Abstract
Preclinical drug discrimination techniques play a significant role in advancing our knowledge of the receptor mechanisms underlying the interoceptive effects of nicotine. Early reports confirmed that nicotinic acetylcholine receptors (nAChRs) are critical for transduction of the nicotine cue. In recent years, advances in molecular biology and the discovery of novel ligands with greater selectively for specific nAChR subtypes have furthered our understanding of these mechanisms. There is now evidence regarding the specific nAChR subtypes involved in nicotine discrimination; in addition, there is also evidence suggesting that other systems (i.e., adenosine, cannabinoid, dopamine, glutamate and serotonin) may play a modulatory role. The neuroanatomical structures mediating the nicotine cue have also begun to be elucidated. However, much remains to be learned about the predictive validity of the drug discrimination procedure, particularly with regard to the relation between interoceptive and reinforcing effects and individual differences in vulnerability to tobacco dependence. Recent data also suggests that the mechanisms involved in the conditional and discriminative stimulus properties of nicotine may be dissociable. Avenues for future research should include assessing the mechanisms of the subjective effects of nicotine withdrawal, factors contributing to individual differences in sensitivity to the nicotine cue, and the role of behavioral factors involved in drug cross-substitution.
Keywords: nicotine, drug discrimination, conditional stimulus, nicotinic acetylcholine receptor
1. Introduction
Drugs of abuse can alter behavior in a number of ways, including strengthening ongoing behavior that precedes their delivery (i.e., reinforcing effects) or guiding behavior between concurrently-available, differentially-reinforced response options with their interoceptive effects. The latter property can be studied with drug discrimination, one of the most commonly used procedures for investigating the neuropharmacology of psychoactive drugs in the behaving animal [1–3]. In contrast to self-administration, which is restricted to investigations of drugs that function as reinforcers, drug discrimination is generally amenable to studying any compound that generates a perceptible internal state that can be associated with appetitive or aversive events in the environment. Thus, one can study the interoceptive cueing effect of nicotine by using nicotine as the training stimulus, or by administering nicotine to subjects trained to recognize a related ligand (e.g., cytisine) thought to act on similar neurotransmitter systems as nicotine. Early investigations in the field used procedures such as the shock-escape T-maze task [4], although the two-lever operant drug discrimination protocol is currently the most widely-used procedure [5]. In this latter method, experimental subjects are trained to earn a reinforcer (e.g., food) by emitting one response (e.g., pressing the left of two levers) following injection of a training drug; a different response is reinforced (e.g., pressing the right of two levers) following placebo injection [6–8]. Thus, subjects are required to first recognize the presence or absence of the training drug cue (often referred to as a discriminative stimulus, or SD), and to then complete the requisite response requirement [e.g., 25 presses on the drug lever] to earn a reinforcer.
More recently, two different variants of a Pavlovian drug discrimination procedure have been used to study the interoceptive stimulus effects of nicotine in rats (see [9] for recent discussion of these protocols). One procedure conceptualizes nicotine as an interoceptive context cue or conditioned stimulus (CS) that signals when intermittent access to liquid sucrose unconditioned stimulus (US) will occur; placebo indicates that sucrose will be withheld. Sucrose delivery occurs regardless of ongoing behavior. Nicotine thereby comes to acquire the ability to evoke anticipatory approach to the goal area (i.e., goal tracking; [10]) relative to the placebo state [11]. The other procedure conceptualizes nicotine as an occasion setter (OS) that signals when a CS-US association is or is not in force. The procedure is similar to the nicotine CS protocol, except that a brief exteroceptive stimulus such as illumination of a cue light is added to nicotine and placebo sessions. If nicotine is trained as a feature positive OS, then sucrose follows each light presentation only on nicotine sessions. If trained as a negative feature, the sucrose occurs only on placebo sessions. The light comes to differentially evoke a goal-tracking CR depending on the drug state [12, 13].
Regardless of the protocol, two types of tests can be conducted once the discrimination has been established. In substitution testing, varying doses of the training drug or a test drug are administered alone to determine the degree to which they generalize to the training drug. In interaction testing, a pretreatment drug is administered in combination with the training drug to determine whether responding controlled by the training drug is altered. Substitution and interaction tests can be used to determine the receptor mechanisms mediating the interoceptive effects of a drug, as a test drug typically substitutes for a training drug within a similar, but not a distinct, pharmacological class. For example, a stimulant drug (e.g., d-amphetamine) would be expected to substitute for the cue of another stimulant such as cocaine, but not for the cue produced by a sedative/hypnotic such as pentobarbital. An important advantage of drug discrimination is that similar cross-species findings are typically obtained when comparing the results of animal and human studies [14]. Thus, the excellent predictive validity and pharmacologic specificity of drug discrimination studies have led to the widespread adoption of these procedures to probe the neuropharmacology of many psychoactive drugs.
The purpose of the present review is to synthesize current knowledge regarding the receptor mechanisms of nicotine action as revealed by drug discrimination studies. A review of the operant drug discrimination based on a literature survey conducted in 2007 has been published recently in book form [15]. Therefore, the focus of the present article will be on recent findings obtained with laboratory animals using Pavlovian procedures, as well as operant methodology. Since the initial work in the field conducted several decades ago [16–18], studies conducted with genetically-altered mice [19] and the discovery of novel ligands with enhanced selectivity for specific receptor subtypes [20, 21], have further illustrated the diverse mechanisms of the interoceptive stimulus properties of nicotine, including recent insights into the role of specific nicotinic receptor subtypes. Work across these areas will be integrated and issues in need of further investigation will be discussed.
2. Acetylcholine
In the central nervous system, acetylcholine (ACh) acts on two distinct classes of receptors, muscarinic acetylcholine receptors (mAChRs) and nicotinic acetylcholine receptors (nAChRs). The mAChR family includes 5 subtypes (M1-M5), each of which is a G protein-coupled metabotropic receptor. The nAChRs are ligand-gated ionotropic pentamers consisting of various combinations of the eight α (α2-α7, α9, and α10) and three β (β2-β4) subunits identified in mammalian brain to date [22].
2.1. Peripheral AChRs
The interoceptive stimulus properties of nicotine appear to be centrally-mediated. The peripheral nAChR agonist methylcarbamylcholine does not substitute for nicotine [23]. The ganglionic blockers hexamethonium and chlorisondamine, which do not readily penetrate the blood-brain barrier, also fail to block nicotine discrimination [11, 13, 24–27], although a single intraventricular administration of chlorisondamine blocks the nicotine cue for up to 4 weeks [27, 28].
2.2. Central AChRs
There is ample evidence indicating that central nAChRs are a critical component of the nicotine cue. The classical central nAChR antagonists mecamylamine and dihydro-β-erythroidine (DHβE) block nicotine discrimination completely without disrupting response rates, whereas the mAChR antagonist atropine fails to block the nicotine cue [11, 13, 21, 27, 29–31]. Nicotine also fails to generalize to the SD effects of the mAChR agonists arecoline, physostigmine, pilocarpine or oxotremorine [32–35] or the SD effects of the mAChR antagonist scopolamine [36]; physostigmine also fails to alter the nicotine cue [37].
Conversely, a variety of nAChR agonists, including 1-acetyl-4-methylpiperazine (AMP), 3-pyridylmethylpyrrolidine, anabasine, anatoxin, cotinine, cytisine, isoarecolone, and nornicotine generalize fully to nicotine in rats [23, 38–45] and/or squirrel monkeys [46]. Nicotine also elicits full substitution for cytisine in rats that have been trained using cytisine as a SD [47]. Combined, these findings indicate that central nAChRs, but not mAChRs, mediate nicotine’s interoceptive stimulus effects. However, each nAChR subtype may not contribute equally to this effect, given the diversity in subunit composition and regional distribution of these receptors.
2.2.1. α4β2* nAChRs
The α4β2* nAChR (note that “*” refers to the possible inclusion of additional native nAChR subunits) is the most abundant heteromeric subtype in mammalian brain, and nicotine binds to α4β2* nAChRs with high affinity [48]. Unlike wild-type (WT) mice, β2 subunit knockout (KO) mice are unable to discriminate nicotine from saline regardless of the training dose and despite extensive training [49]. In addition, the extent to which α4β2 nAChR agonists generalize to the nicotine cue appears to be associated with affinity for α4β2* nAChRs in receptor binding assays and/or the ability to evoke nAChR-mediated [3H]neurotransmitter release in functional assays. Specifically, partial α4β2 agonists such as cytisine, the smoking cessation agent varenicline (Chantix®), and SSR591813 substitute partially for nicotine [50–54], whereas full α4β2 agonists such as ABT-418, ABT-594, A85380, TC2559, epibatidine and 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine (5-IA) substitute fully [51, 55, 56]. Interestingly, the α4β2 nAChR desensitizing agent sazetidine-A also produces full generalization to the nicotine cue [57], consistent with the notion that both nAChR activation and desensitization contribute to the behavioral effects of nicotine [58]. The naturally-occurring nicotine enantiomer, S(−)nicotine, is ~9 times more potent than R(+)nicotine in substitution tests [59], consistent with the greater potency of the former enantiomer at displacing [3H]nicotine binding in rat brain homogenates [60]. Finally, administering nicotine via oral, subcutaneous, or intraperitoneal routes produces equipotent substitution for an oral nicotine SD, although transdermal administration reduces the potency of nicotine by ~1 log unit [52].
In interaction tests, the α4β2 nAChR antagonist DHβE blocks fully the nicotine cue in mice [61] and rats [21, 50, 54, 62, 63], as well as the nicotine-like SD effects of 5-IA [64]. In contrast to mecamylamine, however, the inhibitory effects of competitive nAChR antagonists such as DHβE and erysodine are surmounted by higher doses of nicotine [62, 63, 65]. The partial α4β2 nAChR agonists cytisine, varenicline and SSR591813 also attenuate nicotine discrimination [50, 51], but to a lesser extent than classical antagonists. Thus, α4β2* nAChRs are a critical transduction mechanism for producing the nicotine cue.
