Abstract
Noonan syndrome (NS), Costello syndrome (CS), cardiofaciocutaneous syndrome (CFCS), and LEOPARD syndrome (now also referred to as Noonan syndrome with multiple lentigines or NSML) are clinically overlapping dominant disorders that are caused by mutations in RAS signaling pathway genes. The spectrum of cancer susceptibility in this group of disorders has not been studied in detail. We identified more than 1900 cases of NS, CS, CFCS, or NSML reported in the literature between 1937 to 2010; eighty-eight cancers were reported. The most common cancers reported in 1051 NS subjects were neuroblastoma (n=8), acute lymphoblastic leukemia (n=8), low grade glioma (n=6), and rhabdomyosarcoma (n=6). These associations are biologically plausible, given that somatic RAS pathway mutations are known to occur in these specific cancers. In addition, 40 childhood cases of myeloproliferative disease were described in individuals with NS, several of whom experienced a benign course of this hematologic condition. We confirmed the previously-described association between CS and cancer in 268 reported individuals: 19 had rhabdomyosarcoma, 4 had bladder cancer, and 5 had neuroblastoma. By age 20, the cumulative incidence of cancer was approximately 4% for NS and 15% for CS; both syndromes had a cancer incidence peak in childhood. The cancers described in CFCS and NSML overlapped with those reported in NS and CS. Future epidemiologic studies will be required to confirm the described cancer spectrum and to estimate precise cancer risks.
Keywords: RASopathies, Cancer risk, Mortality
INTRODUCTION
Noonan syndrome (NS, OMIM 163950) is a dominant disorder characterized by short stature, distinct facial features, developmental delay, congenital heart defects and other abnormalities (reviewed in [Romano et al., 2010]). The disorder overlaps clinically with Costello syndrome (CS, OMIM 218040), cardiofaciocutaneous syndrome (CFCS, OMIM 115150), Noonan syndrome with multiple lentigines (NSML, formerly referred to as LEOPARD syndrome: OMIM 151100), neurofibromatosis type 1 (NF1, OMIM 162200), and Legius syndrome (OMIM 611431) [reviewed in [Zenker, 2009].
NS is genetically heterogeneous: germline mutations in the PTPN11, SOS1, KRAS, NRAS, RAF1, BRAF, SHOC2, or MEK1 genes are identified in 70%–75% of affected individuals. Moreover, CBL mutations cause a NS-like phenotype. Almost all patients with CS harbor germline mutations in HRAS, while CFCS is caused by mutations in BRAF, MEK1, or MEK2. NSML is caused by germline mutations in PTPN11, BRAF, or RAF1. All these causative genes encode signaling molecules within the RAS signaling pathway, and have, therefore, been referred to as “the RASopathies” [Martinelli et al., 2010; Niemeyer et al., 2010; Tartaglia and Gelb, 2010]. Note that different mutations in PTPN11, KRAS, BRAF, RAF1, and MEK1 cause more than one of these RASopathies.
The RAS signaling pathway is a major contributor to carcinogenesis, and somatic mutations in PTPN11, KRAS, NRAS, BRAF, and CBL occur in a broad range of malignancies [Downward, 2006; Makishima et al., 2009; Schubbert et al., 2007]. Therefore, it is not surprising that several of the RASopathies have been associated with cancer. However, the cancer rates and spectrum in patients with NS, CFCS, CS, and NSML have not been studied systematically [Hasle, 2009; Kratz, 2009]. It is well documented in case reports and small case series that NS is associated with an often transient myeloproliferative syndrome (MPD) of early childhood resembling juvenile myelomonocytic leukemia (JMML) [Bader-Meunier et al., 1997; Kratz et al., 2005]. Several other reports indicate that patients with CS are at increased risk of developing embryonic rhabdomyosarcoma (RMS), neuroblastoma, and bladder cancer [Gripp, 2005; Gripp et al., 2002]. Despite these associations, the magnitude of the cancer risk in individuals with these syndromes is unknown.
