Abstract
Objective:
Our goal was to examine the daily average consumption (DACON) of oxycodone controlled-release tablets (OxyContin CR)and oxymorphone extended-release tablets (Opana ER) in patients with low back pain.
Study Design:
An observational, retrospective cohort study enrolled patients with multiple prescriptions for oxycodone CR or oxymorphone ER tablets. These patients also had International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for low back pain. Pharmacy prescription medication claims data were obtained from a large commercially insured health plan in the U.S. Mean daily consumption was calculated for a 90-day period.
Methods:
We used descriptive statistics to evaluate patient demographics and health plan characteristics. Univariate analyses were used to examine the data as observed. A generalized linear model with a gamma distribution and log-link function provided a sensitivity measure, adjusting for heterogeneity among patients and the skewed nature of the DACON variable.
Results:
A total of 4,023 patients received oxycodone CR, and 374 patients received oxymorphone ER. The mean age of patients (standard deviation, SD) was 49.0 (11.6) years for oxycodone CR and 47.3 (10.6) years for oxymorphone ER. DACON of oxycodone CR was 3.2 tablets per day, and DACON of oxymorphone ER was 2.7 tablets per day (P < 0.01). Utilization of maximum-strength tablets of oxycodone CR 80 mg was 3.9 tablets per day, which was significantly higher, by one tablet per day, than the utilization of equipotent oxymorphone ER maximum-strength tablets of 40 mg at 2.9 tablets per day (P < 0.01).
Conclusion:
The use of oxycodone CR, measured as mean daily consumption over a 90-day period, was significantly higher than that for oxymorphone ER in these patients, a finding that could have financial implications for health care systems.
INTRODUCTION
Opioid analgesics have long been used to treat moderate-to-severe pain for a variety of non–cancer-related conditions, including those affecting the musculoskeletal system.1–4 The Federation of State Medical Boards has adopted guidelines to promote access to opioid analgesics,5 and the current guidelines of the American Society of Anesthesiologists Practice Guidelines for Chronic Pain Management 6 have identified extended-release opioids as part of a multimodal pain management strategy for patients with neuropathic pain or back pain. In the previous decade, there was an increase in the frequency of diagnosis of non-cancer pain conditions and greater use of opioids to treat them.7
Low back pain occurs in approximately 28% of adults 18 years of age or older and accounts for the second most common symptom reported by individuals during physician office visits.8,9 In one study examining data from 1992 to 2001, opioids were prescribed by primary care physicians 53% of the time for patients with a confirmed diagnosis of back pain, arthritis, or acute musculoskeletal conditions.10 Although opioids do relieve low back pain,11,12 patient management can be complicated by the risk of abuse. A meta-analysis of studies published in 2007 reported that the prevalence of current substance use disorders in chronic back pain patients receiving opioids ranged from 3% to 43%, with a lifetime prevalence as high as 54%.13
The prevalence of low back pain and the frequency with which opioids are prescribed combine to make opioid analgesics a significant contributor to costs of care. In one study, the cost of opioids to treat patients with low back pain represented 48% of the $1,795,375 total cost of the opioid class of drugs for a university-based health plan.14
There has been concern about overutilization of opioid analgesics in the U.S.;15 however, little research to date has examined the real-world use of different long-acting opioids. Yet recent evidence suggests that changes in pharmacy policy have mixed success in reducing utilization of oxycodone (OxyContin, Purdue Pharma).16–18 Patterns of use for long-acting opioids have potential clinical and financial implications as physicians, payers and patients attempt to manage risks and costs of therapy while achieving effective pain relief.
One common measure of utilization is daily average consumption (DACON), which has been used to assess medications for diseases such as diabetes,19,20 hypertension,21 and arthritis.22 DACON can be defined as the number of tablets per day that are dispensed to a patient over a defined period of time. This measure does not necessarily correlate with adherence to therapy, but it can reveal patterns of use in specified populations.
For this study, the measure of DACON provided an opportunity to examine how two opioids in long-acting formulations, with the same prescribing information for twice-daily dosing, differ with respect to usage in patients with low back pain. If utilization is not similar, there could be, at a minimum, economic consequences for pharmacy costs to patients and payers. The objective was to quantify the differences in utilization between controlled-release oxycodone (OxyContin CR) and extended-release oxymorphone (Opana ER, Endo) in a population of patients with low back pain.
