Abstract
Hematopoietic stem cells (HSC) can be harmed by disease, chemotherapy, radiation and normal aging. We now show that damage also occurs in mice repeatedly treated with very low doses of lipopolysaccharide (LPS). Overall health of the animals was good, and there were relatively minor changes in marrow hematopoietic progenitors. However, HSC were unable to maintain quiescence, and transplantation revealed them to be myeloid skewed. Moreover, HSC from treated mice were not sustained in serial transplants and produced lymphoid progenitors with low levels of the E47 transcription factor. This phenomenon was previously seen in normal aging. Screening identified monoclonal antibodies that resolve HSC subsets, and relative proportions of these HSC changed with age and/or chronic LPS treatment. For example, minor CD150Hi CD48− populations lacking CD86 or CD18 expanded. Simultaneous loss of CD150Lo/− CD48− HSC and gain of the normally rare subsets, in parallel with diminished transplantation potential would be consistent with age or Tolllike receptor (TLR) related injury. On the other hand, HSC in old mice differed from those in LPS treated animals with respect to VCAM-1 or CD41 expression, and lacked proliferation abnormalities. HSC can be exposed to endogenous and pathogen derived TLR ligands during persistent low-grade infections. This stimulation might contribute in part to HSC senescence and ultimately compromise immunity.
Introduction
The bone marrow is a target for some pathogens, including cytomegalovirus, parvovirus, dengue virus, hepatitis and HIV (1–5). Animal studies indicate that hematopoiesis can also be affected by C. parvum, pertussis, malaria, influenza, vaccinia virus and immunization (6–11). Depending on the agent, HSC, hematopoietic progenitors and marrow stromal cells can be infected. The same cells can also respond to inflammatory cytokines (12–14).
We discovered another mechanism through which stem and progenitor cells are influenced by pathogen products and endogenous danger signals (15). These cells express Toll-like receptors (TLR), co-receptors and associated signaling molecules. Moreover, several responses were recorded when highly purified cells were exposed to TLR ligands such as LPS under defined culture conditions. HSC were driven into cycle and stimulated to acquire lineage markers while committed myeloid progenitors differentiated even when no exogenous growth and differentiation factors were added. In addition, we observed dramatic changes in lymphoid progenitors; B lymphopoiesis was arrested and CLP were directed to become dendritic cells. Following intra-peritoneal injection, LPS travels quickly to the marrow and engages the TLR4 receptor (15). HSC are then mobilized to the periphery where their differentiation can again be affected by this ligand (16, 17). This ability of stem and progenitor cells to sense pathogen products may be protective, allowing rapid mobilization and generation of cells in the innate immune system. However, it is also possible that this mechanism has pathological consequences in some situations, such as during prolonged systemic exposure to TLR ligands. This would be the case in such conditions as gram-negative periodontitis, sub-acute bacterial endocarditis, and other chronic infectious circumstances (18). Furthermore, TLR4 can be engaged by endogenous ligands and fatty acids, suggesting that HSC might be altered by inflammation associated with tissue damage and obesity (19, 20). This could account for the fact that HSC from some TLR knockout mice have an engraftment advantage over wild type HSC during transplantation (21).
HSC have sufficient potential to replenish all blood cell types for several lifetimes, and numbers of transplantable HSC do not decline (22–24). However, many studies suggest they undergo important age-related changes, including the selective loss of lymphopoietic potential (25–28). This intrinsic tendency for lineage skewing has not been reported in other situations where HSC are injured. For example, a shortening of telomeres causes myeloid skewing, but largely because of systemic factors and HSC remain relatively normal (29, 30). The same is true of animals with naturally occurring DNA damage or defects in DNA repair enzymes (31). That is, the potential to replenish both lymphoid and myeloid lineage cells is compromised. Accelerated HSC senescence has been reported in other stressful conditions that include ionizing radiation and chemotherapy (32, 33). Finally, the marrow can be influenced by cytokines released during systemic infections (13, 14). Again, these seem to be examples in which hematopoietic functions are uniformly compromised.
We now report that repeated exposure to small amounts of LPS is harmful to long-term repopulating stem cells. HSC cycling was elevated and remained so during eight months of serial transplantation within untreated recipients. Initially able to reconstitute multiple hematopoietic lineages, stem cells from LPS-treated mice preferentially lost the ability to generate lymphocytes. This lymphoid versus myeloid bias became more obvious with time, and there was evidence of HSC exhaustion. Some, but not all changes resemble those occurring during aging.
Materials and Methods
Mice
C57BL/6 (CD45.2 alloantigen, Jackson Laboratory, Bar Harbor, ME), aged C57BL/6 (National Institute on Aging), B6-SJL/Ly5.1 (CD45.1 alloantigen, Jackson Laboratory), C57BL/6 × SJL/Ly5.1 F1 (CD45.1 & CD45.2 alloantigens) were bred and maintained in the Laboratory Animal Resource Center at the Oklahoma Medical Research Foundation (Oklahoma City, OK). All mice were 8–15 weeks old, and male and female mice were used without gender discrimination. Experiments were performed in accordance with approved IACUC protocols.
Intraperitoneal LPS Injections
Daily injections containing 6 µg LPS (O55:B5, Sigma, St. Louis, MO) in 200µL PBS, or PBS alone were administered during the 4–6 week treatment period. C57BL/6 mice received LPS, and B6/SJL mice received PBS. Analysis of hematopoietic tissues was performed after 4–6 weeks of injections.
Isolation of cell populations and flow cytometry
Flow cytometry analyses and sorting were performed as previously described (15, 34). Tissue and cell manipulations were performed in HBSS + 5% FCS. Marrow cells were isolated from the long bones of donor mice, and erythrocytes were lysed in NH4Cl− hypotonic solution. Peritoneal cavity (PC) cells were acquired by lavage with 10mL HBSS + 5% FCS. To isolate progenitor populations for culture and transplantation, BM cells were enriched by negative selection by labeling marrow with Ly6G+C/Gr-1 (RB6–8C5), CD11b/Mac-1 (M1/70), TER-119, CD3 (17A2), CD8 (53-6.7), CD19 (1D3), B220 (14.8), and then immunomagnetically depleted with the BioMag goat anti–rat IgG system (Qiagen, Valencia, CA). All cells were treated with Fc-receptor block (2.4G2) prior to fluorescent staining and sorting. After staining marrow with biotin-anti-lineage markers (Gr-1, CD11b, CD19, CD45R/B220, Ter119, CD3, CD8, and NK1.1), anti–cKit (2B8) and anti-Sca-1 (D7, eBioscience, San Diego, CA), anti-IL-7Rα (A7R34, eBioscience), and anti-Flt3 (A2F10. Biolegend) and secondary streptavidin PE-Texas Red (Caltag Laboratories, Burlingame, CA), Lin− populations were sorted using either a MoFlo (DakoCytomation, Ft. Collins, CO) or FACSAria cytometer (BD Biosciences, San Diego, CA) into specific populations. Dead cells were excluded by propidium iodide (PI) staining (Molecular Probes, Eugene, OR). Purification of each subset was achieved by double sorting, and confirmed by post-sort analysis. Cells harvested from sacrificed recipient mice were analyzed to determine the phenotypes of resident or donor cells. Abs included CD3 (145-2C11), B220 (RA3–6B2), CD8 (53–6.7), CD11b (M170), TER-119, Gr-1 (RB6–8C5), IgM (R6–60.2), NK1.1 (PK136), CD19 (1D3), CD48 (HM48-1, Biolegend), CD135/Flt3 (A2F10, Biolegend), CD11c (HL3), CD34 (HM34, Biolegend), FcγRII/III (93), CD150 (TC15-12F12.2, Biolegend), CD86 (GL1), CD18 (C71/16), Vcam-1 (429(MVCAM.A)), CD41 (MWReg30), CD39 (5F2, Biolegend), CD25 (PC61), CD44 (IM7), CD45.1 (A20) and CD45.2 (104). Intracellular staining with FITC-anti-E47 was accomplished by fixation in 100% ethanol, followed by permeabilization in 0.2% Tween 20. After E47 staining, cells were washed 3 times prior to flow cytometry analysis in HBSS +5% FCS. Flow cytometry was performed on a BD LSRII (BD Biosciences, San Jose, CA), and FlowJo software (Treestar, San Carlos, CA) was used for data analysis. All Abs came from BD Pharmingen, unless otherwise stated.
