Abstract
The uptake, bioaccumulation, biotransformation, and risks of nanomaterials (NMs) for the food crops are still not well understood. Very few NMs and plant species have been studied, mainly at the very early growth stages of the plants. Most of the studies, except one with multiwalled carbon nanotubes performed on the model plant Arabidopsis thaliana and another with ZnO nanoparticles (NPs) on ryegrass, reported the effect of NMs on seed germination or 15 day old seedlings. Very few references describe the biotransformation of NMs in food crops and the possible transmission of the NMs to the next generation of plants exposed to NMs is unknown. The possible biomagnification of NPs in the food chain is also unknown.
Keywords: Food crops, Nanomaterials uptake, Genotoxicity, Phytotoxic effects, Biotransformation
1. Introduction
Studies have shown that environmental conditions may influence plant ion concentrations in crop plants (1–3). Under specific growing environment, plants absorb essential and non-essential elements, which above certain concentration may cause toxicity (4). In addition, toxic elements with no known function in biological systems are found to be accumulated in plant tissues, with lethal effects for non-tolerant species (5–9). Once stored within plants, beneficial or toxic elements can be transferred from producers (plants) to consumers. For instance, selenium laden plants can be used to supply selenium deficiencies in ruminants and other animals (10, 11); however, this is still under scrutiny because the borderline between deficiency and toxicity is very narrow (12).
Plants have evolved in the presence of natural nanomaterials (NMs). However, the probability of plant exposure to NMs has increased to a greater extent with the ongoing increasing production and use of engineered nanomaterials (ENMs) in a variety of instruments and goods (13). ENMs can reach the plants through direct application, accidental release, contaminated soil/sediments, or atmospheric fallouts. Though, little is known about the impact of ENMs on food crops and their possible effects in the food chain are unknown (14, 15). A few studies on the toxicity of ENMs have been performed on crop plants such as rape (Brassica napus), radish (Raphanus sativus), lettuce (Lactuca sativa), corn (Zea mays), and cucumber (Cucumis sativus), among others (16–18). These studies and all the most recent publications on the absorption, translocation, accumulation, and biotransformation of ENMs (metal based (MB) and carbon based (CB)) in edible plants are included in this review. Although MB NMs include nanoparticles (NPs, materials with at least two dimensions between 1 and 100 nm) and other NMs (materials with at least one dimension of 100 nm), only reports on NPs will be discussed (19). The reviewed reports on CB NMs include fullerenes and carbon nanotubes (single walled (SWCNTs and multi-walled MWCNTs) (20).
2. Uptake, translocation, and accumulation of NPs into the edible plants
The uptake of CB and MB NMs by plants is a very recent field of study. Most of the data corresponds to the germination stage and cell cultures. Because the protocols for quantification of NPs within tissues are not well defined yet, the discussion of the current literature is more oriented to the effect of the NPs on plants. Among CB NMs, the most studied materials are the fullerene C70, the fullerol (C60(OH)20) and CNTs; while the most studied MB NMs are TiO2, CeO2, FeO, and ZnO NPs. The uptake of other MB NMs such as Au, Ag, Cu, and Fe NPs is also discussed.
2.1 Mode of uptake of nanoparticles by plants
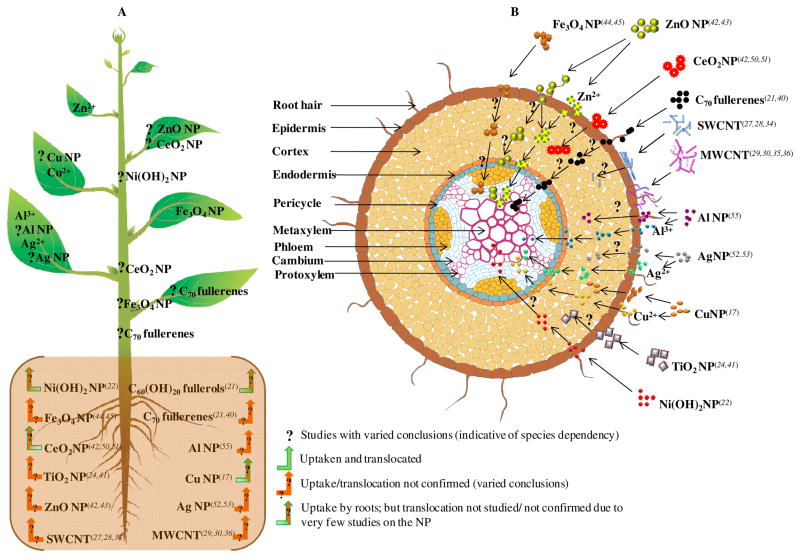
Current literature revealed that the uptake, translocation, and accumulation of NPs depend on the species of plant and the size, type, chemical composition, functionalization, and stability of the NPs. Among the CB NMs, only the fullerene C70 and fullerols were shown to get readily accumulated in plants. Conversely, most of MB NPs were found to be taken up and accumulated in plants, although some conflicting data exists. The selective uptake, biotransformation, and translocation of various nanoparticles by a model plant have been schematically represented in Figure 1. As seen in Fig. 1A, and discussed in the following sections, concluding studies about the absorption and movement of NPs within living plants pertain only to fullerols, Ni(OH)2, and Cu NPs, (21–23). Results for the uptake of other types of NPs are shown in Figure 1B. As shown in this figure, the published data about NPs uptake by plants is still not conclusive.
Figure 1.
Uptake, translocation and biotransformation pathway of various nanoparticles in a plant system. A. Plant showing the selective uptake and translocation of nanoparticles. B. Transverse cross section of the root absorption zone showing the differential nanoparticle interaction on exposure. The superscripts depict the reference cited (Drafted by S. Majumdar).
Several avenues for the uptake of NPs by plant cells have been proposed (Figure 2). Asshown in this figure, the data suggests that NPs can enter plant cells by binding to ca rrier proteins, through aquaporins, ion channels, endocytosis, creating new pores (preferably for CNTs) or by binding to organic chemicals in the environmental media. Due to increased surface area to mass ratio of the NPs as compared to the bulk metals, they are thought to deliver more reactivity with their surroundings. The NPs may form complexes with membrane transporters or root exudates (Figure 2) and subsequently be transported into the plants (24, 25). Most of the MB NPs that have been reported as taken up by plants include elements for which ion transporters have been identified (26). Once inside the cells, NPs may be transported apoplastically or symplastically. They may be transported from one cell to the other through plasmodesmata. However, the exact mechanisms why only some plant species readily take up several NPs are still unknown and remain to be explored.
Figure 2.
Probable modes of cellular uptake of the nanoparticles in a plant cell (Drafted by S. Majumdar).
2.2. Uptake of carbon based nanomaterials by plants
With the recent developments involving CNTs in smart delivery systems of various biomolecules/genes/drugs into the cells, studies are increasingly being performed in an effort to find the uptake and transport mechanism of CNTs into the intact plant cells. Although SWCNTs are too large to penetrate cell walls, Shen et al. (27) showed an evidence of endocytosis-like structure in the plasma membrane in an Arabidopsis thaliana leaf cell. Although not a food crop, but the model plant for studying plant biology, the results obtained with Arabidopsis are deemed extremely relevant and significant to guide further studies with edible plants. Studies with cell suspensions of Nicotiana tabacum cv. Bright Yellow (BY-2) showed that thewater soluble SWCNTs (< 500 nm in length) were found to penetrate the intact cell wall and the cell membrane through fluidic phase endocytosis (28). In addition, it has been reported that MWCNT were taken up by the seeds and root systems of the developed tomato seedlings (29). It was hypothesized that the MWCNTs were able to penetrate the seed coat by creating new pores; thereby, enhancing water uptake. MWCNTs were also visualized initially on the root surface and ultimately piercing the epidermal and root hair cell walls and root cap of wheat seedlings (30). Although the above mentioned studies suggest potential modes of uptake of nanotubes, results are insufficient to conclude whether the nanotubes translocate from the root systems to the aerial parts of the plants. The hydrophobic properties of the MWCNTs render them capable to interact with many organic substances (31). It has been suggested that the very low surface friction of CNTs facilitate the flow of organic substances into the cytoplasm (32). This property has been explored for phytoremediation purposes. Ma and Wang (33) found that C60 at 15 mg L−1 increased the uptake of trichloroethylene by 82% in cottonwood (Populus deltoides) cuttings.
Conversely, Canas et al. (34) found no uptake of SWCNTs and functionalized SWCNTs (F-SWCNTs) by roots of cucumber seedlings after 48 h treatment. However, the SWCNTs were found adhered on the external surface of the main root and secondary roots in the form of nanotube sheets. Other studies reported that the cell walls of rice cell suspension restrict the entry of the MWCNTs into the cellular cytoplasm (35). MWCNTs were reported to form black clumps tightly wrapping around and associating with the cells (36). The clumps were found to increase in number and size with increase in concentration. The CNTs are hypothesized to interact with the proteins and polysaccharides on the cell wall and elicit hypersensitive responses mimicking plant pathogens due to their small size, eventually leading to cell mortality (35–37). This is also supported by identification of non-covalent interactions between CNTs and rice starch (38). This hypersensitive response is considered to be responsible for prevention of the entry of MWCNTs through the plant cell walls.