2.2.2. α6β2* nAChRs
The α6 nAChR subunit is expressed primarily on the soma and axon terminals of midbrain DA neurons [66–68]. The functional significance of α6-containing receptors was unknown until the peptide toxin α-conotoxin MII (α-Ctx MII) was isolated from the marine snail C. magus [69]. The complete loss of [125I]α-Ctx MII binding sites in α6 KO mice [66] spurred research leading to recognition of the critical role of this subtype in regulating basal and nicotine-evoked DA release [68, 70], as well as nicotine self-administration [71]. Although α-Ctx MII cannot be tested systemically because it does not cross the blood-brain barrier, a novel series of N,N’-alkane-diyl-bis-3-picolinium (bAPi) analogs appear to represent the first systemically-effective α6β2 antagonists [21]. Based on initial findings that two bAPi analogs (N,N’-decane-1,10-diyl-bis-picolinium diiodide, bPiDI; and N,N’-dodecane-1,12-diyl-bis-picolinium dibromide, bPiDDB) attenuate nicotine-evoked DA release in nucleus accumbens and nicotine self-administration [72–74], each of the bAPi analogs has been tested for blockade of nicotine discrimination. Although several bAPi analogs selectively inhibit nicotine-induced hyperactivity, none block the SD or CS effects of nicotine [21, 75]. Thus, it appears that while α6β2* nAChRs play a critical role in the DA-mediated reinforcing and stimulant properties of nicotine, this subtype does not seem to be important for the interoceptive stimulus effects of nicotine.
2.2.3. α3β4* nAChRs
There are only a few reports examining the role of α3β4* nAChRs in nicotine discrimination. The α3β4 nAChR agonist WO 03/062224 does not substitute for nicotine or serve as a SD [56]. The N-methyl-D-aspartate (NMDA) glutamate receptor and α3β4 nAChR antagonist dextromethorphan (DXM) also fails to substitute for or alter the SD effects of nicotine [76, 77]. However, DXM partially blocks the CS, and fully blocks the locomotor, effects of nicotine [75]. This antagonism may be mediated by DXM antagonism of NMDA receptors, rather than α3β4* nAChRs, given that pretreatment with MK-801, a noncompetitive NMDA receptor antagonist, fully blocks the CS effects of nicotine [30; see later]. Regardless, these findings indicate a need for more research, as well as improved pharmacological tools, to better assess the involvement (or lack thereof) of α3β4* nAChRs in the nicotine cue.
2.2.4. α7* nAChRs
Although α7* nAChRs are the most abundant homomeric subtype, there is only limited support of a role for these receptors in generating the nicotine stimulus. In contrast to β2 KO mice, α7 KO mice readily learn to discriminate nicotine from saline [78]. In substitution tests, the α7 agonists GTS-21 and WO 01/60821A1 do not substitute for nicotine in rats [56, 78]. In interaction tests, GTS-21 and the α7 antagonist methyllycaconitine (MLA) fail to alter the stimulus effects of nicotine in rats [50, 64, 75, 79, 80]. However, a recent study demonstrated that nicotine substitutes more readily in WT mice than in α7 KO mice trained to discriminate d-amphetamine, and that MLA attenuates the SD effects of nicotine in nicotine-trained WT mice, as well as the SD effects of nicotine and d-amphetamine in WT mice trained to discriminate d-amphetamine [19]. Thus, the d-amphetamine-like SD effects of nicotine may involve α7* nAChRs, although MLA appears to block the nicotine cue in nicotine-trained mice only.
3. Dopamine
Dopamine (DA) is a critical mediator of the reinforcing and stimulant effects of abused drugs, including nicotine [81–85]. The two primary classes of DA receptors are the D1-like (i.e., D1 and D5) and the D2-like (i.e., D2, D3 and D4) receptors. Nicotine initially activates, but then desensitizes, high-affinity β2 subunit-containing nAChRs on DA neurons that evoke release. In addition, a relatively prolonged reduction of inhibitory GABA input, combined with potentiation of excitatory glutamate input to these neurons, promotes DA release in the synapse [83]. While these effects are critical for nicotine self-administration [86], the contribution of DA to the interoceptive stimulus effects of nicotine remains a matter of debate [87, 88].
3.1. D1 receptors
Some evidence supports a role for D1-like DA receptors in nicotine discrimination. The D1 agonists SKF-38393, SKF-81297 and SKF-82958 substitute partially for nicotine using the common 0.4 mg/kg nicotine training dose [29, 89, 90], whereas SKF-82958 substitutes fully in rats trained to discriminate 0.1 mg/kg nicotine [29]. However, when rats are trained in a 3-choice discrimination procedure using nicotine, saline, and SKF 812897 as SD, SKF 81297 alone no longer evokes nicotine-appropriate responding [89]. Although the D1 antagonist SCH-23390 partially attenuates the SD effects of nicotine [91], as well as the generalization of d-amphetamine to nicotine [89], reductions in response rate are also observed (see also [92]), indicating that the attenuation by SCH-23390 may represent nonspecific effects due to behavioral impairment. Thus, only limited findings [29] support the notion that D1 receptors are involved in the SD effects of a low nicotine dose.
3.2. D2 receptors
There are also mixed results on the role of D2 receptors in the nicotine cue. The D2 agonist R(−)-10,11-dihydroxy-N-n-propylnoraporphine hydrochloride (NPA), the mixed D2/3 agonists bromocriptine, (±)-7-hydroxy-N,N-di-n-propyl-2-aminotetralin (7-OH-DPAT) and quinpirole, and the DA autoreceptor antagonist (+)-AJ-76, do not substitute for nicotine [29, 89, 90]. The D2 antagonists haloperidol and spiperone, and the DA release inhibitor CGS 10746B, partially attenuate the nicotine SD in some studies [27, 29, 91, 93], but haloperidol and the nonselective DA antagonist cis-flupentixol are not effective in other studies [94]. Finally, the D2/3 antagonist eticlopride attenuates nicotine-evoked conditioned responding, but only at doses that also decrease activity [92]. Collectively, it appears that the attenuation of nicotine’s interoceptive effect by DA antagonists occurs only at doses that reduce either response rates or general activity, which complicates interpretation [91].
3.3. D3 receptors
There are no results supporting D3 receptor involvement in nicotine discrimination. The D3 agonist PD 128, 907, the D3 partial agonist BP-897, and the D3 antagonists ST 198 and nafadotride do not generalize to, or alter, the responding controlled by the stimulus effects of nicotine [29, 39, 92, 95].
3.4. D4 receptors
There also does not appear to be a role for D4 receptors in the stimulus effects of nicotine. Although the atypical antipsychotic clozapine (an antagonist of D4 receptors) attenuates nicotine discrimination [39], the more selective D4 antagonist U-101,387 fails to alter the SD effects of nicotine [89]. The effect of clozapine may therefore be attributable to its actions on other neurotransmitter systems (e.g., serotonin) that appear to play a greater role in the nicotine cue.
3.5. DA agonists
Stronger evidence for a role of DA in nicotine discrimination is derived from investigations of the effects of nonselective DA agonists (i.e., DA transporter [DAT] inhibitors and DA releasers), although the diverse actions of such compounds precludes speculation on the role of any particular receptor(s).
3.5.1. Dopamine transport inhibitors
The SD effects of several DAT inhibitors (i.e., bupropion, cocaine, GBR 12909 and methylphenidate) have been assessed in nicotine-trained rats. The nonselective monoamine uptake inhibitor cocaine elicits no substitution [26, 96, 97] or partial substitution [89, 98, 99] in nicotine-trained rats, pigeons, or squirrel monkeys. Further, cocaine pretreatment fails to alter the effects of nicotine in nicotine-trained rats [100]. On the other hand, nicotine generalizes fully in rats [23, 101] or rhesus monkeys [102] trained to discriminate cocaine. The cocaine-like SD effects of nicotine are blocked by the nonselective DA receptor antagonist cis-flupentixol [98], although mecamylamine does not alter cocaine discrimination [98, 101]. Thus, the generalization of nicotine to the cocaine cue appears to be mediated by nAChRs and DA, whereas the generalization of cocaine to the nicotine cue is mediated by DA but not nAChRs.
The SD effects of the DAT and norepinephrine transporter (NET) inhibitor bupropion (which also blocks nAChRs [103]), have also been examined in nicotine-trained rats. Bupropion substitutes partially or fully for nicotine in some studies [99, 104–106], but not others [98, 107]. Also, nicotine fully substitutes for bupropion when it is trained as a feature positive OS in rats [104]. The bupropion metabolite S,S-hydroxybupropion, as well as (+) and (−) threo bupropion, elicit partial substitution for the SD effects of nicotine [108]. However, S,S-hydroxybupropion does not substitute for a nicotine CS in rats [104]. In interaction tests, bupropion shifts the nicotine dose-response curve leftward based on one study [106], but not based on another study [107]. There is no shift in the CS effects of nicotine after pretreatment with 5 or 10 mg/kg of bupropion, although 20 mg/kg of bupropion decreases nicotine-controlled responding [104]; similarly, the bupropion metabolite R,R-hydroxybupropion attenuates the SD effects of the nicotine training dose by ~50% [108]. While the nicotine-like SD effects of bupropion are not blocked by mecamylamine [105, 106], the nicotine-like CS effects of bupropion are blocked by the dopamine D1 antagonist SCH-23390 and the D2/3 antagonist eticlopride [104]. Thus, DA receptors appear to regulate the nicotine-like CS effects of bupropion, although nAChRs are not involved in the nicotine-like SD effects of bupropion.
Mixed findings have also been obtained with the DAT and NET inhibitor methylphenidate. Although methylphenidate (1.25–10 mg/kg) does not substitute in rats trained to discriminate 0.3 mg/kg of nicotine, it dose-dependently enhances the SD effects of a lower 0.056 mg/kg nicotine dose [109]. In contrast, 10 mg/kg of methylphenidate substitutes partially in rats trained with 0.2 mg/kg of nicotine as a CS [99].
The nicotine-like SD effects of the selective DAT inhibitor GBR 12909 have also been examined. GBR 12909 produces either partial [91] or full [29] generalization to the SD effects of nicotine. In contrast, GBR 12909 does not substitute for the CS effects of 0.2 mg/kg of nicotine [99]. Interestingly, GBR 12909 does not substitute for the SD effects of nicotine if caffeine (3 mg/ml) is added to the rats’ daily drinking water [29].