This report includes an examination of all identified cases of NS, CS, CFCS and NSML published between 1937 (the earliest identified report) and October 2010, and describes the types and frequencies of cancers that occur in these disorders. Our analysis suggested modest associations between NS and neuroblastoma, acute leukemia, low grade glioma, and RMS. We confirmed the previously described excess of RMS, bladder cancer and neuroblastoma in patients with CS. We estimated that the cancer hazard rate and cumulative incidence was substantially higher in CS than in NS and observed a childhood cancer peak in both syndromes. The cancer types observed in CFCS and NSML overlapped with those observed in NS and CS.
METHODS
We adopted a search strategy that has recently been employed for dyskeratosis congenita, another inherited cancer susceptibility condition [Alter et al., 2009]. In brief, the medical literature was searched for reports on patients with NS, CS, CFCS, or NSML, employing PubMed, using the search terms "Noonan syndrome or Noonan’s syndrome", "Costello syndrome", "CFC syndrome, cardio-facio-cutaneous syndrome, cardio-facio-cuteneous syndrome, or cardiofacio cutaneous syndrome" and "LEOPARD syndrome or LEOPARD," supplemented by review of the bibliographies of each article. Cases were accepted if the reports provided sufficient diagnostic clinical information, and/or the patients had mutations in any of the genes associated with these syndromes. Cases of cancer as defined by the original authors were accepted without verification. Duplicate reports were identified by matching descriptions in the publications or by prior citations. Data for cancer in the general population came from the United States Cancer Registry Surveillance, Epidemiology and End Results (SEER) database [SEER, 2000–2007]. Information for individual literature cases was entered into Microsoft Excel (Microsoft, Redmond, WA, USA) spreadsheets. Data included demographics, physical manifestations of NS, CS, CFCS, or NSML, age at onset of cancer, type of cancer, age at death, and last known age alive. Cause-specific hazards and cumulative incidence curves accounting for competing risks for cancer and death prior to cancer were calculated as described previously using MATLAB [Rosenberg et al., 2003]. Cases were censored if the patients died or follow-up ended before the development of cancer. The outcomes of interest were cancer or death.
RESULTS
Our search strategy led to the compilation of 892 suitable publications describing 1941 cases contributing 23,756 person-years with sufficient information for this analysis (Table I). Of the 1941 patients, 87 had 88 cancers (excluding MPDs), for crude rates of 3.8% (NS), 10.8% (CS), 3.5% (CFCS) and 1.6% (NSML) of reported cases (references for patients with cancer or MPD: Online Supplementary file).
Table I.
Cancer in NS, CS, CFCS, and NSML literature cases
| Syndrome | All cases | Cancer-free | All cancers |
|---|---|---|---|
| Noonan | 1151 | 1106 | 45 pts with 46 cancers (3.9%) |
| Costello | 268 | 239 | 29 (10.8%) |
| Cardofaciocutaneous | 226 | 218 | 8 (3.5 %) |
| Noonan syndrome with multiple lentigines | 296 | 291 | 5 (1.6%) |
Forty-six cases of cancer were reported in 45 subjects with NS (Table II): There were 8 patients with neuroblastoma and 8 with acute lymphoblastic leukemia. The next most frequent neoplasms were 6 with glioma (1 with low-grade hypothalamic glioma, 1 with leptomeningeal dissemination of a low-grade mixed glioneuronal tumor, 1 with bilateral optic nerve pathway gliomas – this patient had deleterious mutations in 2 genes, PTPN11 and NF1, 2 with pilocytic astrocytoma [PA], 1 with glioma unclassified); 6 with RMS (1 botryoid, 5 embryonal); 3 with acute myeloid leukemia (including 1 therapy-related leukemia); 3 with testicular cancer (1 embryonal cell carcinoma, 1 seminoma, 1 Sertoli cell tumor); 2 with non-Hodgkin lymphoma; and 2 with colon cancer. There were single reports of Wilms tumor, hepatoblastoma, Hodgkin disease, chronic myelomonocytic leukemia, chronic lymphocytic leukemia, breast cancer, malignant schwannoma, and bile duct cancer. The ages of the patients at the time of cancer diagnosis were comparable to the ages expected for sporadic cancers.
Table II.
