Abstract
Background
Epidemiologic studies have shown that men with type 2 diabetes have a lower risk of prostate cancer than non-diabetic men. Recently, common variants in two genes, HNF1B and JAZF1, were found to be associated with both of these diseases.
Methods
We examined whether the relationship between HNF1B and JAZF1 variants and decreased prostate cancer risk may potentially be mediated through diabetes in two large prospective studies, the Cancer Prevention Study II Nutrition Cohort and the Prostate, Lung, Colorectal, and Ovarian cancer Screening Trial.
Results
Three HNF1B SNPS, rs11649743, rs4430796, and rs7501939 were associated with decreased risk of prostate cancer and were also associated, with marginal statistical significance, with increased risk of diabetes. The JAZF1 SNPs rs6968704 and rs10486567 were associated with decreased risk of prostate cancer, but were not associated with diabetes. All five SNP–prostate cancer relationships did not substantially differ when the analyses were stratified by diabetic status or when diabetic status was controlled for in the model. Furthermore, the association of diabetes with prostate cancer was not altered when the SNPs were included in the logistic model.
Conclusions
These findings indicate that the HNF1B variants are directly associated with both diabetes and prostate cancer, that diabetes does not mediate these gene variant-prostate cancer relationships, and the relationship between these diseases is not mediated through these gene variants.
Keywords: prostate cancer, diabetes, genes, prospective study
Introduction
Type II diabetes and prostate cancer are complex chronic diseases that result in considerable morbidity and mortality (1). Diabetes is associated with a decreased risk of prostate cancer, suggesting a common biologic link between these two diseases. This association is supported by considerable epidemiologic evidence, including a meta-analysis of 19 studies published between 1971 and 2005 (RR=0.84, 95% CI: 0.76–0.93) (2), and six (3–8) of seven (9) studies published since then.
The recent finding from several genome wide association studies that common allelic variants in the HNF1B (also known as TCF2) and JAZF1 genes are associated with both type II diabetes (10–12) and prostate cancer (11,13,14) further suggests that there is a link between these two diseases (15). Two distinct HNF1B loci have been associated with prostate cancer (11,13,14). One of these loci, represented by the SNPs rs4430796 and rs7501939, has been associated with type II diabetes whereas the second, more recently identified locus, represented by SNP rs11649743, has not been investigated in diabetes. For JAZF1, the prostate cancer-associated SNPs are distinct from those discovered for diabetes (15). Because the HNF1B and JAZF1variants are associated with increased diabetes risk, it is possible that these SNPs may reduce prostate cancer risk specifically through a diabetes-related mechanism. To test this hypothesis, we examined whether diabetes mediates the relationship between HNF1B and JAZF1 variants and prostate cancer risk in two large nested case-control studies. Additionally, whether the association of diabetes with prostate cancer risk results from the association of the SNPs with the two diseases was also investigated.
Materials and Methods
Study Populations
This study utilized participants from two prospective cohort studies, the American Cancer Society’s Cancer Prevention Study-II (CPS-II) Nutrition Cohort, and the Prostate, Lung, Colon and Ovarian (PLCO) Cancer Screening Trial.
The CPS-II Nutrition Cohort is a prospective study of cancer incidence involving approximately 184,000 US adults (86,406 men) between the ages of 50 and 74 enrolled in 1992 and sent follow-up questionnaires in 1997 and every two years afterwards (16). Prostate cancer cases were ascertained from Caucasian men using follow-up through 2003 through self report on the repeated questionnaires and verified through medical records or linkage with state cancer registries or identified through linkage with the National Death Index as having this malignancy as the underlying cause of death. An equal number of controls were matched to the cases on age (±6 months), race and date of blood collection (±6 months) from men who were cancer-free at the time of cancer diagnosis of their matched case (17). A total of 1760 cases and 1775 controls were initially selected. The 1554 cases and 1559 controls for whom information on diabetes status was available were included in this analysis.