The uptake of the CNTs by plants also depends on its dispersion in the experimental media. In natural ecosystems the effect and uptake of NMs by the plants is expected to depend to a great extent on the chemical properties, organic content, and the colloidal properties of the associated soil, sludge, or the sediments. Natural organic matter (NOM) is a collection of heterogeneous organic substances from decomposed living species, which may pose as important factor impacting nanomaterial exposure to plants (21). The hydrophobic moieties of NOM are believed to interact with the hydrophobic carbon of NP surfaces giving rise to a dynamic equilibrium process (39). Lin et al. (40) studied the uptake, accumulation, and the translocation of NOM suspended fullerene C70 and MWCNT in rice plants. He reported the presence of C70 in the form of black aggregates that were more abundant in the seeds and roots, compared to the stems and leaves of the rice seeds. The presence of NOM-C70 aggregates in leaves suggests that they followed the transmission route of water and the nutrients through the xylem. In the matured plants, NOM-C70 were predominantly present in or near the stem’s vascular systems and leaves; whereas, the roots were noted to be devoid of C70 supporting the claim of robust translocation from the roots to the aerial parts of the plant. In contrast, the seeds treated with NOM-MWCNT showed insignificant uptake, with very few aggregates of nanotubes in the vascular system and none in the tissues. The individual fullerene C70 NPs were hypothesized to enter the plant roots through osmotic pressure, capillary forces, pores on cell walls, and intercellular plasmodesmata, or via the highly regulated symplastic route. In a contrasting study, Chen et al. (21) reported that hydrophobic fullerenes C70-NOM blocked the cell wall pores in Allium cepa cell suspensions, resulting in negligible uptake of the NPs by the cells. Whereas, the small size and greater hydrophilicity of the fullerols C60(OH)20 allow their permeability through A. cepa cell walls and eventually accumulates at the interface between cell wall and plasma membrane. Moreover, the C60(OH)20 were found to accumulate between adjacent epidermal cell walls indicating its apoplastic mode of transport in the plant tissues. The above discussed results clearly indicate that CB NMs can be taken up by some edible plants, but more studies are needed to establish the uptake mechanisms and consequences of their accumulation in edible plants species.
2.3. Uptake of metal oxide nanoparticles by plants
2.3.1 Uptake of TiO2 nanoparticles
Although TiO2 NPs are profusely used in daily life products, the report on their uptake and translocation in plants is really limited, especially on food crops. Kurepa et al. (24) reported that an ultra small TiO2 (<5nm) complexed with Alizarin red S nanoconjugate was taken up and translocated by A. thaliana seedlings following tissue and cell specific distribution. He showed that roots of A. thaliana released mucilage that formed a pectin hydrogel capsule surrounding the root which could either inhibit or facilitate the entry of the TiO2 complexed with Alizarin red S or sucrose. Other studies have shown that polysaccharides in mucilage might adsorb and inactivate toxic heavy metals in the rhizosphere or enhance accumulation depending on the plant species (25). Asli and Neumann (41) investigated the uptake and translocation of TiO2 NPs (30 nm) in maize (Zea mays) excised roots having intact apices. The NPs were not seen to be taken up by the root cells, probably due to its large size compared to the size of the pore diameter (6.6 nm) in the root cell wall of maize. The high stability of TiO2 NPs makes the digestion process difficult for TiO2 treated plant samples. Possibly, this is one of the reasons why the number of reports on the uptake of TiO2 by plants is so limited.
2.3.2 Uptake of ZnO nanoparticles
As in the case of other MB NPs, the uptake, translocation and accumulation of ZnO NPs in food crops are not well understood. In addition, most of the studies have been carried out until germination stage, which provided limited information because of the incomplete plant root and vascular system development. Lopez-Moreno (42) investigated the uptake and accumulation of ZnO NPs (8 nm) by soybean (Glycine max) seedlings. These researchers treated the soybean seeds with ZnO NPs in the range of 500–4000 mg L−1. The Zn uptake by the seedlings was significantly higher at 500 mg L−1, perhaps because at this concentration the NPs have lesser aggregation. At high concentrations (1000–4000 mg L−1), an increase in the probability for formation of agglomerates is proposed. This makes passage through the cell pore walls difficult; thereby, reducing uptake and accumulation as understood from the results. These researchers performed x-ray absorption spectroscopy (XAS) analysis of the ZnO NP treated samples. The XAS results showed Zn2+ inside the plant tissues but the spectrum resembled more the one of Zn acetate and nitrate than ZnO NPs. Although ZnO NPs are expected to be a source for Zn2+ found within tissues, the study fails to highlight whether the Zn2+ was contributed by the biotransformation of the ZnO NPs on/in roots. However, it may be hypothesized that root exudates ionized the ZnO NPs on the root surface, as no trace of ZnO NPs were shown by the XAS spectra. But, more elaborative studies need to be performed to confirm the biotransformation of the ZnO NPs and the associated mechanism and factors affecting the ionization. In ryegrass, scanning electron microscopy studies confirmed the adsorption and aggregation of the ZnO NPs to the root surface (43). The high magnification TEM images of the ryegrass root cross-sections also showed the presence of particles in the apoplast, cytoplasm and nuclei of the endodermal cells and the vascular cylinder, presumed to be ZnO NPs. Unfortunately, X-ray absorption studies confirming the presence of the NPs were not presented.
2.3.3 Uptake of iron oxide nanoparticles
Only studies on the edible plants pumpkin and lima beans treated with Fe3O4 NPs were found. Zhu et al. (44) studied the uptake of magnetite (Fe3O4 NP, 20 nm diameter) by pumpkin seedlings in hydroponic conditions using a vibrating sample magnetometer. It was reported that the signal for magnetic NPs were detected in roots, stems, and leaves of pumpkin plants. However, the uptake of the NPs was also seen to depend on the growth medium, because no uptake was observed when grown in soils and reduced uptake when grown on sands. This may be attributed to the adherence of the Fe3O4 NPs to the soil and sand grains. It seems that the uptake also depends on the species of plant because no uptake of Fe3O4 NPs was found to occur in treated lima bean plants (Phaseolus limensis). On the other hand, Wang et al. (45) did not notice any uptake of 25 nm Fe3O4 NPs by the pumpkin plants. It has been hypothesized that it is difficult for the large size NPs to penetrate through the cell walls and transport across the plasma membranes. The cell wall pore sizes vary from 2–20 nm, while the size of ions and water molecules are about 0.28 nm. Thus ions and water find their ways through ion channels and aquaporins, respectively (46–49).
2.3.4 Uptake of CeO2 nanoparticles
It was found in two recent studies that seedlings of soybean, alfalfa (Medicago sativa), corn, and tomato (Lycopersicon esculentum) accumulate Ce in tissues as the external concentration of CeO2 NPs (7 nm) increased (42,50). Interestingly, at 4000 mg CeO2 L−1, the concentration of Ce (mg kg−1 DW biomass) significantly varied between species (≈300 for corn, 462 for soybean, 4000 for tomato, and 6000 for alfalfa). This differential accumulation could be explained by specific differences in root microstructures and the physical and chemical interactions between the NPs and the root exudates in the rhizosphere. This opens up an area of extensive research. X-ray absorption near edge structure (XANES) analysis confirmed the presence of CeO2 NPs in the root samples of all of the tested plant species. However, whether the resemblance of the spectrum of the plant roots was due to the absorption into the roots or binding of the CeO2 nanoparticles to root hairs and exudates was not very clear. In a more recent study Birbaum et al. (51) reported that CeO2 NPs applied as aerosol or suspension on corn leaves, were absorbed by the leaves, but not translocated to new leaves. It was also reported that NPs applied in the irrigation water resulted in no detectable translocation of the NPs within the plant. This could suggest that the absorption and translocation of the nanoceria is species dependent.
2.3.5. Uptake of nickel hydroxide nanoparticles
Only one reference was found about the uptake of Ni(OH)2 NPs. Parsons et al. (22) investigated the uptake and translocation of Ni(OH)2 NPs (8.7 nm) in mesquite (Prosopis sp.). The accumulation and oxidation state of the NPs was studied by x-ray absorption spectroscopy. The x-ray absorption near edge structure (XANES) spectra showed the presence of Ni(OH)2 NPs in roots and shoots of plants treated with uncoated NPs; whereas, plants treated with citrate coated NPs before or after synthesis showed Ni NPs only in roots. Results also showed that none of the treatments reduced plant size or chlorophyll production, but the study was conducted with plants at the seedling stage.
2.4 Uptake of metallic nanoparticles
The uptake and toxicity of NPs may be either due to its small size, surface characteristics, aggregation and associated characteristics or it may also be mimicking and expressing the toxicity associated with element. Stampoulis (52) was the first to report on the uptake of Ag NPs in zucchini (Cucurbita pepo) compared to its corresponding bulk counterpart. The Ag concentration in the plant shoots was found to be on an average 4.7 times higher in the plants exposed to 10–1000 mg L−1 Ag NPs than those treated with bulk Ag powder at similar concentrations. It has been suggested that the greater ion release from Ag NPs is responsible for the greater Ag concentration in the shoots. However, the form of Ag (nanoparticles, aggregates or ionic) inside the plants was not studied. In another study, Brassica juncea plants treated with Ag NPs did not seem to accumulate Ag in any form (53). Corredor et al. (54) reported that carbon-coated Fe NPs applied to the leaf petioles of living pumpkin plants were found only in the epidermal cells close to the application site. No NPs were traced in the cells located distant from the application points or near the xylem.
