Abstract
Allogeneic blood or marrow transplantation (BMT) is potentially curative for a variety of life-threatening nonmalignant hematologic diseases such as paroxysmal nocturnal hemoglobinuria (PNH) and hemoglobinopathies. The application of BMT to treat these disorders is limited by the lack of suitable donors and often end-organ damage from the underlying disease. We treated three patients with thrombotic PNH, one of whom also had sickle cell disease, with a nonmyeloablative, HLA-haploidentical BMT with post-transplant CY. Rapid engraftment without GVHD occurred in two of the patients, including the patient with sickle cell disease. Both patients are disease free with full donor chimerism and require no immunosuppressive therapy, with follow-up of 1 and 4 years, respectively. Nonmyeloablative, HLA-haploidentical BMT with post-transplant CY is a promisingapproac h for patients with life-threatening nonmalignant hematologic disease who lack an HLA-matched sibling donor.
Keywords: paroxysmal nocturnal hemoglobinuria, sickle cell anemia, cyclophosphamide, haploidentical
Introduction
Allogeneic blood or marrow transplantation (BMT) is potentially curative for a variety of life-threatening nonmalignant hematologic diseases including sickle cell disease, aplastic anemia, paroxysmal nocturnal hemoglobinuria (PNH), thalassemia and others. The best results in sickle cell disease are with myeloablative conditioning regimens in children and young adults using HLA-matched siblings;1,2 however, PNH and sickle cell patients often have significant end-organ toxicity that disqualifies them from myeloablative BMT. Reduced-intensity BMT from matched siblings has been successful in PNH,3 but graft rejection has been a major obstacle to this approach in sickle cell disease.4–6
Identifying a suitable donor also limits the application of BMT, especially for sickle cell anemia; fewer than 18% of patients with sickle cell disease have a suitable HLA-matched sibling donor.7 To expand the potential donor pool, umbilical cord blood BMT has been tried for sickle cell disease; however, the high incidence of graft failure has also limited the enthusiasm for this approach.8,9
Related haploidentical BMT is an alternative method for expanding the potential pool of BM donors; any patient shares one HLA haplotype with each biologic parent or child and siblings or half-siblings have a 50% chance of being haploidentical. The disadvantage of this approach has been the high incidence of graft rejection and severe GVHD. Recently, we have shown that haploidentical BMT using nonmyeloablative conditioning and high-dose, post-transplantation CY is associated with low rates of fatal graft failure, infection and severe acute GVHD in patients with hematologic malignancies.10
Here, we report the results of three PNH patients, one of whom also suffered from sickle cell disease, treated with a reduced-intensity allogeneic BM transplant from an HLA-haploidentical donor using post-transplantation high-dose CY to mitigate GVHD.
Patients and methods
Patient characteristics
Patient characteristics are listed in Table 1. All patients gave informed consent for study participation as approved by the Johns Hopkins University Institutional Review Board. Patients 1 and 2 had classical PNH with multiple thromboses requiring thrombolytic therapy and systemic anticoagulation. Despite aggressive anticoagulation both patients experienced progression of their thromboses leading to worsening performance status from Budd–Chiari syndrome. Patient 3 acquired PNH in the setting of sickle cell disease and immune thrombocytopenic purpura. Before acquiring PNH, she experienced more than 10 pain crises a year that required hospitalization and 2–3 pain crises a month that she managed at home. Her immune thrombocytopenia was unresponsive to treatment with prednisone, i.v. Ig, splenectomy, rituximab and danazol. One year before her BMT she was diagnosed with PNH after presenting with multiple bouts of hemoglobinuria associated with back pain, abdominal pain and esophageal spasm distinct from her sickle cell pain. She required multiple RBC and plt transfusions and developed a positive direct antiglobulin test and panreactive HLA Ab with a panel reactive Ab titer of 100. The HLA genotypes for the three patients and their donors are given in Table 2. All three patients were fully mismatched with their donors for HLA-A, B, Cw, DRB1 and DQB1 alleles on the unshared haplotype, except for patient 1, who is homozygous at the HLA-A locus. Because of this homozygosity, there was only a four-allele mismatch in the GVH direction for patient 1.
Table 1.