Types and ages of malignancies in NS, CS, CFCS and NSML literature case (References: Supplementary File)
| Syndrome and Type of cancer (excluding myeloproliferative disease) |
No. of cancers |
Median age, y (range) |
Median age in general population, ya |
Pediatric age peak or range, yb |
|---|---|---|---|---|
| Noonan Syndrome | ||||
| Neuroblastoma | 8 | 0.8 (0.4–4) | 1 | 0–0.5 |
| Acute lymphoblastic leukemia | 8 | 2.3 (0.8–17) | 13 | 2–3 |
| Glioma | 6 | 9.5(1.8–20) | 43 | 0–19c |
| Rhabdomyosarcoma | 6 | 4(1.7–9) | 49 | <5d |
| Acute myeloid leukemia | 3 | 4, 9, 34 | 67 | 0–1 |
| Testicular cancer | 3 | 4, 22, 26 | 34 | - |
| Non Hodgkin lymphoma | 2 | N/A | 67 | - |
| Colon carcinoma | 2 | 70,70 | 72 | - |
| Chronic lymphocytic leukemia | 1 | 56 | 72 | - |
| Wilms tumor | 1 | N/A | 3 | 3 |
| Hepatoblastoma | 1 | 0.1 | 1 | 1 |
| Hodgkin disease | 1 | 35 | 38 | 14 |
| Chronic myelomonocytic leukemia | 1 | 35 | 76 | - |
| Bile duct tumor | 1 | N/A | 71 | - |
| Malignant schwannoma | 1 | 14 | 52 | - |
| Breast Cancer | 1 | 45 | 61 | - |
| Costello Syndrome | ||||
| Rhabdomyosarcoma | 19 | 2.3 (0.5–6.0) | 49 | <5d |
| Neuroblastoma | 5 | 1.4(0–3.0) | 1 | 0–0.5 |
| Bladder cancer | 4 | 13.5(0.8–20) | 73 | - |
| Fibrosarcoma | 1 | 2.5 | 54.5 | - |
| Cardiofaciocutaneous Syndrome | ||||
| Acute lymphoblastic leukemia | 4 | 5.0(1.8–41.0) | 13 | 2–3 |
| Non Hodgkin lymphoma | 2 | 35.1 (0.2–70) | 67 | - |
| Hepatoblastoma | 1 | 2.9 | 1 | 1 |
| Rhabdomy o sarcoma | 1 | 1.7 | 49 | <5d |
| Noonan Syndrome with Multiple Lentigines or LEOPARD Syndrome | ||||
| Acute myeloid leukemia | 2 | 9.5(9–13) | 67 | 0–1 |
| Acute lymphoblastic leukemia | 1 | 8 | 13 | 2–3 |
| Neuroblastoma | 1 | N/A | 1 | 0–0.5 |
| Melanoma | 1 | 60 | 59 | - |
According to [SEER, 2000–2007]
According to [Pizzo and Poplack, 2001; WHO, 2002; WHO, 2007]
This age refers to juvenile pilocytic astrocytoma
This age refers to embryonal rhabdomyosarcoma
We also identified 40 cases of NS (or Noonan-like syndrome) associated MPD in the literature. The disease had a benign course in 16 (40%) and an aggressive course in 6 (15%) cases. Twelve patients underwent hematopoietic bone marrow transplantation. Follow-up information was not available in the remaining cases. Two additional cases of MPD have been described, one in NSML and one in CFCS.
Twenty-nine cases of cancer were reported in 29 subjects with CS (Table II): There were 19 RMS at a median age of 2.3 years (9 embryonal RMS, 1 alveolar RMS, 1 mixed histology, 1 pleomorphic RMS, 1 spindle cell type, and 6 with RMS unclassified). The next most frequent neoplasms were: neuroblastoma (n=5, including 4 with ganglioneuroblastoma); bladder cancer (n=4, including 3 with transitional cell carcinoma, 1 with low-grade papillary bladder carcinoma); and fibrosarcoma (n=1). The ages of the patients at the time of diagnosis of bladder cancer were substantially younger (mean=13.5 years) than the ages expected for sporadic cancers (mean=73 years).
