Abstract
Bungarotoxin present in Bungarus caeruleus (BC) causes life threatening respiratory muscle paralysis. Deep coma and hypokalaemia have been observed in a significant proportion of patients, but the cause is unknown. We postulate the likely mechanism behind these two phenomena. We studied clinical details of two patients admitted with deep coma and performed electroencephalograms (EEG) and brain stem auditory and visual evoked potentials (BAEP and VEP). Daily serum potassium was measured along with urinary potassium excretion as a marker of total extracellular body potassium. Both patients had no brain stem reflexes on admission and the EEG revealed absent alpha and delta activity and presence of dominant theta activity. Alpha rhythm returned on the 3rd day in one patient, while in the other it did not, and the latter patient died on the 13th day due to disseminated intravascular coagulation. BAEP were delayed and VEP were absent in the deceased patient. Both had low serum potassium and low urinary potassium excretion. Replacement of potassium (up to 1.5mmol/kg/day) did not improve serum potassium and urinary potassium excretion. Absent alpha and delta activity in EEG and delayed BAEP and absent VEP are suggestive of a central action of the venom on both the cortical and brain stem neurones. Persistently low serum potassium and reduced urinary potassium excretion are suggestive of intracellular shift as the causative mechanism of hypokalaemia.
Keywords: Deep coma, hypokalaemia, Bungarus caeruleus, EEG, evoked potentials
INTRODUCTION
Bungarus caeruleus (Common Krait) bites are a continuing medical problem in Sri Lanka (Kularatne, 2002; Ariaratnam et al, 2008). These bites rapidly lead to a classical occulo-facio-bulbar and respiratory muscle paralysis that requires mechanical ventilation in about half of all victims (Kularatne, 2002).
Physicians managing Bungarus caeruleus bites frequently encounter patients in states of deep coma mimicking brain death. A prospective study of 210 cases of Bungarus caeruleus bites in the North Central Province of Sri Lanka reported a high incidence of deep coma (Kularatne, 2002). This series reported a state of ‘total unresponsiveness to deep pain or loud voices associated with fixed dilated pupils and absent brain stem and spinal reflexes' in 35 (17%) patients. All of the survivors in this group (22 patients) made a complete neurological recovery after varying periods of mechanical ventilation. Personal communication with many practicing physicians in the Island revealed similar transient and completely reversible deep coma in a significant number of victims of common Bungarus caeruleus in Sri Lanka. This phenomenon may be due to hypoxia secondary to respiratory failure or a direct effect of venom on the cortical and/or brainstem neurons. We assumed that EEG and brainstem auditory and visual evoked potentials will be abnormal if the venom acted on the cortical and brainstem neurones.
Another intriguing phenomenon reported following Bungarus caeruleus bite in Sri Lanka is hypokalaemia (Sellahewa, 1997; Kularatne, 2002). Kularatne reported a high incidence (71%) of hypokalaemia (<3.5mmol/l) in their series (Kularatne, 2002). Author postulated beta adrenergic stimulation and intra-cellular shift of potassium as the potential cause of this phenomenon. A third possibility is loss of potassium in the renal tubules. We postulated that measurement of urinary potassium excretion would be a reliable surrogate marker of renal conservation of potassium. If there is intracellular shifting of potassium, the renal tubules would attempt to conserve potassium and thus, potassium excretion of would be minimal. A third possibility is the loss of potassium in the renal tubules.
There are no published reports on human studies conducted to describe the pathophysiology of these phenomena. The objective of the current report is to postulate two putative mechanisms of deep coma and hypokalaemia following Bungarus caeruleus bites in Sri Lanka.
MATERIALS AND METHODS
Two patients who were transferred in November 2008 to the study hospital following proven bites by Bungarus caeruleus were prospectively followed up until death or discharge. Regular findings of clinical observations of cardiovascular and nervous systems were documented. Daily blood samples were analysed for serum potassium and sodium while the daily urine was collected for the analysis of potassium excretion. Digital electroencephalograms (EEG) and electrocardiograms (ECG) were performed on a daily basis. In patient 1, we performed peripheral nerve conduction studies (NCS), repetitive nerve stimulation studies (RNS), visual (VEP) and brain stem auditory evoked potentials (BAEP) to assess the peripheral and central nerve pathways. Clinical management included replacement of potassium at a rate of 1-1.5mmol/kg/day, replacement of fluids and nutrients.