Intravenous Serial Competitive Transplantations
A chimerism-based transplantation method was developed for these studies. Recipients were 8- to 12-week-old F1 (CD45.1+CD45.2+) mice, lethally irradiated (2 × 650 Rads) in a 137Cs source (Mark I gamma irradiator; J. L. Shepard and Associates, Glendale, CA). Mice were anesthetized with isofluorane (Isosol; Vedco, St Joseph, MO), and cells were infused intravenously by retro-orbital injection. F1 recipients allowed precise demarcation of both competing donor marrow grafts (CD45.1+ control, and CD45.2+ LPS-treated) from residual host cells (CD45.1+CD45.2+ F1). Transplant grafts consisted of equal-part chimeric mixtures containing 2 × 106 nucleated whole marrow cells each from CD45.1+ control- and CD45.2+ LPS-treated mice. Pre-transplant chimeric ratios were confirmed by FACS analysis. Competitive repopulation was assessed at 4 week intervals by peripheral blood analysis, as described below. Primary F1 recipients were sacrificed at 16 weeks, and total, multi-lineage hematopoietic contributions of the competing grafts was assessed in the bone marrow, spleen, thymus, and peritoneal cavities of the F1 recipient mice. Equal numbers of whole marrow nucleated cells collected from one femur of each primary recipient were pooled, and 2 × 106 of these mixed cells were re-transplanted into secondary F1 recipients in similar fashion.
Peripheral Blood Preparations
Mice were briefly anesthetized with isofluorane, and approximately 0.05 mL peripheral blood was obtained from each mouse by retro-orbital bleeding with heparin-coated 10-inch elongated glass pipettes. Heparinized blood from the pipettes was emptied directly into glass centrifuge tubes containing 0.4 mL of a 2% Dextran solution, and mixed thoroughly. The mixture was incubated for 20 minutes at 37° C to allow sedimentation of red blood cells. The upper WBC-enriched phase was retained and cells were washed before brief resuspension in hypotonic NH4Cl− solution as an additional means of removing erythrocytes. The cells were washed in HBSS and stained with immunofluorescent antibodies for analysis by flow cytometry. Complete blood counts were performed using heparin-coated capillary tubes and a cell counter (Serono 9000; Serono-Parker Diagnostics, Allentown, PA).
Cell Cycle Kinetics Analyses
Ki-67: Single cell suspensions of C57BL/6 marrow were fixed and permeabilized utilizing the BD Cytofix/Cytoperm™ kit (BD Biosciences), and stained intracellularly with FITC anti-Ki-67 (B56) or MOPC-21 isotype control. BrdU: Mice were given an intraperitoneal injection of 100µg 5’-Bromo-deoxyuridine in 100µL PBS (BrdU, BD Biosciences), with continual administration of BrdU-enriched drinking water (0.8 mg/mL, Sigma-Aldrich, St. Louis, MO) thereafter during the final 4 days of the injection period. Marrow from a single femur was collected and analyzed by intracellular staining with FITC anti-BrdU (FITC BrdU flow kit; BD Biosciences), and compared to isotype control stains.
Serum-free, Stromal Cell-free Cell Cultures
Sorted cells were cultured in round-bottom 96-well plates (Corning, Inc.) with X-VIVO15 medium (Biowhittaker, Walkersville, MD) containing 1% detoxified bovine serum albumin (Stem Cell Technologies, Vancouver, Canada), 5 × 10−5 M 2-mercaptoethanol (2-ME), 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. Culture medium was enriched with 100 ng/mL FL, 20 ng/mL SCF, 1 ng/mL IL-7, and 20 ng/mL M-CSF in various combinations as indicated in the Results section. DC-lineage promoting conditions included SCF and FL without IL-7. Incubation was maintained at 37°C in a 5% CO2 humidified atmosphere. Cells were fed by replacing half culture volume with fresh media and cytokines every three to four days. Cells were harvested at designated times and stained with mAbs to CD19, B220, CD11c, Ly6c, CD11b/Mac-1, and NK1.1.
Methylcellulose Cultures
Sorted cells were added to 2 mL of Methocult 3434 (Stem Cell Technologies) medium and plated at 100 cells/plate in 35 mm culture dishes. Incubation was carried out as described above, and colonies were counted with a dissecting microscope after 10 days.
Western Blot Analysis of E2A in Pro-B cells
Equal numbers (1.5 × 105 to 5.0 × 105) of sorted pro-B cells (CD19+B220+IgM−CD43+) were lysed in RIPA and separated on 9% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The immunoreactive proteins on the membrane were detected using enhanced chemiluminescence reagents (Amersham, Piscataway, NJ). The antibodies used for probing E2A and α-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistics
The Prism V3.02 software (GraphPad, San Diego, CA) was used for statistical analysis. Unpaired, two-tailed t-test analyses were employed for intergroup comparisons, and p-values were considered significant if less than 0.05.
Isolation of cell populations and flow cytometry
Tissue and cell manipulations were performed in HBSS + 5% FCS. Marrow cells were isolated from the long bones of donor mice, and erythrocytes were lysed in NH4Cl− hypotonic solution. Peritoneal cavity (PC) cells were acquired by lavage with 10mL HBSS + 5% FCS. To isolate progenitor populations for culture and transplantation, BM cells were enriched by negative selection by labeling marrow with Ly6G+C/Gr-1 (RB6–8C5), CD11b/Mac-1 (M1/70), TER-119, CD3 (17A2), CD8 (53-6.7), CD19 (1D3), B220 (14.8), and then immunomagnetically depleted with the BioMag goat anti–rat IgG system (Qiagen, Valencia, CA). All cells were treated with Fc-receptor block (2.4G2) prior to fluorescent staining and sorting. After staining marrow with biotin-anti-lineage markers (Gr-1, CD11b, CD19, CD45R/B220, Ter119, CD3, CD8, and NK1.1), anti–cKit (2B8) and anti-Sca-1 (D7, eBioscience, San Diego, CA), anti-IL-7Rα (A7R34, eBioscience), and anti-Flt3 (A2F10. Biolegend) and secondary streptavidin PE-Texas Red (Caltag Laboratories, Burlingame, CA), Lin− populations were sorted using either a MoFlo (DakoCytomation, Ft. Collins, CO) or FACSAria cytometer (BD Biosciences, San Diego, CA) into specific populations. LSK cells were obtained as Lin−ckitHiSca1+ CLP were sorted as Lin−IL-7Rα+ckitLoSca-1+. CMP were sorted as Lin−Sca-1−ckitHiCD34LoFcγRII/IIILo. Dead cells were excluded by propidium iodide (PI) staining (Molecular Probes, Eugene, OR). Purification of each subset was achieved by double sorting, and confirmed by post-sort analysis. Cells harvested from sacrificed recipient mice were analyzed to determine the phenotypes of resident or donor cells. Abs included CD3 (145-2C11), B220 (RA3–6B2), CD8 (53-6.7), CD11b (M170), TER-119, Gr-1 (RB6–8C5), IgM (R6–60.2), NK1.1 (PK136),CD19 (1D3), CD48 (HM48-1, Biolegend), CD135/Flt3 (A2F10, Biolegend), CD11c (HL3), CD34 (HM34, Biolegend), FcγRII/III (93), CD150 (TC15-12F12.2, Biolegend), CD86 (GL1), CD18 (C71/16), Vcam-1 (429(MVCAM.A)), CD41 (MWReg30), CD39 (5F2, Biolegend), CD25 (PC61), CD44 (IM7), CD45.1 (A20) and CD45.2 (104). Intracellular staining with FITC-anti-E47 was accomplished by fixation in 100% ethanol, followed by permeabilization in 0.2% Tween 20. After E47 staining, cells were washed 3 times prior to flow cytometry analysis in HBSS +5% FCS. Flow cytometry was performed on a BD LSRII (BD Biosciences, San Jose, CA), and FlowJo software (Treestar, San Carlos, CA) was used for data analysis. All Abs came from BD Pharmingen, unless otherwise stated.