Lee et al. (23) investigated the uptake and translocation of Cu NPs in mungbean (Phaseolus radiata) and wheat (Triticum aestivum) in agar growth medium. The study showed that the Cu NPs could cross the cell membrane and agglomerate in the cells. The bioaccumulation factors (amount of Cu in plant dry weight tissue divided by amount of Cu in the growth media) of mungbean and wheat plants exposed to 1000 mg Cu NPs L−1 were 8 and 32 mg kg−1, respectively. Also, a responsive relationship between the bioaccumulated NPs in plant tissues and growth media was observed. Likewise, Doshi et al. (55) investigated the uptake of Al in red kidney beans exposed to 10–10000 mg Al NPs kg−1 (1–100 nm) in soil. Results showed that the Al concentration in the red kidney beans was not significantly different with that of the untreated control. Unfortunately, in both studies (23,55), it was not quantified how much of the Ag or Al content in plant was in the form of NPs. However, the results suggested that the uptake of metallic NPs is species-specific.
There are reports on the reduction of metal ions into NPs and subsequent accumulation of the NPs in edible plants. Gardea-Torresdey et al. (56, 57) found that Au(III) and Ag(I) ions in agar solid growth media got reduced and accumulated as Au and Ag NPs inside alfalfa seedlings. Similarly, biotransformation and accumulation of Ag(I) and Pt(II) ions into Ag and Pt NPs in alfalfa and mustard seedlings were reported (58,59). These studies strongly suggest that Au, Ag, and Pt NPs can get produced/accumulated in alfalfa and mustard seedlings.
2.5 Storage of NPs in plants
There are few issues arising from the observation that nanoparticles are accumulated in plants. One important question that demands attention is how and where within the plants are the absorbed nanoparticles stored. At the present time there are no specific studies addressing this gap. Available literatures indicated vaguely that nanoparticles are found in the plant’s cells and tissues. Although one of the abovementioned studies with SWCNTs in N. tobacum plant cell suspensions, found their fate in vacuoles (SWNTs- FITC) as well as cytoplasmic strands (SWCNT- DNA) (22). A study on the reduction of Ag ions into Ag NPs and its accumulation in alfalfa showed that Ag NPs could accumulate on the surface of root cell organelles (58). However, Gardea-Torresdey et al. (57) showed the presence of Ag NPs in alfalfa stems. One more issue corollary to the accumulation of NPs in edible plants is their transmission to the plant’s next generation. Lin et al. (40) reported that C70 were detected, though much less frequently, in the leaf tissues of second generation rice plants. If NPs are found in the second generation plants, there is the possibility that these plants become adapted and more responsive, accumulating more of the respective NPs. Another important issue is the bioavailability of the accumulated NPs to the next trophic level, e.g. in humans and ruminants. There are studies showing that NPs in algae and tobacco are transmitted to the next trophic level (20, 60).
3. Cellular toxicity, genotoxicity and transmission of ENMs in the edible plants
Characteristics such as small size, shape and larger surface area to mass ratio of NPs have created a new dimension in current science and technology with their varied applications in medicine, drug development, and many more applications. On the other hand, the propensity of the NPs to cross cell barriers and their interactions with intracellular structures owing to their small size and high surface reactivity, contribute to potential cellular and genetic toxicity by the induction of oxidative stress (61–62).
Very few studies have been conducted on the genetic response rendered by the NPs on edible plants. Plants with long and low number of chromosomes have been considered to be excellent cytogenetic systems with a wide range of genetic endpoints, from gene mutation to mitotic and meiotic chromosome aberrations, alterations in ploidy, sister chromatid exchanges and DNA damage (63).
Tan et al. (35,36) reported that MWCNTs reduced the cell density on cultured rice cell suspensions in a dose dose-dependent manner. At lower concentration of MWCNTs the cell death was predominantly noted to be caused by apoptosis; however at higher concentration cell mortality was attributed to necrosis identified by leakage of cytoplasmic content and membrane disruption. On the better side, the rice cells in the suspensions were observed to demonstrate self-defense response, when exposed to MWCNTs, by sacrificing a small population of cells that aggregates with the NPs and precipitates. This protected the remainder of the cells in culture (35).
The studies by Shen et al. (27) in rice and Arabidopsis protoplast cells well established that the nano size and the concentration of the SWCNTs were responsible for potential cytotoxicity. Abundant endonucleolytic cleavage of DNA was evident in the Arabidopsis cells proving the genotoxic potential of the SWCNT in plant systems. Other studies (32) have shown that in A. cepa, the more water soluble and small sized fullerol C60(OH)20 produced more cell damage than the fullerene C70 suspended in natural organic matter (C70-NOM). The cell damage was also attributed to the aggregation of the NPs leading to blockage of the apoplastic pathway, which is a probable route of uptake of these NPs in the plant tissues. Lin et al. (40)studied the generational transmission of C 70-NOM in rice plants and established the presence of black aggregates of C70 in the leaves of the second generation of the plants treated with the fullerenes only in their first generation. Although the study at the molecular level was not included, the data highly suggest that the first generation plants had taken up and genetically transmitted the NPs to its next generation.
Studies on the cytotoxicity of metal oxide NPs only include a recent report by Lopez-Moreno et al. (42) with CeO2 and ZnO NPs in soybean seedlings. The genotoxicity of both NPs were investigated by detecting new DNA bands using the random amplified polymorphic DNA (RAPD) assay. A new DNA band in the RAPD profile of soybean roots treated with ZnO NPs at 4000 mg L−1 was detected. According to Lopez-Moreno et al. (42) the toxicity may rise either due to the interaction of the DNA with the Zn ions leached out from the ZnO NPs or with its direct interaction with the ZnO NPs. But the absence of ZnO NPs in plant tissues, as shown by the XANES results, failed to conclude on the potent reason behind genotoxic response in soybean. On the other hand, the RAPD profile of soybean roots treated with CeO2 at 2000 and 4000 mg L−1 showed four and three new bands, respectively. Thus, cubic CeO2 NPs (7nm) were shown to affect the genetic stability to a greater extent in comparison with a slightly larger sized hexagonal ZnO NPs (8 nm). Apart from the size and shape of the NPs, the differential genotoxic response may also be attributed to the fact that CeO2 NPs were shown by the XANES spectra within the tissues and, unlike Ce, Zn is an essential element used in several biochemical processes, as established by several researchers (64–67).
TiO2 NPs (~100 nm in size) were found to be genotoxic as well as cytotoxic in plant systems (68). In their study, Ghosh et al. (68) found an initial increase in the extent of DNA damage in Allium cepa (~ 3.5 fold increase at 4 mM concentration) followed by gradual decrease until the highest selected concentration (10 mM). The decrease was explained by the precipitation of nanomaterials at high concentration. The genotoxic potential of TiO2 in this plant was confirmed by comet assays and DNA laddering technique. Presence of chromosomal aberrations and interphase micronuclei in A. cepa plants validated the occurrence of cellular fragmentation in the previous cell cycle. The genotoxic and the cytotoxic effects observed were well correlated with the results of the generation of superoxide radicals resulting in lipid peroxidation in the A. cepa cells.
The literature on the cytotoxicity of metallic NPs on edible plants only includes Ag and Fe NPs. In A. cepa root meristem cells, Ag NPs have been reported to possess mitodepressive, mitoclassic and clastogenic properties (63,69). Dose dependant decrease has been noticed in the frequency of mitotic index in the Ag NP treated A. cepa cells from 60.30% (control) to 27.62% (100 mg Ag NPs L−1) (63). Babu et al. (69) noted dose as well as duration-dependent decrease in mitotic index in response to Ag NPs in A. cepa root meristems. This mitodepressive and cytotoxic response may be attributed to the inhibition of DNA synthesis at S-phase of the cell cycle (70). Kumari et al. (63) on treating the A. cepa cells with varying concentrations of Ag NPs noticed different kinds of chromosomal aberrations like stickiness, chromosomal breaks, gaps, disturbed metaphase and cell wall disintegration at different doses. In a similar in vivo cytogenetic assay carried out by Babu et al. (69), various types of chromosomal and mitotic abnormalities such as fragments, C-metaphase, sticky metaphase, laggard chromosomes, anaphasic bridge and disturbed anaphase were identified in the treated meristem cells. The interaction of Ag NPs with tubulin-SH group may probably be responsible for the ineffective mitotic spindle function (70). As explained by Kumari et al. (63), the stickiness in the metaphase and anaphase stages may be attributed to degradation or depolymerization of chromosomal DNA or by intermingling of inter chromosomal chromatin fibers, which leads to sub chromatid connections between chromosomes (71–73). The induction of chromosomal breaks and micronuclei by Ag NPs indicates the clastogenic potential of the xenobiotic, which may lead to a loss of genetic material (74). Thus, these may be regarded as an endpoint of irreversible genotoxicity on the chromosomes. Although the genotoxicty of the Ag NP is well established, the causative mechanism has not been reflected in this study. Moreover, no studies have been made to clarify if the toxicity is caused by the Ag NPs themselves or the Ag(II) ions released from the NPs in the external/biological media.