Patient characteristics
| Patient | Age (years)/sex |
Duration of PNH (years) |
PNH granulocytes |
Sites of thrombosis | LDH (U/l) | WBC | Hgb | Platelets | Karnofsky score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 27/male | 15 | 87% | Hepatic vein, portal vein, | 2315 | 2120 | 7.8 | 73 000 | 50 |
| 2 | 37/male | 7 | 99% | Saggital vein, internal jugular vein, hepatic vein, pulmonary embolism, inferior vena cava | 709 | 4200 | 7.2 | 84 000 | 30 |
| 3 | 33/female | 1 | 50% | None | 1536 | 3860 | 10.7 | 6000 | 30 |
Abbreviations: LDH = lactate dehydrogenase; PNH = paroxysmal nocturnal hemoglobinuria.
Table 2.
Donor/recipient HLAalleles
| Patient/donor | HLA class I alleles |
HLA class II alleles |
Allele level MM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | Cw1 | Cw2 | DRB1-1 | DRB1-2 | DQB1-1 | DQB1-2 | GVH | HVG | |
| Patient 1 | 201 | 201 | 702 | 2705 | 0401/09N | 102 | 401 | 701 | 304 | 303 | 4 | 5 |
| Donor—father | 201 | 3101 | 1302 | 4001 | 0401/09N | 304 | 401 | 404 | 304 | 302 | ||
| Patient 2 | 3001 | 3201 | 1302 | 3801 | 602 | 1203 | 901 | 1301 | 0303/12 | 603 | 5 | 5 |
| Donor—brother | 3001 | 2402 | 1302 | 3503 | 602 | 0401/09N | 901 | 1501 | 0303/12 | 0602/19 | ||
| Patient 3 | 301 | 6802 | 702 | 0705/06 | 702 | 1505 | 1501 | 1102 | 0602/19 | 0301/09 | 5 | 5 |
| Donor—mother | 301 | 3001 | 702 | 4201 | 702 | 1701/02/03 | 1501 | 302 | 0602/19 | 402 | ||
HLA alleles were defined through a combination of sequence-based typing and reverse sequence-specific oligonucleotide hybridization. Mismatched alleles on the unshared donor haplotype are indicated in the shaded boxes. All donors are fully mismatched in the direction of rejection (Host vs graft) at all HLA loci tested. Because patient 1 is homozygous at the HLA-A locus, there are only four allele mismatched in the graft vs host vector.
BM transplant conditioning regimen
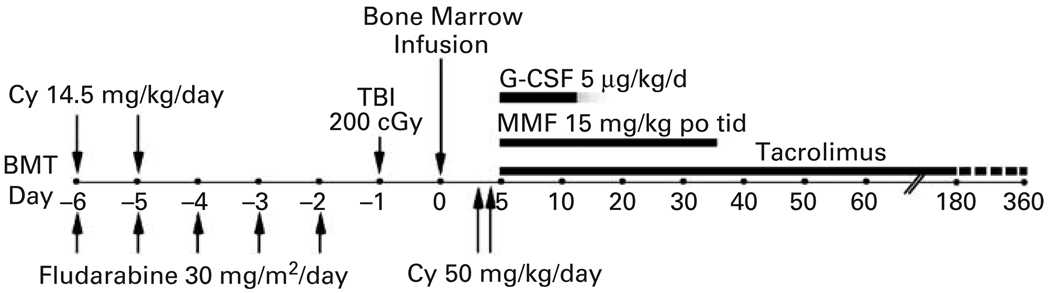
All patients received conditioning therapy and post-transplant GVHD immunosuppression as previously described.10,11 Briefly, i.v. CY 14.5 mg/kg/day was administered on days −6 and −5, fludarabine 30 mg/m2/day i.v. on days −6 to −2 followed by 200 cGy of TBI on day −1 (Figure 1). The marrow allograft contained 1.38 × 108 nucleated cells/kg (buffy coat prepared on a Gambro apheresis instrument for RBC depletion because of major ABO incompatibility), and 4.57 × 106 CD34 positive cells/kg in patient 1; 4.49 × 108 nucleated cells/kg and 5.40 × 106 CD34 positive cells/kg in patient 2; and 4.57 × 108 nucleated cells/kg and 4.44 × 106 CD34 positive cells/kg in patient 3. On days 3 and 4 post transplant, 50 mg/kg CY was administered over 90 min together with Mesna (80% of CY dose in four divided doses over 8 h) by i.v. infusion. The patients received mycophenolate mofetil (Cellcept; Roche Laboratories, Nutley, NJ, USA) 15 mg/kg p.o. t.i.d. from day 4 to 35 and tacrolimus (Prograf; Fujisawa, Deerfield, IL, USA) from day 4 to day 180 or 360. Tacrolimus was initiated at a dose of 1 mg i.v. daily, adjusted to achieve a therapeutic level of 5–15 ng/ml, and then converted to oral form until discontinuation. Filgrastim (Neupogen, Amgen, Thousand Oaks, CA, USA), 5 µg/kg/day was administered by s.c. injection starting on day 1 and continuing until recovery of neutrophils to >1000/µl for 3 days. Prophylactic anti-microbial therapy was started on day −6 and included norfloxacin 400 mg p.o. twice daily, fluconazole 400 mg p.o. daily, appropriate prophylaxis for Pneumocystis carinii pneumonia and valacyclovir, 500 mg p.o. thrice daily, as described previously. All patients were treated in the ambulatory transplant clinic.