Serum Cytokine Analyses
Non-heparinized peripheral blood samples were obtained from mice 2 hours after the daily i.p. LPS injection. Blood samples were allowed to clot for 1 hour at room temperature, then briefly centrifuged to optimize serum separation. 100µL aliquots of serum were assayed for TNFα abundance by ELISA (R & D Systems, Minneapolis, MN). The PE-based Cytometric Bead Array (BD Biosciences) was also employed for simultaneous analysis of multiple serum cytokines (TNFα, IFNγ, IL-6, GM-CSF, IL-10 and IL-12p70), according to manufacturer instructions.
Bone Density Analyses
The right proximal tibiae were scanned by micro-CT (mCT-20, Scanco Medical, Bassersdorf, Switzerland), as described previously (See Anginot, A., et al. PLoS. ONE. 2, e585 (2007). Briefly, the proximal tibiae were fixed in PBS plus 4% paraformaldehyde over night at 4°C and then stored in 70% ethanol. The bones were placed in a 17-mm holder and an image consisting of 200 slices at 9mm voxel size through a region of 1.08 mm in all three axes was generated. Evaluation was done on 120 slices initiating 0.1 mm from the lowest point of the growth plate. A 3D cubical voxel model of bone was built and the following calculations were made: relative bone volume (BV/TV), trabecular number (Tb.N), thickness (Tb.Th), spacing (Tb.Sp), connectivity density (Conn. Dens.) and cortical thickness (Ct. Th).
Results
A model for chronic TLR stimulation
There is no evidence that TLR are needed to build the hematopoietic system under normal steady-state conditions (35). However, we hypothesized that chronic exposure might have longer-term consequences and sought conditions where mice could be safely exposed to a defined TLR ligand for extended periods of time. Following a published protocol, animals were implanted with time-release pellets calibrated to deliver ∼3.5 µg of LPS/ day (36). Although this treatment was well tolerated, the method gave variable mouse to mouse hematopoietic responses. We subsequently found that daily LPS injections of 6 µg/day worked well when delivered for 4–6 weeks, as treatment caused no noticeable wasting or morbidity. Complete blood counts and thymus cellularity were normal at that time (Table 1, and data not shown). While serum ELISA analyses during the first week of injection revealed slight increases in levels of TNFα, no abnormalities were found after four weeks of treatment in TNFα, IFNγ, IL-6, GM-CSF, IL-10 or IL-12p70 (not shown).
Table 1.
Relatively minor changes in hematopoietic cells in low-dose LPS-treated mice
| Cells | Control | LPS |
|---|---|---|
| Total BM (1 femur) | 23.6 ±3.7 × 106 | 24.7 ±4.0 × 106 |
| Total SPL | 1.61 ±0.3 × 108 | 2.8 ±0.9 × 108* |
| Total Thymus | 1.33 ±0.3 × 108 | 1.01 ±0.2 × 108 |
| # Myeloid / femur | 9.76 ±0.03 × 106 | 13.5 ±0.08 × 106* |
| # Pre-B / femur | 6.58 ±0.1.8 × 105 | 2.5 ±0.9 × 105* |
| # cDC / femur | 3.5 ±0.2 × 104 | 5.9 ±1.1 × 104* |
| # B cells / spleen | 6.0 ±2.1 × 10′ | 11.7 ±0.8 × 10′* |
| # cDC / spleen | 3.0 ±0.9 × 106 | 5.6 ±1.1 × 106* |
| # Myeloid / spleen | 25.0 ±6.8 × 106 | 34.5 ±4.8 × 106* |
cDC were gated as CD19−B220−CD11b+ CD11c+
Myeloid cells were gated as CD11b+ Gr-1+
Pre-B were gated as B220+ CD43−IgM−/lo
Representative of 4 experiments with at least 4 mice per group per experiment
p<0.05 as determined by t-test
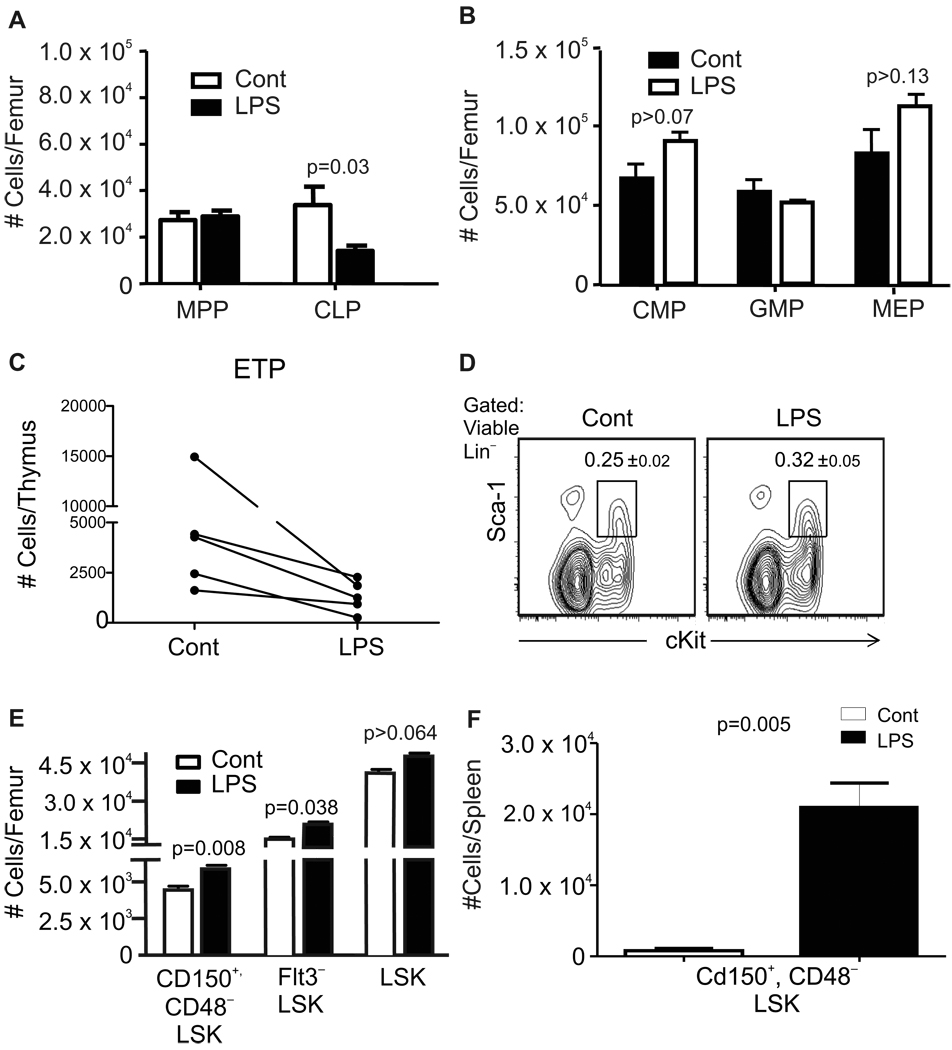
Although bone densities were significantly reduced (Supplemental Fig. 1A), total numbers of marrow nucleated cells were unchanged in LPS-treated mice after 4–6 weeks of daily injection (Table 1). However, percentages of CD11 b+Gr-1+ myeloid cells and CD45R/B220−CD19−CD11 b+CD11 c+ dendritic cells were slightly elevated (1.4 and 1.7 fold, respectively) and there were reciprocal reductions in B220+CD19+ B-lineage lymphocytes (Table 1 and data not shown). The largest depletions were seen in numbers of B220+CD43−IgM− pre-B and newly formed B cells. Numbers of lineage-marker negative (Lin−) Sca-1+cKitLoIL-7Rα+ common lymphoid progenitors (CLP) were significantly reduced while Lin−Sca-1+cKitHi (LSK) Flt3+ multipotent progenitors (MPP), Lin−Sca-1−cKitHiFcγRII/IIILoCD34Lo common myeloid progenitors (CMP), Lin−Sca-1− cKitHiFcγRII/III+CD34+ granulocyte-monocyte progenitor (GMP) and Lin− Sca-1− cKitHi FcγRII/III− CD34− megakaryocyte-erythroid progenitors (MEP) were unchanged relative to untreated mice (Fig. 1A,B and Supplemental Fig. 1B). Numbers of early thymocyte progenitors (ETP) gated as Lin- c-KitHi CD44Hi CD25− were reduced an average of 73% (43–90% ) in LPS treated thymuses as compared to control animals (Fig. 1C). Our analyses then focused on HSC-containing subsets of bone marrow. The Sca-1 surface antigen is known to be a member of the cytokine-inducible Ly-6 gene family (37). Consistent with our findings that inflammatory cytokines were not elevated during the final weeks of treatment, Sca-1 densities and numbers of cells in the primitive LSK fraction were normal in LPS treated mice (Fig. 1D, E). Long-term repopulating HSC represent only a small fraction of LSK, so we employed more rigorous analyses using Flt3−LSK (38, 39) and Lin−Sca-1+cKitHi CD48− CD150+ (40) definitions for HSC (Supplemental Fig. 1B). Absolute numbers of such long-term repopulating stem cells in chronically stimulated marrow were significantly elevated in three independent experiments (Fig. 1E). It has been reported that TLR stimulation increases numbers of HSC in the periphery (16). We found that animals sacrificed after the last treatment had enlarged spleens and absolute numbers of CD150+ CD48− LSK defined HSC in that site were elevated an average of 28 fold (Fig. 1F).