The water based magnetic NPs (50–300 μl/L) coated with perchloric acid have been reported to decrease the nucleic acid level in the cells of corn revealing an inhibitory effect on biosynthesis (75). In a related study, treatment with magnetite NPs coated with β-cyclodextrin (C42H70O35) as stabilizer resulted in increased total prophase, metaphase, anaphase and telophase at high volume fractions of the fluid, coupled with linear increase in mitotic index with increasing volume fraction of magnetite NPs (75). The coatings on the magnetic NPs prevent close approach of the nanomagnetic cores, reducing aggregation via Van der Waals or magnetic attractions. Răcuciu and Creangă (76) hypothesized that the ferrophase might have penetrated the nuclear membrane and the extra nuclear- DNA from the chloroplasts is the one of the most probable target of magnetic fluids. The magnetic NPs were also reported to induce chromosomal aberrations and perturbation of the proliferative capacity.
It is not clear from the abovementioned studies whether the genotoxicity in plants is caused by the NPs themselves or their biotransformation within the plants. Therefore, more research needs to be focused on the differences in toxicity of MB NPs with their respective bulk counterparts, the increased concentration of the element if it is an essential micronutrient, and the effect of the ions produced inside or outside the organism exposed to the NPs. Moreover, the factors associated with the varied toxicological responses of different plant species to different NPs have not, yet, been well explored.
4. Factors affecting the toxicity of NPs in the edible plants
The toxicity of NPs in edible plants has been discussed in several reviews (77–80). Studies revealed that not all plants treated with NPs manifested toxicity effects, in fact more studies revealed positive or no consequential effects in plants (Table 1). However, caution must be observed when making conclusions about the effect of particular NPs. Physiological and visual toxicological effects in plants might not be a sensitive indicator of toxicity. Studies at proteomic, genomic, and metabolic levels are needed.
Table 1.
Positive or no consequential effects of nanoparticles in food crops
| Nanoparticle | Particle size (nm) | Plant | Concentration (mg L−1) | Observed toxicity | Reference |
|---|---|---|---|---|---|
| Zero valent Fe | - | Flax, Red clover, White clover, Meadow fescue | 100, 250, 500 | No effect on germination | (82) |
| Barley, Ryegrass | 100, 250 | No effect on germination | (82) | ||
| Al | Radish, Rape, Lettuce, Corn, Cucumber | 2000 | No effect on germination | (16) | |
| 1–100 | Red kidney beans, Ryegrass | 10, 100, 1000, 10000 | No observed toxicity | (55) | |
| - | Radish, Rape | 2000 | Improved root growth | (16) | |
| Ag | 20 | Flax | 20, 40, 60, 80, 100 | No effect on the germination | (82) |
| 2 | Cucumber, Lettuce | 62, 100, 116 | Low to zero toxicity | (18) | |
| Au | 10 | Cucumber, Lettuce | 62, 100, 116 | Positive effect on germination index | (18) |
| Si | - | Zucchini | 1000 | No effect on the germination | (52) |
| Cu | - | Lettuce | 0.013% (w/w) | No effect on the germination; improved shoot/root ratio | (84) |
| Dodecanethiol functionalized Au | - | Lettuce | 0.013% (w/w) | No effect on the germination; improved shoot/root ratio | (84) |
| Pd entrapped in Al(OH)2 matrix | - | Lettuce | 0.013–0.066% (w/w) | No effect on the germination; improved shoot/root ratio | (84) |
| 3-amino functionalized SiO2 | - | Lettuce | 0.013–0.066% (w/w) | No effect on the germination; improved shoot/root ratio | (84) |
| CeO2 | 7 | Corn, Alfalfa, Soybean | 500, 1000, 2000, 4000 | Significantly increased root and stem growth | (50) |
| <25 | Wheat | 100 | (30) | ||
| ZnO | 8 | Soybean | 500 | Increased root growth | (42) |
| Al2O3 | Radish, Rape, Ryegrass, Lettuce, Corn, Cucumber | 2000 | No effect on germination | (16) | |
| Fe3O4 | 20 | Pumkin | 500 | No toxic effect | (44) |
| 7 | Cucumber, Lettuce | 62, 100, 116 | Low to zero toxicity | (18) | |
| TiO2 | <100 | Wheat | 100 | (30) | |
| Nanoanatase (TiO2) | 4–6 | Spinach | 0.25% | Enhanced rca mRNA expressions (51%), protein levels (42%), activity of Rubisco activase, Rubisco carboxylation, the rate of photosynthetic carbon reaction, single plant dry weight, chlorophyll content | (91) |
| 5 | Spinach | 0.25% | Improved spinach growth related to N2 fixation by TiO2 | (92) | |
| 5 | Spinach | 0.25% | Improved light absorbance, transformation from light energy to electron energy, and active chemical energy, and promoted carbon dioxide assimilation | (93) | |
| Rutile (TiO2) | - | Spinach (naturally aged) | 0.25–4% | Increased germination and germination and vigor indexes, plant dry weight, chlorophyll formation, ribulosebisphosphate carboxylase/oxygenase activity, photosynthetic rate | (86) |
| - | Spinach | 0.25–4% | Promoted photosynthesis, the rate of evolution of oxygen in the chloroplasts was accelerated | (87) | |
| Ni(OH)2 | 8.7 | Mesquite | 2 | No effect | (22) |
| Mixture of SiO2/TiO2 | Soybean | Increased germination and shoot growth Increased nitrate reductase activity Increased absorption and utilization of water/fertilizer Enhanced antioxidant system |
(85) | ||
| Mixture of Au/Cu | - | Lettuce | 0.013% (w/w) | No effect on the germination; improved shoot/root ratio | (84) |
| Multi-wallled carbon nanotube | - | Tomato | 10–40 | Significant increase in germination rate, fresh biomass, and length of stem; significantly enhanced moisture content inside tomato seeds | (29) |
| - | Radish, Rape, Ryegrass, Lettuce, Corn, Cucumber | 2000 | No effect on germination | (16) | |
| - | Ryegrass | 2000 | Increased root length | (16) | |
| - | Zucchini | No effect on the germination | (52) | ||
| internal dimension: 110–170 | Wheat | 100 | No significant effect on root or shoot growth | (30) | |
| Single-wallled carbon nanotube | 8 | Onion, Cucumber | 104, 315, 1750 | Significantly increased root length | (34) |
| 8 | Cabbage, Carrot, Lettuce | 104, 315, 1750 | No effect | (34) | |
| Functionalized single-walled carbon nanotube | 8 | Cabbage, Carrot, Tomato, Onion, Lettuce | 9, 56, 315, 1750 | No effect | (34) |
Currently, there is no definite mechanism on the toxicity of NPs in plants. Many researchers believe that the observed toxicity of NPs in plants is based on plant-NP physical interactions. The presence of NPs on the root surface could alter the surface chemistry of the root such that it affects on how the roots interact with its environment (34). Studies revealed that plant development is negatively affected because NPs clog the root openings and both hydraulic conductivity and nutrient uptake in roots are inhibited (41).
Studies on NPs in food crops revealed that various factors influence toxicity, and that at present, no general trend could be made yet. Based on several studies, the following are the principal factors that influenced toxicity in agricultural food crops: concentration of NPs, particle size and specific surface area, physicochemical properties of NPs, plant species, plant age/life cycle stage, growth media, NP stability, and dilution agent (81).
The size of seeds could render more sensitivity to NPs exposure (34, 82). This is because a large-seeded species (e.g. cucumber) have a lower surface to volume ratio than a small-seeded species (e.g. tomato). SWNTs showed higher toxicity effects in the smaller-seeded species (lettuce, onion, and tomato) than in large-seeded species (cucumber) (34, 80). However, a clear effect of the size of seeds on the toxicity of nanoparticles in plants cannot be confirmed at this time (16, 23, 50).
Lee et al. (23) reported that mungbean was more sensitive than wheat to Cu NPs toxicity probably due to differences in root anatomy since xylem structures determine the speed of water transport, and different xylem structures may demonstrate different uptake kinetics of nanoparticle(78). Mungbean is a dicot with one large primary root and several smaller lateral roots while wheat is a monocot with numerous small roots without a primary root. However, generalization on whether the toxicity is based on dicot or monocot classification cannot be made (16,50, 82).
Solvent was reported to affect the toxicity of nanoparticles. In the study conduct ed by Barrena et al. (18), it was observed that the effect of nanoparticles-solvents was sometimes more significant than that of the nanoparticles themselves. Three MB NPs (Au, Ag, and Fe3O4) demonstrated low to zero toxic effects on lettuce and cucumber, and effects could be primarily due to the presence of stabilizers. The very small size of nanoparticles is believed to cause higher toxicity in plants. This was generally observed in many studies. Various sizes of silver NPs were tested and result showed that Agcolloid (0.6–2 nm) had greater toxicity in flax, barley and ryegrass than Ag NPs of 5 and 20 nm (82). The particle surface characteristic was also important factor in nanoparticle toxicity (83).
