. In this study all patients started with adalimumab (40 mg subcutaneously every other week) 24 h after the baseline scans. Three patients used maximally tolerable methotrexate at a stable dosage (10–25 mg/week). The others received adalimumab monotherapy. The use of concomitant non-steroidal anti-inflammatory drugs was permitted if stable for at least 1 month prior to baseline and was kept stable throughout the study. All patients provided written informed consent and approval was granted by the local medical ethics committee.
Isolation and labelling of monocytes
Isolation and labelling of monocytes was performed as described previously (see supplementary text).
Scintigraphy and signal calculations
Scintigraphy and signal calculations were done as described previously (see supplementary text). This procedure was done at day −14, baseline and day 14.
Statistical analysis
Data were analysed using the Wilcoxon signed ranks test to determine significant changes from baseline. Correlations were calculated using the Spearman's rank correlation coefficient. Values are expressed as median and IQR. The calculations were performed with SPSS 16.0 for Windows.
Results
Patient characteristics
Half of the patients were IgM rheumatoid factor positive and all had erosive disease. Their median age was 53 (48.5–57) years and median disease duration was 162 (119–273) months. Individual patient characteristics are shown in table 1.
Table 1.
Baseline patient characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| TJC of 28 joints | 4 | 6 | 25 | 16 | 12 | 23 | 4 | 17 |
| SJC of 28 joints | 3 | 6 | 4 | 12 | 6 | 20 | 15 | 7 |
| VAS general disease activity | 47 | 19 | 87 | 71 | 59 | 89 | 70 | 67 |
| ESR (mm/h) | 35 | 6 | 97 | 14 | 10 | 43 | 58 | 16 |
| C reactive protein (mg/l) | 4.6 | 7.2 | 85.6 | 5.9 | 5.1 | 17.5 | 34.7 | 3.0 |
| DAS28 | 4.75 | 3.58 | 7.58 | 5.91 | 5.06 | 7.82 | 6.03 | 5.3 |
| Sex (0=male) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| Age (years) | 59 | 53 | 39 | 58 | 47 | 53 | 53 | 54 |
| Disease duration (months) | 114 | 264 | 132 | 42 | 456 | 168 | 276 | 156 |
| Rheumatoid factor (1=positive) | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 |
| Erosive disease (1=yes) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
DAS28, disease activity score in 28 joints; ESR, erythrocyte sedimentation rate; SJC, swollen joint count; TJC, tender joint count; VAS, visual analogue scale.
Monocyte influx is stable and not markedly decreased early after initiation of adalimumab treatment
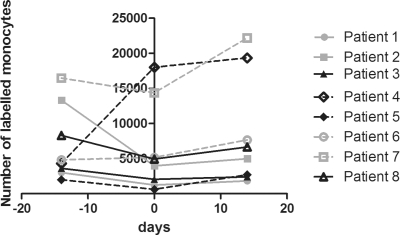
Scans were made 14 days prior to the start of adalimumab treatment, at baseline and 14 days after the start of adalimumab treatment (see supplementary figure 1). The number of labelled monocytes in the joint of interest per patient was comparable 1, 2 and 3 h after re-infusion. This was true for day −14, baseline and day 14 (see supplementary table). Monocyte influx per patient at 3 h postinfusion is shown in figure 1. Of interest, there was no significant change in monocyte influx 1, 2 and 3 h after re-infusion from day −14 to day 1, which was before the start of adalimumab treatment (p=0.33, p=0.67, p=0.21 respectively). The heterogeneity between patients is consistent with changes in synovial macrophages that were found in serial biopsies of patients treated with placebo.14 Importantly, the influx of monocytes did not decrease after re-infusion of monocytes comparing day 14 to baseline, indicating that adalimumab treatment did not affect the influx of monocytes early after initiation of treatment (figure 1).
Figure 1.
Number of monocytes in joint of interest 3 h post re-infusion per patient at day −14, 1 and 14.
Clinical benefit of adalimumab treatment
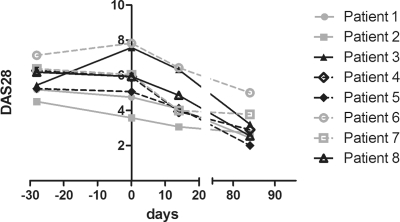
The change in DAS28 from screening to day 84 is shown in figure 2. The median DAS28 at baseline was 5.9 (4.8–7.2). The DAS28 did not change significantly between screening and baseline (p=0.58). There was a statistically significant decrease in median DAS28 from baseline to day 14 of 1.2 (0.72–1.8, p=0.01). At day 84 the median DAS28 had decreased to 2.8 (2.5–3.6) (figure 2). Three patients were European League Against Rheumatism (EULAR) moderate responders to treatment and five were EULAR good responders. There was no correlation between the decrease in DAS28, erythrocyte sedimentation rate or C reactive protein and the change in monocyte influx into the joints at 1, 2 or 3 h after re-infusion from baseline to 14 days (see supplementary figure 2).
