INTRODUCTION
Among the leading causes of cancer-related deaths, prostate cancer is second to lung cancer, but it is the most frequently occurring noncutaneous cancer among men in the U.S.1 Therapies for prostate cancer depend on age, rate of the cancer’s growth, the specific benefits and drawbacks of treatment, and stage of disease. Patients older than 70 years of age with a slowly growing, localized tumor may be placed on active surveillance. Patients with localized disease, for whom watchful waiting is inadequate, can undergo surgical removal of the prostate or radiation therapy. Even with these options, the disease recurs in 20% to 30% of patients. For more advanced cases and for cancer that has spread beyond the prostate gland, androgen-deprivation therapy is commonly used with or without surgery.2,3
Medical management comprising androgen-deprivation therapy involves agents that decrease the body’s production of, or block the activity of, testosterone. Decreasing testosterone production is achieved by gonadotropin-releasing hormone (GnRH) analogues, such as leuprolide acetate (Lupron, TAP) and goserelin acetate implant (Zoladex, Astra-Zeneca). Blocking the hormone’s activity can be achieved by antiandrogens such as flutamide (Eulexin, Schering), bicalutamide (Casodex, AstraZeneca), and nilutamide (Nilandron, Sanofi-Aventis).4,5
If hormone-deprivation therapies fail, chemotherapeutic agents, such as taxanes (e.g., docetaxel [Taxotere, Sanofi-Aventis]), are the next best option.2–5 Failure of both hormonal therapies and chemotherapy agents leaves only a palliative option for patients with relapsed disease.3,4 Therefore, a more effective yet relatively nontoxic systemic therapy for prostate cancer is warranted.
For more than 25 years, immunological or vaccine-type treatments have been investigated to meet this need with a goal of providing therapeutic benefits without creating deleterious effects. Sipuleu-cel-T (Provenge, Dendreon) is the first agent that may meet this need.6–8
CLINICAL PHARMACOLOGY
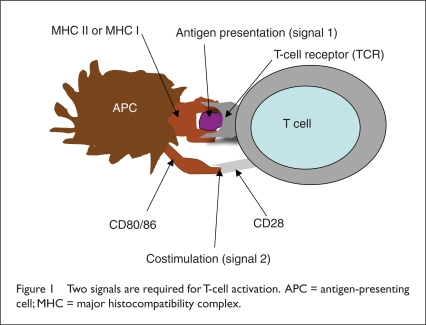
The development of sipuleucel-T vaccine was based on the concept of antigen-presenting cells (APCs). These cells “present” antigens in a form that T cells can recognize. APCs are a group of white blood cells (WBCs) that include dendritic cells, macrophages, and B lymphocytes (B cells). These cells express major histocompatibility complex (MHC) class II and MHC class I molecules, which can stimulate CD4+ T-helper cells and CD8+ T cytotoxic cells, respectively. Invaders are first engulfed and brought inside the cell. They are then broken down into their antigens and are moved to the cell surface, where they are recognized by T-cell receptors (Figure 1).
Figure 1.
Two signals are required for T-cell activation. APC = antigen-presenting cell; MHC = major histocompatibility complex.
When there is a match between the T cell and the antigen, the T cell is stimulated and starts to produce several cytokines and other chemical messengers that include, but are not limited to, interleukin-12 (IL-12), granulocyte–macrophage colony-stimulating factor (GM–CSF), tumor necrosis factor–alpha (TNF-α), cytotoxic T cells, and B cells. Cytotoxic T cells are especially effective in destroying abnormal body cells, including cancerous cells.9–11
Among the APCs, dendritic cells are considered the most potent antigen presenters, capable of initiating antitumor responses from both naive and memory T cells.12 Dendritic cells can internalize, process, and subsequently display foreign antigens to B and T lymphocytes and are critical for priming a cytotoxic T lymphocyte (CTL)–mediated immune response.9–13
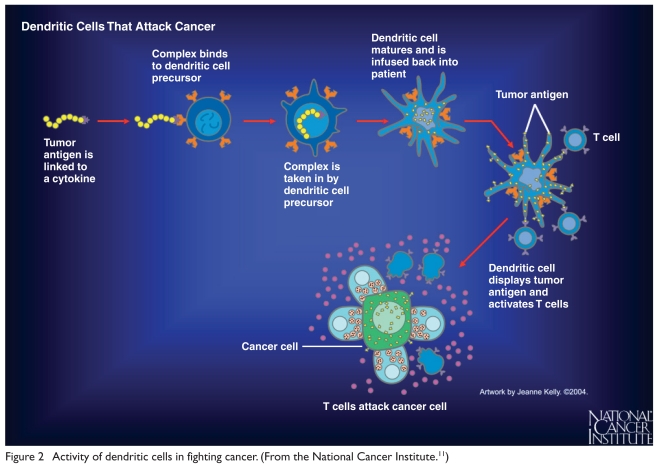
Using this information, scientists were able to make sipuleucel-T by harvesting APCs and dendritic cells from humans. The cells are centrifuged, submitted for culture, activated, and then returned to the patient as an immune modulator or vaccine (Figure 2).12–15
Figure 2.