The concentration of nanoparticle is another major factor affecting toxicity in food crops. Based on the macroscopic standard phytotoxicity tests (germination/root elongation or vigor test), it is possible to conclude that food crops tested required high concentrations of nanoparticles (1000–4000 mg L−1) before toxic effects begin to manifest (Table 2). Zero valent Fe nanoparticles completely inhibited germination of ryegrass, flax and barley at very high concentrations (2000 and 5000 mg L−1). Likewise, ZnO NPs at 1000 mg L−1 caused death of almost all living cells at the root tip of ryegrass (43). Cu NPs reduced root and seedling growth of wheat only at a relatively higher concentration (< 200 mg L−1). These observations raise important considerations in future toxicity studies. First, high concentration of NPs may not be realistic since it is not commonly found in the natural environment. Second, if high concentration is already required for NPs to exhibit toxicity at hydroponic set up, a much higher concentration might be needed to cause toxicity in the natural environment like soil. In fact, toxicity studies conducted in different soil media required higher amount of NPs to induce toxicity in plants (55, 82, 84). Lastly, most plants showed visible signs of recuperation from NP toxicity indicating that toxicity was temporary.
Table 2.
Negative effects of nanoparticles on different food crops
| Nanoparticle | Particle size (nm) | Food crop | Concentration (mg/L) | Growth media | Observed toxicity | Reference |
|---|---|---|---|---|---|---|
| Zero valent Fe | - | Flax, Barley, Ryegrass | 2000, 5000 | Aqueous suspension | Completely inhibited germination | (82) |
| - | Barley | 300 | Sandy soil | Reduced germination | (82) | |
| - | Flax, Barley, Ryegrass | >1500 | Sandy and clay soil | No germination | (82) | |
| Ag (colloid) | 0.6–2 | Ryegrass | 10 | Aqueous suspension (0.1% v/v Tween 20) | Reduced germination (20%) | (82) |
| Ryegrass | 20 | Aqueous suspension (0.1% v/v Tween 20) | Reduced germination (50%) | (82) | ||
| Flax, Ryegrass | 10 | Aqueous suspension (0.1% v/v Tween 20) | Reduced shoot length | (82) | ||
| Barley, Ryegrass, Flax | 20 | Aqueous suspension (0.1% v/v Tween 20) | Reduced shoot length | (82) | ||
| Ag | 5 | Barley | 10 | Aqueous suspension (0.1% v/v Tween 20) | Reduced germination | (82) |
| Flax, Barley | 10 | Aqueous suspension (0.1% v/v Tween 20) | Reduced shoot length | (82) | ||
| Ag | 20 | Barley | 10 | Aqueous suspension (0.1% v/v Tween 20) | Reduced germination | (82) |
| Barley | 10 | Aqueous suspension (0.1% v/v Tween 20) | Reduced shoot length | (82) | ||
| Barley, Ryegrass | 20 | Aqueous suspension (0.1% v/v Tween 20) | Reduced shoot length | (82) | ||
| Ag | 100 | Zucchini | 100, 500, 1000 | 25% Hoagland solution | Reduced transpiration (41–79%) | (52) |
| 100 | Zucchini | 500, 1000 | 25% Hoagland solution | Reduced biomass (57–71%) | (52) | |
| Ag | <100 | Onion | 100 | Aqueous suspension | Decreased mitosis; disturbed metaphase; sticky chromosome; cell wall disintegration and breaks | (63) |
| Cu | - | Mungbean | <200 | Agar culture media | Reduced seedling growth | (23) |
| - | Mungbean | 800 | Agar culture media | Reduced shoot growth | (23) | |
| - | Wheat | <200 | Agar culture media | Reduced root and seedling growth | (23) | |
| 50 | Zucchini | 1000 | 25% Hoagland solution | Reduced biomass (90%) | (52) | |
| 50 | Zucchini | 1000 | Aqueous suspension | reduced root growth | (52) | |
| Si | 10 | Zucchini | 1000 | Aqueous suspension (0.2% sodium dodecyl sulfate) | Completely inhibited germination | (52) |
| Al | - | Ryegrass | 2000 | Aqueous suspension | Decreased root length | (16) |
| - | Ryegrass | 2000 | Aqueous suspension | Reduced germination | (16) | |
| - | Corn, Lettuce | 2000 | Aqueous suspension | Reduced root length | (16) | |
| Zn | - | Radish, Rape, Ryegrass, Lettuce, Corn, Cucumber | 2000 | Aqueous suspension | Highly reduced root growth | (16) |
| ZnO | 9–37 (mean: 19±7) | Ryegrass | 1000 | 1 strength hoagland solution | Reduced biomass, shrank root tips, epidermis and rootcap were broken, highly vacuolated and collapsed cortical cells | (43) |
| - | Corn | 2000 | Aqueous suspension | Reduced germination | (16) | |
| - | Radish, Rape, Ryegrass, Lettuce, Corn, Cucumber | 2000 | Aqueous suspension | Highly reduced root growth | (16) | |
| 5 | Zucchini | 1000 | 25% Hoagland solution | Reduced biomass (78–90%) | (52) | |
| 8 | Soybean | 2000, 4000 | Aqueous suspension | Decreased root growth | (50) | |
| CeO2 | 7 | Alfalfa | 1000, 2000 | Aqueous suspension | Slightly reduced shoot growth | (50) |
| Tomato | 2000 | Aqueous suspension | Significantly reduced shoot growth (30%) | (50) | ||
| Cucumber | 2000 | Aqueous suspension | Reduced shoot growth (20%) | (50) | ||
| Maize | 500, 1000, 2000 | Aqueous suspension | Significantly reduced shoot growth (30%) | (50) | ||
| Alfalfa | 500 | Aqueous suspension | Significantly reduced the biomass | (50) | ||
| Maize | 500–2000 | Aqueous suspension | Reduced germination | (50) | ||
| Maize | 4000 | Aqueous suspension | Reduced root growth | (50) | ||
| Tomato, Cucumber | 2000 | Aqueous suspension | Reduced germination | (50) | ||
| Tomato | 1000–2000 | Aqueous suspension | Reduced root growth | (50) | ||
| Alfalfa | 2000–4000 | Aqueous suspension | Reduced root growth | (50) | ||
| Soybean | 2000 | Aqueous suspension | Reduced germination | (50) | ||
| Al2O3 | 13 | Maize, Cucumber, Carrots, Cabbage | 2000 | Aqueous suspension | Reduced root growth | (83) |
| - | Corn | 2000 | Aqueous suspension | Reduced root length | (16) | |
| Single-walled carbon nanotube | 1.19 (major), 18, 722 nm | Rice | 400 | Half strength Murashige and Skoog basal medium | Delayed flowering, decreased yield | (40) |
| 8 | Tomato | 104, 315, 1750 | Suspension (35:65 w/w CNT:poly-3-aminobenzoic acid) | Most sensitive in root reduction | (34) | |
| Functionalized carbon nanotube | 8 | Lettuce | 104, 315, 1750 | Suspension (35:65 w/w CNT:poly-3-aminobenzoic acid) | Reduced root length at longer exposure | (34) |
| Multi-wallled carbon nanotube | - | Zucchini | 1000 | 25% Hoagland solution | Reduced biomass (38%) | (52) |
| - | Lettuce | 2000 | Aqueous suspension | Reduced root length | (16) | |
| Diameter range: 10–30 | Rice | 20, 40, 80 | Murashige and Skoog basal medium | Chromatin condensed inside the cytoplasm and caused cell death, plasma membrane detachment from cell wall and cell shrinkage | (36) | |
| TiO2/Inorganic bentonite clay | 30/1–60 | Maize | 300, 1000 | 0.1 strength hoagland solution | Inhibited hydraulic conductivity, leaf growth, and transpiration | (41) |
The above mentioned studies showed that NP phytotoxicity studies are at the very beginnings. Studies must be conducted for a longer duration to investigate if plants that initially exhibited toxicity will recover after sometime. Toxicity studies that will cover one whole life cycle of plant similar to that conducted by Lin et al. (37) are much desired to determine the long term effect of nanoparticle toxicity. It may also be recommended to conduct toxicity studies in soil media since NPs are likely to react with the constituents of environmental matrices that will enhance, lessen or modify the toxic effect of NPs. Also, since germination, and root and shoot growth assays appeared to be poor indicators for phytotoxicity studies of NPs, toxicity indicators based on biological markers, plant defense mechanism, changes in plants integrity at cellular or genetic level tested periodically during the plant’s life cycle would be more appropriate. Ma et al. (78) pointed out that one of the most urgent needs in plant-NP interaction studies is to determine what the genetic response of plants is and what genes are up-regulated/down-regulated in plant exposed to NPs. Furthermore, elucidation of mechanisms of toxicity and genotoxicity remains an unexplored field of study.