3.5.2. Dopamine releasers
There are conflicting reports regarding whether DA releasers produce nicotine-like stimulus effects. For example, d-amphetamine produces partial [11, 26, 89, 110] or full [111] substitution for the SD or CS effects of nicotine in rats; d-amphetamine also substitutes partially in nicotine-trained C57BL/6 mice [112]. In contrast, d-amphetamine does not substitute for 0.4 mg/kg nicotine trained as a feature positive OS [113, 114]. Methamphetamine also elicits partial substitution for nicotine [94], whereas cathinone elicits full substitution [93]. On the other hand, the nonselective DA agonist apomorphine does not substitute for nicotine [26]. In tests of cross-substitution in stimulant-trained rats, nicotine either fails to substitute [115, 116], or substitutes only partially [47, 113], for d-amphetamine or cathinone [93]. However, nicotine substitutes fully for methamphetamine [94], and for the SD effects of a low 0.3 mg/kg dose of d-amphetamine [101]. The α4β2 nAChR partial agonist cytisine [47] and the nicotine metabolite nornicotine [38] also evoke partial substitution for d-amphetamine. Interestingly, the nicotine-like SD effects of d-amphetamine are not blocked by haloperidol [89], although haloperidol attenuates the partial substitution of methamphetamine [94]. The DA release inhibitor CGS 10746B also prevents the partial substitution of cathinone for nicotine [93]. On the other hand, mecamylamine and hexamethonium do not alter the SD effects of d-amphetamine [101] or methamphetamine [94], although mecamylamine (but not haloperidol) blocks the substitution of nicotine for methamphetamine [94]. Finally, nicotine potentiates the SD effects of d-amphetamine in d-amphetamine-trained rats [117]. While there clearly is not a complete overlap in the SD effects of nicotine and stimulant drugs, the stimulant-like SD effects of nicotine involve both DA and nAChRs, whereas the nicotine-like SD effects of stimulant drugs are mediated primarily by DA.
4. Norepinephrine
There is limited evidence supporting a role for NE in the interoceptive stimulus effects of nicotine. Although one early study found that depleting central NE levels with the tyrosine hydroxylase inhibitor α-methyl-para-tyrosine (AMPT) attenuates the discriminative stimulus effects of nicotine [118], AMPT also depletes DA, making it difficult to conclude that blockade depends specifically on NE depletion. Further, the NET inhibitor desipramine [89], the β2 adrenergic agonist clenbuterol [41], and the α2 adrenergic antagonists agmatine [119] and yohimbine [89], do not substitute for nicotine. Further, the α1 adrenergic antagonist dibenamine [120], the α2 adrenergic antagonist agmatine [119], and the nonselective β adrenergic antagonist propranolol [120] do not alter nicotine’s SD effects. Although the NET inhibitor atomoxetine does not substitute for the CS effects of nicotine, atomoxetine blocks partially the nicotine CS without altering activity [99]. This observation has also been extended to the NET inhibitor reboxetine (R.A. Bevins, unpublished data). Thus, NE does not appear to be a component of the SD effects of nicotine, although atomoxetine and reboxetine partially attenuate the CS property of nicotine at doses that do not produce nonspecific behavioral impairments.
5. Serotonin
Some early attempts to determine the role of serotonin (5-hydroxytryptamine; 5-HT) in the SD effects of nicotine produced negative results. Depletion of central 5-HT levels with para-chlorophenylalanine does not alter rats’ ability to discriminate nicotine from saline [118]. The mixed 5-HT receptor antagonist methergoline does not alter the nicotine cue [120], and the mixed 5-HT receptor agonist quipazine does not substitute for nicotine [26]. More recently, nicotine was shown to generalize partially to a compound SD composed of both the 5-HT releaser fenfluramine and the DA releaser phentermine, but not when fenfluramine or phentermine alone is used as the training stimulus [121]. Fenfluramine alone also does not substitute for nicotine [89]. In contrast to these results, however, there is recent evidence supporting a role for specific 5-HT receptor subtypes in modulating the nicotine cue.
5.1. 5-HT1A receptors
The 5HT1A agonist (±)-8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT) dose-dependently attenuates nicotine-appropriate responding [122], although the 5HT1A partial agonist buspirone does not alter the nicotine cue [123].
5.2. 5-HT2A/C receptors
There appears to be a role for 5-HT2A/c receptors in the interoceptive stimulus effects of nicotine. The 5-HT2A antagonist R-(+)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol (M100,907), the 5-HT2A/C agonist (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane HCl (DOI), the 5-HT2C receptor antagonist 6-chloro-5-methyl-1-to indoline (SB 242,084), and the 5-HT2C agonists Ro-60–0175 and (7bR, 10aR)-1,2,3,4,8,9,10,10a–octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole (WAY 163,909) lack nicotine-like SD effects [124]. However, DOI and 1-(4-bromo-2, 5-dimethoxyphenyl)-2-aminopropane (DOB) attenuate the nicotine cue [122, 124]. The 5-HT2A/C antagonists ketanserin or M100,907, but not SB 242,084, reverse the DOI blockade [122, 124], whereas the blockade by Ro-60–0175- or WAY 163,909 is reversed by SB 242,084, but not by M100,907 [124]. The 5-HT2C agonists 6-chloro-2-(1-piperazinyl) pyrazine hydrochloride (MK 212) and Ro-60–0175 also attenuate responding controlled by nicotine, although response rates were decreased at the highest test dose of each drug [122, 124, 125]. Similarly, the generalization to nicotine by the α4β2 nAChR agonist 5-IA is also blocked partially by WAY 163,909 and completely by DOI and Ro 60–0175 [124]. Interestingly, chronic nicotine treatment in ICR mice reduces cortical 5-HT2A receptor density, an effect that was suggested to reflect a potential 5-HT2A antagonist effect of nicotine [126]. If such a notion is true, it appears that the ability of 5-HT2A/C agonists to attenuate the SD effects of nicotine may be due to reversal of nicotine-induced 5-HT2A receptor antagonism, providing further support of a 5-HT mechanism in the stimulus effects of nicotine.
5.3. 5-HT3 receptors
A limited number of studies have failed to support a role for 5-HT3 receptors in the nicotine cue, as the 5-HT3 antagonists ICS-205930, MDL 72,222, and ondansetron each fail to alter nicotine-maintained responding [123, 127].
5.4. 5-HT6 receptors
One study [124] demonstrated that the 5-HT6 antagonist MS-245 does not substitute for nicotine alone. However, pretreatment with MS-245 prior to the ED50 dose of nicotine elicits full generalization, suggesting 5-HT6 antagonism augments the discriminative cue of a low nicotine dose [128].
6. γ-aminobutyric acid
There have been few investigations of a potential role for γ-aminobutyric acid (GABA) in the stimulus effects of nicotine. The benzodiazepine site GABAA positive modulator chlordiazepoxide, the GABAA agonist topiramate, and the GABAB agonist baclofen do not generalize to nicotine or alter its stimulus effects [26, 113, 129, 130; but see section 15 below for an interesting exception). While the noncompetitive GABAA antagonist picrotoxin does not elicit nicotine-appropriate responding in rats [26], the benzodiazepine site GABAA partial inverse agonist ethyl-β-carboline-3-carboxylate elicits partial or full substitution in squirrel monkeys trained to discriminate intravenous nicotine from saline [131]. Withdrawal from 21 days of chronic nicotine treatment elicits partial generalization to the discriminative stimulus effects of the GABAA antagonist pentylenetetrazole (PTZ) [132], although nicotine itself does not generalize to PTZ [133]. Interestingly, the substitution of nicotine withdrawal for PTZ is reversed by the GABAA positive modulator diazepam, but not mecamylamine [132]. These results suggest a relatively limited role of GABA in the cueing effect of nicotine, although the interoceptive cue of nicotine withdrawal may reflect, in part, a reduction in GABA transmission.
7. Glutamate
The role of glutamate has also not been addressed fully. The N–methyl-D-aspartate (NMDA) ionotropic glutamate receptor subtype channel blockers dextromethorphan (DXM), MK-801 and memantine, and the metabotropic 5 glutamate receptor (mGluR5) subtype antagonist MPEP, do not generalize to nicotine in substitution tests, although memantine and MPEP, but not MK-801 or DXM, produce a slight attenuation in responding controlled by the nicotine SD [76, 77, 134]. In contrast, using a CS procedure in nicotine-trained rats, MPEP produces no effect in substitution or interaction tests, although MK-801 blocks nicotine-evoked responding at doses that do not alter general activity [92]. Finally, nicotine does not generalize to an NMDA SD [135]. Thus, results to date do not appear to support a primary role for glutamate in the discriminative stimulus properties of nicotine, although NMDA and/or mGluR5 glutamate receptors may play a minor role. Further, the results reported in [92] suggest the possibility that NMDA receptors play a greater role in the CS properties of nicotine, although further work is needed before conclusions can be accepted fully.
8. Cannabinoids
The role of endogenous cannabinoids and the CB1 and CB2 cannabinoid receptor subtypes in the stimulus effects of nicotine has been addressed in several recent investigations.
8.1. Endogenous cannabinoids
The endogenous cannabinoid anandamide, the anandamide uptake/fatty acid amide hydrolase (FAAH) inhibitor AM-404, and the FAAH inhibitor URB 597 lack nicotine-like discriminative stimulus effects, although combinations of AM-404 + anandamide or URB 597 + anandamide produce modest leftward shifts in the nicotine dose-response curve [64]. On the other hand, in rats trained to discriminate the CB1 agonist Δ9-tetrahydrocannabinol (Δ9-THC; 3 mg/kg) from vehicle, a combination of nicotine (0.1–0.56 mg/kg) + URB-597 (0.3 mg/kg) evokes ~75% Δ9-THC-appropriate responding that is reversed by the CB1 antagonist/inverse agonist SR141716 (rimonabant) [136].
8.2. CB1/CB2 receptors
Three studies have shown that SR141716 does not substitute for, or alter, the SD effects of nicotine in nicotine-trained rats [64, 137, 138]. In contrast, SR141716 partially blocks conditioned responding evoked by a nicotine CS [139]. The non-selective cannabinoid CB1/2 receptor agonist CP 55,940 substitutes partially for the nicotine CS and enhances conditioned responding evoked by a lower nicotine dose. Interestingly, SR141716 dose-dependently attenuates the generalization of nicotine in d-amphetamine-trained rats without disrupting response rates [137]. In rats trained to discriminate Δ9-THC from vehicle, nicotine (0.1–0.56 mg/kg) alone does not elicit Δ9-THC-appropriate responding but a low 0.1 mg/kg dose of nicotine enhances the discriminative stimulus effects of Δ9-THC, an effect reversed by co-administration of either SR141716 or mecamylamine with nicotine [136]. Finally, the mixed CB1/2 agonists CP 55,940 and WIN 55,212, and the CB2 antagonist SR144528 lack nicotine-like discriminative stimulus properties in substitution tests [64]. Collectively, it appears that cannabinoid receptor activation does not produce a nicotine-like cue, but can augment the effects of nicotine.