Activity of dendritic cells in fighting cancer. (From the National Cancer Institute.11)
MECHANISM OF ACTION
The precise mechanism of action is unknown. Sipuleucel-T, an autologous cellular immunological agent, is thought to work through APCs to stimulate T-cell immune response targeted against prostatic acid phosphatase (PAP), an antigen that is highly expressed in most prostate cancer cells.6,10,15,17
PRODUCTION
Sipuleucel-T is composed of recombinant antigen protein, which must be incubated with the patient’s isolated APCs ex vivo. The procedure starts at the physician’s office, the blood-collection center, or the laboratory, where blood containing the APCs is collected by leukapheresis. The patient is sent home and is asked to return to the infusion center when the vaccine becomes available. The blood is sent to Dendreon’s manufacturing facility in New Jersey. At the facility, the harvested APCs are incubated with recombinant fusion protein antigen, which contains both PAP and GM–CSF. This process activates the APCs, which are now ready to fight the cancerous prostate cells. The activated, antigen-loaded APCs are transported back to the infusion center and are infused into the patient. The complete process takes up to four days. Because Dendreon is the only manufacturer for this vaccine, transportation, sterility, and stability of the cells must be ensured before the process begins. Only a few doctors and 50 centers are using this therapy.4–18
INDICATION AND USAGE
Sipuleucel-T is intended for patients with metastatic, symptomatic, or minimally symptomatic, castrate-resistant (hormone-refractory) prostate cancer.15
CLINICAL TRIALS
Phase 3 Studies
The FDA approval for sipuleucel-T was based on three pivotal phase 3 studies (Table 1). Overall survival rates were consistent across multiple subgroups. An analysis showed that the time to disease progression (TTP) did not meet statistical significance in any of these phase 3 studies.
Table 1.
Summary of Overall Survival and Time to Disease Progression in Various Clinical Trials
| Parameter | D9901 | D9902A | Integrated Analysis D9901 and D9902A | IMPACT Study D9902B | ||||
|---|---|---|---|---|---|---|---|---|
| Sipuleucel-T (N = 82) | Placebo (N = 45) | Sipuleucel-T (N = 65) | Placebo (N = 33) | Sipuleucel-T (N = 147) | Placebo (N = 78) | Sipuleucel-T (N = 341) | Placebo (N = 171) | |
| Median survival (CI), months | 25.9 | 21 | 19 | 15.7 | 23.2 | 18.9 | 25.8 | 21.7 |
| Hazard ratio* (CI)b | 1.71 (1.13–2.58) | 1.27 (0.78–2.07) | 1.50 (1.10–2.05) | 0.775a (0.614–0.979) | ||||
| Overall survival, P valuec | P = 0.010 | P = 0.331 | P = 0.011 | P = 0.032a | ||||
| Median time to disease progression (CI), weeks | 11 | 9.1 | 10.9 | 9.9 | 11.1 | 9 | 14.6 | 14.4 |
| Hazard ratio* (CI) | 1.45 (0.99–2.11) | 1.09 (0.69–1.70) | 1.26 (0.95–1.68) | 0.92 (0.75–1.12) | ||||
| Overall TTP, P value | P = 0.052 | P = 0.719 | P = 0.111 | P = 0.40 | ||||
CI = confidence interval; IMPACT = IMmunotherapy for Prostate AdenoCarcinoma Treatment; ln = natural logarithm; TTP = time to disease progression.
The hazard ratio expresses the risk in patients treated with placebo divided by the risk for patients treated with sipuleucel-T. Therefore, a hazard ratio greater than 1 indicates a greater risk for patients treated with placebo relative to sipuleucel-T.