The PLCO Cancer Screening Trial is a large, randomized controlled trial of approximately 155,000 adults (76,693men) that began in 1993 and ended in 2001 which was designed to evaluate selected methods for the early detection of four cancers (18). Incident prostate cancer cases were ascertained through annually mailed questionnaires to participants and verified through medical records. 1175 prostate cancer cases were selected from the eligible Caucasian men in the screening arm of the study and 1157 control subjects were matched to the cases by age at cohort entry (5-year intervals), time since initial screening (1-year time window), and calendar year of cohort entry identified by incidence density sampling (17). Of those, 1175 cases and 1100 controls had information on diabetes status and were included in the present analysis.
Assessment of diabetes
For CPS-II, men were classified as diabetic if they reported they had ever been diagnosed with diabetes in 1982 (when enrolled in the baseline CPS-II cohort) or 1992 and if they reported they used insulin in the 1992. Diabetes status for PLCO participants was assessed by self-report at enrollment as having this medical condition. For both studies, the questionnaires did not distinguish between type I and type II diabetes and diabetes status was not updated during the follow-up period. A total of 407 cases from the two studies (210 from CPS-II and 197 from PLCO) were identified as diabetics.
Genotyping
Data on 11 tag SNPs in HNF1B and JAZF1 genes were available for both the PLCO and CPS-II subjects as a component of the Cancer Genetic Markers of Susceptibility (CGEMS) study (13). The 4 tag SNPs in HNF1B were rs3094509, rs11649743, rs4430796, and rs7501939 while the 6 tag SNPs in JAZF1 were rs17155987, rs215, rs6968704, rs10486567, rs995367, rs10486570, and rs6945134. None of these tag SNPs significantly (p < 0.05) departed from Hardy-Weinberg proportions among controls. The genotype for rs864745 in the JAZF1 gene was imputed in the PLCO subjects using the observed genotypes, the HapMap CEPH European reference panel, and the MACH imputation program (http://sph.umich.edu/csg/abecasis/MACH/) to allow investigation of a region of this gene not genotyped through CGEMS.
Statistical Analysis
Odds ratios (OR) and 95% confidence intervals (CI) for the association of various SNPs with prostate cancer and diabetes were determined using unconditional logistic regression in which a continuous variable for the number of minor alleles (0, 1, or 2) was entered into the regression model (1 df test). For PLCO, the analyses were adjusted for age (in five year intervals), study center, and three eigenvectors to control for population