9. Adenosine
The role of adenosine in nicotine discrimination has been examined using the nonselective adenosine receptor antagonist caffeine, as well as with ligands selective for the A1 and A2A adenosine receptor subtypes. In rats provided with normal drinking water or with drinking water containing caffeine, there are no differences in the rate of acquisition, nicotine dose-effects, or mecamylamine blockade of nicotine discrimination, although several DAergic compounds generalize to nicotine in rats with normal water, but not when caffeine is added [29]. Further, the putative DA release inhibitor CGS 10746B attenuates the nicotine cue in rats with normal water, but not in rats with caffeine added to the drinking water [29]. Chronic exposure to 0.25 mg/ml of caffeine, but not 1.0 mg/ml of caffeine, also enhances acquisition of learning to discriminate nicotine from saline [29]. In substitution tests in nicotine-trained rats, depending on the study, caffeine either lacks nicotine-like stimulus effects [140], substitutes partially [141] or fully [113]. Caffeine also potentiates the effects of a low nicotine dose in interaction tests [140]. Finally, the A1 adenosine receptor antagonist CPT and the A2A adenosine receptor antagonist MSX-3 also produce partial generalization to nicotine, while shifting the nicotine dose-response curve leftward [141].
10. Opioids
The role of opioid systems has not been addressed extensively. The nonselective opioid receptor agonist morphine does not substitute for nicotine [41, 111], and the µ opioid receptor antagonists naloxone and naltrexone do not block the nicotine cue [142]. In contrast, the stimulus effects of nicotine trained as a positive OS are fully blocked by the µ opioid receptor antagonist naloxone [13]. However, the doses required for such blockade (2 to 6 mg/kg) are higher than those typically used to block the stimulus effects of morphine, and leave open the possibility for a non-specific account of this antagonism. Further, rats can discriminate between the µ opioid receptor agonist fentanyl and nicotine [143], indicating that these drugs produce distinct discriminative stimulus effects.
11. Ion channel blockers
One study demonstrated that the dihydropyridine Ca2+ channel blocker isradipine reduces levels of nicotine-appropriate responding elicited by nicotine by ~50%, although effects on response rate were not reported [127].
12. Monoamine oxidase
Tobacco smoke also delivers psychoactive compounds that inhibit monoamine oxidase (monoamine oxidase inhibitors; MAOIs) in human smokers [144], and recent evidence has shown that MAOIs can dramatically increase nicotine self-administration in rodents [145–148]. Two studies have examined the discriminative stimulus effects of MAOIs in nicotine-trained rats [89, 149]. The selective MAOB inhibitor deprenyl produces a maximum of ~20% nicotine-appropriate responding in substitution testing (deprenyl was not examined in interaction tests; [89]). The selective MAOA inhibitor clorgyline, the selective MAOB inhibitor pargyline, and the nonselective MAOAB inhibitor phenelzine also do not evoke nicotine-appropriate responding (with the exception of 17 mg/kg of phenelzine, which produces ~43% nicotine-appropriate responding, although response rates were suppressed; [149]). In interaction testing, 10 mg/kg of phenelzine enhances the effects of a low 0.056 mg/kg dose of nicotine and prolongs the time course effect of the 0.3 mg/kg nicotine training dose [149]. Thus, these findings suggest that concurrent inhibition of each MAO isozyme can potentiate the nicotine cue, although the underlying mechanisms are unknown.
13. Neuroanatomical mediation of the nicotine cue
Drug discrimination has also been used to elucidate the brain regions mediating the stimulus effects of nicotine. The involvement of cholinergic-innervated regions such as dorsal hippocampus (HPC), mesencephalic reticular formation (MRF), and medial habenula (mHB) in the nicotine cue has been investigated. Local infusion of nicotine into the MRF or HPC substitutes partially for nicotine, and systemic mecamylamine eliminates completely the substitution of intra-HPC nicotine and attenuates the substitution by intra-MRF nicotine [150]. Intra-HPC administration of nicotine substitutes in rats trained to discriminate systemic nicotine [151]. Interestingly, substitution for intra-HPC nicotine is obtained in normal rats, but not in rats treated with 6-hydroxydopamine as neonates [152]. In contrast, others have found no substitution of intra-HPC nicotine [153, 154]. Those latter studies also reported that intra-HPC mecamylamine fails to block responding controlled by the systemic nicotine cue. Finally, nicotine infused into the mHB fails to elicit nicotine-appropriate responding [155].
The involvement of DA neurons and terminal fields in the ventral tegmental area (VTA), nucleus accumbens (NAcc), and medial prefrontal cortex (mPFC) have also been investigated. Intra-VTA nicotine does not evoke nicotine-appropriate responding and intra-VTA mecamylamine does not block responding controlled by the systemic nicotine cue in one report [153], although partial substitution with intra-VTA nicotine was obtained in another report [154]. Partial to full substitution for systemic nicotine is obtained with intra-NAcc nicotine [153, 154, 156], and intra-NAcc mecamylamine blocks responding controlled by systemic nicotine [153, 156]. However, intra-NAcc nicotine did not substitute for nicotine in another report [151]. Finally, intra-mPFC nicotine has been shown to substitute for systemic nicotine [155].
Together, it appears that the MRF, VTA, NAcc and mPFC, but not HPC or mHB, play a role in mediating the cueing effects of nicotine in operant procedures. The reasons for the discrepant findings among some studies are unknown, although it is possible that differences in the nicotine training doses used, and/or subtle variations in the location of brain infusion sites, may play a role. The brain regions mediating the Pavlovian CS properties of nicotine also warrant investigation.
14. Individual differences
There is a large body of literature indicating that individual difference variables play important roles in drug abuse vulnerability [157–159]. However, little work has been conducted to examine the potential for these variables to influence the interoceptive stimulus properties of drugs. Some evidence suggests that the drug discrimination procedure is amenable to studying individual differences. For example, genetic factors play a role, as Lewis rats acquire a nicotine-saline discrimination at a dose of 0.4 mg/kg, whereas a higher dose of nicotine (0.9 mg/kg) is needed with Fischer-344 rats; in addition, the ED50 value for nicotine generalization is lower for Lewis rats than for Fischer-344 rats [160]. There is also evidence that nicotine is generalized to an ethanol stimulus in alcohol-preferring rats to a greater extent than in alcohol-nonpreferring rats [156]. There is also evidence that inbred C57BL/6 mice are more sensitive to nicotine than DBA/2 mice [162]. Regarding sex differences, nicotine substitutes for a PTZ stimulus more readily in male and ovariectomized female rats than in intact female rats [163]. Further, environmental differences are also important, as preliminary results suggest that rats raised in an enriched condition (EC) are less sensitive to the discriminative stimulus effects of nicotine compared to rats raised in an impoverished condition (IC), and that EC rats are more sensitive to mecamylamine attenuation of the nicotine cue than IC rats [164]. Given that IC rats also showed a greater density of [125I]epibatidine-sensitive nAChR binding sites in the VTA [164], it is possible that individual differences in nAChR expression could predict sensitivity to nicotine’s SD effects. Based on recent findings showing that baseline expression of α4β2* nAChRs predicts motivation to self-administer nicotine under a PR schedule in squirrel monkeys [165], future research should also attempt to address the potential role of differential baseline expression of nAChR subtypes in acquisition of, and/or sensitivity to, nicotine’s interoceptive stimulus properties.
15. Functional rather than pharmacological substitution
As described previously, an important strength of drug discrimination as a tool for studying neuropharmacological processes is that control of responding is relatively specific to the training drug. For instance, when nicotine is trained as a feature positive OS, neither amphetamine nor chlordiazepoxide substitutes for the nicotine stimulus [113]. Notably, recent research has shown that substitution crosses pharmacological classes and occurs as a result of shared learning history [114]. In that latter study, rats had nicotine trained as a feature positive OS to indicate when a discrete 15-sec cue (e.g., illumination of a light) would be followed by sucrose. In the same rats, chlordiazepoxide signaled when a separate and distinct stimulus (e.g., white noise) would be followed by sucrose. In the placebo (saline) state, the white noise and the light were presented but never reinforced. Not surprisingly, under these conditions, the discrete stimulus evoked a robust conditioned response when tested in the drug state in which it had been previously reinforced (i.e., nicotine:light and chlordiazepoxide:noise in this example). More importantly, the ability to evoke responding transferred to the other stimulus. Thus, nicotine is able to substitute for chlordiazepoxide, and vice versa, with the discrete stimulus evoking ~100% conditioned responding that it had never been trained with. Notably, this transfer is not the result of intermixed training with 2 different drug states producing a drug versus no-drug discrimination, as no responding is seen in substitution tests with amphetamine. Future research will need to examine the generality of such a finding, as well as the necessary learning conditions to establish generalization across drug classes. For instance, a similar outcome does not appear to occur when two drug states (rather than placebo) are used in the two-lever drug discrimination task. Perhaps having the incorrect lever present on all sessions with its stimulus and response controlling properties under extinction prevents such an effect. Alternatively, does the same US (sucrose in [114]) need to be used for both drug states trained as a positive feature, or is it sufficient to use a US within the same class of outcomes (appetitive or aversive)?
16. Summary and future directions
The drug discrimination procedure has been an important tool for elucidating the receptor mechanisms that mediate the interoceptive effects of nicotine. The critical initial transduction mechanism of the nicotine cue is activation of high-affinity β2 subunit nAChRs, although it appears that α4, rather than α6, is the complementary subunit involved; a role for other nAChR subunits has yet to emerge. However, since activation of α4β2* nAChRs modulates the activity of many neurotransmitter systems [166], it seems likely that the mechanisms underlying the interoceptive stimulus effects of nicotine are complex. Accordingly, recent findings suggest that adenosine, cannabinoid, and 5-HT systems are important elements of the nicotine cue; in addition, dopamine and glutamate may also be involved. Ongoing technical advances in the use of neurochemical techniques such as fast-scan cyclic voltammetry or rapid microdialysis could perhaps eventually be used to measure behavior and neurotransmitter fluctuations concurrently as a way of further refining our understanding of the neural correlates mediating the stimulus effects of nicotine.