The hazard ratio and P value are based on the Cox model, adjusted for prostate-specific antigen (ln) and lactic dehydrogenase (ln) and stratified by bisphosphonate use, number of bone metastases, and primary Gleason grade.
The hazard ratio is based on the unadjusted Cox model (not prespecified).
The P value is based on a log-rank test (not pre-specified).
Small et al. (D9901)13
In the first reported phase 3, double-blind, placebo-controlled clinical trial, D9901, immune response data were collected from men with androgen-independent prostate cancer. A total of 127 patients were randomly assigned, in a 2:1 ratio, to receive sipuleucel-T (n = 82) or placebo (n = 45) every two weeks. The placebo patients who were observed to have disease progression were switched to sipuleucel-T. Survival for all patients was evaluated for 36 months. At the time of the data analysis, 115 patients were found to have progressive disease.
The median TTP for sipuleucel-T was 11.7 weeks, compared with 10 weeks for placebo (P = 0.052). The hazard ratio (HR) was 1.45, with a 95% confidence interval (CI) of 0.99 to 2.11. There was no significant delay in TTP for the sipuleucel-T group as a whole (P = 0.061), but a significant difference in TTP was seen in patients with a Gleason score of 7 or less.
Median survival differed significantly for sipuleucel-T (25.9 months), compared with placebo (21.4 months), for a difference of 4.5 months (P = 0.01). Treatment remained a strong independent predictor of overall survival after adjustments were made for prognostic factors (P = 0.002; HR, 2.12; 95% CI, 1.31–3.44). An eightfold increase in T-cell proliferation was shown for the sipuleucel-T patients, compared with the placebo group (16.9 vs. 1.99, respectively; P = 0.001).
At 36 months, 34% of sipuleucel-T patients were alive, compared with 11% of placebo patients, for a significant threefold improvement in overall survival fraction (P = 0.001). Sipuleucel-T therapy was well tolerated. This study suggested that sipuleucel-T might provide survival advantages to patients with asymptomatic hormone-refractory prostate cancer.
Higano et al. (D9901/D9902A)14
An integrated analysis of D9901 and D9902A studies was performed. D9902A was part one of a two-stage phase 3 trial (D9902), which was started concurrently with D9901. D9902 was a double-blind, placebo-controlled study of patients with metastatic, asymptomatic, hormone-refractory prostate cancer. The men were randomly assigned to receive three doses of either sipuleucel-T or placebo.
After 98 patients were recruited, the study was stopped because findings from the previous study (D9901) did not reveal any statistically significant benefit of sipuleucel-T over placebo in TTP (P = 0.033). However, a benefit was noted in a subgroup of patients with a Gleason score of 7 or less. This first part of the study, without regard to the Gleason score, was designated D9902A. The study protocol was amended to focus on patients with a Gleason score of 7 or less and was continued as D9902B. This decision was again reversed after about two years to include patients with a Gleason score greater than 7.
In an integrated analysis of D9901 and D9902A, 225 patients were randomly assigned to receive sipuleucel-T (n = 147) or placebo (n = 78). The treated patients experienced a significant 33% reduction in the risk of death (P = 0.011), compared with a reduction of 15% in the placebo group.
Treatment also resulted in a 21% reduction in the risk of disease progression (P = 0.111). Only seven of the 147 patients (4.8%) in the sipuleucel-T arm had a reduction of 25% or greater in prostate-specific antigen (PSA) levels; however, none of the 78 patients in the placebo group did.
After adjustments were made for imbalances in baseline prognostic factors, post-study treatment chemotherapy use, and non-prostate cancer–related deaths, the treatment effect remained strong. The correlation between a measure of potency, CD54 up-regulation, and overall survival was supported by evidence of sipuleucel-T’s activity.
Adverse drug effects (AEs) were similar in men receiving sipuleucel-T (98.6%) and placebo (96.1%). These AEs, which were well tolerated, minimal, and short-lived, included chills, pyrexia, headache, asthenia, dyspnea, vomiting, and tremor. The study demonstrated a favorable risk–benefit ratio for sipuleucel-T.
Kantoff et al. (D9902B, IMPACT)18
D9902B, also called IMPACT (Immunotherapy for Prostate AdenoCarcinoma Treatment), was a randomized, double-blind, placebo-controlled study of 512 men with metastatic, hormone-refractory prostate cancer. Initially, the targeted patients were those with a Gleason score of 7 or less. Later this criterion was changed to include patients with a Gleason score higher than 7. Patients received intravenous (IV) sipuleucel-T (n = 341) or placebo (n = 171) at two-week intervals, for a total of three doses.