Eight cases of cancer were reported in 8 subjects with CFCS (Table II): There were 4 acute lymphoblastic leukemias. The next most frequent neoplasms were non-Hodgkin lymphoma (n=2); hepatoblastoma (n=1); and embryonal RMS (n=1; there has been debate in the literature whether this patient with RMS had CS rather than CFCS). Five cases of cancer were reported in 5 subjects with NSML (Table II), including 2 acute myeloid leukemias; 1 acute lymphoblastic leukemia; 1 neuroblastoma; and 1 melanoma.
For NS, the cumulative incidence of cancer by age 20 years was 4% (95% CI: 3% – 6%) versus 10% (95% CI: 7 – 12%) for cancer-free death [Figure 1a]. Hence, 14% of NS subjects had developed a cancer or died prior to developing one by age 20 years. Noonan syndrome associated MPDs were excluded from this analysis because of their often benign course. For CS, the corresponding cumulative incidences were 15% (95% CI: 10 – 21%) and 12% (95% CI: 8 – 16%), respectively, and 27% had developed cancer or died [Fig 1b]. For NS, the annual cause-specific hazard (incidence rate per year among subjects who are still susceptible) of cancer showed an early peak during infancy of approximately 0.6%/year (with a large margin of error) [Figure 1c]. Subsequently, the cancer risk was stable at ~0.25%/year (also with a large margin of error).
Figure 1. Cumulative incidence and annual hazard rates and by age.
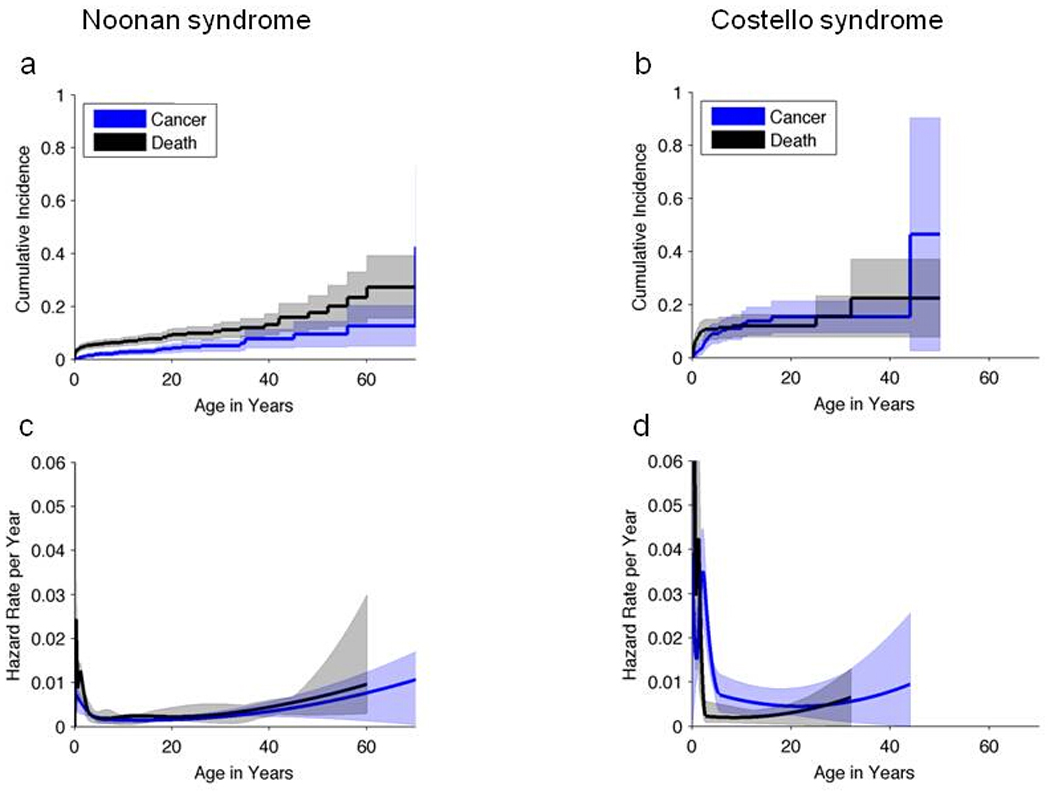
(a, b) Cumulative incidence for cancer and cancer-free death by age in patients with Noonan syndrome (a) and Costello syndrome (b) and 95% CIs (shaded regions). (c, d) Annual hazard rates (incidence rate per year among subjects who are still susceptible) of cancer-free death or cancer, and 95% point-wise confidence envelopes (shaded regions) for Noonan syndrome (c) and Costello syndrome (d).