Figure 2.
DAS28 from screening to day 84.
Discussion
The results of this mechanistic study indicate that CD14 monocyte influx into the synovium is not decreased 2 weeks after the start of adalimumab treatment. Earlier, TNF blockade was shown not to induce apoptosis in the synovium early after initiation of treatment, although the possibility cannot be excluded that more long-term treatment would lead to a more pro-apoptotic state, being a result of dampening of inflammation rather than its cause.1 This suggests that the rapid decrease in synovial macrophage numbers observed after anti-TNF treatment cannot be explained by an immediate effect on monocyte influx, as previously hypothesised. These data strengthen the recent observation that monocytes migrate towards the inflamed RA synovium at a slow macrophage-replacement rate,12 which already suggested that blockade of monocyte migration would be insufficient to explain the rapid reduction of macrophage numbers found after anti-TNF treatment. Consistent with the hypothesis that TNF blockade does not interfere with high levels of monocyte influx into the synovial compartment, numbers of peripheral blood monocytes were not increased after initiation of infliximab treatment.7 This lack of reduction in monocyte influx is in contrast with previous work showing a reduction of labelled neutrophils into affected joints after treatment with TNFα blockers.4 8 Of note, there is a clear difference in replacement rate for neutrophils compared to monocytes, as large numbers of neutrophils traffic into the synovial fluid.15 In addition, the difference might be explained in part by the differential use of adhesion molecules between neutrophils and monocytes. Very late antigen-4/vascular cell adhesion molecule-1 (VLA-4/VCAM-1) dependent rolling is seen mostly in monocytes, whereas adhesion of neutrophils is very much dependent on lymphocyte function-associated antigen 1/inter cellular adhesion molecule-1.16 The reduced serum levels of sICAM-1 but not sVCAM-1 (reflecting expression of adhesion molecules by endothelial cells) after administration of infliximab could perhaps explain the sustained influx of monocytes while neutrophil influx is reduced.17
In contrast to sVCAM-1 levels in peripheral blood, VCAM-1 expression in the synovial tissue is reduced after anti-TNF treatment.3 7 VCAM-1 is especially highly expressed by fibroblast-like synoviocytes, and may play a crucial role in retention and survival of infiltrating monocytes/macrophages.18 Similarly, ICAM-1 expression is decreased in the tissue after TNF blockade. Thus, decreased expression of adhesion molecules3 as well as chemokines4 in the tissue may facilitate macrophage egress from the synovium, resulting in decreased macrophage numbers in the synovial tissue. In line with increased cell egress after anti-TNF treatment, it was found that anti-TNF therapy results in increased lymphatic vessel formation in the synovium.19
Taken together, these data suggest that at this early time point the anti-inflammatory effect of treatment with TNF blockers can only partly be explained by downregulation of vascular adhesion molecules on endothelial cells and subsequent reduction in migration of inflammatory cells into the joint. Anti-TNF treatment may diminish RA disease activity by decreasing inflammatory cell retention in the synovial tissue, for example, by reducing integrins expressed by fibroblast-like synoviocytes. Accordingly, treatments aimed at integrins such as anti-LFA-1 (efatizumab) and anti-VLA-4 were shown to be effective treatment in inflammatory diseases, although serious infectious side effects were reported.20 21
In conclusion, this study indicates that treatment with adalimumab does not reduce the influx of monocytes into the synovium 2 weeks after the start of treatment. In previous work early reduction in synovial inflammation after TNF blockade could not be explained by apoptosis induction at the site of inflammation. Based on these studies, it is hypothesised that efflux of inflammatory cells is a major contributor to the rapid reduction of cellularity after initiation of anti-TNF antibody therapy.
Acknowledgments
The authors thank the clinical team of the Division of Clinical Immunology and Rheumatology, and Nuclear Medicine and the staff of the Laboratory for Stem Cell Transplantation at Sanquin Research for their assistance.
Footnotes
Funding This study was funded by Millennium: The Takeda Oncology Company.