At 36 months, a survival benefit of 4.1 months was noted with sipuleucel-T; median survival was 25.8 months with sipuleucel-T and 21.7 months with placebo. Overall survival was significantly prolonged for sipuleucel-T patients (P = 0.032; HR = 0.775) at the time of cutoff. Results were consistent in multiple patient groups.
An updated analysis performed after the death of 349 patients at an estimated follow-up of 36.5 months showed that sipuleucel-T’s treatment effect remained significant (HR = 0.751; P = 0.012).
AEs that were noted in other studies were also commonly observed in IMPACT, including chills, pyrexia, headache, influenza-like illness, and myalgia. These effects were generally mild, occurring within one day of the infusion and resolving within one to two days. The authors concluded that sipuleucel-T was effective in prolonging survival.
Supportive Phase 1 and 2 Studies
Burch et al.7 and Small et al.12
Phase 1 and 2 clinical trials of sipuleucel-T (APC 8015), designed to assess safety, dosing and immunological response, showed short-lived fever, chills, myalgia, pain, fatigue, urinary incontinence urgency, and nocturia. The studies also revealed a drop in circulating PSA levels after sipuleucel-T was received. T cells drawn from patients after infusions of sipuleucel-T, but not before, could be stimulated in vitro by GM–CSF (P = 0.0004) and by PAP (P = 0.0001), demonstrating broken tolerance against the two normal proteins. However, injections of placebo did not influence the reactivity of T cells against GM–CSF or PAP.7
In a phase 2 study, TTP correlated with development of an immune response to PAP and with the dose of dendritic cells received.12 Small et al. concluded that sipuleucel-T was safe, breaking tolerance to the tissue antigen PAP, and they recommended further research on clinical efficacy data.
Adverse Reactions7,12,14,15,18
Of 601 prostate cancer patients who underwent leukapheresis at least once and who were evaluated for AEs, 98.3% of those in the sipuleucel-T group and 96% in the placebo group reported an AE. The most common AEs in the sipuleucel-T group that occurred more than 10% of the time included chills, fatigue, fever, back pain, nausea, joint aches, headaches, citrate toxicity, paresthesia, vomiting, anemia, constipation, oral paresthesia, pain in the extremities, dizziness, muscle aches, asthenia, and diarrhea (Table 2). These AEs occurred within the first few days of treatment and dissipated within one or two days. About 67.4% of AEs were mild to moderate in severity and were related to the infusion. Severe or life-threatening AEs occurred in 27.6% of sipuleucel-T patients and in 28.4% of the placebo group.
Table 2.
Incidence of Adverse Events Occurring in 10% or More of Patients Receiving Sipuleucel-T (Provenge)
| Adverse Event |
Sipuleucel-T (N = 601) N (%) |
Controls (N = 303) N (%) |
|---|---|---|
| Chills | 319 (53.1) | 33 (10.9) |
| Fatigue | 247 (41.1) | 105 (34.7) |
| Fever | 188 (31.3) | 29 (9.6) |
| Back pain | 178 (29.6) | 87 (28.7) |
| Nausea | 129 (21.5) | 45 (14.9) |
| Joint pain | 118 (19.6) | 62 (20.5) |
| Headache | 109 (18.1) | 20 (6.6) |
| Citrate toxicity | 89 (14.8) | 43 (14.2) |
| Paresthesia | 85 (14.1) | 43 (14.2) |
| Vomiting | 80 (13.3) | 23 (7.6) |
| Anemia | 75 (12.5) | 34 (11.2) |
| Constipation | 74 (12.3) | 40 (13.2) |
| Pain | 74 (12.3) | 30 (6.6) |
| Oral paresthesia | 74 (13.3) | 43 (14.2) |
| Pain in extremity | 73 (12.1) | 40 (13.2) |
| Dizziness | 71 (11.8) | 34 (11.2) |
| Muscle aches | 71 (11.8) | 17 (5.6) |
| Asthenia | 65 (10.8) | 20 (6.6) |
| Diarrhea | 60 (10.0) | 34 (11.2) |
Fatalities occurred in 3.3% of treated men and in 3.5% of controls. Back pain and chills were the most common severe or fatal AEs reported with sipuleucel-T. Other serious AEs reported in 24% of sipuleucel-T patients and in 25% of placebo patients included infusion reactions, cerebrovascular events, rhabdomyolysis, myasthenia gravis, myositis, and tumor flare. Overall, there was no increased risk of AEs with sipuleucel-T compared with placebo.