Figure 1. Slight perturbation of hematopoiesis during TLR ligand exposure.
Mice were given daily 6µg injections of LPS, or PBS for 4–6 weeks before harvest of bone marrow and flow cytometry analyses (Gating illustrated in Supplemental Fig. 1B). A, Multipotent progenitors (MPP) defined as Lin−Sca-1+cKitHiFlt3+ and Lin−Sca-1+cKitLoIL-7Rα+ common lymphoid progenitors (CLP) were evaluated in the same samples. B, Lin−Sca-1−cKitHiCD34LoFcγRII/IIILo common myeloid progenitors (CMP), Lin−Sca-1−cKitHiFcγRII/III+CD34+ granulocyte-monocyte progenitor (GMP) and Lin− Sca-1− cKitHi FcγRII/III− CD34− megakaryocyte-erythroid progenitors (MEP) were enumerated in separate experiments. Additional values are given in Table 1, and it is noteworthy that numbers of total nucleated cells in the marrow were unaffected. These data are representative of four independent experiments with 4–6 mice per group, and are given as mean values ± SEM. C, Early thymocyte progenitors gated as Lin− c-KitHi CD44Hi CD25− cells were enumerated and the results from five independent experiments are shown as individual plots. D, The interferon regulated Sca-1 antigen was not up-regulated on cells in the primitive LSK fraction of bone marrow. E, Absolute numbers of cells in the HSC enriched Flt3−LSK fraction and the Lin− Sca-1+ KitHiCD48− CD150+ stem cell subset were significantly increased, although this was not the case for the larger LSK category. F, Absolute numbers of Lin− Sca-1+ KitHiCD48−CD150+ stem cells in the spleen are shown for one of three independent experiments that gave very similar results (p=<0.005).
Therefore, mice can tolerate repeated exposure to very low doses (approximately 1% of the LD50) of LPS. The treatment caused modest increases in HSC numbers as well as depressions in B and T lymphoid progenitors. Expansion of HSC in the spleen could reflect mobilization from the marrow or stimulation in the periphery.
Chronic TLR ligation alters cell division and differentiation potential of primitive hematopoietic cells
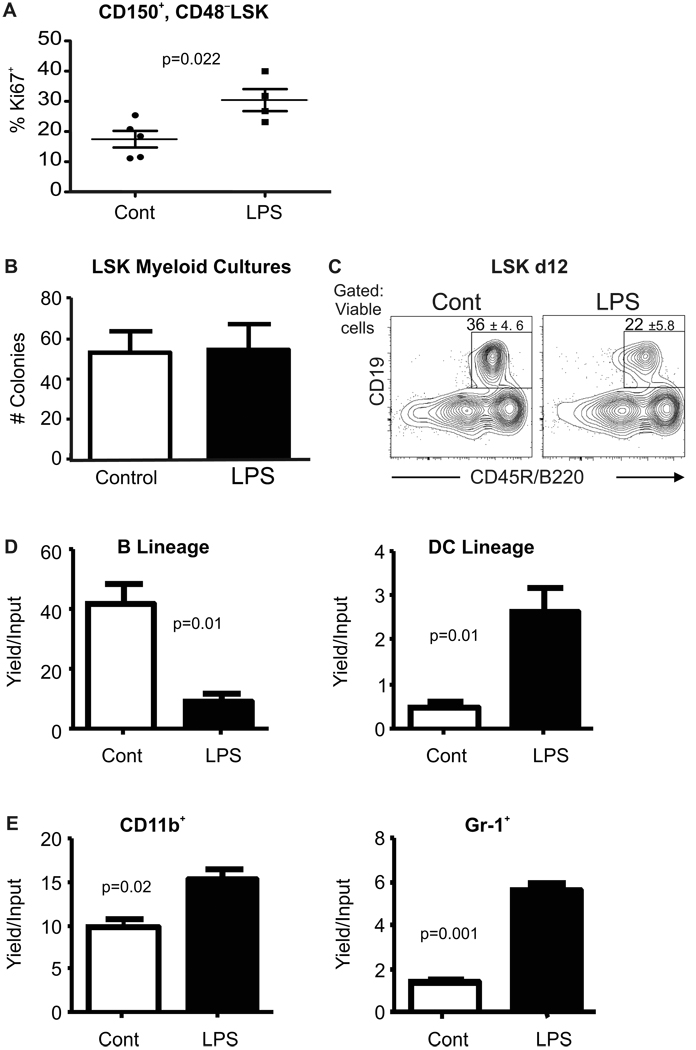
Assessments were then made of cycle status in HSC. Fig. 2A shows small but statistically significant elevations in percentages of CD150+ CD48− LSK for the intracellular Ki-67 antigen, and representative staining is given in Supplemental Fig. 2A. Similar results were obtained when HSC were more generously gated as Flt3−LSK (data not shown). Furthermore, increased numbers of Flt3− LSK incorporated 5-Bromo-deoxyuridine (BrdU) during the last four days of LPS treatment, indicating a persistently elevated cycling rate for HSC-enriched cells chronically exposed to LPS (Supplemental Fig. 2B). The differentiation potential of primitive cells in the LSK subset was then assessed with two types of culture conditions. LSK sorted from marrow of LPS-treated mice and placed into myeloid-promoting Methocel cultures were unaffected in their ability to form myelo-erythroid colonies (Fig. 2B). In contrast, we observed that the same cells displayed a slight bias against formation of CD19+ B-lineage lymphocytes in lymphoid-supporting liquid cultures (Fig. 2C). That is, approximately 25% fewer lymphocytes were generated per input progenitor isolated from treated versus control bone marrow (not shown).
Figure 2. Primitive hematopoietic cells are altered by chronic LPS exposure.
A, Cell cycling activity in CD150+ CD48− LSK in control and LPS treated marrow was assessed by flow cytometry for the Ki-67 proliferation antigen. B, LSK were sorted from mice at the end of LPS or PBS exposure and placed in myeloid supporting Methocel cultures. Average numbers of colonies were counted 10 days later. C, The same fractions were placed in defined, lymphoid supporting liquid cultures for 12 days before flow cytometry. Boxes show percentages of B220+CD19+ lymphocytes that were generated; (p=0.02). This data is representative of that found in four independent experiments with 5–8 mice per group. All error values depict SEM. D, Common lymphoid progenitors (CLP) defined as in Supplemental Fig. 1B were recovered from the two groups of mice after 4–6 weeks of chronic exposure and sorted to high purity. These were placed in defined lymphoid supporting cultures (see Experimental Procedures) for 8 days before harvest and flow cytometry. Yields of CD19+B220+ B-lineage lymphocytes and CD11c+ dendritic cells ±SEM per input progenitor were calculated. E, Common myeloid progenitors (CMP) sorted as shown in Supplemental Fig. 1B were placed in myeloid supporting cultures and their potential for generation of myeloid marker bearing cells was determined 8 days later by flow cytometry. Absolute yields of myeloid cells ±SEM per input progenitor are shown. Similar results were obtained in four independent experiments with at least four culture wells per group. Statistical significance was determined by t-test analysis.