stratification. In CPS-II, the analyses were adjusted for age (±6 months), race and date of blood collection (±6 months). Summary ORs and 95% CIs were determined based on a weighted average of the risk estimates of the component studies. Homogeneity of the component studies was tested using the Q statistic (17). If the p-value for the test statistic was ≥0.05, the pooled estimate from a fixed effects model was used. Otherwise, the pooled estimate from a random effects model was used. The Q statistic was also used to determine the p for heterogeneity for the SNP-prostate cancer associations stratified by diabetes status.
Results
An inverse association of diabetes with prostate cancer risk has been found in a number of prospective studies, including CPS-II (19) and PLCO (4). This association was reproduced in the subset of participants in these studies used here (CPS-II OR=0.68, 95% CI: 0.51, 0.90; PLCO OR= 0.58, 95% CI: 0.43, 0.78).
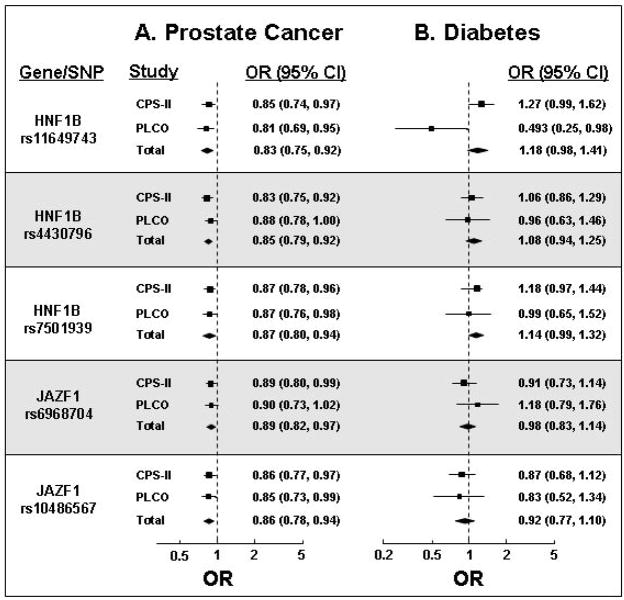
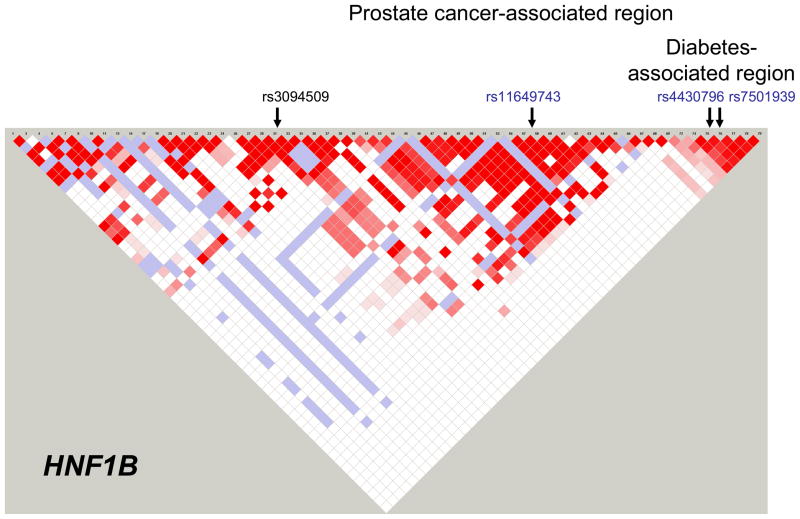
We found that three HFN1B SNPs (rs11649743, rs4430796, and rs7501939) were significantly associated with decreased risk of prostate cancer (Figure 1A), as previously reported (13,14). The rs4430796 and rs7501939 SNPs were in strong linkage disequilibrium (LD, D′=1.0, r2=0.77) but others were not (pairwise D′≤0.12, r2≤0.09) (Figure 2). These three HNF1B SNPs were associated with an increased risk of diabetes (Figure 1B), although these results were not statistically significant.
Figure 1.
Per allele odds ratios (OR) for associations of the minor alleles for 3 HNF1B SNPs and 2 JAZF1 SNPs with either prostate cancer (A) or diabetes (B). Summary estimates for each SNP are indicated by the diamonds. The minor allele nucleotides are: HNF1B rs11649743; A, HNF1B rs4430796; G, HNF1B rs7501939: T, JAZF1 rs6968704; C, and JAZF1 rs10486567; G.
Figure 2.
Linkage disequilibrium (LD) matrix for the HNF1B gene. The relative positions of the four SNPs investigated in this study are shown. The three SNPs in blue were found to be significantly associated prostate cancer risk. The general area in which the diabetes-associated SNPs are located is under the diabetes heading. The area in which the prostate cancer-associated SNPs reside is larger and encompasses the diabetes-associated SNPs.
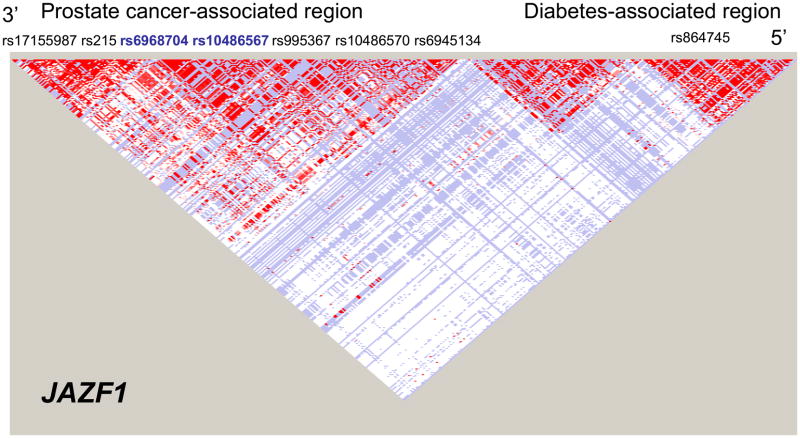
The JAZF1 SNPs rs6968704 and rs10486567 were in high LD (D′=1.0, r2=0.68) (Figure 3) and were associated with a decreased risk of prostate cancer (Figure 1A). As expected though, neither of these SNPs was associated with diabetes risk (Figure 1B). Other JAZF1 SNPS examined were not associated with risk of prostate cancer, or with risk of diabetes.