For CS, the cause-specific cancer hazard also showed an early childhood peak of ~2-3%/year (also with a large margin of error) prior to the age of 5 years. Subsequently, the cancer risk was stable at around 0.7%/year (also with a large margin of error) [Fig 1d]. Note that the early childhood cancer hazard appears to be greater for CS than NS [Fig 1c, d] due to the high risk of RMS in young children with CS.
DISCUSSION
This is the first comprehensive review with identification of most literature cases of NS, CS, CFCS or NSML and quantitative analysis of annual hazard rates and cumulative incidences of cancer and cancer-free death. The most common cancers in NS were neuroblastoma, low grade glioma (e.g., PA), RMS, and acute leukemia. These associations are biologically plausible as RAS pathway mutations are known to be relevant at the somatic level in all 4 malignancies. Somatic NRAS or PTPN11 mutations occur at low frequencies in neuroblastoma [Bentires-Alj et al., 2004; Moley et al., 1991]. Pilocytic astocytoma is strongly associated with neurofibromatosis type 1 (~15% of cases in early-to-mid childhood), a RASopathy that is caused by germline mutations in the NF1 gene coding for the negative RAS regulator neurofibromin. Moreover, the majority of sporadic PA harbor a somatic tandem duplication leading to a rearrangement of BRAF, a RAS effector gene [Jones et al., 2008; Pfister et al., 2008]. Somatic mutations in NRAS, KRAS, or PTPN11 occur in both acute lymphoblastic and myeloid leukemia [Case et al., 2008; Tartaglia et al., 2004; Tartaglia et al., 2005]. It is noteworthy that two patients with NS and leukemia displayed loss of the wild-type PTPN11 allele in tumor cells [Chantrain et al., 2007; Karow et al., 2007], providing additional evidence for the hypothesis that the germline mutations were involved in the pathogenesis of these leukemias. Finally, somatic RAS pathway mutations are a common event in embryonal RMS [Kratz et al., 2007; Martinelli et al., 2009].
The most common cancers in CS were RMS, neuroblastoma, and bladder cancer. Somatic mutations in HRAS have been shown to play a role in the pathogenesis of embryonal RMS, which is the most common type associated with CS [Kratz et al., 2007]. The HRAS gene is located on chromosome 11p15, a region that shows uniparental isodisomy (UPD) in both sporadic and CS associated embryonal RMS [Kratz et al., 2007]. We speculate that a germline mutation in HRAS may provide a growth advantage to cells that also acquire UPD of this chromosomal region, thus promoting RMS formation. In addition, somatic HRAS mutations are a major event in sporadic bladder cancer [Jebar et al., 2005], providing one possible explanation for why these tumors occur in subjects with CS. It is of note that epithelial bladder cancer is almost never observed in children; CS is the only known genetic condition that predisposes individuals to bladder carcinoma at a young age. The observation that no leukemias were observed in patients with CS may be due to the fact that HRAS plays a minor role in these malignancies [Schubbert et al., 2007].
The number of cancers identified in patients with CFCS and NSML were too small to draw conclusions. However, based on the observation that the cancer types observed in CFCS and NSML overlap with those observed in the other syndromes (Table II), we hypothesize that the germline mutations contributed to CFCS- and NSML-associated malignancies. Studies with larger sample sizes will be needed to further clarify this possibility.
For both CS and NS, there was an early-childhood peak in cancer incidence, followed by stable rates of cancer thereafter. The cancer rate was higher in CS than in NS. This is consistent with biochemical evidence that the CS mutations exhibit stronger biological effects and overlap with somatic mutations observed in certain cancers [Schubbert et al., 2007]. In both cohorts, there was also a considerable risk of death in the absence of cancer (e.g., death due to cardiomyopathy or respiratory failure). It is not known whether these mortality rates from literature review reflect the contemporary experience.