METHODS
Scope of the Study
We conducted a retrospective, observational study of commercially insured patients taken from a large managed health care plan in the U.S. Data regarding the study population were drawn from the i3 InVision Data Mart data set, which contained aggregated medical claims and prescription drug information reported to United Healthcare for the period January 1, 2006, through September 30, 2009. The number of covered lives during the 36-month period at any particular point in time was approximately 15 million. The population was diverse geographically across the U.S.
Coverage included medical and pharmacy benefits, as well as plan options that allowed for different levels of copayments and deductibles, but both oxycodone CR and oxymorphone ER were subject to the same formulary tier status and quantity limits. De-identified patient data were used in accordance with the Health Insurance Portability and Accountability Act (HIPAA). Approval by the institutional review board was not required.
Sample Selection and Characteristics
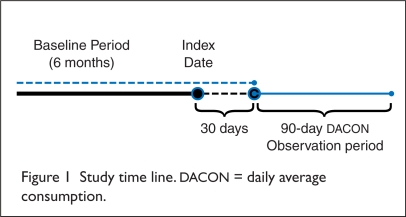
As shown in Figure 1, an index date for each patient was defined as the date of the first prescription claim for either oxycodone CR or oxymorphone ER; patients had to have at least a 30-day supply of the study drug at least one month before the DACON observation period in order to avoid capturing titration utilization patterns at the initiation of therapy. Prescription claims totaling a minimum of a 90-day supply of the study drug were required during the DACON observation period, as three months is consistent with definitions for chronic pain.23,24 Thus, utilization would be within the labeled indication for both opioids of “use for an extended period of time.”25,26
Figure 1.
Study time line. DACON = daily average consumption.
Patients included in the analysis had to have continuous insurance coverage for the six months before and after the start of the DACON observation period for the purpose of identifying exclusion criteria diagnoses. They also had to have at least one diagnosis of low back pain during that time, following the list of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, developed at the University of Washington in Seattle (Table 1).10
Table 1.
Classification of Patients With Low Back Pain
Patients with low back pain were identified using International Classification of Diseases, Ninth Revision, Clinical Modification codes (ICD-9-CM) as follows:
|
Patients were retained in the study cohort only if they did not switch to the other study drug during the 90-day DACON observation period. There were no other limitations on the use of other short-acting or long-acting opioids.
Patients were excluded from the study if they were younger than 18 years of age or pregnant (ICD-9-CM 761.5x, V22.xx, V72.40, and V2.32), given that opioid utilization might be more limited in these populations. Patients with cancer (ICD-9-CM 140-239) were also excluded because of the potential for increased opioid utilization unrelated to back pain.
Average Daily Opioid Consumption
DACON for each patient was calculated by dividing the number of tablets dispensed during the 90-day observation period by 90. From these amounts, overall DACON for each of the two opioids was calculated, as was that for the highest dosage strength and all lower dosage strengths for each opioid. This approach allowed the separation of prescribed doses that would require multiple tablets of the highest dosage strength from doses that could be achieved with a single tablet.
Comparing the utilization of the highest tablet strengths of each opioid requires that these highest strengths be equipotent. We determined the equivalence of potency of the oxycodone CR 80-mg tablet and the oxymorphone ER 40-mg tablet on the basis of the 2:1 (oxycodone CR/oxymorphone ER) dosage conversion ratio. The ratio was derived from a study of patients with low back pain that examined the efficacy and safety of oxymorphone ER compared with placebo. Oxycodone CR was the active control.12 Both drugs demonstrated similar analgesia that was superior to that of placebo. The relative dose of oxymorphone ER (79.4 mg/day) was approximately half that of oxycodone CR (155 mg/day).
Statistical Analysis
We analyzed demographic variables for age and sex of the patients, as well as plan type and region in each group, descriptively using either chi-square tests or an independent t-test. Univariate analyses to compare mean differences between oxycodone CR and oxymorphone ER use were conducted with t-tests. We performed multivariate analyses using generalized linear models with a gamma distribution and log-link function to adjust for the observed heterogeneity among patients. The dependent variable was DACON.