Purified hematopoietic progenitors respond to TLR ligands in defined liquid cultures (15, 34), and we found similar changes in chronically LPS treated mice. That is, CLP lost the potential to generate CD19+ B-lineage cells and had increased propensity for producing CD11c+ dendritic cells (Fig. 2D, Supplemental Fig. 2C). Also, CMP from bone marrow of chronically stimulated mice were prone to generate cells with higher surface densities of the CD11b and/or Gr-1 myeloid cell markers (Fig. 2E, Supplemental Fig. 2D). Thus, low-grade TLR stimulation increased numbers of phenotypically defined HSC, caused some of them to enter cell cycle and have reduced lymphopoietic potential.
Chronic TLR stimulation causes durable changes in long-term repopulating HSC
Experiments involving transplantation of TLR knockout bone marrow indicated that these receptors have important functions on long-term repopulating HSC (21). Furthermore, the above data shows that repeated exposure to LPS interfered with quiescence and skewed differentiation of the HSC rich fractions away from the adaptive immune system. It was unclear if these abnormalities were persistent or would resolve with time, so formal assessments of HSC function were made in competitive transplantation experiments.
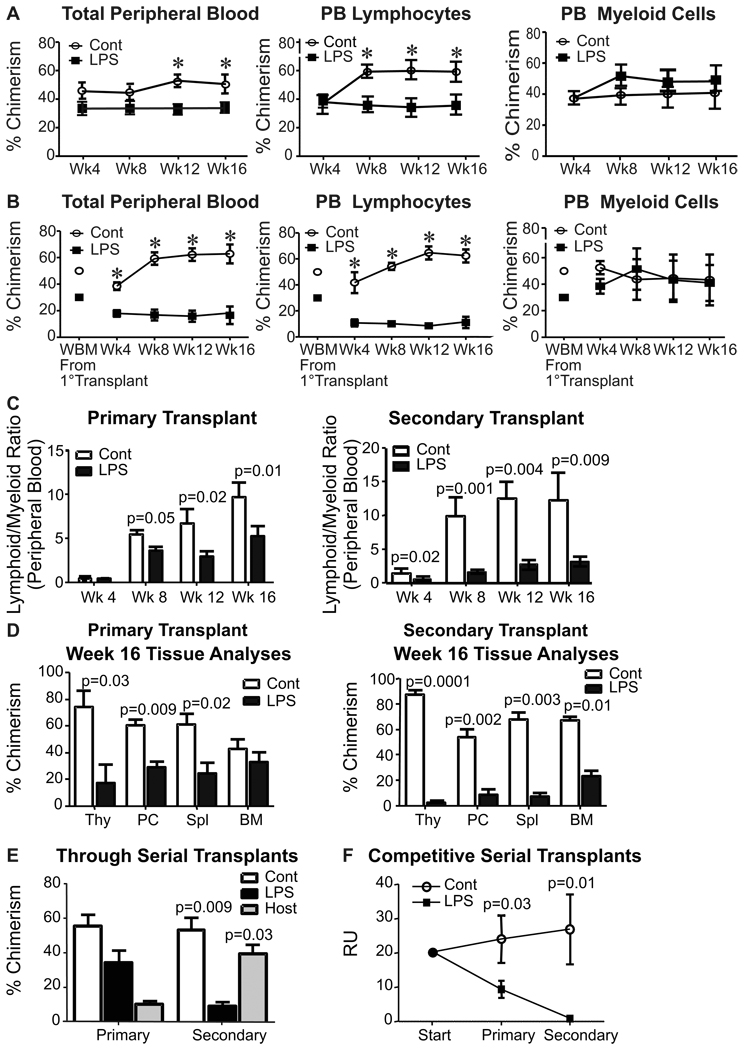
Equal numbers of control (CD45.1+) and LPS-treated (CD45.2+) bone marrow cells were transplanted together into lethally irradiated (CD45.2 X CD45.1) F1 mice (Fig. 3). This should have given a slight (1.3 fold) advantage to the treated HSC (Fig. 1E, above). However, HSC derived from the control marrow dominated with respect to peripheral blood chimerism, and particularly with respect to CD11b−Gr-1− CD19+ CD3+ lymphocytes (Fig. 3A). In contrast, the two donors contributed equally to production of large CD11b+ peripheral blood myeloid cells, and this myeloid bias increased when marrow cells were re-transplanted for an additional 16 weeks (Fig. 3B). Lymphoid/myeloid ratios reflected enduring, heritable myeloid bias and lymphoid deficiency in HSC originating from marrow chronically exposed to LPS (Fig. 3C).
Figure 3. Low-grade inflammation causes age-related changes in long-term HSC.
Competitive serial marrow transplants were constructed as described in the Experimental Procedures. A, Peripheral blood was analyzed at 4 week intervals to determine hematopoietic contributions of PBS and LPS treated marrow after primary transplantation. Total peripheral blood chimerism is shown in the left panel, while CD11b−Gr-1−CD3+/CD19+ lymphocytes and CD3−CD19−CD11b+ myeloid chimerism is indicated in the middle and right panels, respectively. B, Secondary transplants were initiated with chimeric marrow from primary transplants, as indicated. C, The same data was used to calculate lymphoid to myeloid cell ratios of donor type cells in peripheral blood from primary, as well as secondary transplants. D, Tissues recovered from these secondary transplant recipients at 16 weeks were analyzed for percentages of chimerism by flow cytometry. E, Marrow recovered was studied by flow cytometry to determine what proportions of cells in the primitive LSK fraction were derived from the original LPS treated animals, the PBS treated control mice or radio-resistant host mice. F, Repopulating units (RU) of control (CD45.1+) and LPS-treated (CD45.2+) marrow were calculated through serial transplantations initiated with equal numbers of competing whole marrow cells to determine the ability of both donor types to repetitively maintain their RU as an indication of HSC self-renewal capacity (41). This data represents pooled averages of total bone marrow chimerism after 16-week serial transplantation interval analyses from four independent experiments. Each one was conducted with not less than six recipient animals per group. Error bars depict SEM and asterisks reflect statistically significant differences.
A thorough analysis of hematopoietic chimerism was performed with tissues harvested from the recipients 16 weeks after each transplant (Fig. 3D). Marrow cells originating in chronically treated mice became progressively defective in repopulating lymphocytes in the thymus, peritoneal cavity, spleen and marrow.
As noted above, low grade TLR ligation did not immediately change the myeloid potential of LSK (Fig. 2B above), and the same was found in Methocel culture experiments using LSK harvested from primary transplant recipients (Supplemental Fig. 3A). However, CD45.2+ LSK recovered from secondary transplants had increased ability to form colonies in this assay compared to CD45.1+ controls (Supplemental Fig. 3A). As was the case for the initial marrow donors, we found statistically significant increases in cycling Ki-67+ Flt3−LSK cells recovered from primary and secondary transplant recipients (Supplemental Fig. 3B).
Analysis of the primitive LSK fraction of marrow in recipients also suggested that stem cells harvested from LPS treated donors were compromised in long-term self-renewal potential (Fig. 3E). This was apparent even in the primary transplants, and cells originating from LPS treated animals (CD45.2+) competed poorly with control donor (CD45.1+) stem cells. Secondary transplants revealed that LPS-treated stem cells were defective even relative to radio-resistant host F1 (CD45.1+CD45.2+) stem cells (Fig. 3E). This was also apparent in terms of repopulating units (RU) (41). While these values were unchanged when control marrow was transplanted, there was an 88% reduction on secondary transplantation of LPS treated HSC (Fig. 3F). All of these results indicate that chronic LPS exposure permanently harms self-renewal capacity and functional integrity of HSC.