Using published literature as the source of epidemiologic data to assess cancer risk in patients with RASopathies has major inherent limitations including publication bias and diagnostic drift. Publication bias can result in overestimation of risk, due to over-reporting of cases with cancer and under-reporting of cases without cancer [Alter et al., 2009]. Recognition of NS, CS, CFCS, or NSML in patients with or without cancer may be limited to the subsets with a more severe phenotype, and thus, cases occurring in persons with a milder phenotype may be overlooked. Many of the reports did not include all the details required to confirm the syndrome diagnosis; for this analysis, we accepted the classification provided by the authors.
Another limitation of the literature is the possibility that duplicated reports could not be identified as such. In addition, the malignancies could not be independently validated. Finally, diagnostic conventions related to the syndromes may have changed over time, so that the case reports represent a different mixture over time of the true underlying disorders. For example, molecular confirmation of the syndromic diagnoses became available only within the last decade, and mutation results were reported in a small fraction of cases reviewed here. Despite all these limitations, the literature provides important information on the spectrum of cancers in patients with genetic conditions, and estimates of relative frequencies and ages [Alter et al., 2009]. For example, prior literature reviews have been informative regarding the recognition that cancer is an important feature of CS [Gripp, 2005; Gripp et al., 2002].
CONCLUSIONS
The evidence suggests that NS and CS (and possibly CFCS and NSML) are truly cancer-prone syndromes; the magnitude of the cancer risk is greater in CS than in NS; and the types of cancer partially overlap. Validation of these results is needed and definitive estimates of cancer risk will emerge as prospective syndrome-specific cohorts are assembled, analyzed, and followed. The development of cohort studies for these conditions that prospectively enroll patients and follow them over time in conjunction with biobanks will be invaluable in the next iteration of cancer predisposition in the RASopathies. Although there is no data in support of a cancer screening program for patients with RASopathies our finding may lead to an increased clinical awareness.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute. We are grateful to Drs. Blanche P. Alter and Mark H. Greene for their insightful comments. We thank Dr. William F. Anderson for his statistical support related to SEER-derived median ages of cancer in the general population.
Biographies
Christian Kratz is a pediatric hematologist/oncologist and Principal Investigator at the Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. He studies cancer predisposition syndromes.
Suthee Rapisuwon worked as a visiting fellow in the National Cancer Institute’s Clinical Genetics Branch in 2010. He is currently enrolled in the combined internal medicine and genetics residency program at Case Western Reserve University, Cleveland, Ohio.
Helen Reed worked as a summer student in the National Cancer Institute’s Clinical Genetics Branch in 2010. She is currently completing her Master of Public Health at the University of California, Berkley.
Henrik Hasle is a Professor of pediatrics and pediatric oncology in the Department of Pediatrics, Aarhus University Hospital Skejby, Aarhus, Denmark. He studies childhood myeloid malignancies and cancer-prone syndromes.
Philip Rosenberg is a Senior Investigator in the Biostatics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. He is an expert on cancer rates and trends.