Explanatory variables included the study drug, tablet strengths, age, sex, and the Charlson Comorbidity Index (CCI), a proxy measure that assigns weights for 19 chronic conditions.27,28 SAS version 9.1 (SAS Institute, Inc., Cary, N.C.) and Stata version 10.1 (StataCorp, College Station, Tex.) were used to analyze the data.
RESULTS
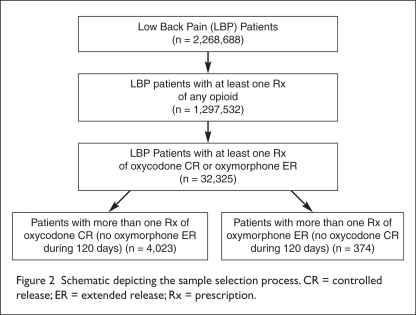
We identified a total of 2,268,688 patients with low back pain (Figure 2). Applying the inclusion and exclusion criteria produced final cohorts of 4,023 patients in the oxycodone CR group and 374 patients in the oxymorphone ER group (see Figure 2). Demographic findings (Table 2) revealed no significant differences between the groups; however, the mean age of oxycodone CR patients was 1.7 years older than that of the oxymorphone ER patients (P < 0.01), and geographical distribution reflected that of the patients in the database, favoring the southern region of the U.S. (P < 0.01).
Figure 2.
Schematic depicting the sample selection process. CR = controlled release; ER = extended release; Rx = prescription.
Table 2.
Demographic and Plan Characteristics
| Characteristic | Oxycodone CR Group (n = 4,023) | Oxymorphone ER Group (n = 374) | P Value* |
|---|---|---|---|
| Mean (SD) age on index date: | 49.0 (11.6) | 47.3 (10.6) | <0.01 |
| Women n (%) | 1,986 (49.4) | 195 (52.1) | 0.31 |
| Region n (%) | |||
| Northeast | 383 (9.5) | 24 (6.4) | |
| Midwest | 1,016 (25.3) | 78 (20.9) | |
| South | 1,808 (44.9) | 212 (56.7) | |
| West | 816 (20.3) | 60 (16.0) | <0.01 |
| Health Plan n (%) | |||
| HMO | 497 (12.4) | 51 (13.6) | |
| PPO | 428 (10.6) | 42 (11.2) | |
| POS | 2,436 (60.6) | 229 (61.2) | |
| Others | 816 (20.3) | 52 (13.9) | 0.58 |
| Charlson Comorbidity Index n (%) | |||
| CCI = 0 | 1,290 (32.1) | 116 (31.0) | |
| CCI = 1 | 359 (8.9) | 43 (11.5) | |
| CCI = 2 | 1,169 (29.1) | 118 (31.6) | |
| CCI ≥ 3 | 1,205 (30.0) | 97 (25.9) | 0.16 |
Pearson chi-square and t-tests were used to compare proportions by drug groups and mean difference, respectively.
CR = controlled release; ER = extended release; HMO = health maintenance organization; PPO = preferred provider organization; POS = point of service; SD = standard deviation.
DACON of oxycodone CR was higher than that for oxymorphone ER in all comparisons (Table 3). Mean DACON values ranged from 2.7 for oxymorphone ER at the lower strengths to 3.9 for oxycodone CR at the highest strength. The greatest difference between drugs was at the highest tablet strength; patients used one more tablet of oxycodone CR per day. The mean difference for all strengths was 0.5 more tablets of oxycodone CR dispensed per day.
Table 3.
Univariate Analysis
| Low Back Pain, Mean (SD), Tablets* |
Oxycodone CR |
Oxymorphone ER |
Difference in DACON (Tablets/Day) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Highest strengths† | 688 | 3.9 (2.4) | 91 | 2.9 (1.3) | 1.0 |
| Lower strengths† | 3,335 | 3.1 (1.6) | 283 | 2.6 (1.2) | 0.5 |
| Overall† | 4,023 | 3.2 (1.8) | 374 | 2.7 (1.2) | 0.5 |
Highest tablet strength: oxycodone CR = 80 mg and oxymorphone ER = 40 mg; lower tablet strengths: oxycodone CR < 80 mg and oxymorphone ER < 40 mg.
† t-tests established statistically significant differences in daily average consumption (DACON) between oxycodone CR and oxymorphone ER across all categories (P < 0.01).