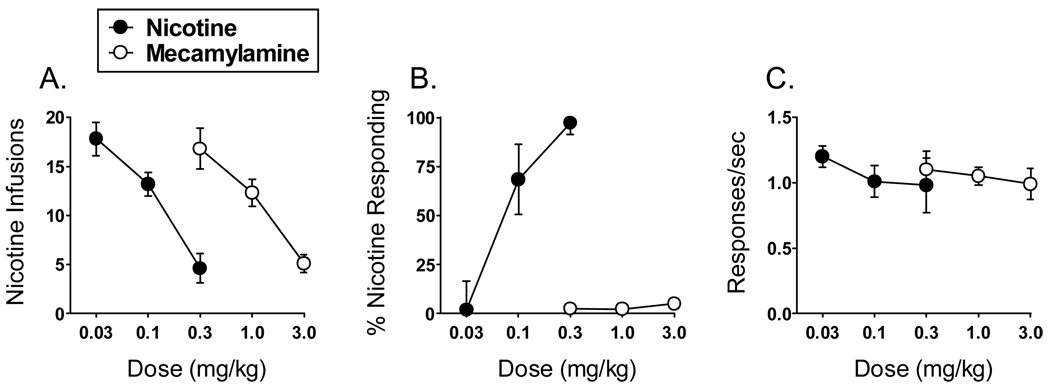
The drug discrimination procedure has also been employed in medication development. A particularly useful application of the procedure has been the characterization of nAChR agonists and antagonists for the treatment of tobacco dependence. For example, rats pretreated with a nAChR agonist (i.e., nicotine) or nAChR antagonist (i.e., mecamylamine) show similar decreases in rates of nicotine self-administration; thus, a drug that decreases nicotine self-administration may due so by acting either as a “substitute” agonist or as a “blocking” antagonist. Thus, it is important to also evaluate novel nAChR ligands in the drug discrimination procedure in order to identify the mechanism underlying decreases in nicotine intake. As shown in Fig. (1), drug discrimination dissociates the effects of nAChR agonists and antagonists more readily than self-administration (M.T. Bardo and T.E. Wooters, unpublished observations); nicotine and mecamylamine each decrease the number of nicotine infusions earned in a dose-related manner (Fig. 1A), whereas only nicotine elicits a dose-dependent increase in nicotine-appropriate responding in rats trained to discriminate nicotine from saline (Fig. 1B). Interestingly, however, compounds from several drug classes that do not alter the SD effects of nicotine have been shown to reduce nicotine self-administration. Notable examples of this phenomenon include the DA antagonists SCH23390 and spiperone [86], the CB1 receptor antagonist/inverse agonist SR141716 [137] (but see [139]), the GABAB agonist baclofen [177] and the putative α6β2 nAChR antagonists bPiDI and bPiDDB [21, 72, 74]. A number of other drugs that either generalize to or augment the SD effects of nicotine have been shown to increase nicotine self-administration; these include bupropion [167], GBR-12909 [168], methamphetamine [167], methylphenidate [109] and nonselective MAO inhibitors [145, 148]. Thus, while the SD and reinforcing effects of nicotine are clearly dissociable, drugs that produce nicotine-like interoceptive effects appear to reliably increase nicotine self-administration, although this notion requires further evaluation.
Fig. (1).
Effects of the nAChR agonist nicotine and the nAChR antagonist mecamylamine on nicotine self-administration and nicotine discrimination. Panel A demonstrates that nicotine (0.03–0.3 mg/kg, SC; 15-min pretreatment interval [PTI]) and mecamylamine (0.3–3.0 mg/kg, SC; 15-min PTI) both decrease the (mean ± SEM) number of nicotine (0.03 mg/kg/infusion) infusions earned in rats (n = 6) trained to self-administer nicotine under a fixed ratio 5 (FR5) reinforcement schedule during daily 60-min sessions. Panel B demonstrates that nicotine (0.03–0.3 mg/kg, SC; 5-min PTI), but not mecamylamine (0.3–3.0 mg/kg, SC; 15-min PTI), produces a dose-dependent increase in the percentage of nicotine-appropriate responses in rats (n = 6) trained to discriminate nicotine (0.3 mg/kg, SC) from saline under a FR10 reinforcement schedule during daily 15-min sessions. Panel C illustrates the effects of nicotine and mecamylamine on response rates (responses/sec) corresponding to the data presented in panel B.
Drug discrimination techniques may also prove amenable to investigate of the subjective effects of nicotine withdrawal. Drug discrimination has been used widely to characterize the discriminative effects of withdrawal from d-amphetamine [169], benzodiazepines [170, 171], opioids [172, 173], and Δ9-THC [174]. Although studies of nicotine dependence/withdrawal have relied primarily on somatic (e.g., somatic signs) or affective (e.g., conditioned place aversion, intracranial self-stimulation) measures, it seems plausible that rats could learn to discriminate injections of a nAChR antagonist (e.g., mecamylamine) following acute nicotine pretreatment or during chronic nicotine exposure. In fact, rats are able to discriminate mecamylamine following acute pretreatment with nicotine, but not saline [51]. In addition, the partial α4β2 nAChR agonist SSR591813 substitutes partially for mecamylamine, and attenuates the mecamylamine cue, when administered after nicotine and prior to mecamylamine [51]. It would be of interest to determine whether clinically-available smoking cessation agents (e.g., bupropion and varenicline) also attenuate the discriminative stimulus effects of nicotine withdrawal, and whether or not such results predict a compound’s ability to alter nicotine self-administration. Such a procedure could potentially provide further insight into the receptor mechanisms involved in nicotine withdrawal. Finally, the involvement of the corticotrophin-releasing factor [175] and hypocretin/orexin [176] systems in nicotine reinforcement has been demonstrated recently; thus, the possibility that such systems also mediate the interoceptive effects of nicotine could help to clarify the nature of the relation between the interoceptive and reinforcing properties of nicotine.
Acknowledgements
The authors received support from USPHS grants F31 DA023853, R21 DA023951, R01 DA018114, and U19 DA017548 while preparing the manuscript. We acknowledge the assistance of Anthony S. Rauhut, Ph.D., for collecting some of the data presented herein, and Dustin J. Stairs, Ph.D., for sharing recent findings from his laboratory.
References
- 1.Appel JB, West WB, Buggy J. LSD, 5-HT (serotonin), and the evolution of a behavioral assay. Neurosci Biobehav Rev. 2004;27:693–701. doi: 10.1016/j.neubiorev.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Balster RL. Drug abuse potential evaluation in animals. Br J Addict. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson T, Pickens R. Stimulus properties of drugs. New York: Plenum Press; 1971. [Google Scholar]
- 4.Overton DA. In: Drug discrimination: applications to drug abuse research. Glennon RA, Jarbe TUC, Frankenheim J, editors. Washington, DC: U.S. DHHS; 1991. pp. 5–24. [Google Scholar]
- 5.Colpaert FC. In: Methods of assessing the reinforcing properties of abused drugs. Bozarth MA, editor. New York: Springer-Verlag; 1987. pp. 341–372. [Google Scholar]
- 6.Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 7.Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1196. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- 8.Stolerman IP. Drug discrimination. In: van Haaren F, editor. Methods in behavioral pharmacology. Amsterdam: Elsevier; 1993. pp. 217–243. [Google Scholar]
- 9.Bevins RA. Altering the motivational function of nicotine through conditioning processes. Nebr Symp Motiv. 2009;55:111–129. doi: 10.1007/978-0-387-78748-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (“goal tracking”) in rats. Learn Motiv. 1979;10:295–312. [Google Scholar]
- 11.Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- 12.Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM. Characterization of nicotine's ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology (Berl) 2006;184:470–481. doi: 10.1007/s00213-005-0079-3. [DOI] [PubMed] [Google Scholar]
- 13.Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol. 2004;15:183–194. [PubMed] [Google Scholar]
- 14.Kamien JB, Bickel WK, Hughes JR, Higgins ST, Smith BJ. Drug discrimination by humans compared to nonhumans: current status and future directions. Psychopharmacology (Berl) 1993;111:259–270. doi: 10.1007/BF02244940. [DOI] [PubMed] [Google Scholar]
- 15.Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol. 2009:295–333. doi: 10.1007/978-3-540-69248-5_11. [DOI] [PubMed] [Google Scholar]
- 16.Morrison CF, Stephenson JA. Nicotine injections as the conditioned stimulus in discrimination learning. Psychopharmacologia. 1969;15:351–360. doi: 10.1007/BF00403710. [DOI] [PubMed] [Google Scholar]
- 17.Rosecrans JA. Nicotine as a discriminative stimulus to behavior: its characterization and relevance to smoking behavior. NIDA Res Monogr. 1979:58–69. [PubMed] [Google Scholar]
- 18.Schechter MD, Rosecrans JA. C.N.S. effect of nicotine as the discriminative stimulus for the rat in a T-maze. Life Sci. 1971;10:821–832. doi: 10.1016/0024-3205(71)90037-3. [DOI] [PubMed] [Google Scholar]
- 19.Quarta D, Naylor CG, Barik J, Fernandes C, Wonnacott S, Stolerman IP. Drug discrimination and neurochemical studies in alpha7 null mutant mice: tests for the role of nicotinic alpha7 receptors in dopamine release. Psychopharmacology (Berl) 2009;203:399–410. doi: 10.1007/s00213-008-1281-x. [DOI] [PubMed] [Google Scholar]
- 20.Damaj MI, Glassco W, Marks MJ, et al. Pharmacological investigation of (+)- and (−)-cis-2,3,3a,4,5,9b–hexahydro-1-methyl-1H–pyrrolo-[3,2-h]isoq uinoline, a bridged-nicotine analog. J Pharmacol Exp Ther. 1997;282:1425–1434. [PubMed] [Google Scholar]
- 21.Dwoskin LP, Wooters TE, Sumithran SP, et al. N,N'-Alkane-diyl-bis-3-picoliniums as nicotinic receptor antagonists: inhibition of nicotine-evoked dopamine release and hyperactivity. J Pharmacol Exp Ther. 2008;326:563–576. doi: 10.1124/jpet.108.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Ann Rev Pharmacol Toxicol. 2007;47:699–727. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 23.Desai RI, Barber DJ, Terry P. Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behav Pharmacol. 1999;10:647–656. doi: 10.1097/00008877-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Damaj MI, Creasy KR, Grove AD, Rosecrans JA, Martin BR. Pharmacological effects of epibatidine optical enantiomers. Brain Res. 1994;664:34–40. doi: 10.1016/0006-8993(94)91950-x. [DOI] [PubMed] [Google Scholar]
- 25.Hazell P, Peterson DW, Laverty R. Brief communication. Inability of hexamethonium to block the discriminative stimulus (SD) property of nicotine. Pharmacol Biochem Behav. 1978;9:137–140. doi: 10.1016/0091-3057(78)90025-4. [DOI] [PubMed] [Google Scholar]