REFERENCES
- Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader-Meunier B, Tchernia G, Mielot F, Fontaine JL, Thomas C, Lyonnet S, Lavergne JM, Dommergues JP. Occurrence of myeloproliferative disorder in patients with Noonan syndrome. J Pediatr. 1997;130:885–889. doi: 10.1016/s0022-3476(97)70273-7. [DOI] [PubMed] [Google Scholar]
- Bentires-Alj M, Paez JG, David FS, Keilhack H, Halmos B, Naoki K, Maris JM, Richardson A, Bardelli A, Sugarbaker DJ, Richards WG, Du J, Girard L, Minna JD, Loh ML, Fisher DE, Velculescu VE, Vogelstein B, Meyerson M, Sellers WR, Neel BG. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64:8816–8820. doi: 10.1158/0008-5472.CAN-04-1923. [DOI] [PubMed] [Google Scholar]
- Case M, Matheson E, Minto L, Hassan R, Harrison CJ, Bown N, Bailey S, Vormoor J, Hall AG, Irving JA. Mutation of genes affecting the RAS pathway is common in childhood acute lymphoblastic leukemia. Cancer Res. 2008;68:6803–6809. doi: 10.1158/0008-5472.CAN-08-0101. [DOI] [PubMed] [Google Scholar]
- Chantrain CF, Jijon P, De Raedt T, Vermylen C, Poirel HA, Legius E, Brichard B. Therapy-related acute myeloid leukemia in a child with Noonan syndrome and clonal duplication of the germline PTPN11 mutation. Pediatr Blood Cancer. 2007;48:101–104. doi: 10.1002/pbc.20527. [DOI] [PubMed] [Google Scholar]
- Downward J. Signal transduction. Prelude to an anniversary for the RAS oncogene. Science. 2006;314:433–434. doi: 10.1126/science.1134727. [DOI] [PubMed] [Google Scholar]
- Gripp KW. Tumor predisposition in Costello syndrome. Am J Med Genet C Semin Med Genet. 2005;137C:72–77. doi: 10.1002/ajmg.c.30065. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Scott CI, Jr, Nicholson L, McDonald-McGinn DM, Ozeran JD, Jones MC, Lin AE, Zackai EH. Five additional Costello syndrome patients with rhabdomyosarcoma: proposal for a tumor screening protocol. Am J Med Genet. 2002;108:80–87. doi: 10.1002/ajmg.10241. [DOI] [PubMed] [Google Scholar]
- Hasle H. Malignant diseases in Noonan syndrome and related disorders. Horm Res. 2009;72 Suppl 2:8–14. doi: 10.1159/000243773. [DOI] [PubMed] [Google Scholar]
- Jebar AH, Hurst CD, Tomlinson DC, Johnston C, Taylor CF, Knowles MA. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, Collins VP. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow A, Steinemann D, Gohring G, Hasle H, Greiner J, Harila-Saari A, Flotho C, Zenker M, Schlegelberger B, Niemeyer CM, Kratz CP. Clonal duplication of a germline PTPN11 mutation due to acquired uniparental disomy in acute lymphoblastic leukemia blasts from a patient with Noonan syndrome. Leukemia. 2007;21:1303–1305. doi: 10.1038/sj.leu.2404651. [DOI] [PubMed] [Google Scholar]
- Kratz C. Myeloproliferative Disease and Cancer in Persons with Noonan Syndrome and Related Disorders. In: Zenker M, editor. Noonan Syndrome and Related Disorders - A Matter of Deregulated Ras Signaling. Karger: Basel; 2009. pp. 119–127. [Google Scholar]
- Kratz CP, Niemeyer CM, Castleberry RP, Cetin M, Bergstrasser E, Emanuel PD, Hasle H, Kardos G, Klein C, Kojima S, Stary J, Trebo M, Zecca M, Gelb BD, Tartaglia M, Loh ML. The mutational spectrum of PTPN11 in juvenile myelomonocytic leukemia and Noonan syndrome/myeloproliferative disease. Blood. 2005;106:2183–2185. doi: 10.1182/blood-2005-02-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Steinemann D, Niemeyer CM, Schlegelberger B, Koscielniak E, Kontny U, Zenker M. Uniparental disomy at chromosome 11p15.5 followed by HRAS mutations in embryonal rhabdomyosarcoma: lessons from Costello syndrome. Hum Mol Genet. 2007;16:374–379. doi: 10.1093/hmg/ddl458. [DOI] [PubMed] [Google Scholar]