A similar experimental design was used to assess the differentiation potentials of HSC highly enriched as CD150+ CD48− LSK before transplantation and marrow analysis (Supplemental Figure 4). Although the sorting procedure might have normalized some of the LPS induced changes in HSC subsets (see below), HSC derived from treated mice displayed strong myeloid bias in three of five recipient mice.
Thus, residence in chronically LPS treated mice diminished the ability of long-term HSC to sustain their numbers through serial transplantation, and lymphopoietic potential was selectively lost while myeloid output was maintained. These characteristics were durable, with no signs of recovery through two rounds of transplantation in untreated recipient mice over a period of eight months.
Some, but not all, hematopoietic changes resemble those described for normal aging
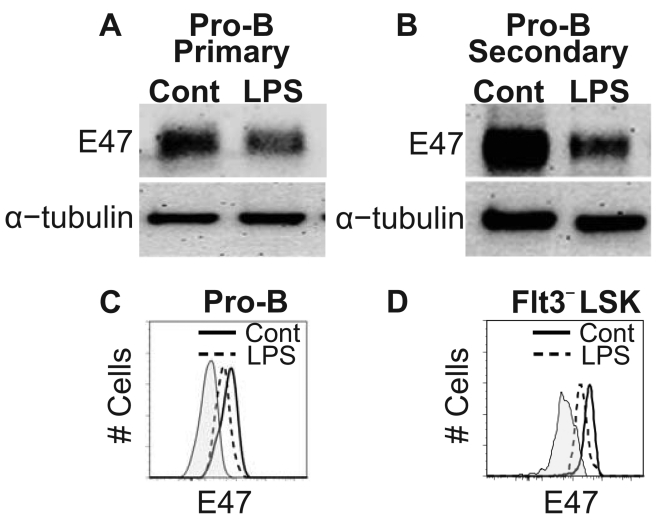
The myeloid skewing we observed in LPS treated mice has been shown to be a characteristic of old mice (25–28), so we investigated other possible similarities. Members of the E protein family of transcription factors have critical roles at early lymphoid progenitor, multipotent progenitor and stem cell stages (42–46). Importantly, it has been demonstrated that levels of the E47 transcription factor protein markedly decline in CD19+B220+CD43+IgM− pro-B cells during aging (47). Therefore, we sorted pro-B cells according to origin from primary or secondary transplant recipients and then evaluated them by western blot analyses (Fig. 4A, B). E47 protein levels were significantly reduced in pro-B cells generated from marrow originating in LPS-treated mice. A similar assessment was made of pro-B cells, as well as the HSC containing Flt3−LSK subset of secondary transplants using flow cytometry (Fig. 4C, D). Again, there was significant depression of E47 levels. Mean fluorescence intensity (MFI) values ±SEM for pro-B cells generated from control and LPS-treated marrow in secondary transplants were 425 ±81 and 175 ±19 (p=0.02), respectively. Values for Flt3−LSK generated from control and LPS-treated marrow were 2610 ±358 and 1780 ±180 (p=0.04), respectively. Thus, pro-B cells generated after two successive, four month transplants of treated bone marrow had undergone a change previously described in aged mice. Furthermore, the depression of E47 was apparent even in the Flt3−LSK subset.
Figure 4. Chronic TLR stimulation leads to decreased E2A protein abundance in pro-B and primitive cells.
Western blot analysis comparing E47 protein levels in pro-B cells generated from control or LPS treated marrow after primary (A) and secondary (B) transplants. α-tubulin served as an internal protein control for this assay. The data is representative of three independent experiments per time point. Two independent flow cytometry analyses from separate laboratories assessed intracellular E47 protein levels in individual pro-B (C) and HSC-enriched Flt3−LSK cells (D) after secondary transplantation. Plots are representative of 3 independent experiments with 5–6 mice per analysis. Statistical significance was determined by t-test analysis.
Recent papers revealed that functionally distinct HSC can be partially resolved by immunostaining and that many HSC in aged mice have high levels of CD150 (48– 50). We found that total numbers of CD150+ CD48− LSK were elevated by chronic LPS treatment (Fig. 1E, above), this was most apparent for the brightest CD150+ cells and mean fluorescence intensities (MFI) for CD150 were always increased (Fig. 5A,B and Fig. 6 below). Additional informative antibodies were identified by microarray analysis and screening (not shown). While CD86 (B7.2) stained most HSC in normal, young mice, a negative population expanded with LPS treatment and aging (Fig. 6). Similar, but not identical staining patterns were obtained with antibodies to CD18 (integrin β chain, Fig. 6). Reductions in percentages of CD86+ or CD18+ cells were significant when HSC were liberally gated as Flt3− LSK or CD48− LSK (Fig. 6 and data not shown), but in each case the changes were greatest for the CD150Hi HSC.
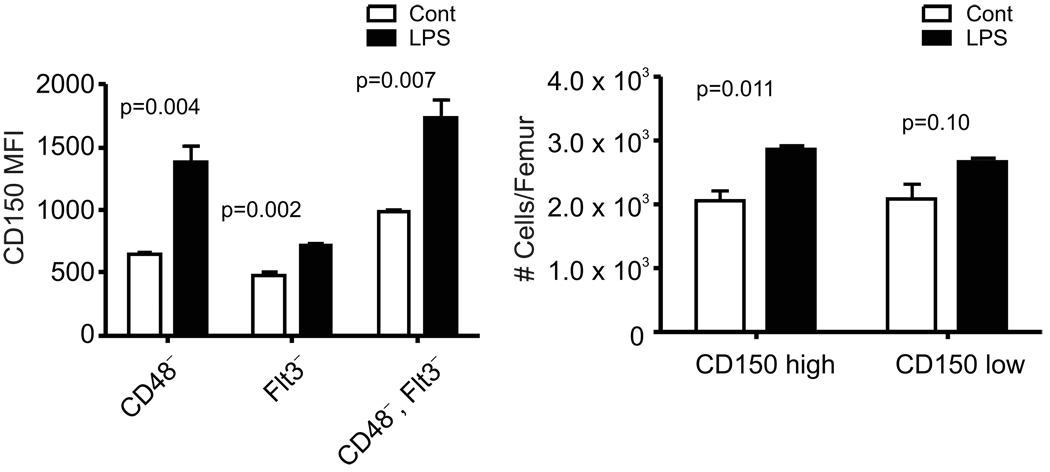
Figure 5. Repeated exposure to LPS increases densities of CD150 and numbers of CD150Hi HSC in bone marrow.
Marrow was harvested from mice given daily injections of saline or LPS for four-six weeks. Subsets of HSC in the LSK gate illustrated in Supplemental Fig. 1B were resolved on the basis of CD48 and/or Flt3 expression. A, Densities of CD150 are reflected in mean fluorescence intensities (MFI). B, HSC identified as CD150+ CD48− LSK were subdivided into CD150Lo and CD150Hi fractions (See Supplemental Fig. 1B) that were enumerated in control and LPS treated mice.
Figure 6. Monoclonal antibody staining resolves HSC subsets altered by LPS treatment and/or aging.
The indicated antibodies were used to characterize CD48− LSK in marrow of mice repeatedly injected with LPS or allowed to age naturally. Note that both of these conditions resulted in a shift to high CD150 expression on HSC (see also Fig. 5). These results are representative of more than three independent experiments with each reagent. Additionally, CD41 staining was confirmed with two different fluorochromes (PE or FITC). Mean percentages of labeled cells ± S.E. from one of three representative experiments are given within the boxes.
Staining with those reagents suggest strong similarities between chronic LPS treatment and old age. However, a distinct VCAM-1− HSC population appeared in LPS treated mice, but not aged mice (Fig. 6). VCAM-1 is a cell adhesion ligand associated with HSC homing (51). MFI values for the CD39 ectoenzyme were also significantly reduced in LPS treated, but not aged mice (not shown). While the CD41 integrin chain has typically been used to study HSC in early embryos (52), we observed significant staining of adult HSC with a CD41 antibody labeled with two different fluorochromes (Fig. 6 and data not shown). Although there was no change with LPS treatment, HSC staining for CD41 became much more homogeneous with aging.