Figure 1.
Treatment schema. CY = cyclophosphamide; MMF = mycofenolate mofitil; TBI = total body irradiation.
Chimerism analyses
At monthly intervals, nucleated cells were isolated from the marrow or peripheral blood or T cells (CD3-positive) and granulocytes (CD33-positive) were sorted from peripheral blood by flow cytometry. Percentages of donor–host chimerism for recipients of sex-mismatched BMT were determined by fluorescein in situ hybridization (FISH)12 using probes for X and Y chromosomes. For recipients of sex-matched BMT, chimerism was based on RFLP13 or PCR analysis of variable nucleotide tandem repeats14 unique to donors or recipients.15
Results
Patient 1
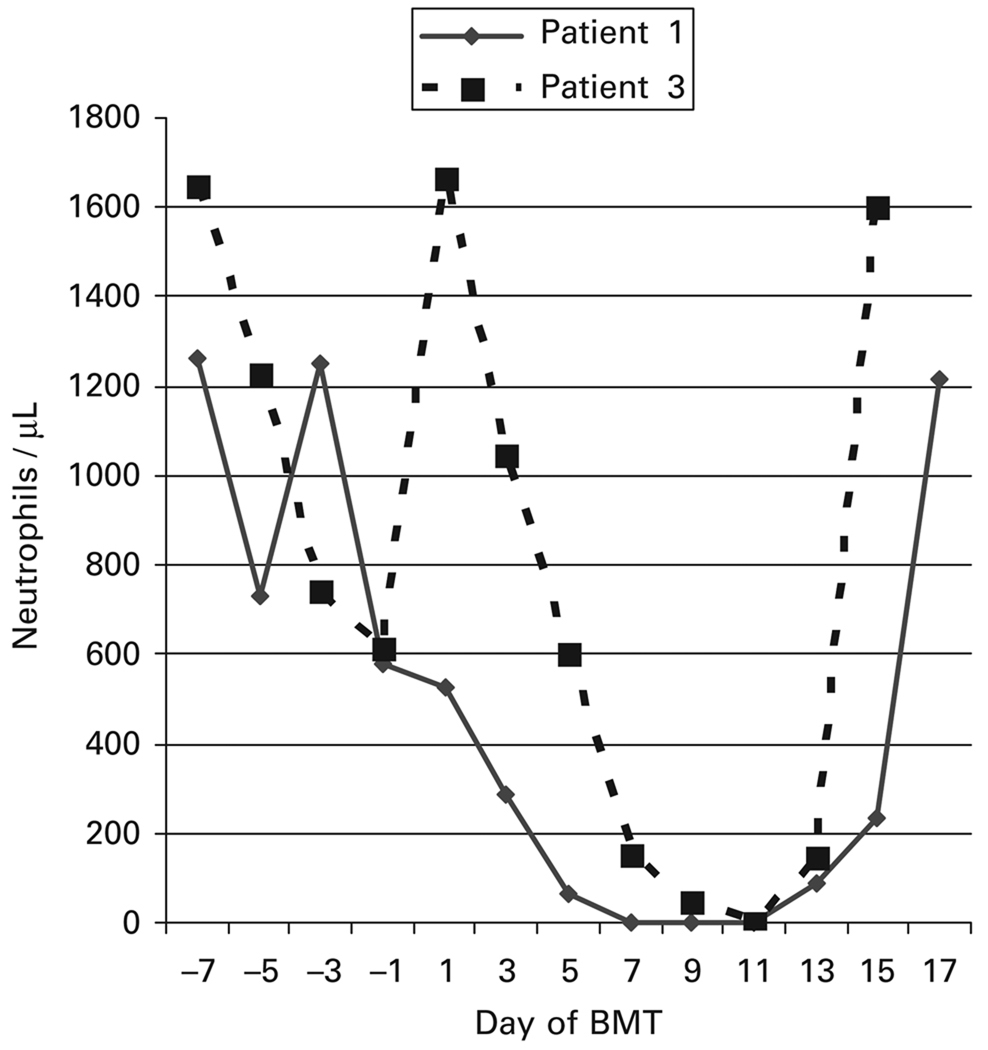
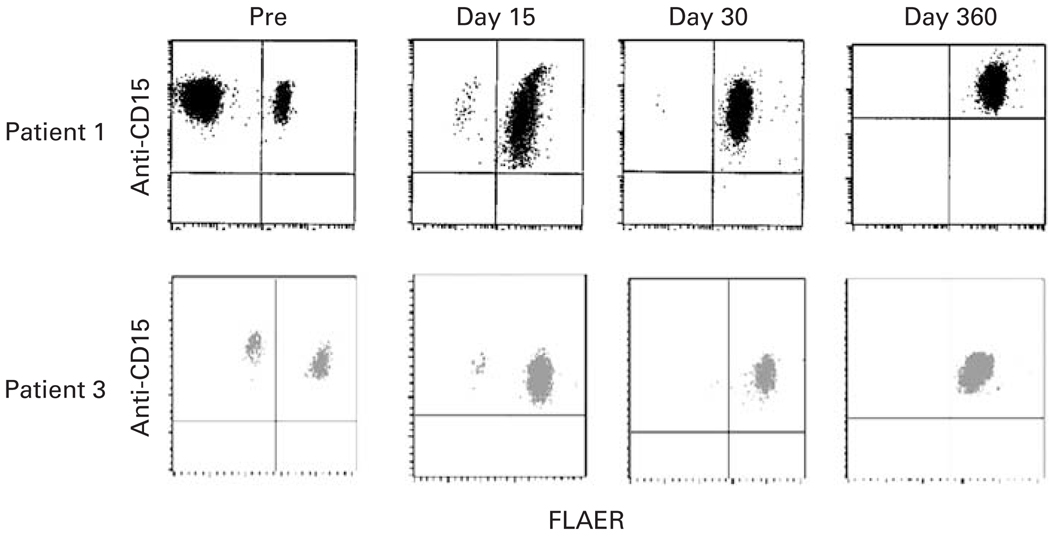
On day −1 through day 5 after BMT, the patient was admitted to the hospital for a PNH crisis that manifested with abdominal pain, hemoglobinuria, nausea, vomiting and fever lasting 6 days. He was readmitted for neutropenic fever on days 11–14. Blood cultures grew viridans Streptococci and he was treated with a 14-day course of vancomycin. The patient experienced rapid hematopoietic recovery (Figure 2). His ANC reached 500/µl on day 16 and he achieved RBCs and plt transfusion independence on days 23 and 22, respectively. Full-donor chimerism was documented on day 30 after BMT. By day 30, his PNH clone had regressed (Figure 3), his anticoagulation was discontinued, and all PNH manifestations had resolved. His tacrolimus was discontinued on day 360. The patient had no GVHD. At 10 months post transplant, he developed varicella zoster of his right trigeminal nerve that resolved after treatment with acyclovir. He remains in complete hematologic remission without evidence of PNH 48 months post transplant.
Figure 2.
Neutrophil recovery after haploidentical BMT.
Figure 3.
Dual-color display of peripheral blood granulocytes from patients 1 and 3 after staining with anti-CD15 PE and FLAER. Granulocytes were analyzed before (pre) and at days 15, 30 and 360, after BMT.
Patient 2
The patient was admitted to the hospital on day −4 of his transplant for Candida krusei sepsis. Despite the use of broad-spectrum antibacterial and antifungal antibiotics the patient developed multiorgan failure and expired from Candida krusei sepsis 8 days after his HLA-haploidentical BMT. There was no evidence of engraftment at the time of death.