- 26.Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- 27.Stolerman IP, Kumar R, Reavill C. Discriminative stimulus effects of cholinergic agonists and the actions of their antagonists. Psychopharmacol Ser. 1988;4:32–43. doi: 10.1007/978-3-642-73223-2_3. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Reavill C, Stolerman IP. Nicotine cue in rats: effects of central administration of ganglion-blocking drugs. Br J Pharmacol. 1987;90:239–246. doi: 10.1111/j.1476-5381.1987.tb16845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasior M, Shoaib M, Yasar S, Jaszyna M, Goldberg SR. Acquisition of nicotine discrimination and discriminative stimulus effects of nicotine in rats chronically exposed to caffeine. J Pharmacol Exp Ther. 1999;288:1053–1073. [PubMed] [Google Scholar]
- 30.Murray JE, Bevins RA. Acquired appetitive responding to intravenous nicotine reflects a Pavlovian conditioned association. Behav Neurosci. 2009;123:97–108. doi: 10.1037/a0013735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosecrans JA. Nicotine as a discriminative stimulus: a neurobiobehavioral approach to studying central cholinergic mechanisms. J Subst Abuse. 1989;1:287–300. [PubMed] [Google Scholar]
- 32.Jung M, Costa L, Shearman GT, Kelly PH. Discriminative stimulus properties of muscarinic agonists. Psychopharmacology (Berl) 1987;93:139–145. doi: 10.1007/BF00179923. [DOI] [PubMed] [Google Scholar]
- 33.Locke KW, Gorney B, Cornfeldt M, Fielding S. Characterization of the discriminative stimulus effects of physostigmine in the rat. J Pharmacol Exp Ther. 1989;250:241–246. [PubMed] [Google Scholar]
- 34.Meltzer LT, Rosecrans JA. Discriminative stimulus properties of arecoline: a new approach for studying central muscarinic receptors. Psychopharmacology (Berl) 1981a;75:383–387. doi: 10.1007/BF00435858. [DOI] [PubMed] [Google Scholar]
- 35.Jung M, Perio A, Worms P, Soubrie P. Pharmacological characterization of the physostigmine stimulus in rats. Psychopharmacology (Berl) 1988;95:553–555. doi: 10.1007/BF00172975. [DOI] [PubMed] [Google Scholar]
- 36.Tang AH, Franklin SR. Discriminative stimulus properties of physostigmine in rats. Eur J Pharmacol. 1988;153:97–104. doi: 10.1016/0014-2999(88)90592-4. [DOI] [PubMed] [Google Scholar]
- 37.Meltzer LT, Rosecrans JA. Nicotine and arecoline as discriminative stimuli: involvement of a non-cholinergic mechanism for nicotine. Pharmacol Biochem Behav. 1988;29:587–593. doi: 10.1016/0091-3057(88)90024-x. [DOI] [PubMed] [Google Scholar]
- 38.Bardo MT, Bevins RA, Klebaur JE, Crooks PA, Dwoskin LP. (−)-Nornicotine partially substitutes for (+)-amphetamine in a drug discrimination paradigm in rats. Pharmacol Biochem Behav. 1997;58:1083–1087. doi: 10.1016/s0091-3057(97)00303-1. [DOI] [PubMed] [Google Scholar]
- 39.Brioni JD, Kim DJ, O'Neill AB, Williams JE, Decker MW. Clozapine attenuates the discriminative stimulus properties of (−)-nicotine. Brain Res. 1994;643:1–9. doi: 10.1016/0006-8993(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 40.Chance WT, Kallman MD, Rosecrans JA, Spencer RM. A comparison of nicotine and structurally related compounds as discriminative stimuli. Br J Pharmacol. 1978;63:609–616. doi: 10.1111/j.1476-5381.1978.tb17273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl) 1989;97:295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- 42.Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C. Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology (Berl) 1983;81:54–60. doi: 10.1007/BF00439274. [DOI] [PubMed] [Google Scholar]
- 43.Reavill C, Spivak CE, Stolerman IP, Waters JA. Isoarecolone can inhibit nicotine binding and produce nicotine-like discriminative stimulus effects in rats. Neuropharmacology. 1987;26:789–792. doi: 10.1016/0028-3908(87)90244-9. [DOI] [PubMed] [Google Scholar]
- 44.Reavill C, Waters JA, Stolerman IP, Garcha HS. Behavioural effects of the nicotinic agonists N-(3-pyridylmethyl)pyrrolidine and isoarecolone in rats. Psychopharmacology (Berl) 1990;102:521–528. doi: 10.1007/BF02247135. [DOI] [PubMed] [Google Scholar]
- 45.Stolerman IP, Albuquerque EX, Garcha HS. Behavioural effects of anatoxin, a potent nicotinic agonist, in rats. Neuropharmacology. 1992;31:311–314. doi: 10.1016/0028-3908(92)90182-o. [DOI] [PubMed] [Google Scholar]
- 46.Takada K, Swedberg MD, Goldberg SR, Katz JL. Discriminative stimulus effects of intravenous l-nicotine and nicotine analogs or metabolites in squirrel monkeys. Psychopharmacology (Berl) 1989;99:208–212. doi: 10.1007/BF00442809. [DOI] [PubMed] [Google Scholar]
- 47.Chandler CJ, Stolerman IP. Discriminative stimulus properties of the nicotinic agonist cytisine. Psychopharmacology (Berl) 1997;129:257–264. doi: 10.1007/s002130050188. [DOI] [PubMed] [Google Scholar]
- 48.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- 49.Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- 50.Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 51.Cohen C, Bergis OE, Galli F, et al. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J Pharmacol Exp Ther. 2003;306:407–420. doi: 10.1124/jpet.103.049262. [DOI] [PubMed] [Google Scholar]
- 52.Craft RM, Howard JL. Cue properties of oral and transdermal nicotine in the rat. Psychopharmacology (Berl) 1988;96:281–284. doi: 10.1007/BF00216050. [DOI] [PubMed] [Google Scholar]
- 53.LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91:461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Brioni JD, Kim DJ, Brodie MS, Decker MW, Arneric SP. ABT-418: discriminative stimulus properties and effect on ventral tegmental cell activity. Psychopharmacology (Berl) 1995;119:368–375. doi: 10.1007/BF02245851. [DOI] [PubMed] [Google Scholar]
- 56.Smith JW, Mogg A, Tafi E, et al. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- 57.Xiao Y, Woolverton WL, Sahibzada N, Yasuda RP, Kellar KJ. Pharmacological properties of sazetidine-A, a desensitizer of alpha4beta2 nicotinic acetylcholine receptors. San Diego: Neuroscience Meeting Planner; 2007. Program No. 574.6/J11. [Google Scholar]
- 58.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It’s not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meltzer LT, Rosecrans JA, Aceto MD, Harris LS. Discriminative stimulus properties of the optical isomers of nicotine. Psychopharmacology (Berl) 1980;68:283–286. doi: 10.1007/BF00428116. [DOI] [PubMed] [Google Scholar]
- 60.Martin BR, Aceto MD. Nicotine binding sites and their localization in the central nervous system. Neurosci Biobehav Rev. 1981;5:473–478. doi: 10.1016/0149-7634(81)90017-8. [DOI] [PubMed] [Google Scholar]
- 61.Gommans J, Stolerman IP, Shoaib M. Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology. 2000;39:2840–2847. doi: 10.1016/s0028-3908(00)00130-1. [DOI] [PubMed] [Google Scholar]
- 62.Shoaib M, Zubaran C, Stolerman IP. Antagonism of stimulus properties of nicotine by dihydro-beta-erythroidine (DHbetaE) in rats. Psychopharmacology (Berl) 2000;149:140–146. doi: 10.1007/s002139900348. [DOI] [PubMed] [Google Scholar]
- 63.Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharmacology (Berl) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- 64.Zaniewska M, McCreary AC, Przegalinski E, Filip M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2006;540:96–106. doi: 10.1016/j.ejphar.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 65.Mansbach RS, Chambers LK, Rovetti CC. Effects of the competitive nicotinic antagonist erysodine on behavior occasioned or maintained by nicotine: comparison with mecamylamine. Psychopharmacology (Berl) 2000;148:234–242. doi: 10.1007/s002130050047. [DOI] [PubMed] [Google Scholar]
- 66.Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grady SR, Salminen O, Laverty DC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 69.Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- 70.Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- 71.Pons S, Fattore L, Cossu G, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology (Berl) 2006;184:426–434. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- 73.Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. The effects of a novel nicotinic receptor antagonist N,N-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB) on acute and repeated nicotine-induced increases in extracellular dopamine in rat nucleus accumbens. Neuropharmacology. 2007;52:755–763. doi: 10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 74.Wooters TE, Ross JT, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effects of the nicotinic acetylcholine receptor antagonist, N,N’-decane-1,10-diyl-bis-3-picolinium diiodide, on nicotine self-administration and food-maintained responding in rats. College on the Problems of Drug Dependence Program Book. 2008:13. [Google Scholar]
- 75.Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA. Nicotinic acetylcholine antagonism of the conditional stimulus effects of nicotine. Eur J Pharmacol. doi: 10.1016/j.pbb.2009.09.012. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wright MJ, Jr, Vann RE, Gamage TF, Damaj MI, Wiley JL. Comparative effects of dextromethorphan and dextrorphan on nicotine discrimination in rats. Pharmacol Biochem Behav. 2006;85:507–513. doi: 10.1016/j.pbb.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zakharova ES, Danysz W, Bespalov AY. Drug discrimination analysis of NMDA receptor channel blockers as nicotinic receptor antagonists in rats. Psychopharmacology (Berl) 2005;179:128–135. doi: 10.1007/s00213-004-2067-4. [DOI] [PubMed] [Google Scholar]
- 78.Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor alpha 7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–371. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 79.van Haaren F, Anderson KG, Haworth SC, Kem WR. GTS-21, a mixed nicotinic receptor agonist/antagonist, does not affect the nicotine cue. Pharmacol Biochem Behav. 1999;64:439–444. doi: 10.1016/s0091-3057(99)00054-4. [DOI] [PubMed] [Google Scholar]