5. Biotransformation of NPs in the edible plants
Very few references were found about the biotransformation of NPs in plants. The differential biotransformation of ZnO and CeO2 NPs in soybean was investigated by Lopez-Moreno et al. (42). CeO2 NPs were taken up by soybean and did not undergo biotransformation. In the case of ZnO, results revealed that Zn was found inside the soybean plant only at Zn2+ oxidation state. Since no ZnO NPs were detected inside the plant and the Zn2+ ions released from ZnO NPs in the hydroponic solution was too low (8–25 mg L−1 for the concentrations of 500–4000 mg L−1) to cause a spike in Zn concentration inside the plant, it was hypothesized that ZnO NPs became transformed on the root surface. Recent studies in our laboratory on the biotransformation of ZnO NPs in mesquite also indicated that the nanoparticles were transformed on/in the root surface. An interesting XAS study by Parsons et al. (22) showed that mesquite plants treated with Ni(OH)2 NPs had Ni(OH)2 in the roots but the XAS spectra showed Ni2+ in the shoots and leaves, demonstrating the biotransformation of the Ni(OH)2 NPs by mesquite. Stampoulis et al. (52) reported that Ag was found at a greater concentration in zucchini grown in Ag NPs than those cultivated in Ag bulk solution. Similarly, a study showed an increase in Al concentration in ryegrass treated with Al NPs (55) and Cu concentration in mungbean and wheat treated with Cu NPs (23). However, no test was done to determine whether the Ag, Al or Cu in the plant existed as NPs or ions; thus, no definite conclusion could be made based on the reported studies.
In the case of other nanoparticles (C70, SWCNTs, MWCNTs, Fe3O4, and TiO2 NPs), biotransformation was not observed (37, 40, 44, 52). These studies revealed that NPs can undergo biotransformation or just get accumulated in plants. There are limited studies in this field that more questions on mechanism of biotransformation, specific storage site within the plant, transmission of NPs to the plant’s next generation, long term effect on the genetic integrity of the plant, transfer of NPs in the food chain, among others, are still in need of research. Beyond that, it remains to be elucidated if those entities experiencing/non-experiencing biotransformation have an adverse or beneficial effect on animal and human health.
6. Effects of the NPs in edible plant species
6.1 Carbon nanomaterials
When discharged into environmental matrices, CNTs could be stabilized by natural organic matter by averting the hydrophobicity (39), with unknown consequences on the ecosystem.
According to the study of Lin and Xing (16), MWCNTs were reported to have no significant effect on seed germination of rape, radish, lettuce, corn and cucumber at 2000 mg L−1 after 5 days of treatment. Similarly, no phytotoxic symptoms or increased physiological response were reported in the study of Wild and Jones (30)on living wheat roots. In tomato seeds, MWCNTs at 10–40 mg L−1 accelerated seed germination, increased germination percentage rate and vegetative mass with respect to control (no CNTs) (29). This may be attributed to the increased water uptake induced by the CNTs as previously explained. On the other hand, SWCNTs and poly-3-aminobenzenesulfonic acid functionalized SWCNTs adversely affected root elongation in tomato. The result was explained by the high concentration of CNTs observed around the base of the apical meristem (where root elongation occurs) of tomato roots (34). Lin (37) reported that the C70 and MWCNT (400 mg L−1) suspended in natural organic matter inhibited rice plants reproduction by delaying flowering by at least one month. Interestingly, it was also found that exposure to the MWCNT induced a self-defense and hypersensitive response in the rice cells (35,36). Lin et al. (37) suggested that the presence of metallic impurities (residual metals used as catalysts for the synthesis of CNTs) may also be a factor contributing to the toxicity of MWCNTs.
6.2 Metal oxide nanoparticles
Studies on the toxicity of metal oxide NPs on crop plants are limited. So far, only Fe3O4, CeO2, SiO2, TiO2, and ZnO, have been studied in a few plant species. Furthermore, very few reports include plant life cycle studies. Accumulation of TiO2 NPs in maize root cell walls was accompanied by a significant reduction in the cell pore diameter (41). This was shown to reduce the hydraulic conductivity in the primary roots; thereby, leading to reduced transpiration and leaf growth. This is attributed to the physical interaction between the colloidal particles and a physical inhibition of the apoplastic flow through the cell walls, rather than toxic effects. In soybean, a mixture of SiO2 and TiO2 NPs increased the nitrate reductase activity, enhancing the uptake of water and fertilizer, stimulating the antioxidant system (85). In spinach, TiO2 NPs were reported to increase chlorophyll formation, photosynthesis, and plant dry weight, among others (86, 87)
It seems that ZnO NPs do not affect seed germination in soybean even at very high concentration of 4000 mg L−1 (42). In addition, experimental data has shown that soybean root elongation was promoted at 500 mg ZnO NPs L−1 but reduced at higher concentrations (42). This could be attributed to an excess of Zn ions released by the NPs or to an interaction between the NPs and the root surface. The interaction of ZnO NPs with plants could be influenced by the species of plants, as reported by Lin and Xing (43). Besides the effect on seedlings’ growth, ZnO NPs have been associated to cortical cells highly vacuolated and collapsed along with the shrinking and partial death of the vascular cells (43).
In contrast to ZnO NPs, the nanoceria were found to reduce seed germination in alfalfa, soybean, tomato and cucumber at high concentration (4000 mg L−1) (50). But corn germination was significantly reduced even at the minimum concentration of 500 mg L−1. Corn was seen to be more sensitive to ZnO and CeO2 NPs, demonstrating toxicity symptoms even at concentration that were not found to significantly affect other food and forage crops (16, 50). Ce is generally precipitated as cerium oxide in cell walls and intercellular spaces of epidermal and cortical walls, and not in the growing zones. Thus, the authors hypothesized that the oxidative stress in the growing zone is reduced; promoting root growth. On the other hand, CeO2 was found to inhibit root growth significantly in tomato and alfalfa seedlings at higher concentrations. This can be correlated with the very high amount of Ce found in the tissues of these plants (4000 mg Ce kg−1 DW for tomato and 6000 mg Ce kg−1 DW for alfalfa), which is not comparable to that of cucumber and soybean (≈400 and 462 mg Ce kg−1 DW). This may be responsible for inducing negative effects on the root growth in tomato and alfalfa.
The toxicity of NPs depends upon the particle surface characteristics. For instance, alumina NPs were shown to inhibit root growth in corn, cucumber, soybean, cabbage and carrot(83). However, phenanthrene (changed the surface characteristics of the alumina NPs) mitigated the effect on cucumber root growth inhibition caused by uncapped alumina NPs (88).
The iron oxide NPs have been found to increase soybean pod and leaf dry weight (89). Also, Fe oxide NPs have been reported as facilitators for iron and photosynthates transfer to the leaves of peanut(90). In the case of pumpkin, Fe oxide NPs increased root elongation (45), which was attributed to the Fe dissolution. Although there was positive/no significant negative effect on pumpkin plants, the Fe3O4 NPs were found to induce oxidative stress and higher antioxidative enzyme activity than the bulk Fe3O4 particles (45). The Fe3O4 NPs adsorbed on the root surface or absorbed by the roots were thought to disturb the metabolic activities in roots, leading to local instability of the cell wall and/or membrane, eventually producing oxidative stress.
6.3 Metallic nanoparticles
References on the effect of metallic NPs are scarce. According to Stampoulis (52), Si NPs at 1000 mg L−1, in the presence of sodium dodecyl sulfate (SDS, surfactant to suspend the NPs in the solution), completely inhibited the germination of zucchini seeds (Cucurbita pepo); whereas, Si NPs in the absence of SDS, resulted in 80% germination. Reduction in germination was also noted in the plants treated with MWCNT and Ag NPs, after excluding the additive effect on the inhibition of the SDS itself. As described in Tables 1 and 2, the effects of NPs thus depend upon the plant species, types of NPs, concentration, size, aggregation, functionalization, and experimental conditions including temperature and time, and method of exposure (seeds/seedlings/cell suspensions) (90–93).
This literature review has confirmed that the knowledge on plant toxicity of ENMs is at the foundation stage. Practically, there are no concluding studies on the nanotoxicity; however, with the limited pieces of information, the new field of nanoecotoxicology has emerged to address the effects of ENMs on the living components of ecosystems (94).
Acknowledgments
This material is based upon work supported by the National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number DBI-0830117. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the Environmental Protection Agency. This work has not been subjected to EPA review and no official endorsement should be inferred. The authors also acknowledge the US Department of Energy under proposal # 31406, the USDA grant number 2008-38422-19138, the Toxicology Unit of the BBRC (NIH NCRR Grant # 2G12RR008124-16A1), and the NSF Grant # CHE-0840525. J. L. Gardea-Torresdey acknowledges the Dudley family for the Endowed Research Professorship in Chemistry.