Patient 3
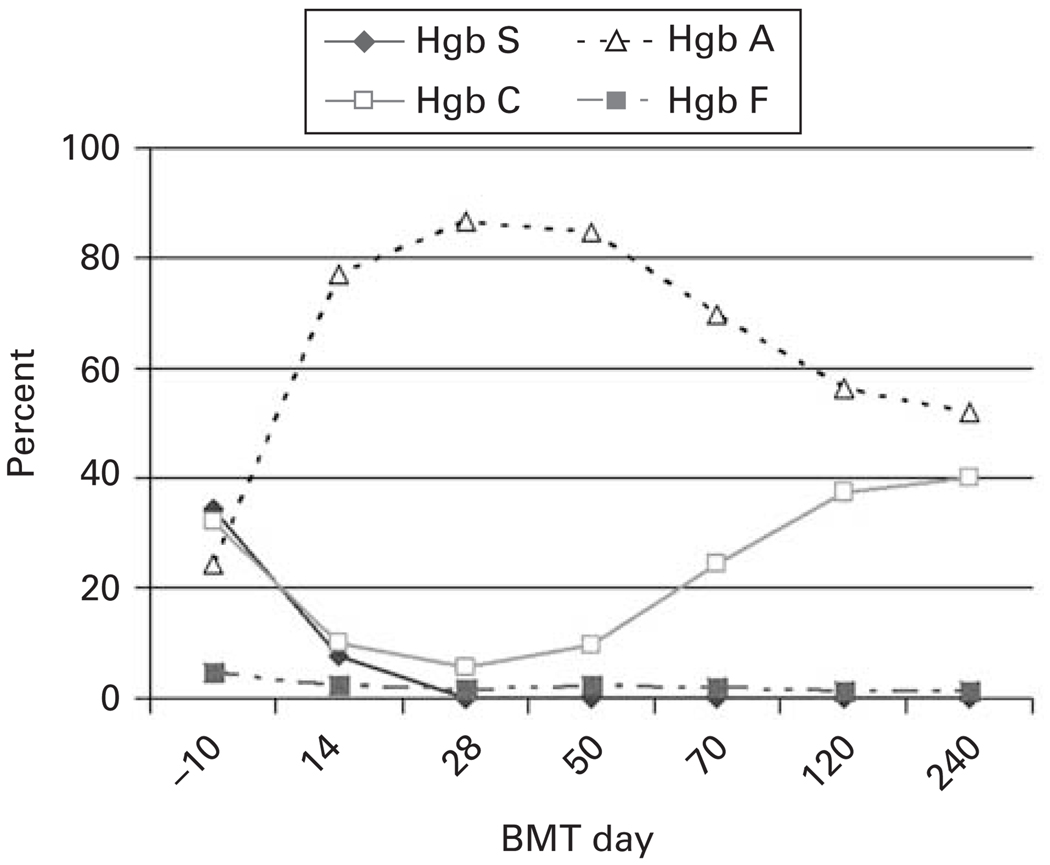
The patient was admitted for her conditioning regimen, due to pain from frequent sickle and PNH crises and discharged 18 days after her BMT. She required patient-controlled analgesia with hydromorphone for pain and broad-spectrum antibiotics for febrile neutropenia. Her ANC reached 500/µl on day 14 and she achieved RBC and plt transfusion independence on days 26 and 17, respectively. RFLP analysis of chimerism on day 30 after BMT showed 90% donor DNA; by day 60 after BMT no patient DNA was detectable. By day 30 after BMT, greater than 99% of her granulocytes were expressing glycosylphosphatidylinositol-anchored proteins and by day 45 Hb S was undetectable (Figures 3b and 4). Her tacrolimus was discontinued on day 180. She is alive and well 1 year after BMT with no GVHD. Her RFLP shows no patient DNA and her sickle cell disease and PNH are in CR. Her donor was heterozygous for Hb C. Accordingly, her most recent Hb variant analysis reveals 52% Hb A, 41% Hb C and 1% Hb F.
Figure 4.
Hb variants from patient 3 before and after BMT.