CR = controlled release; DACON = daily average consumption; ER = extended release; SD = standard deviation.
A generalized linear model was applied to measure any effect of the demographic variables in conjunction with the choice of drug and tablet strength. The bias-adjusted means and standard deviations (SDs) were consistent with the univariate data analysis (Table 4). This modeling confirms that DACON was not affected by age, sex, or the Charlson Comorbidity Index, but it was positively associated with the choice of oxycodone CR (P < 0.01) and with the use of the highest tablet strength (P < 0.01) (Table 5).
Table 4.
Generalized Linear Model, Adjusted by Age, Sex, and Comorbidities
| Low Back Pain Population |
Oxycodone CR (n = 4,023) |
Oxymorphone ER (n = 374) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Highest strength*† | 3.9 | 0.1 | 3.2 | 0.0 |
| Lower strengths† | 3.1 | 0.0 | 2.5 | 0.0 |
| Overall† | 3.2 | 0.3 | 2.7 | 0.3 |
Highest tablet strength: oxycodone CR = 80 mg, oxymorphone ER = 40 mg; lower tablet strengths: oxycodone CR < 80 mg, oxymorphone ER < 40 mg.
Statistically significant differences across all tablet strengths for utilization between oxycodone CR and oxymorphone ER (P < 0.01).
CR = controlled release; ER = extended release; SD = standard deviation.
Table 5.
Results of the Generalized Linear Model
| Coefficient | Standard Error | PValue | |
|---|---|---|---|
| Constant | 1.145 | 0.036 | < 0.01 |
| Drug | 0.197 | 0.029 | < 0.01 |
| Tablet strength | –0.222 | 0.021 | < 0.01 |
| Years of deviation from mean age | –0.001 | 0.001 | 0.149 |
| Sex | –0.004 | 0.016 | 0.824 |
| CCI = 1 | 0.004 | 0.032 | 0.909 |
| CCI = 2 | 0.007 | 0.021 | 0.756 |
| CCI ≥ 3 | 0.028 | 0.024 | 0.243 |
CCI = Charlson Comorbidity Index.
DISCUSSION
Our study examined variations in daily average consumption (DACON) of oxycodone CR and oxymorphone ER tablets in a large health care insurance plan. DACON is a simple utilization measurement calculated in terms of tablets per day, and it varies with respect to manufacturer dosing recommendations and physician prescribing practices. In this case, both oxycodone CR and oxymorphone ER are recommended at twice-daily dosing; thus, the expected DACON would theoretically be two tablets. The mean DACON calculations for both of these opioids at all dosage strengths exceeded two tablets per day; however, the calculated value for oxymorphone ER was closer to the expected level, with patients using 0.5 tablet more of oxycodone CR per day than oxymorphone ER for all strengths.
Prescribed doses higher than oxycodone CR 80 mg or oxymorphone ER 40 mg would require multiple tablets and could therefore exceed a DACON of two tablets while remaining consistent with the prescribing information. For this reason, the highest tablet strengths were analyzed separately from the lower strengths. At these equipotent highest strengths, patients used one more oxycodone CR tablet per day.
Because the dose of an opioid analgesic must be individualized for each patient, the variance in DACON from two tablets could reflect an individual patient’s titration phase. However, clinical trials with these drugs have generally permitted one month or less for titration.29,30 For this reason, a one-month run-in period was built into the study to avoid capturing initial dosing titration. In this analysis, a prescribed asymmetrical dosing regimen of two tablets in the morning and one at night would be reflected as a DACON of three tablets.
There are several theoretical explanations as to why DACON exceeded two tablets per day and why it was higher for oxycodone CR than for oxymorphone ER. First, there is variability in the analgesic duration of effect based on differences in release characteristics. Although pharmacokinetic evaluations of the two drugs have been designed differently, a pharmacokinetic study of oxycodone CR produced a biphasic curve in which 38% of the drug was released in the first 37 minutes;31 this phenomenon has not been observed for oxymorphone ER.32
Research published in 2010 also indicates a difference in subjective effects (e.g., euphoria) between these two drugs,33 which may lead to differences in noncompliant use by patients or others, as the abuse and diversion of opioids, especially oxycodone CR, are well documented.33,34
Finally, differences in utilization could reflect the effect of polymorphisms or drug–drug interactions in drug metabolism. Oxycodone is eliminated through the cytochrome P450 (CYP) 2D6 pathway,25 whereas oxymorphone’s biotransformation occurs via glucoronidation.26 A retrospective study found a 26% prevalence of CYP 2D6 drug–drug exposures among ambulatory osteoarthritis patients using oxycodone CR.35 Any of these possibilities or a combination of factors might help to explain the elevated levels of oxycodone utilization.