References
- 1.Taiz L, Zeiger E. Plant physiology. 2. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 2.Kinnersley RP, Scott LK. Aerial contamination of fruit through wet deposition and particulate dry deposition. J Environ Radioact. 2001;52:191–213. doi: 10.1016/s0265-931x(00)00033-3. [DOI] [PubMed] [Google Scholar]
- 3.Isla R, Aragues R. Yield and plant ion concentrations in maize (Zea mays L.) subject to diurnal and nocturnal saline sprinkler irrigations. Field Crops Res. 2010;116:175–183. [Google Scholar]
- 4.Ke W, Xiong Z-T, Chen S, Chen J. Effects of copper and mineral nutrition on growth, copper accumulation and mineral element uptake in two Rumex japonicas populations from a copper mine and an uncontaminated field sites. Environ Exp Bot. 2007;59:59–67. [Google Scholar]
- 5.Bondada BR, Tu S, Ma LQ. Absorption of foliar-applied arsenic by the arsenic hyperaccumulating fern (Pteris vittata L.) Sci Total Environ. 2004;332:61–70. doi: 10.1016/j.scitotenv.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Michel H, Zuverza-Mena N, Parsons JG, Dokken KM, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL. Speciation and coordination of arsenic in two phenotypes of the desert plant species Chilopsis linearis. Phytochemistry. 2009;70:540–545. doi: 10.1016/j.phytochem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Lopez ML, Peralta-Videa JR, Parsons JG, Gardea-Torresdey JL, Duarte-Gardea M. Effect of indole-3-acetic acid, kinetin, and ethylenediaminetetraacetic acid on plant growth and uptake and translocation of lead, micro, and macronutrients in alfalfa plants. Int J Phytorem. 2009;11:131–149. doi: 10.1080/15226510802378434. [DOI] [PubMed] [Google Scholar]
- 8.Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int J Biochem Cell Biol. 2009;41:1665–1677. doi: 10.1016/j.biocel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Arias J, Peralta-Videa JR, Ellzey JT, Viveros MN, Ren M, Mokgalaka-Matlala NS, Castillo-Michel H, Gardea-Torresdey JL. Plant growth and metal distribution in tissues of Prosopis juliflora-velutina grown on chromium contaminated soil in the presence of Glomus deserticola. Environ. Sci Technol. 2010;44:7272–7279. doi: 10.1021/es1008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whanger PD. Selenocompounds in plants and animals and their biological significance. J Am College Nutrit. 2002;21:223–232. doi: 10.1080/07315724.2002.10719214. [DOI] [PubMed] [Google Scholar]
- 11.Hefnawy AG, Tórtora-Pérez JL. The importance of selenium and the effects of its deficiency in animal health. Small Rumin Res. 2010;89:185–192. [Google Scholar]
- 12.Zhu Y-G, Pilon-Smits EAH, Zhao F-J, Williams PN, Meharg AA. Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009;14:436–442. doi: 10.1016/j.tplants.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Pan B, Xing B. Manufactured nanoparticles and their sorption of organic chemicals. Adv Agr. 2010;108:137–181. [Google Scholar]
- 14.Darlington TK, Neigh AM, Spencer MT, Nguyen OT, Oldenburg SJ. Nanoparticle characteristics affecting environmental fate and transport through soil. Environ Toxicol Chem. 2009;28:1191–1199. doi: 10.1897/08-341.1. [DOI] [PubMed] [Google Scholar]
- 15.Pidgeon N, Harthorn BH, Bryant K, Rogers-Hayden T. Deliberating the risks of nanotechnologies for energy and health applications in the United States and United Kingdom. Nat Nanotech. 2009;4:95–98. doi: 10.1038/nnano.2008.362. [DOI] [PubMed] [Google Scholar]
- 16.Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Lee WM, An YJ, Yoon H, Kweon HS. Toxicity and bioavailability of copper nanoparticles to terrestrial plants Phaseolus radiatus (mungbean) and Triticum aestivum (wheat); plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem. 2008;27:1915–1921. doi: 10.1897/07-481.1. [DOI] [PubMed] [Google Scholar]
- 18.Barrena R, Casals E, Colon J, Font X, Sanchez A, Puntes V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere. 2009;75:850–857. doi: 10.1016/j.chemosphere.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 19.Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead J. R.Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ Toxicol Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 20.Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen R, Ratnikova TA, Stone MB, Lin S, Lard M, Huang G, Hudson JS, Ke PC. Differential uptake of carbon nanoparticles by plant and mammalian cells. Small. 2010;6:612–617. doi: 10.1002/smll.200901911. [DOI] [PubMed] [Google Scholar]
- 22.Parsons JG, Lopez ML, Gonzalez CM, Peralta-Videa JR, Gardea-Torresdey JL. Toxicity and biotransformation of uncoated and coated nickel hydroxide nanoparticles on mesquite plants. Environ Toxicol Chem. 2010;29:1146–1154. doi: 10.1002/etc.146. [DOI] [PubMed] [Google Scholar]
- 23.Lee WM, An YJ, Yoon H, Kweon HS. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Nanomat Environ. 2008;27:1915–1921. doi: 10.1897/07-481.1. [DOI] [PubMed] [Google Scholar]
- 24.Kurepa J, Paunesku T, Vogt S, Arora H, Rabatic BM, Lu J, Wanzer MB, Woloschak GE, Smalle JA. Uptake and distribution of ultrasmall anatase TiO2 alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. doi: 10.1021/nl903518f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe T, Misawa S, Hiradate S, Osaki M. Root mucilage enhances aluminum accumulation in Melastoma malabathricum, an aluminum accumulator. Plant Signal Behavior. 2008;3:603–605. doi: 10.4161/psb.3.8.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall JL, Williams LE. Transition metal transporters in plants. J Exp Bot. 2003;54:2601–2613. doi: 10.1093/jxb/erg303. [DOI] [PubMed] [Google Scholar]
- 27.Shen CX, Zhang QF, Li J, Bi FC, Yao N. Induction of programmed cell death in Arabidopsis and Rice by single-wall carbon nanotubes. Am J Bot. 2010;97:1–8. doi: 10.3732/ajb.1000073. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Chen B, Wang Q, Shi X, Xiao Z, Lin J, Fang X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009;9:1007–1010. doi: 10.1021/nl803083u. [DOI] [PubMed] [Google Scholar]
- 29.Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano. 2009;3:3221–3227. doi: 10.1021/nn900887m. [DOI] [PubMed] [Google Scholar]
- 30.Wild E, Jones KC. Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environ Sci Technol. 2009;43:5290–5294. doi: 10.1021/es900065h. [DOI] [PubMed] [Google Scholar]
- 31.Yang K, Zhu L, Xing B. Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ Sci Technol. 2006;40:1855–1861. doi: 10.1021/es052208w. [DOI] [PubMed] [Google Scholar]
- 32.Whitby M, Quirke N. Fluid flow in carbon nanotubes and nanopipes. Nat Nanotechnol. 2007;2:87–94. doi: 10.1038/nnano.2006.175. [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Wang C. Fullerene nanoparticles affect the fate and uptake of trichloroethylene in phytoremediation systems. Environ Eng Sci. 2010;27:989–992. [Google Scholar]
- 34.Canas JE, Long M, Nations S, Vadan R, Dai L, Luo M, Ambikapathi R, Lee EH, Olszyk D. Effects of functionalized and nonfunctionalized single-walled carbon-nanotubes on root elongation of select crop species. Nanomat Environ. 2008;27:1922–1931. doi: 10.1897/08-117.1. [DOI] [PubMed] [Google Scholar]
- 35.Tan XM, Fugetsu B. Multi-walled carbon nanotubes interact with cultured rice cells: Evidence of a self-defense response. J Biomed Nanotechnol. 2007;3:285–288. [Google Scholar]
- 36.Tan XM, Lin C, Fugetsu B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon. 2009;47:3479–3487. doi: 10.1016/j.jhazmat.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Lin C, Fugetsu B, Su Y, Watari F. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J Hazard Mater. 2009;170:578–583. doi: 10.1016/j.jhazmat.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Casey A, Farrell GF, McNamara M, Byrne HJ, Chambers G. Interaction of carbon nanotubes with sugar complexes. Synth Metals. 2005;153:357. [Google Scholar]
- 39.Hyung H, Fortner JD, Hughes JB, Hong KJ. Natural Organic Matter Stabilizes Carbon Nanotubes in the Aqueous Phase. Environ Sci Technol. 2007;41:179–184. doi: 10.1021/es061817g. [DOI] [PubMed] [Google Scholar]
- 40.Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small. 2009;5:1128–1132. doi: 10.1002/smll.200801556. [DOI] [PubMed] [Google Scholar]
- 41.Asli S, Neumann M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant, Cell Environ. 2009;32:577–584. doi: 10.1111/j.1365-3040.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Moreno ML, De La Rosa G, Hernandez-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol. 2010;44:7315–7320. doi: 10.1021/es903891g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin D, Xing B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol. 2008;42:5580–5585. doi: 10.1021/es800422x. [DOI] [PubMed] [Google Scholar]
- 44.Zhu H, Han J, Xiao JQ, Jin Y. Uptake, translocation, and accumulation of manufactured iron oxide by pumpkin plants. J Environ Monit. 2008;10:713–717. doi: 10.1039/b805998e. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Kou X, Pei Z, Xiao JQ, Shan X, Xing B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology. doi: 10.3109/17435390.2010.489206. [DOI] [PubMed] [Google Scholar]