Discussion
We demonstrated previously that high-dose CY has the potential to eradicate both autoimmunity16–18 and alloimmunization.19 High-dose CY is not toxic to primitive hematopoietic stem cells because they possess high levels of aldehyde dehydrogenase, an enzyme that confers resistance to the drug.20,21 However, high-dose CY can be quite toxic to lymphocytes, especially recently activated T cells. Extensive studies in the mouse suggest that Allo-SCT induces the bidirectional activation and proliferation of alloreactive, that is, host-vs-graft and graft-vs-host reactive, T cells, and that these recently activated T cells are more susceptible than resting T cells to being killed by high-dose CY.22 Consistent with this hypothesis is the finding that CYA, which blocks T-cell activation, also blocks high-dose CY-induced tolerance to histocompatibility Ag.23 Thus, high-dose post-transplantation CY is selectively immunosuppressive, but not myeloablative. Here, we used high-dose CY beginning 3 days after HLA-haploidentical BMT in three PNH patients (one of whom also had sickle cell disease) in an attempt to mitigate GVHD. In spite of the high level of HLA mismatch between the hosts and donors, two of three patients, including the patient with sickle cell disease, achieved rapid hematopoietic engraftment with no GVHD. To our knowledge, this is the first successful report of reduced intensity, HLA-haploidentical BMT in patients with PNH or sickle cell disease.
All three patients in this trial were treated before eculizumab was commercially available; furthermore, none of these patients would have been eligible for the eculizumab trials. Now that eculizumab is FDA approved, the role of BMT in PNH may be decreasing.24–26 Nevertheless, BMT offers the only potential for cure for PNH patients and may still be considered for those with life-threatening thrombosis.
In conclusion, we have shown that nonmyeloablative, HLA-haploidentical BMT with post-transplant CY can eradicate PNH. Furthermore, we demonstrate that nonmyeloablative HLA-haploidentical BMT has the potential to cure sickle cell anemia. Reduced-intensity HLA-haploidentical BMT with post-transplant CY can be administered to patients with compromised performance status and organ function. Two of three patients tolerated the procedure extremely well even though all three patients in this study had a Karnofsky performance status of 50 or below and significant end-organ disease. Moreover, there was no GVHD in the two evaluable patients suggesting that the post-transplantation CY was effective in mitigating GVHD. Successful application of HLA-haploidentical BMT for hemoglobinopathies would greatly expand the pool of suitable donors offering a greater percentage of patients the prospect for cure. Further investigation of this promising approach in patients with life-threatening hemoglobinopathies is planned.
References
- 1.Panepinto JA, Walters MC, Carreras J, Marsh J, Bredeson CN, Gale RP, et al. Matched-related donor transplantation for sickle cell disease: report from the center for international blood and transplant research. Br J Haematol. 2007;137:479–485. doi: 10.1111/j.1365-2141.2007.06592.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–2756. doi: 10.1182/blood-2007-03-079665. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi Y, McCoy JP, Jr, Carvallo C, Rivera C, Igarashi T, Srinivasan R, et al. In vitro and in vivo evidence of PNH cell sensitivity to immune attack after nonmyeloablative allogeneic hematopoietic cell transplantation. Blood. 2004;103:1383–1390. doi: 10.1182/blood-2003-04-1281. [DOI] [PubMed] [Google Scholar]
- 4.van BK, Bartholomew A, Stock W, Peace D, Devine S, Sher D, et al. Fludarabine-based conditioning for allogeneic transplantation in adults with sickle cell disease. Bone Marrow Transplant. 2000;26:445–449. doi: 10.1038/sj.bmt.1702518. [DOI] [PubMed] [Google Scholar]
- 5.Iannone R, Casella JF, Fuchs EJ, Chen AR, Jones RJ, Woolfrey A, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant. 2003;9:519–528. doi: 10.1016/s1083-8791(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 6.Horan JT, Liesveld JL, Fenton P, Blumberg N, Walters MC. Hematopoietic stem cell transplantation for multiply transfused patients with sickle cell disease and thalassemia after low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte globulin. Bone Marrow Transplant. 2005;35:171–177. doi: 10.1038/sj.bmt.1704745. [DOI] [PubMed] [Google Scholar]