- 80.Brioni JD, Kim DJ, O'Neill AB. Nicotine cue: lack of effect of the alpha 7 nicotinic receptor antagonist methyllycaconitine. Eur J Pharmacol. 1996;301:1–5. doi: 10.1016/0014-2999(96)00010-6. [DOI] [PubMed] [Google Scholar]
- 81.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 82.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 83.Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- 84.Palmatier MI, Bevins RA. Examination of GABAergic and dopaminergic compounds in the acquisition of nicotine-conditioned hyperactivity in rats. Neuropsychobiology. 2002;45:87–94. doi: 10.1159/000048682. [DOI] [PubMed] [Google Scholar]
- 85.Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 1991;104:171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- 87.Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 88.Shoaib M. Is dopamine important in nicotine dependence? J Physiol Paris. 1998;92:229–233. doi: 10.1016/s0928-4257(98)80024-7. [DOI] [PubMed] [Google Scholar]
- 89.Mansbach RS, Rovetti CC, Freedland CS. The role of monoamine neurotransmitter systems in the nicotine discriminative stimulus. Drug Alcohol Depend. 1998;52:125–134. doi: 10.1016/s0376-8716(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 90.Reavill C, Stolerman IP. Interaction of nicotine with dopaminergic mechanisms assessed through drug discrimination and rotational behaviour in rats. J Psychopharmacol. 1987;1:264–273. doi: 10.1177/026988118700100408. [DOI] [PubMed] [Google Scholar]
- 91.Corrigall WA, Coen KM. Dopamine mechanisms play at best a small role in the nicotine discriminative stimulus. Pharmacol Biochem Behav. 1994;48:817–820. doi: 10.1016/0091-3057(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 92.Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561:91–94. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schechter MD, Meehan SM. Dopaminergic mediation of the stimulant generalization of nicotine. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:835–845. doi: 10.1016/0278-5846(93)90064-y. [DOI] [PubMed] [Google Scholar]
- 94.Gatch MB, Flores E, Forster MJ. Nicotine and methamphetamine share discriminative stimulus effects. Drug Alcohol Depend. 2008;93:63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- 96.de la Garza R, Johanson CE. Discriminative stimulus properties of cocaine in pigeons. Psychopharmacology (Berl) 1985;85:23–30. doi: 10.1007/BF00427317. [DOI] [PubMed] [Google Scholar]
- 97.Takada K, Hagen TJ, Cook JM, Goldberg SR, Katz JL. Discriminative stimulus effects of intravenous nicotine in squirrel monkeys. Pharmacol Biochem Behav. 1988;30:243–247. doi: 10.1016/0091-3057(88)90452-2. [DOI] [PubMed] [Google Scholar]
- 98.Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 2003;167:335–343. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- 99.Reichel CM, Linkugel JD, Bevins RA. Nicotine as a conditioned stimulus: impact of attention-deficit/hyperactivity disorder medications. Exp Clin Psychopharmacol. 2007;15:501–509. doi: 10.1037/1064-1297.15.5.501. [DOI] [PubMed] [Google Scholar]
- 100.Damaj MI, Slemmer JE, Carroll FI, Martin BR. Pharmacological characterization of nicotine's interaction with cocaine and cocaine analogs. J Pharmacol Exp Ther. 1999;289:1229–1236. [PubMed] [Google Scholar]
- 101.Cunningham CS, Polston JE, Jany JR, Segert IL, Miller DK. Interaction of lobeline and nicotinic receptor ligands with the discriminative stimulus properties of cocaine and amphetamine. Drug Alcohol Depend. 2006;84:211–222. doi: 10.1016/j.drugalcdep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 102.Garza RD, Johanson CE. The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav. 1983;19:145–148. doi: 10.1016/0091-3057(83)90323-4. [DOI] [PubMed] [Google Scholar]
- 103.Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- 104.Wilkinson JL, Li C, Bevins RA. Pavlovian drug discrimination with bupropion as a feature positive occasion setter: substitution by methamphetamine and nicotine, but not cocaine. Addict Biol. 2009;14:165–173. doi: 10.1111/j.1369-1600.2008.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiley JL, Lavecchia KL, Martin BR, Damaj MI. Nicotine-like discriminative stimulus effects of bupropion in rats. Exp Clin Psychopharmacol. 2002;10:129–135. doi: 10.1037//1064-1297.10.2.129. [DOI] [PubMed] [Google Scholar]
- 106.Young R, Glennon RA. Nicotine and bupropion share a similar discriminative stimulus effect. Eur J Pharmacol. 2002;443:113–118. doi: 10.1016/s0014-2999(02)01554-6. [DOI] [PubMed] [Google Scholar]
- 107.Shoaib M, Sidhpura N, Shafait S. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology (Berl) 2003;165:405–412. doi: 10.1007/s00213-002-1277-x. [DOI] [PubMed] [Google Scholar]
- 108.Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003;474:85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- 109.Wooters TE, Neugebauer NM, Rush CR, Bardo MT. Methylphenidate enhances the abuse-related behavioral effects of nicotine in rats: intravenous self-administration, drug discrimination, and locomotor cross-sensitization. Neuropsychopharmacology. 2008a;33:1137–1148. doi: 10.1038/sj.npp.1301477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chance WT, Murfin D, Krynock GM, Rosecrans JA. A description of the nicotine stimulus and tests of its generalization to amphetamine. Psychopharmacology (Berl) 1977;55:19–26. doi: 10.1007/BF00432812. [DOI] [PubMed] [Google Scholar]
- 111.Stolerman IP. Discriminative stimulus effects of nicotine in rats trained under different schedules of reinforcement. Psychopharmacology (Berl) 1989;97:131–138. doi: 10.1007/BF00443427. [DOI] [PubMed] [Google Scholar]
- 112.Varvel SA, James JR, Bowen S, Rosecrans JA, Karan LD. Discriminative stimulus (DS) properties of nicotine in the C57BL/6 mouse. Pharmacol Biochem Behav. 1999;63:27–32. doi: 10.1016/s0091-3057(98)00262-7. [DOI] [PubMed] [Google Scholar]
- 113.Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology. 2005;30:731–741. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- 114.Palmatier MI, Bevins RA. Occasion setting by drug states: Functional equivalence following similar training history. Behav Brain Res. 2008;195:260–270. doi: 10.1016/j.bbr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ho BT, Huang JT. Role of dopamine in d-amphetamine-induced discriminative responding. Pharmacol Biochem Behav. 1975;3:1085–1092. doi: 10.1016/0091-3057(75)90021-0. [DOI] [PubMed] [Google Scholar]
- 116.Schechter MD, Rosecrans JA. D-amphetamine as a discriminative cue: drugs with similar stimulus properties. Eur J Pharmacol. 1973;21:212–216. doi: 10.1016/0014-2999(73)90228-8. [DOI] [PubMed] [Google Scholar]
- 117.Druhan JP, Fibiger HC, Phillips AG. Influence of some drugs of abuse on the discriminative stimulus properties of amphetamine. Behav Pharmacol. 1991;2:391–393. [PubMed] [Google Scholar]
- 118.Schechter MD, Rosecrans JA. Nicotine as a discriminative stimulus in rats depleted of norepinephrine or 5-hydroxytryptamine. Psychopharmacologia. 1972;24:417–429. doi: 10.1007/BF00402536. [DOI] [PubMed] [Google Scholar]
- 119.Zaniewska M, McCreary AC, Sezer G, Przegalinski E, Filip M. Effects of agmatine on nicotine-evoked behavioral responses in rats. Pharmacol Rep. 2008;60:645–654. [PubMed] [Google Scholar]
- 120.Stolerman IP, Pratt JA, Garcha HS, Giardini V, Kumar R. Nicotine cue in rats analysed with drugs acting on cholinergic and 5-hydroxytryptamine mechanisms. Neuropharmacology. 1983;22:1029–1037. doi: 10.1016/0028-3908(83)90021-7. [DOI] [PubMed] [Google Scholar]
- 121.Shoaib M, Baumann MH, Rothman RB, Goldberg SR, Schindler CW. Behavioural and neurochemical characteristics of phentermine and fenfluramine administered separately and as a mixture in rats. Psychopharmacology (Berl) 1997;131:296–306. doi: 10.1007/s002130050296. [DOI] [PubMed] [Google Scholar]
- 122.Batman AM, Munzar P, Beardsley PM. Attenuation of nicotine's discriminative stimulus effects in rats and its locomotor activity effects in mice by serotonergic 5-HT2A/2C receptor agonists. Psychopharmacology (Berl) 2005;179:393–401. doi: 10.1007/s00213-004-2035-z. [DOI] [PubMed] [Google Scholar]
- 123.Stolerman IP, Garcha HS. Failure of 5-HT3 antagonists and other drugs to block the nicotine discriminative stimulus. In: Harris LS, editor. Problems of Drug Dependence 1994, NIDA Research Monograph 141. Rockville: U.S. Department of Health and Human Services; p. 67. [Google Scholar]
- 124.Zaniewska M, McCreary AC, Przegalinski E, Filip M. Effects of the serotonin 5-HT2A and 5-HT2C receptor ligands on the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2007;571:156–165. doi: 10.1016/j.ejphar.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 125.Quarta D, Naylor CG, Stolerman IP. The serotonin 2C receptor agonist Ro-60-0175 attenuates effects of nicotine in the five-choice serial reaction time task and in drug discrimination. Psychopharmacology (Berl) 2007;193:391–402. doi: 10.1007/s00213-007-0802-3. [DOI] [PubMed] [Google Scholar]
- 126.Hayslett RL, Tizabi Y. Effects of donepezil, nicotine and haloperidol on the central serotonergic system in mice: implications for Tourette’s syndrome. Pharmacol Biochem Behav. 2005;81:879–886. doi: 10.1016/j.pbb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 127.Schechter MD, Meehan SM. Further evidence for the mechanisms that may mediate nicotine discrimination. Pharmacol Biochem Behav. 1992;41:807–812. doi: 10.1016/0091-3057(92)90231-4. [DOI] [PubMed] [Google Scholar]