Figure 3.
LD matrix for the JAZF1 gene. The relative positions of the seven SNPs investigated in this study are shown under the prostate cancer heading towards the 3” end of the gene. The two SNPs in blue were found to be significantly associated prostate cancer risk. The diabetes-associated SNP rs864745 is near the 5’ end of this gene.
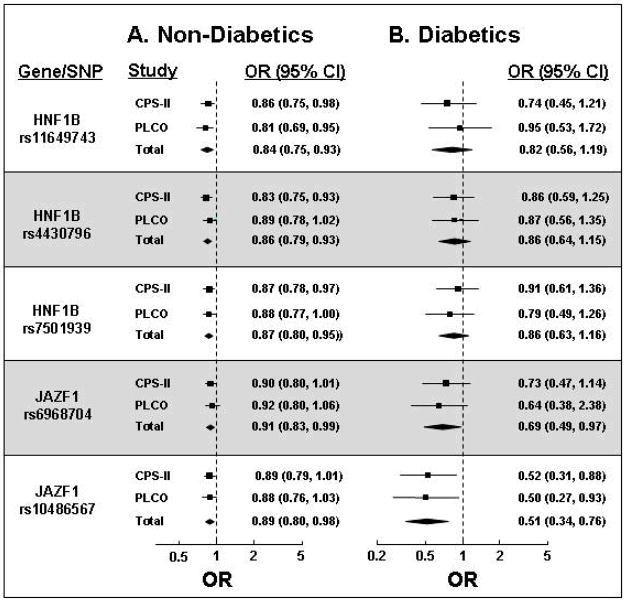
To determine if the associations of HNF1B and JAZF1 variants with prostate cancer were mediated by diabetes, risk estimates for the SNP-prostate cancer relationship among diabetics and non-diabetics separately were determined and compared. As shown in Figure 4, the associations of four of five SNPs with prostate cancer were not substantially different according to diabetic status (p-heterogeneity >0.05 for rs11649743, rs4430796, rs7501939, rs6968704). The variant at JAZF1 rs10486567 was associated with a lower risk of prostate cancer in diabetic men (OR=0.51, 95% CI: 0.34, 0.76) than in non-diabetic men (OR=0.89, 95% CI: 0.80, 0.98, p-heterogeneity =0.009). Risk estimates for all 5 SNPS remained unchanged when we adjusted for diabetic status (data not shown).
Figure 4.
Odds ratios for associations of the minor alleles for 3 HNF1B SNPs and 2 JAZF1 SNPs with prostate cancer. The results from the analysis in (A) excluded cases with diabetes while those in (B) are limited only to cases with diabetes. Summary estimates for each SNP are indicated by the diamonds.
To consider the possibility that the associations of the HNF1B and/or JAZF1 variants with both diabetes and prostate cancer resulted in an apparent relationship between these two diseases, we investigated the influence of the various genotypes on the diabetes association with prostate cancer. Diabetes was associated with decreased risk of prostate cancer in the CPS-II and PLCO studies (combined OR=0.63, 95% CI: 0.51, 0.77). These risk estimates were unchanged when the genotypes of the HNF1B and JAZF1 SNPs were included in the model (combined OR=0.64, 95% CI: 0.52, 0.79)
Discussion
Several prospective studies, including CPS-II (19) and PLCO (4), have found that men with diabetes have a 20–30% lower risk of prostate cancer compared to men without diabetes (2–7). Using combined results from CPS-II and PLCO, we confirmed that three SNPs in HNF1B and 2 SNPs in JAZF1 were significantly associated with a decreased risk of prostate cancer (13,14). The two JAZF1 SNPs, which are not in LD with those previously associated with diabetes, were not associated with this disease in our study. However, the three HNF1B SNPs, which represent two distinct loci, were marginally associated with an increased risk of diabetes. These findings, particularly for the rs4430796 and rs7501939 SNPs which have previously been shown to be associated with increased risk of diabetes (10–12), likely lack statistical significance because they were obtained using only 407 diabetics. The other HNF1B SNP, rs11649743, has not previously been associated with diabetes risk. Thus, our findings suggest for the first time that there are two distinct loci in the HNF1B gene associated with increased risk of diabetes.