STUDY LIMITATIONS
Our findings should be considered within the context of several limitations. The sample size of patients receiving oxymorphone ER was substantially smaller than that for oxycodone CR, reflecting the relative market shares for the two drugs within the health plan from which the data were analyzed.
The research objectives covered 90 days, but observed DACON levels might change from the initiation of therapy to points further along the continuum of care. In addition, data extracted from a large database and compiled from several insurance products in the U.S. can be subject to errors, including omissions, inaccurate information, and other possible mistakes.
As with all retrospective claims database analyses, there was no randomization of the oxycodone CR and oxymorphone ER patient populations studied within the i3 InVision Data Mart database. Further, despite the use of multivariate analyses to correct for differences in patient characteristics, such as demographics and comorbidities between the two groups, other differences may exist.
The study could not evaluate patients’ experience of pain, and no comparison of the effectiveness of the two products could be made from these results. The additional use of multiple, long-acting tablets to conduct dose escalations in response to increasing pain severity or tolerance would not be separable in this analysis.
Given these limitations, the fact that utilization of two long-acting opioids can differ in a patient population for whom opioid analgesics are a frequent therapeutic choice implies that assumptions about equivalent utilization should not be made for the class.
RECOMMENDATIONS FOR FUTURE STUDY
Areas for future research could include examining the impact that differences in utilization levels might have on clinical outcomes and health care expenditures. Although our study did not measure health outcomes, an emerging body of research has tied increased medical consequences and costs to daily consumption of higher doses of opioids among patients receiving chronic opioid therapy.36,37
From a cost-of-therapy perspective, switching the 688 patients in this study from the highest strength of oxycodone CR to the equivalent highest strength of oxymorphone ER would generate $217,985 per month in savings at wholesale acquisition costs (Table 6).
Table 6.
Calculations of Cost Differences For Highest Strengths of Oxycodone CR And Oxymorphone ER per Month In the Univariate Analysis*
| 688 patients received oxycodone CR 80 mg | |
| × DACON of 3.9 | |
| × 30 days | |
| × wholesale acquisition cost, $10.83 |
|
| Total = $874,007 | |
| 688 patients switched to oxymorphone ER 40 mg | |
| × DACON of 2.9 | |
| × 30 days | |
| × $10.96 |
|
| Total = $656,022 | |
| $874,007 – $656,022 = $217,985 | |
First Databank wholesale acquisition costs per tablet, as of April 10, 2010, were $10.83 for oxycodone CR 80 mg and $10.96 for oxymorphone ER 40 mg.
DACON = daily average consumption.
Additional research could focus on evaluating the full costs and outcomes of treatment for a defined population using claims data to be supplemented with information from patient medical charts or electronic medical records.
CONCLUSION
Daily average consumption (DACON) of oxycodone CR was one tablet per day more at the highest tablet strengths compared with oxymorphone ER in patients with low back pain. Chronic opioid therapy for non-cancer pain continues to exert significant pressure on health care costs; therefore, careful assessment by prescribers of the utilization patterns and attributes of individual long-acting opioids is merited, just as it is for decision-makers responsible for pharmacy policy.
Acknowledgments
We wish to thank Pi-Chin Lai, MS, for programming contributions; Chunmay Fu, MS, for quality control support; and Kent Summers, PhD, for valuable comments in the preparation of the manuscript.
Footnotes
Disclosure. Dr. Berner, Ms. Thomson, Dr. Hartry, Dr. Puenpatom, and Dr. Ben-Joseph are employed at Endo Pharmaceuticals and report that they have received assistance from the company in preparing the article. Dr. Szeinbach reports that she received financial support from Endo in drafting portions of the manuscript.