- Makishima H, Cazzolli H, Szpurka H, Dunbar A, Tiu R, Huh J, Muramatsu H, O'Keefe C, Hsi E, Paquette RL, Kojima S, List AF, Sekeres MA, McDevitt MA, Maciejewski JP. Mutations of e3 ubiquitin ligase cbl family members constitute a novel common pathogenic lesion in myeloid malignancies. J Clin Oncol. 2009;27:6109–6116. doi: 10.1200/JCO.2009.23.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli S, De Luca A, Stellacci E, Rossi C, Checquolo S, Lepri F, Caputo V, Silvano M, Buscherini F, Consoli F, Ferrara G, Digilio MC, Cavaliere ML, van Hagen JM, Zampino G, van der Burgt I, Ferrero GB, Mazzanti L, Screpanti I, Yntema HG, Nillesen WM, Savarirayan R, Zenker M, Dallapiccola B, Gelb BD, Tartaglia M. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am J Hum Genet. 2010;87:250–257. doi: 10.1016/j.ajhg.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli S, McDowell HP, Vigne SD, Kokai G, Uccini S, Tartaglia M, Dominici C. RAS signaling dysregulation in human embryonal Rhabdomyosarcoma. Genes Chromosomes Cancer. 2009;48:975–982. doi: 10.1002/gcc.20702. [DOI] [PubMed] [Google Scholar]
- Moley JF, Brother MB, Wells SA, Spengler BA, Biedler JL, Brodeur GM. Low frequency of ras gene mutations in neuroblastomas, pheochromocytomas, and medullary thyroid cancers. Cancer Res. 1991;51:1596–1599. [PubMed] [Google Scholar]
- Niemeyer CM, Kang MW, Shin DH, Furlan I, Erlacher M, Bunin NJ, Bunda S, Finklestein JZ, Sakamoto KM, Gorr TA, Mehta P, Schmid I, Kropshofer G, Corbacioglu S, Lang PJ, Klein C, Schlegel PG, Heinzmann A, Schneider M, Stary J, van den Heuvel-Eibrink MM, Hasle H, Locatelli F, Sakai D, Archambeault S, Chen L, Russell RC, Sybingco SS, Ohh M, Braun BS, Flotho C, Loh ML. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat Genet. 2010;42:794–800. doi: 10.1038/ng.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister S, Janzarik WG, Remke M, Ernst A, Werft W, Becker N, Toedt G, Wittmann A, Kratz C, Olbrich H, Ahmadi R, Thieme B, Joos S, Radlwimmer B, Kulozik A, Pietsch T, Herold-Mende C, Gnekow A, Reifenberger G, Korshunov A, Scheurlen W, Omran H, Lichter P. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo PA, Poplack DG. Principles and practice of pediatric oncology. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Romano AA, Allanson JE, Dahlgren J, Gelb BD, Hall B, Pierpont ME, Roberts AE, Robinson W, Takemoto CM, Noonan JA. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2010;126:746–759. doi: 10.1542/peds.2009-3207. [DOI] [PubMed] [Google Scholar]
- Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- SEER’s 17 Registries Database from 2000–2007
- Tartaglia M, Gelb BD. Disorders of dysregulated signal traffic through the RAS-MAPK pathway: phenotypic spectrum and molecular mechanisms. Ann N Y Acad Sci. 2010;1214:99–121. doi: 10.1111/j.1749-6632.2010.05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Martinelli S, Cazzaniga G, Cordeddu V, Iavarone I, Spinelli M, Palmi C, Carta C, Pession A, Arico M, Masera G, Basso G, Sorcini M, Gelb BD, Biondi A. Genetic evidence for lineage-related and differentiation stage-related contribution of somatic PTPN11 mutations to leukemogenesis in childhood acute leukemia. Blood. 2004;104:307–313. doi: 10.1182/blood-2003-11-3876. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Martinelli S, Iavarone I, Cazzaniga G, Spinelli M, Giarin E, Petrangeli V, Carta C, Masetti R, Arico M, Locatelli F, Basso G, Sorcini M, Pession A, Biondi A. Somatic PTPN11 mutations in childhood acute myeloid leukaemia. Br J Haematol. 2005;129:333–339. doi: 10.1111/j.1365-2141.2005.05457.x. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Organization Classification of Tumours: Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. [Google Scholar]
- WHO. WHO Classofication of Tumours of the Central Nervous System. Lyon: IARC Press; 2007. [Google Scholar]
- Zenker M. Genetic and pathogenetic aspects of Noonan syndrome and related disorders. Horm Res. 2009;72 Suppl 2:57–63. doi: 10.1159/000243782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