- 128.Young R, Bondareva T, Wesolowska A, Young S, Glennon RA. Effect of the 5-HT(6) serotonin antagonist MS-245 on the actions of (−)nicotine. Pharmacol Biochem Behav. 2006;85:170–177. doi: 10.1016/j.pbb.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 129.Le Foll B, Wertheim CE, Goldberg SR. Effects of baclofen on conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neurosci Lett. 2008a;443:236–240. doi: 10.1016/j.neulet.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Le Foll B, Justinova Z, Wertheim CE, Barnes C, Goldberg SR. Topiramate does not alter nicotine or cocaine discrimination in rats. Behav Pharmacol. 2008b;19:13–20. doi: 10.1097/FBP.0b013e3282f3cf84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takada K, Hagen TJ, Cook JM, Goldberg SR, Katz JL. Discriminative stimulus effects of intravenous nicotine in squirrel monkeys. Pharmacol Biochem Behav. 1988;30:243–247. doi: 10.1016/0091-3057(88)90452-2. [DOI] [PubMed] [Google Scholar]
- 132.Harris CM, Emmett-Oglesby MW, Robinson NG, Lal H. Withdrawal from chronic nicotine substitutes partially for the interoceptive stimulus produced by pentylenetetrazol (PTZ) Psychopharmacology (Berl) 1986;90:85–89. doi: 10.1007/BF00172876. [DOI] [PubMed] [Google Scholar]
- 133.Shearman G, Lal H. Discriminative stimulus properties of pentylenetetrazol and bemegride: some generalization and antagonism tests. Psychopharmacology (Berl) 1979;64:315–319. doi: 10.1007/BF00427516. [DOI] [PubMed] [Google Scholar]
- 134.Kim DJB, Brioni JD. Modulation of the discriminative stimulus properties of nicotine by diazepam and ethanol. Drug Dev Res. 1995;34:47–54. [Google Scholar]
- 135.Grech DM, Willetts J, Balster RL. Pharmacological specificity of N-methyl-D-aspartate discrimination in rats. Neuropharmacology. 1993;32:349–354. doi: 10.1016/0028-3908(93)90155-v. [DOI] [PubMed] [Google Scholar]
- 136.Solinas M, Scherma M, Tanda G, Wertheim CE, Fratta W, Goldberg SR. Nicotinic facilitation of delta9-tetrahydrocannabinol discrimination involves endogenous anandamide. J Pharmacol Exp Ther. 2007;321:1127–1134. doi: 10.1124/jpet.106.116830. [DOI] [PubMed] [Google Scholar]
- 137.Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 138.Le Foll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport. 2004;15:2139–2143. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- 139.Murray JE, Wells NR, Lyford GD, Bevins RA. Investigation of endocannabinoid modulation of conditioned responding evoked by a nicotine CS and the Pavlovian stimulus effects of CP 55,940 in adult male rats. Psychopharmacology. 2009 doi: 10.1007/s00213-009-1572-x. PMID: 19495728. [DOI] [PubMed] [Google Scholar]
- 140.Gasior M, Jaszyna M, Munzar P, Witkin JM, Goldberg SR. Caffeine potentiates the discriminative-stimulus effects of nicotine in rats. Psychopharmacology (Berl) 2002;162:385–395. doi: 10.1007/s00213-002-1113-3. [DOI] [PubMed] [Google Scholar]
- 141.Justinova Z, Ferre S, Barnes C, et al. Effects of chronic caffeine exposure on adenosinergic modulation of the discriminative-stimulus effects of nicotine, methamphetamine, and cocaine in rats. Psychopharmacology (Berl) 2009;203:355–367. doi: 10.1007/s00213-008-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Romano C, Goldstein A, Jewell NP. Characterization of the receptor mediating the nicotine discriminative stimulus. Psychopharmacology (Berl) 1981;74:310–315. doi: 10.1007/BF00432737. [DOI] [PubMed] [Google Scholar]
- 143.Koek W, Slangen JL. The role of fentanyl training dose and of the alternative stimulus condition in drug generalization. Psychopharmacology (Berl) 1982;76:149–156. doi: 10.1007/BF00435269. [DOI] [PubMed] [Google Scholar]
- 144.Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 145.Guillem K, Vouillac C, Azar MR, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guillem K, Vouillac C, Azar MR, et al. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24:3532–3540. doi: 10.1111/j.1460-9568.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- 147.Villegier AS, Lotfipour S, Belluzzi JD, Leslie FM. Involvement of alpha1-adrenergic receptors in tranylcypromine enhancement of nicotine self-administration in rat. Psychopharmacology (Berl) 2007a;193:457–465. doi: 10.1007/s00213-007-0799-7. [DOI] [PubMed] [Google Scholar]
- 148.Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007b;52:1415–1425. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 149.Wooters TE, Bardo MT. The monoamine oxidase inhibitor phenelzine enhances the discriminative stimulus effect of nicotine in rats. Behav Pharmacol. 2007;18:601–608. doi: 10.1097/FBP.0b013e3282eff0d5. [DOI] [PubMed] [Google Scholar]
- 150.Meltzer LT, Rosecrans JA. Investigations on the CNS sites of action of the discriminative stimulus effects of arecoline and nicotine. Pharmacol Biochem Behav. 1981b;15:21–26. doi: 10.1016/0091-3057(81)90332-4. [DOI] [PubMed] [Google Scholar]
- 151.Shoaib M, Stolerman IP. Brain sites mediating the discriminative stimulus effects of nicotine in rats. Behav Brain Res. 1996;78:183–188. doi: 10.1016/0166-4328(95)00245-6. [DOI] [PubMed] [Google Scholar]
- 152.Rosecrans JA, Chance WT, Schechter MD. The discriminative stimulus properties of nicotine, d-amphetamine and morphine in dopamine depleted rats. Psychopharmacol Commun. 1976;2:349–356. [PubMed] [Google Scholar]
- 153.Ando K, Miyata H, Hironaka N, Tsuda T, Yanagita T. The discriminative effects of nicotine and their central sites in rats. Yakubutsu Seishin Kodo. 1993;13:129–136. [PubMed] [Google Scholar]
- 154.Miyata H, Ando K, Yanagita T. Brain regions mediating the discriminative stimulus effects of nicotine in rats. Ann N Y Acad Sci. 2002;965:354–363. doi: 10.1111/j.1749-6632.2002.tb04177.x. [DOI] [PubMed] [Google Scholar]
- 155.Miyata H, Ando K, Yanagita T. Medial prefrontal cortex is involved in the discriminative stimulus effects of nicotine in rats. Psychopharmacology (Berl) 1999;145:234–236. doi: 10.1007/s002130051054. [DOI] [PubMed] [Google Scholar]
- 156.Miyata H, Ando K, Yanagita T. [Studies on the involvement of the nucleus accumbens in the discriminative effects of nicotine in rats] Nippon Yakurigaku Zasshi. 1991;98:389–397. doi: 10.1254/fpj.98.5_389. [DOI] [PubMed] [Google Scholar]
- 157.Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 158.Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2008.11.011. PMID: 19136222. [DOI] [PubMed] [Google Scholar]
- 159.Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 160.Philibin SD, Vann RE, Varvel SA, et al. Differential behavioral responses to nicotine in Lewis and Fischer-344 rats. Pharmacol Biochem Behav. 2005;80:87–92. doi: 10.1016/j.pbb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 161.Gordon TL, Meehan SM, Schechter MD. P and NP rats respond differently to the discriminative stimulus effects of nicotine. Pharmacol Biochem Behav. 1993;45:305–308. doi: 10.1016/0091-3057(93)90243-m. [DOI] [PubMed] [Google Scholar]
- 162.Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- 163.Jung ME, Wallis CJ, Gatch MB, Lal H. Sex differences in nicotine substitution to a pentylenetetrazol discriminative stimulus during ethanol withdrawal in rats. Psychopharmacology (Berl) 2000;149:235–240. doi: 10.1007/s002130000392. [DOI] [PubMed] [Google Scholar]
- 164.Stairs DJ, Bockman CS, Fosdick J, Mittelstet B, Schwarzkopf L. Enrichment-induced differences in nicotine drug discrimination in rats is nicotinic receptor-mediated. College on Problems of Drug Dependence Program Book. 2009:30. [Google Scholar]
- 165.Le Foll B, Chefer SI, Kimes AS, Shumway D, Stein EA, Mukhin AG, Goldberg SR. Baseline expression of alpha4beta2* nicotinic acetylcholine receptors predicts motivation to self-administer nicotine. Biol Psychiatry. 2009;65:714–716. doi: 10.1016/j.biopsych.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Barik J, Wonnacott S. Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol. 2009:173–177. doi: 10.1007/978-3-540-69248-5_7. [DOI] [PubMed] [Google Scholar]
- 167.Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl) 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- 168.Coen KM, Adamson KL, Corrigall WA. Medication-related pharmacological manipulations of nicotine self-administration in the rat maintained on fixed- and progressive-ratio schedules of reinforcement. Psychopharmacology (Berl) 2009;201:557–568. doi: 10.1007/s00213-008-1321-6. [DOI] [PubMed] [Google Scholar]
- 169.Barrett RJ, Caul WF, Smith R. Withdrawal, tolerance, and sensitization to dopamine mediated interoceptive cues in rats trained on a three-lever drug-discrimination task. Pharmacol Biochem Behav. 2005;81:1–8. doi: 10.1016/j.pbb.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 170.McMahon LR, Javors MA, France CP. Changes in relative potency among positive GABA(A) receptor modulators upon discontinuation of chronic benzodiazepine treatment in rhesus monkeys. Psychopharmacology (Berl) 2007;192:135–145. doi: 10.1007/s00213-006-0692-9. [DOI] [PubMed] [Google Scholar]
- 171.France CP, Gerak LR. Discriminative stimulus effects of flumazenil in rhesus monkeys treated chronically with chlordiazepoxide. Pharmacol Biochem Behav. 1997;56:447–455. doi: 10.1016/s0091-3057(96)00226-2. [DOI] [PubMed] [Google Scholar]
- 172.Becker GL, Gerak LR, Koek W, France CP. Antagonist-precipitated and discontinuation-induced withdrawal in morphine-dependent rhesus monkeys. Psychopharmacology (Berl) 2008;201:373–382. doi: 10.1007/s00213-008-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.France CP, Woods JH. Discriminative stimulus effects of naltrexone in morphine-treated rhesus monkeys. J Pharmacol Exp Ther. 1989;250:937–943. [PubMed] [Google Scholar]
- 174.McMahon LR. Discriminative stimulus effects of the cannabinoid CB1 antagonist SR 141716A in rhesus monkeys pretreated with Delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 2006;188:306–314. doi: 10.1007/s00213-006-0500-6. [DOI] [PubMed] [Google Scholar]
- 175.George O, Ghozland S, Azar MR, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]