REFERENCES
- 1.World Health Organization . WHO Treatment Guidelines on Non-malignant Pain in Adults. 2008. Available at: www.who.int/medicines/areas/quality_safety/Scoping_WHOGuide_non-malignant_pain_adults.pdf. Accessed August 25, 2010. [Google Scholar]
- 2.Kalso E, Laurie A, Dellemijn PLI, et al. Recommendations for using opioids in chronic non-cancer pain. Eur J Pain. 2003;7:381–386. doi: 10.1016/S1090-3801(02)00143-X. [DOI] [PubMed] [Google Scholar]
- 3.Paulose-Ram R, Hirsh R, Dillon C, et al. Prescription and non-prescription analgesic use among the U.S. adult population: Results from the Third National Health and Nutrition Examination Survey (NHANES III) Pharmacoepidemiol Drug Saf. 2003;12:315–326. doi: 10.1002/pds.755. [DOI] [PubMed] [Google Scholar]
- 4.Portenoy RK, Farrar JT, Backonja M, et al. Long-term use of controlled-release oxycodone for noncancer pain: Results of a 3-year registry study. Clin J Pain. 2007;23(4):287–299. doi: 10.1097/AJP.0b013e31802b582f. [DOI] [PubMed] [Google Scholar]
- 5.Federation of State Medical Boards: Model policy for the use of controlled substances for the treatment of pain, 2004. Available at: www.fsmb.org/pdf/2004_grpol_Controlled_Substances.pdf. Accessed August 25, 2010.
- 6.Guidelines for Chronic Pain Management An Updated Report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810–833. doi: 10.1097/ALN.0b013e3181c43103. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan MD, Edlund MJ, Ming-Yu Fan. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: The TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics . Health, United States, 2007 with Chartbook on Trends in the Health of Americans. Hyattsville, Md: U.S. Dept of Health and Human Services, Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 9.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain: Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine. 1995;20:11–19. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Vogt MT, Kwoh K, Cope DK, et al. Analgesic usage for low back pain: Impact on health care costs and service use. Spine. 2005;30(9):1075–1081. doi: 10.1097/01.brs.0000160843.77091.07. [DOI] [PubMed] [Google Scholar]
- 11.Jamison R, Raymond S, Slawsby E, et al. Opioid therapy for chronic non-cancer back pain: A randomized prospective study. Spine. 1998;23:2591–2600. doi: 10.1097/00007632-199812010-00014. [DOI] [PubMed] [Google Scholar]
- 12.Hale ME, Dvergsten C, Gimbel J. Efficacy and safety of oxymorphone extended-release in chronic low back pain: Results of a randomized, double blind, placebo- and active-controlled study. J Pain. 2005;6(1):21–28. doi: 10.1016/j.jpain.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Martell B, O’Connor P, Kerns R, et al. Systematic review: Opioid treatment for chronic back pain. Prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 14.Olsen Y, Baumit GL, Ford DE. Opioid prescriptions by U.S. primary care physicians from 1992 to 2001. J Pain. 2006;7(4):225–235. doi: 10.1016/j.jpain.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Katz N, Adams E, Chilcoat H, et al. Challenges in the development of prescription opioid abuse-deterrent formulations. Clin J Pain. 2007;238:648–660. doi: 10.1097/AJP.0b013e318125c5e8. [DOI] [PubMed] [Google Scholar]
- 16.Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(9):573–580. doi: 10.1097/MLR.0b013e31816493fb. [DOI] [PubMed] [Google Scholar]
- 17.Katz MH, Kotabe S. Decreasing use of controlled-release oxycodone: Response to Morden, et al. Med Care. 2008;46(9):1002. doi: 10.1097/MLR.0b013e3181858edc. [DOI] [PubMed] [Google Scholar]
- 18.Holzworth A. Impact of an OxyContin formulary change on member opioid utilization and prescriber practices. Poster presented at the Academy of Managed Care Pharmacy, 22nd Annual Meeting & Showcase; San Diego. April 7–10, 2010. [Google Scholar]
- 19.McAdam-Marx C, Bouchard J, Brixner DI. Comparison of daily insulin dose and other anti-diabetic medications usage for type 2 diabetes patients treated with an analog basal insulin. Curr Med Res Opin. 2010;26(1):191–201. doi: 10.1185/03007990903432470. [DOI] [PubMed] [Google Scholar]