- 7.Mentzer WC, Heller S, Pearle PR, Hackney E, Vichinsky E. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am J Pediatr Hematol Oncol. 1994;16:27–29. [PubMed] [Google Scholar]
- 8.Adamkiewicz TV, Szabolcs P, Haight A, Baker KS, Staba S, Kedar A, et al. Unrelated cord blood transplantation in children with sickle cell disease: review of four-center experience. Pediatr Transplant. 2007;11:641–644. doi: 10.1111/j.1399-3046.2007.00725.x. [DOI] [PubMed] [Google Scholar]
- 9.Walters MC. Cord blood transplantation for sickle cell anemia: bust or boom? Pediatr Transplant. 2007;11:582–583. doi: 10.1111/j.1399-3046.2007.00757.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell PV, Luznik L, Symons H, Jones RJ, Gooley T, Harrington E, et al. Favorable outcome of patients with relapsed Hodgkin lymphoma after nonmyeloablative hematopoietic cell transplantation using related haploidentical donors. Blood. 2006;108:894a–895a. [Google Scholar]
- 11.O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using post transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 12.Crescenzi B, Fizzotti M, Piattoni S, La SR, Matteucci C, Carotti A, et al. Interphase FISH for Y chromosome, VNTR polymorphisms, and RT-PCR for BCR-ABL in the monitoring of HLA-matched and mismatched transplants. Cancer Genet Cytogenet. 2000;120:25–29. doi: 10.1016/s0165-4608(99)00245-9. [DOI] [PubMed] [Google Scholar]
- 13.Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 14.Sreenan JJ, Pettay JD, Tbakhi A, Totos G, Sandhaus LM, Miller ML, et al. The use of amplified variable number of tandem repeats (VNTR) in the detection of chimerism following bone marrow transplantation. A comparison with restriction fragment length polymorphism (RFLP) by Southern blotting. Am J Clin Pathol. 1997;107:292–298. doi: 10.1093/ajcp/107.3.292. [DOI] [PubMed] [Google Scholar]
- 15.Van Deerlin VM, Leonard DG. Bone marrow engraftment analysis after allogeneic bone marrow transplantation. Clin Lab Med. 2000;20:197–225. [PubMed] [Google Scholar]
- 16.Brodsky RA, Sensenbrenner LL, Jones RJ. Complete remission in acquired severe aplastic anemia following high-dose cyclophosphamide. Blood. 1996;87:491–494. [PubMed] [Google Scholar]
- 17.Brodsky RA, Petri M, Smith BD, Seifter EJ, Spivak JL, Styler M, et al. Immunoablative high-dose cyclophosphamide without stem cell rescue for refractory severe autoimmune disease. Ann Intern Med. 1998;129:1031–1035. doi: 10.7326/0003-4819-129-12-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky RA, Sensenbrenner LL, Smith BD, Dorr D, Seaman PJ, Karp JE, et al. Durable treatment-free remission following high-dose cyclophosphamide for previously untreated severe aplastic anemia. Ann Intern Med. 2001;135:477–483. doi: 10.7326/0003-4819-135-7-200110020-00006. [DOI] [PubMed] [Google Scholar]
- 19.Brodsky RA, Fuller AK, Ratner LE, Leffell MS, Jones RJ. Elimination of alloantibodies by immunoablative high-dose cyclophosphamide. Transplantation. 2001;71:482–484. doi: 10.1097/00007890-200102150-00025. [DOI] [PubMed] [Google Scholar]
- 20.Hilton J. Role of aldehyde dehydrogenase in cyclophosphamide-resistant L1210 leukemia. Cancer Res. 1984;44:5156–5160. [PubMed] [Google Scholar]
- 21.Jones RJ, Barber JP, Vala MS, Collector MI, Kaufmann SH, Ludeman SM, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 22.Mayumi H, Umesue M, Nomoto K. Cyclophosphamide-induced immunological tolerance: an overview. Immunobiology. 1996;195:129. doi: 10.1016/S0171-2985(96)80033-7. [DOI] [PubMed] [Google Scholar]
- 23.Nomoto K, Eto M, Yanaga K, Nishimura Y, Maeda T. Interference with cyclophosphamide-induced skin allograft tolerance by cyclosporin A. J Immunol. 1992;149:2668–2674. [PubMed] [Google Scholar]
- 24.Hillmen P, Young NS, Schubert J, Brodsky RA, Socie G, Muus P, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 25.Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 26.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]