- 46.Woehlecke H, Ehwald R. Characterization of size-permeation limits of cell walls and porous separation materials by highperformance size-exclusion chromatography. J Chromatogr A. 1995;708:263–271. [Google Scholar]
- 47.Carpita N, Sabularse D, Montezinos D, Delmer DP. Determination of the pore size of cell walls of living plant cells. Science. 1979;205:1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- 48.Carpita N. Limiting diameters of pores and the surface structure of plant cell walls. Science. 1982;218:813–814. doi: 10.1126/science.218.4574.813. [DOI] [PubMed] [Google Scholar]
- 49.Rondeau-Mouro C, Defer D, Leboeuf E, Lahaye M. Assessment of cell wall porosity in Arabidopsis thaliana by NMR spectroscopy. Int J Biol Macromol. 2008;42:83–92. doi: 10.1016/j.ijbiomac.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Moreno ML, De La Rosa G, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem. 2010;58:3689–3693. doi: 10.1021/jf904472e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birbaum K, Brogiolli R, Schellenberg M, Martinoia E, Stark WJ, Gunther D, Limbach L. No evidence for cerium dioxide nanoparticle translocation in maize plants. Environ Sci Technol. doi: 10.1021/es101685f. [DOI] [PubMed] [Google Scholar]
- 52.Stampoulis D, Sinha SK, White JC. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 2009;43:9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- 53.Haverkamp RG, Marshall AT. The mechanism of metal nanoparticle formation in plants: limits on accumulation. J Nanopart Res. 2009;11:1453–1463. [Google Scholar]
- 54.Corredor E, Testillano PS, Coronado MJ, Gozalez-Melendi P, Fernandez-Pacheco R, Marquina C, Ibarra MR, de la Fuente J, Rubiales D, Perez de Luque A, Risueno MC. Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biolog. 2009;9 doi: 10.1186/1471-2229-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doshi R, Braida W, Christodoulatos C, Wazne M, O’Connor G. Nano-aluminum: transport through sand columns and environmental effects on plants and soil communities. Environ Res. 2008;106:296–303. doi: 10.1016/j.envres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Yacaman MJ. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano lett. 2002;2:397–401. [Google Scholar]
- 57.Gardea-Torresdey JL, Gomez E, Peralta-Videa J, Parsons JG, Troiani HE, Yacaman MJ. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19:1357–1361. [Google Scholar]
- 58.Bali R, Siegele R, Harris AT. Biogenic Pt uptake and nanoparticle formation in Medicago sativa and Brassica juncea. J Nanopart Res. doi: 10.1007/s11051-010-9904-7. [DOI]
- 59.Harris AT, Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopart Res. 2008;10:691–695. [Google Scholar]
- 60.Judy JD, Unrine JM, Bertsch PM. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ Sci Technol. 2011;45:776–781. doi: 10.1021/es103031a. [DOI] [PubMed] [Google Scholar]
- 61.Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F. Genotoxicity investigations on nanomaterials: Methods, preparation and characterization of test material, potential artifacts and limitations- Many questions, some answers. Mut Res. 2009;681:241–258. doi: 10.1016/j.mrrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Kovacic P, Somanathan R. Biomechanisms of nanoparticles (toxicants, antioxidants and therapeutics): Electron transfer and reactive oxygen species. J Nanosci Nanotechnol. 2010;10:1–12. doi: 10.1166/jnn.2010.3028. [DOI] [PubMed] [Google Scholar]
- 63.Kumari M, Mukherjee A, Chadrasekaran N. Genotoxicity of silver nanoparticle in Allium cepa. Sci Total Environ. 2009;407:5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Yang R, Yu M, Bai F, Li C, Wang ZL. Cellular level biocompatibility and biosafety of ZnO nanowires. J Phys Chem C. 2008;112:20114–20117. [Google Scholar]
- 65.Zang JF, Li CM, Cui XQ, Wang JX, Sun XW, Dong H, Sun CQ. Tailoring zinc oxide nanowires for high performance amperometric glucose sensor. Electroanalysis. 2007;19:1008–1014. [Google Scholar]
- 66.Wang JX, Sun XW, Wei A, Lei Y, Cai XP, Li CM, Dong ZL. Zinc oxide nanocomb biosensor for glucose detection. Appl Phys Lett. 2006;88:233106–233106-3. [Google Scholar]
- 67.Wei A, Sun XW, Wang JX, Lei Y, Cai XP, Li CM, Dong ZL, Huang W. Enzymatic glucose biosensor based on ZnO nanorod array grown by hydrothermal decomposition. Appl Phys Lett. 2006;9:123902–123902-3. [Google Scholar]
- 68.Ghosh M, Bandyopadhyay M, Mukherjee A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophies levels: Plant and human lymphocytes. Chemosphere. 2010 doi: 10.1016/j.chemosphere.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 69.Babu K, Deepa M, Shankar SG, Rai S. Effect of nano-silver on cell division and mitotic chromosomes: A prefatory siren. Internet J Nanotechnol. 2008;2:2. http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijnt/vol2n2/silver.xml.
- 70.Kuriyama R, Sakai H. Role of tubulin-SH group in polymerization to microtubules. J Biochem. 1974;76:651–654. doi: 10.1093/oxfordjournals.jbchem.a130609. [DOI] [PubMed] [Google Scholar]
- 71.Sudhakar R, Gowda KNN, Venu G. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 2001;66:235–239. [Google Scholar]
- 72.McGill M, Sen P, Hsu TC. Effect of ethidium bromide on mitosis and chromosomes. A possible material basis for chromosome stickiness. Chromosoma. 1974;47:157–167. doi: 10.1007/BF00331803. [DOI] [PubMed] [Google Scholar]
- 73.Klasterska I, Natarajan AT, Ramel C. An interpretation of the origin of subchromatid aberrations and chromosome stickiness as a category of chromatid aberrations. Hereditas. 1976;83:153–162. doi: 10.1111/j.1601-5223.1976.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 74.Raun C, Lilum J. Application of micronucleus test in Vicia faba root tips in the rapid detection of mutagenic environmental pollutants. Chin J Environ Sci. 1992;4:56–58. [Google Scholar]
- 75.Răcuciu M, Creangă DE. Biocompatible magnetic fluid nanoparticles internalized in vegetal tissue. Rom Journ Phys. 2009;54:115–124. [Google Scholar]
- 76.Răcuciu M, Creangă DE. Cytogenetical changes induced by -cyclodextrin coated nanoparticles in plant seeds. Rom Journ Phys. 2009;54:125–131. [Google Scholar]
- 77.Monica RC, Cremonini R. Nanoparticles and higher plants. Caryologia. 2009;62:161–165. [Google Scholar]
- 78.Ma X, Geiser-Lee J, Deng Y, Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408:3053–3061. doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 79.Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar S. Nanoparticle material delivery to plants. Plant Sci. 2010;179:154–163. [Google Scholar]
- 80.Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- 81.Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol. 2010;44:1962–1967. doi: 10.1021/es902987d. [DOI] [PubMed] [Google Scholar]
- 82.El-Temsah YS, Joner EJ. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol. 2010 doi: 10.1002/tox.20610. [DOI] [PubMed] [Google Scholar]
- 83.Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Shah V, Belozerova I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009;197:143–148. [Google Scholar]
- 85.Lu CM, Zhang CY, Wen JQ, Wu GR, Tao MX. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002;21:168–172. (in Chinese) [Google Scholar]
- 86.Zheng L, Hong F, Lu S, Liu C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res. 2005;104:83–91. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]
- 87.Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res. 2005;105:269–279. doi: 10.1385/BTER:105:1-3:269. [DOI] [PubMed] [Google Scholar]
- 88.Murashov V. Comments on “Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles” by Yang, L., Watts, D.J., Toxicology Letters, 2005; 158, 122–132. Toxicol Lett. 2006;164:185–187. doi: 10.1016/j.toxlet.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Sheykhbaglou R, Sedghi M, Shishevan MT, Sharifi RS. Effects of nano-iron oxide particles on agronomic traits of soybean. Notulae Scientia Biologicae. 2010;2:112–113. [Google Scholar]
- 90.Liu XM, Zhang FD, Zhang SQ, He XS, Fang R, Feng Z, Wang Y. Effects of nano-ferric oxide on the growth and nutrients absorption of peanut. Plant Nutr Fert Sci. 2010;11:14–18. [Google Scholar]
- 91.Gao F, Hong F, Liu C, Zheng L, Su M, Wu X, Yang F, Wu C, Yang P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol Trace Elem Res. 2006;111:239–253. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- 92.Yang F, Liu C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P. The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res. 2007;119:77–88. doi: 10.1007/s12011-007-0046-4. [DOI] [PubMed] [Google Scholar]
- 93.Linglan M, Chao L, Chunxiang Q, Sitao Y, Jie L, Fengqing G, Fashui H. Rubisco activase mRNA expression in spinach: modulation by nanoanatase treatment. Biol Trace Elem Res. 2008;122:168–178. doi: 10.1007/s12011-007-8069-4. [DOI] [PubMed] [Google Scholar]
- 94.Baun A, Hartmann NB, Grieger K, Kusk KO. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17:387–395. doi: 10.1007/s10646-008-0208-y. [DOI] [PubMed] [Google Scholar]