- 20.Borah BJ, Darkow T, Bouchard J, et al. A comparison of insulin use, glycemic control, and health care costs with insulin detemir and insulin glargine in insulin-naïve patients with type 2 diabetes. Clin Ther. 2009;31(3):623–631. doi: 10.1016/j.clinthera.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Jan SA, Patel JV, Welz J, Ishak P. A retrospective database analysis of prescribing patterns for specific angiotensin receptor blockers. Drug Benefit Trends. 2005;17:23–29. [Google Scholar]
- 22.Schnitzer TJ, Kong SX, Mitchell JH, et al. An observational, retrospective, cohort study of dosing patterns for rofecoxib and celecoxib in the treatment of arthritis. Clin Ther. 2003;25(12):3162–3172. doi: 10.1016/s0149-2918(03)90100-7. [DOI] [PubMed] [Google Scholar]
- 23.Turk D, Okifuji A. Pain terms and taxonomies of pain. In: Fishman S, Ballantyne J, Rathmell J, editors. Bonica’s Management of Pain. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 24.Thielke S, Simoni-Wastila L, Edlund M, et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med. 2010;11:248–256. doi: 10.1111/j.1526-4637.2009.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.OxyContin (oxycodone HCl controlled-release tablets), prescribing information . Stamford, Conn: Purdue Pharma; 2010. Available at: www.purduepharma.com/pi/prescription/oxycontin.pdf. Accessed August 25, 2010. [Google Scholar]
- 26.Opana ER (oxymorphone HCl extended-release tablets), prescribing information . Chadds Ford, Pa: Endo Pharmaceuticals; 2008. Available at: www.endo.com/pdf/Opana_ER_PI.pdf. Accessed August 25, 2010. [Google Scholar]
- 27.Hall WH, Ramachandran R, Narayan S, et al. An electronic application for rapidly calculating Charlson co-morbidity score. BMC Cancer. 2004;4:94. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic co-morbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Hale M, Fleischman R, Salzman R, et al. Efficacy and safety of controlled-release versus immediate-release oxycodone: Randomized, double-blinded evaluation in patients with chronic back pain. Clin J Pain. 1999;15(3):179–183. doi: 10.1097/00002508-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Watson C, Moulin D, Watt-Watson J, et al. Controlled-release oxycodone relieves neuropathic pain: A randomized controlled trial in painful diabetic neuropathy. Pain. 2003;105:71–78. doi: 10.1016/s0304-3959(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 31.Mandema JW, Kaiko RF, Oshlack B, et al. Characterization and validation of a pharmacokinetic model for controlled-release oxycodone. Br J Clin Pharmacol. 1996;42:747–756. doi: 10.1046/j.1365-2125.1996.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams M, Adhieh H. Pharmacokinetics and dose-proportionality of oxymorphone extended-release and its metabolites: Results of a randomized cross-over study. Pharmacotherapy. 2004;24(4):468–476. doi: 10.1592/phco.24.5.468.33347. [DOI] [PubMed] [Google Scholar]
- 33.Sellers E, Schoedel KA, McMorn S, et al. Oral intact extended-release oxymorphone is associated with less liking and lower positive subjective effects than equianalgesic doses of oral intact controlled-release oxycodone in healthy nondependent recreational opioid users. J Pain. 2010;11(4):S21. [Google Scholar]
- 34.Katz N, Panas L, Kim ML, et al. Usefulness of prescription monitoring programs for surveillance analysis of schedule II opioid prescription data in Massachusetts, 1996–2006. Pharmaco-epidemiol Drug Saf. 2010;19:115–123. doi: 10.1002/pds.1878. [DOI] [PubMed] [Google Scholar]
- 35.Pergolizzi JV, Jr, Labhsetwar SA, Puenpatom RA, et al. Prevalence of exposure to potential CYP450 pharmacokinetic drug–drug interactions among patients with chronic low back pain taking opioids. Pain Practice. 2010 Aug 26; doi: 10.1111/j.1533-2500.2010.00413.x. (online). [DOI] [PubMed] [Google Scholar]
- 36.White A, Birnbaum H, Mareva M, et al. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm. 2005;11(6):469–479. doi: 10.18553/jmcp.2005.11.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders K, Dunn K, Merrill J, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25(4):310–315. doi: 10.1007/s11606-009-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn K, Saunders K, Rutter C, et al. Opioid prescriptions for chronic pain and overdose. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]