Competing interests Millennium: The Takeda Oncology Company participated in the design of the study, monitored the clinical study and participated in the analysis and interpretation of the clinical study.
Ethics approval This study was conducted with the approval of the Academic Medical Center/University of Amsterdam.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008;117:244–79 [DOI] [PubMed] [Google Scholar]
- 2.Goedkoop AY, Kraan MC, Picavet DI, et al. Deactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapy: a prospective single-centre study. Arthritis Res Ther 2004;6:R326–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tak PP, Taylor PC, Breedveld FC, et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum 1996;39:1077–81 [DOI] [PubMed] [Google Scholar]
- 4.Taylor PC, Peters AM, Paleolog E, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum 2000;43:38–47 [DOI] [PubMed] [Google Scholar]
- 5.Goedkoop AY, Kraan MC, Teunissen MB, et al. Early effects of tumour necrosis factor alpha blockade on skin and synovial tissue in patients with active psoriasis and psoriatic arthritis. Ann Rheum Dis 2004;63:769–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smeets TJ, Kraan MC, van Loon ME, et al. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum 2003;48:2155–62 [DOI] [PubMed] [Google Scholar]
- 7.Wijbrandts CA, Remans PH, Klarenbeek PL, et al. Analysis of apoptosis in peripheral blood and synovial tissue very early after initiation of infliximab treatment in rheumatoid arthritis patients. Arthritis Rheum 2008;58:3330–9 [DOI] [PubMed] [Google Scholar]
- 8.den Broeder AA, Wanten GJ, Oyen WJ, et al. Neutrophil migration and production of reactive oxygen species during treatment with a fully human anti-tumor necrosis factor-alpha monoclonal antibody in patients with rheumatoid arthritis. J Rheumatol 2003;30:232–7 [PubMed] [Google Scholar]
- 9.Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum 2009;60:1210–21 [DOI] [PubMed] [Google Scholar]
- 10.Haringman JJ, Gerlag DM, Zwinderman AH, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:834–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennink RJ, Thurlings RM, van Hemert FJ, et al. Biodistribution and radiation dosimetry of 99mTc-HMPAO-labeled monocytes in patients with rheumatoid arthritis. J Nucl Med 2008;49:1380–5 [DOI] [PubMed] [Google Scholar]
- 12.Thurlings RM, Wijbrandts CA, Bennink RJ, et al. Monocyte scintigraphy in rheumatoid arthritis: the dynamics of monocyte migration in immune-mediated inflammatory disease. PLoS ONE 2009;4:e7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24 [DOI] [PubMed] [Google Scholar]
- 14.Gerlag DM, Haringman JJ, Smeets TJ, et al. Effects of oral prednisolone on biomarkers in synovial tissue and clinical improvement in rheumatoid arthritis. Arthritis Rheum 2004;50:3783–91 [DOI] [PubMed] [Google Scholar]
- 15.Youssef PP, Cormack J, Evill CA, et al. Neutrophil trafficking into inflamed joints in patients with rheumatoid arthritis, and the effects of methylprednisolone. Arthritis Rheum 1996;39:216–25 [DOI] [PubMed] [Google Scholar]
- 16.Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7:678–89 [DOI] [PubMed] [Google Scholar]
- 17.Paleolog EM, Hunt M, Elliott MJ, et al. Deactivation of vascular endothelium by monoclonal anti-tumor necrosis factor alpha antibody in rheumatoid arthritis. Arthritis Rheum 1996;39:1082–91 [DOI] [PubMed] [Google Scholar]
- 18.Carter RA, Wicks IP. Vascular cell adhesion molecule 1 (CD106): a multifaceted regulator of joint inflammation. Arthritis Rheum 2001;44:985–94 [DOI] [PubMed] [Google Scholar]
- 19.Polzer K, Baeten D, Soleiman A, et al. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann Rheum Dis 2008;67:1610–16 [DOI] [PubMed] [Google Scholar]
- 20.Keeley KA, Rivey MP, Allington DR. Natalizumab for the treatment of multiple sclerosis and Crohn's disease. Ann Pharmacother 2005;39:1833–43 [DOI] [PubMed] [Google Scholar]
- 21.Lotti T, Chimenti S, Katsambas A, et al. Efficacy and safety of efalizumab in patients with moderate-to-severe plaque psoriasis resistant to previous anti-psoriatic treatment: results of a multicentre, open-label, phase IIIb/IV trial. Arch Drug Inf 2010;3:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]