Thus, persistent TLR stimulation causes HSC in young mice to acquire some, but not all properties of HSC in mice maintained for two years under pathogen-free conditions. Expansion of rare CD150Hi HSC subsets in parallel with functional defects is consistent with some type of irreversible injury.
Discussion
The expression of pathogen receptors on hematopoietic cells may be life sparing in some circumstances, such as when production of innate effector cells is boosted during infections. However, these new findings show that HSC are injured by persistent exposure of mice to small amounts of a single TLR ligand. Thus, low-grade host/pathogen interactions may be detrimental to stem cells over time.
Administering very low doses of LPS for an extended period was done to avoid major inflammation and generalized stress. This protocol might reflect conditions in chronic infections such as gram-negative periodontitis and sub-acute bacterial endocarditis (18). The fact that thymus cellularity and blood counts were normal indicates that the LPS treatment was well tolerated. However, bone densities were reduced and other subtle changes were occurring after four to six weeks of LPS treatment. Previous culture experiments showed that differentiation of myeloid progenitors is promoted, and lymphoid progenitors are directed to become dendritic cells by exposure to TLR ligands (15, 34). Similar changes were seen in the marrows of low-dose LPS treated mice. That is, we found elevated percentages of myeloid lineage and dendritic cells. In addition, progenitors isolated from those animals were clearly deficient with respect to lymphopoietic potential.
Although phenotypically defined HSC increased in LPS treated mice, their repopulating and lymphopoietic potentials dramatically declined. While it is clear that this reflects stem cell injury, we do not know if they were direct and/or indirect targets of TLR ligands. Sub-endosteal osteoblasts are thought to be essential components of HSC niches, and experimental manipulations affecting these cells influence HSC (53, 54). Osteoblast precursors could be affected by cytokines released during inflammation, or express TLR that would allow them to be direct LPS targets (55). In addition to osteoblastic niches, HSC reside in perivascular areas of the bone marrow and recirculate throughout the body (17, 40). LPS-induced chronic inflammation leads to bone remodeling, and mobilizes additional hematopoietic cells to the periphery (16, 56). Extended mobilization would have the effect of reducing their residence time in supporting niches.
Selective loss of lymphopoietic potential in HSC and progenitors has also been seen in studies of normal aging, and could conceivably contribute to immunosenescence (25–27, 57). The incidence of myeloid versus lymphoid leukemias is also age-related (58). The cause of lineage skewing is unknown, but could involve stable epigenetic changes (59, 60). We now report that long-term competence of HSC to produce B and T lymphocytes was severely compromised in low-dose LPS treated mice. Given that eight months had elapsed since LPS treatment, these changes may result from epigenetic regulation.
Chromatin modifications could result in altered patterns of transcription factor abundance, and helix-loop-helix (HLH) family proteins are attractive candidates. E2A degradation occurs through a MAPK dependent ubiquitination process (61), and the same mechanism may explain the reduced protein levels in lymphocytes of aged mice (62). Recent studies suggest that members of the E2A family have pivotal importance in the earliest hematopoietic stages (42–46). That is, control of net HLH activity permits appropriate lymphoid/myeloid fate decisions, maintenance of stem cell quiescence, and ultimately long-term repopulation capability of HSC. Subnormal levels of E2A transcription factor proteins in pro-B cells correlate with aging (47). We found that the same was true for stem and pro-B cells made from LPS treated marrow in long-term transplant recipients.
Although rare, HSC are surprisingly heterogeneous, and subsets have been described to be deeply quiescent, latent, intermediate term repopulating, myeloid biased, balanced or lymphoid biased (48, 50, 63–66). The degree of overlap between subpopulations is far from clear, but a series of recent reports suggest that flow cytometry analyses can be highly informative (48–50). For example, high CD150 density in young mice correlates with high self-renewal potential and myeloid skewing. CD150Hi CD48− HSC expand during normal aging and are myeloid biased. This could mean that proportions of specialized subsets change over time (67). However, all HSC in old animals differ from those in young mice with respect to homing and self renewal (24, 49).
We found that TLR stimulation elicited some, but not all, of these changes in HSC subsets. The density of Slamf1/CD150 was significantly elevated in HSC from chronically LPS treated mice. While not to the extent seen in aging, absolute numbers of CD150Hi HSC were always significantly increased. At the same time, normally minor populations of CD150Hi CD48− HSC lacking either the T cell co-stimulatory CD86 antigen or the CD18 (integrin β2) expanded. It is interesting that marrow stromal cells express the CD28 counter-receptor for CD86 (68), and it will be important to learn if either of these molecules are associated with HSC functions. Their absence corresponded with poor self-renewal and lymphopoietic potential in parallel with depletion of CD150Lo/− CD48− HSC. Transplantation experiments suggest that CD150Hi CD48− HSC can give rise to CD150Lo and CD150− subsets with lymphopoietic potential (49, 50, 69). It seems possible that these primordial HSC become injured and unable to make the transition to lymphopoietic cells as a result of age or TLR stimulation.
Some HSC recovered from persistently LPS treated animals were in cell cycle, but this is not a characteristic of HSC in aged mice (31, 59, 70) and data not shown). We found that two additional parameters could be used to discriminate HSCs in these situations. The VCAM-1 cell adhesion molecule was down-regulated by LPS treatment, but not aging. Reciprocally, the CD41 integrin was uniformly up-regulated on old, but not TLR stimulated HSC.
We conclude that HSC in chronically LPS treated animals are functionally compromised and have some, but not all, age-related characteristics. This and other recent studies suggest that monoclonal antibody staining as well as transcription patterns can be informative about functionally specialized HSC subsets and serve as biomarkers of normal aging. It should now be possible to determine what population changes correlate with loss of HSC integrity and develop new strategies to protect them. More aggressive management of chronic infections could ensure the competency of HSC to replenish the immune system.
Supplementary Material
Acknowledgments
We thank Tara Khamphanthala for technical assistance, Jacob Bass and Dr. Diana Hamilton for cell sorting, Dr. Xiao-Hong Sun for reagent contributions and scientific consultation, Beverly Hurt for graphics assistance, and Shelli Wasson for editorial assistance.