The different relationships of the SNPs from the two genes with the prostate cancer and diabetes, in which the HNF1B SNPs are associated with both but the JAZF1 SNPs are only associated with prostate cancer, provide a useful comparison when probing how these genes influence the association between the two diseases. For HNF1B, the nature of the associations of the SNPs with the two diseases, in which common variants are associated with an increased risk of diabetes and a decreased risk of prostate cancer, mirrors the inverse association between diabetes and prostate cancer risk. Therefore, the possibility that the SNP-prostate cancer relationship is mediated by diabetes requires consideration. If this were the case, then the association of the HNF1B SNPs with prostate cancer should be substantially reduced or eliminated when diabetics are excluded from the analysis or when adjustment is made for diabetic status. We found that these associations persisted despite such exclusions and adjustments. The associations of the JAZF1 SNPs with prostate cancer, which shouldn’t be influenced by diabetes status, were also maintained after the same exclusions or adjustments. Although we cannot entirely rule out the possibility of an indirect association (20), our findings are consistent with the HNF1B variants being directly associated with prostate cancer. In addition, the association of diabetes with prostate cancer was not changed when the HNF1B genotypes were included in the model, suggesting that the diabetes-prostate cancer relationship is not mediated through these gene variants. Thus, our findings suggest that the diabetes-prostate cancer association is not explained by the associated SNPs.
Understanding the expression and function of the HNF1B and JAZF1 gene products should help to explain their involvement in prostate cancer and diabetes and may shed light on the relationship between these two diseases. Both proteins are transcription factors that regulate the expression of numerous genes involved in a variety of cellular functions. HNF1B plays a key role in the development and function of several organs, particularly the pancreas (21) and kidney (22). It regulates the expression of numerous genes in the tissues in which it is expressed (23), but whether it alters levels of the various metabolic and hormonal factors that may influence prostate cancer risk in diabetic men is unknown. JAZF1 is also a transcription factor that acts as a transcriptional repressor of an orphan nuclear receptor known as TR4 (also known as TAK1 or NR2C2) (24) that contributes to the regulation of blood levels of glucose (25) and IGF-1 (26). JAZF1 polymorphisms have been associated with human height (27), further supporting a role for this gene in the regulation of growth and metabolism. Thus, it appears feasible that genetic variation in JAZF1 and HNF1B, may influence prostate cancer risk by altering the levels of hormones or growth-related proteins previously suggested to mediate the association between diabetes and this cancer.
The main strength of this study is the availability of men for whom both prostate cancer and diabetes status has been defined within two well-characterized, large cohorts. This allowed us to examine the association of the HNF1B and JAZF1 SNPs with both diseases in the same population. The limitations include the relatively low number of prostate cancer cases with diabetes, which limited the power of the stratified analyses. Additional limitations are the use of self-reported diabetes status and fact that diabetes status was not updated during the follow-up period. However, a recent study found that almost 80% of self-reports of diabetes were valid (28), suggesting that misclassification of men as diabetic was minimal. The use of self-reported diabetes status also did not separate men with type I diabetes from those with type II diabetes and could result in some misclassification of disease status. However, this may be minimal because a previous study reported that approximately 95% of all diabetes cases in the US population with similar age distribution as in CPS-II and PLCO are type II (29). Some misclassification could also arise because diabetes status was not updated during the follow-up period. This could potentially reduce the attenuation of the association of the SNPs with prostate cancer when testing whether diabetes status mediated this relationship. However, since few men are likely to be misclassified because of this and there was no attenuation of this association when diabetes status was included in the model, the lack of updating does not appear to influence the conclusions drawn from this study.
In summary, we have shown that the association of the HNF1B SNPs with prostate cancer is not mediated by diabetes status and that these genetic variants are not responsible for the association of diabetes with reduced prostate cancer risk. We also found that the HNF1B SNP rs11649743, which represents a distinct locus from the original one associated with prostate cancer (11,13,14), may be associated with increased risk of diabetes. If this association holds up to replication in larger diabetes populations, then there will be two separate loci in the HNF1B gene associated with both prostate cancer and diabetes. The JAZF1 SNPs associated with prostate cancer are not associated with diabetes. Further research into the functions of the HNF1B and JAZF1 transcription factors and the influence of the relevant SNPs on these functions is needed to clearly define the involvement of these genes in diabetes and prostate cancer.