PRECAUTIONS AND CONTRAINDICATIONS
Nearly all patients (98.3%) who received sipuleucel-T experienced an AE; most of these events (71.2%) were infusion-related. To control such reactions, medications such as acetaminophen and diphenhydramine can be administered prior to the infusion. If a reaction occurs, the infusion may be stopped to perform appropriate medical management.14,15,17
Sipuleucel-T is not routinely tested for transmissible infectious diseases; therefore, health care professionals should use universal precautions when handling the leukapheresis materials. No studies of drug interactions with sipuleucel-T have been performed. There are no known contraindications.
Because of the low representation of non-Caucasian patients in clinical trials, the drug’s safety and efficacy based on race cannot be confirmed. However, the evaluation of safety of sipuleucel-T in patients older than 65 years of age, compared with younger patients, revealed no apparent differences in the product’s safety. In an analysis of survival in 488 patients, 382 (78.3%) were 65 years of age and older. The median survival time was 23.4 months for sipuleucel-T patients older than 65 years of age (95% CI, 22.0–27.1) and 17.3 months for controls (95% CI, 13.5–21.5).14,15,18
DOSAGE AND ADMINISTRATION
The sipuleucel-T dose is a minimum of 50 million autologous CD54+ cells activated with PAP or GM–CSF, suspended in 250 mL of lactated Ringer’s solution USP, in a sealed infusion bag designated for each patient. The vaccine is given every two weeks for 60 minutes in three doses. If a dose is missed, an additional leukapheresis procedure is necessary to continue and complete the treatment.12,15,18
Before the infusion, it is important to match each patient’s information on the infusion bag with the Cell Product Disposition Form sent by the manufacturer. The form contains patient identifiers, expiration dates, and the time. The infusion should be given before the expiration date indicated on the form.
The bag contents are slightly cloudy, with a cream to pink color. Small clumps of cellular material should disperse with gentle mixing. Sipuleucel-T should not be used if the clumps do not disperse after gentle mixing or if the infusion bag has a leak. The bag should remain insulated with polyurethane until the time of infusion. If the bag containing sipuleucel-T is left at room temperature for more than three hours, it should be discarded.14,15
COST ANALYSIS19–21
Sipuleucel-T treatment consists of three infusions at approximately two-week intervals for one month. The cost for a complete course of treatment of three infusions is $93,000, which includes the cost of administration. For the extended survival period of 4.1 months with sipuleucel-T, the average monthly expenditure is $22,683 per month of added median survival. By comparison, docetaxel, the drug of choice for metastatic, hormone-refractory prostate cancer, is administered every three weeks for a total of 10 cycles. The cost of one cycle of docetaxel approaches $4,000, or a total of $40,000 for all 10 cycles.19–21
The median extended survival period for prostate cancer patients receiving docetaxel is 2.4 months, or an average cost of $16,667 per month of added survival. This cost does not take into consideration the serious AEs associated with docetaxel (neutropenia, infections, anemia, nausea, and vomiting). These AEs can lead to extended treatments and, therefore, increase the total cost of care, whereas common AEs with sipuleucel-T are infusion-related and short-lived.
Quality of life has been reported to be superior with sipuleucel-T, compared with docetaxel, the standard of care for hormone-refractory prostate cancer (Table 3). In an analysis of expenses for an additional month of median survival advantage,21 sipuleucel-T was significantly more expensive than docetaxel for managing AEs.
Table 3.
Comparison of Sipuleucel-T (Provenge) and Docetaxel (Taxotere) in Quality of Life
| Sipuleucel-T | Docetaxel | |
|---|---|---|
| Treatment period | 1 month | 7 months |
| Common adverse events | Fever and chills for 3 days | Hair loss |
| Percentage of patients hospitalized for adverse events | 1.2% | >26% |
| Percentage of patients stopping treatment because of adverse events | 1% | 11% |
| Percentage of patients dying owing to adverse events | None | 1% to 3% |
REIMBURSEMENT
The high cost of sipuleucel-T ($93,000) has attracted the scrutiny of federal agencies involved with health care spending (formulary decision-making), even though the drug is comparable with other high-cost cancer treatments, as previously discussed. The Centers for Medicare and Medicaid Services (CMS) initiated a 12-month process, called the National Coverage Assessment (NCA), to decide under which circumstances it would cover the expensive treatment.