This work was supported by grants AI020069, AI058162, AI069024 (P.W.K.), F30AG031646 (B.L.E.) and AI079047 (L.A.B.) from the National Institutes of Health. P.W.K. holds the William H. and Rita Bell Endowed Chair in Biomedical Research.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J. Exp. Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtzman GJ, Platanias L, Lustig L, Frickhofen N, Young NS. Feline parvovirus propagates in cat bone marrow cultures and inhibits hematopoietic colony formation in vitro. Blood. 1989;74:71–81. [PubMed] [Google Scholar]
- 3.Nakao S, Lai CJ, Young NS. Dengue virus, a flavivirus, propagates in human bone marrow progenitors and hematopoietic cell lines. Blood. 1989;74:1235–1240. [PubMed] [Google Scholar]
- 4.Zeldis JB, Mugishima H, Steinberg HN, Nir E, Gale RP. In vitro hepatitis B virus infection of human bone marrow cells. J. Clin. Invest. 1986;78:411–417. doi: 10.1172/JCI112591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS. Pathog. 2008;4:e1000215. doi: 10.1371/journal.ppat.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monette FC, Morse BS, Howard D, Niskanen E, Stohlman F., Jr Hemopoietic stem cell proliferation and migration following Bordetella pertussis vaccine. Cell Tissue Kinet. 1972;5:121–129. doi: 10.1111/j.1365-2184.1972.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 7.Jyonouchi H, Kincade PW, Good RA. Immunosuppression of marrow B lymphocytes by administration of corynebacterium parvum in mice. J. Immunol. 1981;127:2502–2507. [PubMed] [Google Scholar]
- 8.Nagaoka H, Gonzalez-Aseguinolaza G, Tsuji M, Nussenzweig MC. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J. Exp. Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrow P, Hou S, Gloster S, Ashton M, Hyland L. Virus infection-associated bone marrow B cell depletion and impairment of humoral immunity to heterologous infection mediated by TNF-alpha/LTalpha. Eur. J. Immunol. 2005;35:524–532. doi: 10.1002/eji.200425597. [DOI] [PubMed] [Google Scholar]
- 10.Singh P, Yao Y, Weliver A, Broxmeyer HE, Hong SC, Chang CH. Vaccinia virus infection modulates the hematopoietic cell compartments in the bone marrow. Stem Cells. 2008;26:1009–1016. doi: 10.1634/stemcells.2007-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat. Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- 15.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, Bovio I, Akira S, Kumagai Y, Moldawer LL. Cutting Edge: Bacterial Infection Induces Hematopoietic Stem and Progenitor Cell Expansion in the Absence of TLR Signaling. J. Immunol. 2010;184:2247–2251. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, Von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000;13:547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichii M, Shimazu T, Welner RS, Garrett KP, Zhang Q, Esplin BL, Kincade PW. Functional diversity of stem and progenitor cells with B lymphopoietic potential. Immunol. Rev. 2010;237:10–21. doi: 10.1111/j.1600-065X.2010.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J. Exp. Med. 1982;156:1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang Y, Van ZG, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nature Medicine. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann. N. Y. Acad. Sci. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 28.Warren LA, Rossi DJ. Stem cells and aging in the hematopoietic system. Mech. Ageing Dev. 2009;130:46–53. doi: 10.1016/j.mad.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat. Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 30.Song Z, Wang J, Guachalla LM, Terszowski G, Rodewald HR, Ju Z, Rudolph KL. Alterations of the systemic environment are the primary cause of impaired B and T lymphopoiesis in telomere-dysfunctional mice. Blood. 2010;115:1481–1489. doi: 10.1182/blood-2009-08-237230. [DOI] [PubMed] [Google Scholar]
- 31.Rossi DJ, Seita J, Czechowicz A, Bhattacharya D, Bryder D, Weissman IL. Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle. 2007;6:2371–2376. doi: 10.4161/cc.6.19.4759. [DOI] [PubMed] [Google Scholar]
- 32.Meng A, Wang Y, Van ZG, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–5419. [PubMed] [Google Scholar]
- 33.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welner RS, Pelayo R, Nagai Y, Garrett KP, Wuest TR, Carr DJ, Borghesi LA, Farrar MA, Kincade PW. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112:3753–3761. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson NS, Young LJ, Kupresanin F, Naik SH, Vremec D, Heath WR, Akira S, Shortman K, Boyle J, Maraskovsky E, Belz GT, Villadangos JA. Normal proportion and expression of maturation markers in migratory dendritic cells in the absence of germs or Toll-like receptor signaling. Immunol. Cell Biol. 2008;86:200–205. doi: 10.1038/sj.icb.7100125. [DOI] [PubMed] [Google Scholar]
- 36.Smith BJ, Lerner MR, Bu SY, Lucas EA, Hanas JS, Lightfoot SA, Postier RG, Bronze MS, Brackett DJ. Systemic bone loss and induction of coronary vessel disease in a rat model of chronic inflammation. Bone. 2006;38:378–386. doi: 10.1016/j.bone.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 37.LeClair KP, Palfree RGE, Flood PM, Hammerling U, Bothwell A. Isolation of a murine Ly-6 cDNA reveals a new multigene family. EMBO J. 1986;5:3227–3234. doi: 10.1002/j.1460-2075.1986.tb04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Perry SS, Zhao Y, Nie L, Cochrane SW, Huang Z, Sun XH. Id1, but not Id3, directs long-term repopulating hematopoietic stem cell maintenance. Blood. 2007;110:2351–2360. doi: 10.1182/blood-2007-01-069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dias S, Mansson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity. 2008;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Q, Kardava L, St LA, Martincic K, Varnum-Finney B, Bernstein ID, Milcarek C, Borghesi L. E47 controls the developmental integrity and cell cycle quiescence of multipotential hematopoietic progenitors. J. Immunol. 2008;181:5885–5894. doi: 10.4049/jimmunol.181.9.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semerad CL, Mercer EM, Inlay MA, Weissman IL, Murre C. E2A proteins maintain the hematopoietic stem cell pool and promote the maturation of myelolymphoid and myeloerythroid progenitors. Proc. Natl. Acad. Sci. USA. 2009;106:1930–1935. doi: 10.1073/pnas.0808866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cochrane SW, Zhao Y, Welner RS, Sun XH. Balance between Id and E proteins regulates myeloid-versus-lymphoid lineage decisions. Blood. 2009;113:1016–1026. doi: 10.1182/blood-2008-06-164996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van der Put E, Frasca D, King AM, Blomberg BB, Riley RL. Decreased E47 in senescent B cell precursors is stage specific and regulated posttranslationally by protein turnover. J. Immunol. 2004;173:818–827. doi: 10.4049/jimmunol.173.2.818. [DOI] [PubMed] [Google Scholar]
- 48.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J. Exp. Med. 2010 doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc. Natl. Acad. Sci. USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR, Yoder MC. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 53.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 54.Fleming HE, Janzen V, Lo CC, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gimble JM, Pietrangeli CE, Henley A, Dorheim MA, Silver J, Namen AE, Takeichi M, Goridis C, Kincade PW. Characterization of Murine Bone Marrow and Spleen Derived Stromal Cells: Analysis of Leukocyte Marker and Growth Factor mRNA Transcript Levels. Blood. 1989;74:303–311. [PubMed] [Google Scholar]
- 56.Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3:484–492. doi: 10.1016/j.stem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Signer RA, Montecino-Rodriguez E, Witte ON, McLaughlin J, Dorshkind K. Age-related defects in B lymphopoiesis underlie the myeloid dominance of adult leukemia. Blood. 2007;110:1831–1839. doi: 10.1182/blood-2007-01-069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS. Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nie L, Xu M, Vladimirova A, Sun XH. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 2003;22:5780–5792. doi: 10.1093/emboj/cdg567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King AM, Van der PE, Blomberg BB, Riley RL. Accelerated Notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased ERK MAPK activation. J. Immunol. 2007;178:3521–3529. doi: 10.4049/jimmunol.178.6.3521. [DOI] [PubMed] [Google Scholar]
- 63.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, MacDonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 64.Benveniste P, Frelin C, Janmohamed S, Barbara M, Herrington R, Hyam D, Iscove NN. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell. 2010;6:48–58. doi: 10.1016/j.stem.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- 66.Kent DG, Copley MR, Benz C, Wohrer S, Dykstra BJ, Ma E, Cheyne J, Zhao Y, Bowie MB, Zhao Y, Gasparetto M, Delaney A, Smith C, Marra M, Eaves CJ. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113:6342–6350. doi: 10.1182/blood-2008-12-192054. [DOI] [PubMed] [Google Scholar]
- 67.Muller-Sieburg C, Sieburg HB. Stem cell aging: survival of the laziest? Cell Cycle. 2008;7:3798–3804. doi: 10.4161/cc.7.24.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray PK, Stephan RP, Apilado RG, Lill-Elghanian DA, Lee KP, Saha B, Witte PL. Expression of CD28 by bone marrow stromal cells and its involvement in B lymphopoiesis. J. Immunol. 2002;169:2292–2302. doi: 10.4049/jimmunol.169.5.2292. [DOI] [PubMed] [Google Scholar]
- 69.Papathanasiou P, Attema JL, Karsunky H, Xu J, Smale ST, Weissman IL. Evaluation of the long-term reconstituting subset of hematopoietic stem cells with CD150. Stem Cells. 2009;27:2498–2508. doi: 10.1002/stem.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Attema JL, Pronk CJ, Norddahl GL, Nygren JM, Bryder D. Hematopoietic stem cell ageing is uncoupled from p16INK4A-mediated senescence. Oncogene. 2009;28:2238–2243. doi: 10.1038/onc.2009.94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.