References
- 1.Heron MP. Deaths: Leading Causes for 2004. National Vital Statistics Reports. 2007;56(5):1–92. [PubMed] [Google Scholar]
- 2.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 3.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2008;124(6):1398–1403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leitzmann MF, Ahn J, Albanes D, Hsing AW, Schatzkin A, Chang SC, Huang WY, Weiss JM, Danforth KN, Grubb RL, 3rd, Andriole GL. Diabetes mellitus and prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Causes Control. 2008;19(10):1267–1276. doi: 10.1007/s10552-008-9198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calton BA, Chang SC, Wright ME, Kipnis V, Lawson K, Thompson FE, Subar AF, Mouw T, Campbell DS, Hurwitz P, Hollenbeck A, Schatzkin A, Leitzmann MF. History of diabetes mellitus and subsequent prostate cancer risk in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2007;18(5):493–503. doi: 10.1007/s10552-007-0126-y. [DOI] [PubMed] [Google Scholar]
- 6.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, Kristal AR. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 7.Velicer CM, Dublin S, White E. Diabetes and the risk of prostate cancer: the role of diabetes treatment and complications. Prostate Cancer Prostatic Dis. 2007;10(1):46–51. doi: 10.1038/sj.pcan.4500914. [DOI] [PubMed] [Google Scholar]
- 8.Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. Association of diabetes with prostate cancer risk in the Multiethnic cohort. Am J Epidemiol. 2009;169(8):937–946. doi: 10.1093/aje/kwp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce BL, Plymate S, Ostrander EA, Stanford JL. Diabetes mellitus and prostate cancer risk. Prostate. 2008;68(10):1126–1132. doi: 10.1002/pros.20777. [DOI] [PubMed] [Google Scholar]
- 10.Winckler W, Weedon MN, Graham RR, McCarroll SA, Purcell S, Almgren P, Tuomi T, Gaudet D, Bostrom KB, Walker M, Hitman G, Hattersley AT, McCarthy MI, Ardlie KG, Hirschhorn JN, Daly MJ, Frayling TM, Groop L, Altshuler D. Evaluation of common variants in the six known maturity-onset diabetes of the young (MODY) genes for association with type 2 diabetes. Diabetes. 2007;56(3):685–693. doi: 10.2337/db06-0202. [DOI] [PubMed] [Google Scholar]
- 11.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39(8):977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 12.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, Ardlie K, Bostrom KB, Bergman RN, Bonnycastle LL, Borch-Johnsen K, Burtt NP, Chen H, Chines PS, Daly MJ, Deodhar P, Ding CJ, Doney AS, Duren WL, Elliott KS, Erdos MR, Frayling TM, Freathy RM, Gianniny L, Grallert H, Grarup N, Groves CJ, Guiducci C, Hansen T, Herder C, Hitman GA, Hughes TE, Isomaa B, Jackson AU, Jorgensen T, Kong A, Kubalanza K, Kuruvilla FG, Kuusisto J, Langenberg C, Lango H, Lauritzen T, Li Y, Lindgren CM, Lyssenko V, Marvelle AF, Meisinger C, Midthjell K, Mohlke KL, Morken MA, Morris AD, Narisu N, Nilsson P, Owen KR, Palmer CN, Payne F, Perry JR, Pettersen E, Platou C, Prokopenko I, Qi L, Qin L, Rayner NW, Rees M, Roix JJ, Sandbaek A, Shields B, Sjogren M, Steinthorsdottir V, Stringham HM, Swift AJ, Thorleifsson G, Thorsteinsdottir U, Timpson NJ, Tuomi T, Tuomilehto J, Walker M, Watanabe RM, Weedon MN, Willer CJ, Illig T, Hveem K, Hu FB, Laakso M, Stefansson K, Pedersen O, Wareham NJ, Barroso I, Hattersley AT, Collins FS, Groop L, McCarthy MI, Boehnke M, Altshuler D. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40(5):638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J, Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willet WC, Cussenot O, Valeri A, Andriole GL, Crawford ED, Tucker M, Gerhard DS, Fraumeni JF, Jr, Hoover R, Hayes RB, Hunter DJ, Chanock SJ. Multiple loci identified in a genome-wide association study of prostate cancer. Nature Genet. 2008;40(3):310–315. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Zheng SL, Wiklund F, Isaacs SD, Purcell LD, Gao Z, Hsu FC, Kim ST, Liu W, Zhu Y, Stattin P, Adami HO, Wiley KE, Dimitrov L, Li T, Turner AR, Adams TS, Adolfsson J, Johansson JE, Lowey J, Trock BJ, Partin AW, Walsh PC, Trent JM, Duggan D, Carpten J, Chang BL, Gronberg H, Isaacs WB, Xu J. Evidence for two independent prostate cancer risk-associated loci in the HNF1B gene at 17q12. Nat Genet. 2008;40(10):1153–1155. doi: 10.1038/ng.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frayling TM, Colhoun H, Florez JC. A genetic link between type 2 diabetes and prostate cancer. Diabetol. 2008;51(10):1757–1760. doi: 10.1007/s00125-008-1114-9. [DOI] [PubMed] [Google Scholar]
- 16.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, Feigelson HS, Thun MJ. The American Cancer Society Cancer Prevention Study II Nutrition Cohort. Cancer. 2002;94:2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 17.Rothman KJ, Greenland S. Modern Epidemiology. 2. Baltimore: Lippincott, Williams and Wilkens; 1998. [Google Scholar]
- 18.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21(6 Suppl):251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol. 2005;161:147–152. doi: 10.1093/aje/kwh334. [DOI] [PubMed] [Google Scholar]
- 20.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiol. 1992;3(2):143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Yamagata K. Regulation of pancreatic beta-cell function by the HNF transcription network: lessons from maturity-onset diabetes of the young (MODY) Endocr J. 2003;50(5):491–499. doi: 10.1507/endocrj.50.491. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Bohn S, Ryffel GU. The HNF1beta transcription factor has several domains involved in nephrogenesis and partially rescues Pax8/lim1-induced kidney malformations. Eur J Biochem. 2004;271(18):3715–3728. doi: 10.1111/j.1432-1033.2004.04312.x. [DOI] [PubMed] [Google Scholar]
- 23.Senkel S, Lucas B, Klein-Hitpass L, Ryffel GU. Identification of target genes of the transcription factor HNF1beta and HNF1alpha in a human embryonic kidney cell line. Biochim Biophys Acta. 2005;1731(3):179–190. doi: 10.1016/j.bbaexp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Fujino S, Nakanishi G, Kim YS, Jetten AM. TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4. Nucleic Acids Res. 2004;32(14):4194–4204. doi: 10.1093/nar/gkh741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu NC, Lin WJ, Kim E, Collins LL, Lin HY, Yu IC, Sparks JD, Chen LM, Lee YF, Chang C. Loss of TR4 orphan nuclear receptor reduces phosphoenolpyruvate carboxykinase-mediated gluconeogenesis. Diabetes. 2007;56(12):2901–2909. doi: 10.2337/db07-0359. [DOI] [PubMed] [Google Scholar]
- 26.Collins LL, Lee YF, Heinlein CA, Liu NC, Chen YT, Shyr CR, Meshul CK, Uno H, Platt KA, Chang C. Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci U S A. 2004;101(42):15058–15063. doi: 10.1073/pnas.0405700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson A, Marroni F, Hayward C, Franklin CS, Kirichenko AV, Jonasson I, Hicks AA, Vitart V, Isaacs A, Axenovich T, Campbell S, Dunlop MG, Floyd J, Hastie N, Hofman A, Knott S, Kolcic I, Pichler I, Polasek O, Rivadeneira F, Tenesa A, Uitterlinden AG, Wild SH, Zorkoltseva IV, Meitinger T, Wilson JF, Rudan I, Campbell H, Pattaro C, Pramstaller P, Oostra BA, Wright AF, van Duijn CM, Aulchenko YS, Gyllensten U. Common variants in the JAZF1 gene associated with height identified by linkage and genome-wide association analysis. Hum Mol Genet. 2009;18(2):373–380. doi: 10.1093/hmg/ddn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, Kempainen S, Simin L, Robinson JG, Safford MM, Tinker LT, Phillips LS. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5(3):240–247. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]