The decision memo for national coverage of sipuleucel-T was expected as early as March 30, 2011; a complete coverage analysis is expected to be completed by June 30, 2011. All local subcontractors of the CMS, however, have already decided to cover sipuleucel-T, and many of them are already paying for it. These coverage inclusions and exclusions mirror the FDA-approved label and entry criteria for the pivotal trial. This makes it highly unlikely that the CMS will decide against reimbursing for sipuleucel-T when its draft for coverage is released.22
CONCLUSION
Sipuleucel-T (Provenge) is a personalized vaccine encompassing patients’ ex vivo processed dendritic cells that express a key tumor antigen (prostatic acid phosphatase). In late-stage, randomized trials, this drug showed a statistically significant extended survival of at least 4.1 months and an overall survival of about 20 months, when compared with placebo. Men receiving sipuleucel-T experienced a 22.5% overall reduced risk of death compared with the control group.
D9901, the first phase 3 study, was comparable in design to D9902B/IMPACT in the evaluation of metastatic, asymptomatic, hormone-refractory prostate cancer, demonstrating a similar advantage in survival. The approval of sipuleucel-T represents a new option in the care of men with advanced prostate cancer.12,14,18,21
Footnotes
Disclosure: The authors report that they have no financial or commercial/industrial relationships to disclose in regard to this article.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Kasper DL, Braunwald E, et al., editors. Harrison’s Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008. pp. 594–600. [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Ross RW, Kantoff PW. Hormone-refractory prostate cancer: Choosing the appropriate treatment option. Oncology (Williston Park) 2007;21:185–193. [PubMed] [Google Scholar]
- 5.Newling DW, Dennis L, Vermeylen K. Orchiectomy versus goserelin and flutamide in the treatment of newly diagnosed metastatic prostate cancer: Analysis of the criteria for evaluation used in the European Organization for Research and Treatment of Cancer. Genitourinary Group Study, 30853. Cancer (Phila) 1993;72:3793–3798. doi: 10.1002/1097-0142(19931215)72:12+<3793::aid-cncr2820721706>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Bok RA. Treatment of prostate cancer: Therapeutic potential of targeted immunotherapy with APC8015: Therapeutics and clinical risk management. 2008;4(1):79–85. doi: 10.2147/tcrm.s905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burch PA, Breen JK, Buckner JC, et al. 2000. Priming tissue-specific C cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 8.Patel PH, Kockler DR. Sipuleucel-T: A vaccine for metastatic, asymptomatic, androgen-independent prostate cancer. Ann Pharmacother. 2008;42(1):91–98. doi: 10.1345/aph.1K429. [DOI] [PubMed] [Google Scholar]
- 9.Risk M, Corman JM. The role of immunotherapy in prostate cancer: An overview of current approaches in development. Rev Urol. 2009;11(1):17–27. [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy G, Tjoa B, Ragde H, et al. Phase I clinical trial: T-cell therapy for prostate cancer using autologous, dendritic cells pulsed with HLA-A0201–specific peptides from prostate specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Understanding Cancer Series: The Immune System. National Cancer Institute. Available at: www.cancer.gov/cancer-topics/understandingcancer/immunesystem/Page34. Accessed February 25, 2011. [Google Scholar]
- 12.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 13.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 14.Higano CS, Schellhammer PF, Eric J, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 15.Provenge (sipuleucel-T) suspension for intravenous infusion, prescribing information. Seattle, Wash.: Dendreon Corp.; 2010. Available at: www.dendreon.com/prescribing-information.pdf. Accessed November 15, 2010.
- 16.Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: A phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 17.Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20(3):241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 19.Force R, Pugmire B, Culbertson V, et al. A taxane total cost of care analysis in metastatic breast cancer: Results of private payor claims data (Abstract 1076) Cancer Res. 2009 2009 Dec 15;69(24 Suppl 3) online. [Google Scholar]
- 20.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 21.Miller D. Dendreon’s Provenge costs the same as chemotherapy. Minyanville Media, July 29, 2010. Available at: www.minyanville.com/businessmarkets/articles/dendreon-provenge-cost-taxotere-sanofi-aventis/7/29/2010/id/29371. Accessed November 5, 2010.
- 22.Mulkhy N. Medicare already paying for Provenge for some patients: National coverage analysis still important. Medscape Medical News, January 6, 2011. Available at: www.medscape.com/viewarticle/735366. Accessed February 2, 2011.