RESULTS
Both patients were presented to the local hospital with the killed specimen of Bungarus caeruleus. Both patients were treated with polyvalent antivenom (20 vials of Lyophilised, Enzyme refined, Equine Snake Venom Antiserum; (Vins Bioproducts Limited® ) raised against Indian Cobra, Common Krait, Russell's Viper and Saw Scaled Viper) at the local hospital, before being transferred to the study hospital for further care.
Patient 1
A 43-year-old man was bitten by a Bungarus caeruleus while he was sleeping on the ground and was admitted to the local hospital within one hour of the bite. He arrived with a normal Glasgow Coma Scale (GCS), peripheral oxygen saturation of 100% and normal blood pressure. Within an hour after admission, he developed respiratory failure and was intubated.
He was then transferred to the study hospital. On arrival at the study hospital, his GCS was 3/15, Blood pressure 90/70 mmHg and pulse rate of 80/min. He did not respond to loud commands or deep pain. His pupils were dilated (6mm) and were not reacting to light. All brain stem and spinal reflexes were absent. Maintenance of mechanical ventilation did not require any form of sedation. Admission blood gases revealed: pH 7.332, PCO2 26.7,PO2 233,HCO3 13.8 and BE 9.5. Blood pressure and metabolic acidosis responded promptly to administration of intravenous fluids.
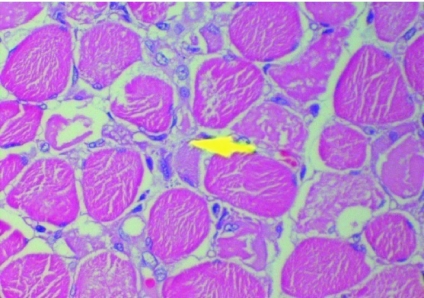
Over the next 4 days, his cortical functions improved from a flicker of movement of the eye lids to deep pain to movement of hands to verbal commands (GCS 10/15). On day 7, he developed features of disseminated intravascular coagulation (DIC) with bleeding from mucous membranes, thrombocytopenia and the chest X ray revealed evidence of Adult Respiratory Distress Syndrome (ARDS). Maintenance of oxygenation became increasingly difficult and he died of an asystolic cardiac arrest 13 days after the bite. Post-mortem examination revealed macroscopic evidence of ARDS. Histology of the diaphragm revealed evidence of extensive muscle necrosis (Figure 1).
Figure 1.
Hematoxylin & Eosin section of diaphragmatic muscle. Arrow indicates an area of necrosis.
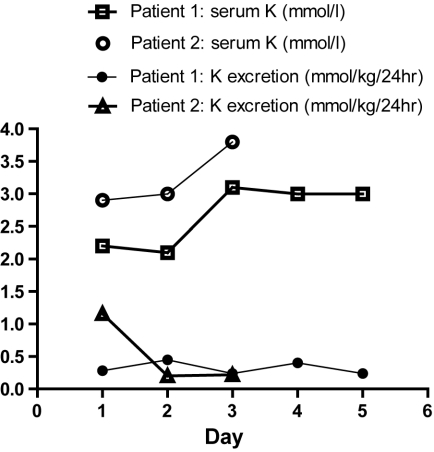
Admission blood samples were analyzed for serum potassium and sodium. Serum Potassium was found to be persistently low (2.2, 2.1, 3.1, 3 and 3mmol/l on admission, days 2,3,4 and 5 respectively) despite replacement of potassium at a rate of 1-1.5mmol/kg/day. Twenty four hour urinary potassium excretion was persistently lower than the normal 1-1.5mmol/kg/day (Figure 2).
Figure 2.
Daily serum potassium and urinary potassium excretion. In patient 1, serum potassium remained below normal (3.5mmol/l) throughout while urinary potassium excretion remained below normal daily excretion (1-1.5mmol/kg/day). In patient 2, serum potassium normalized on day 3 while the urinary excretion remained low.
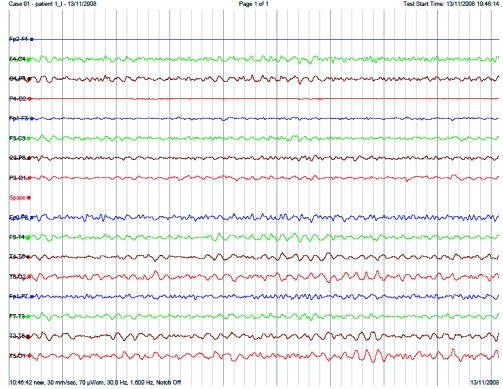
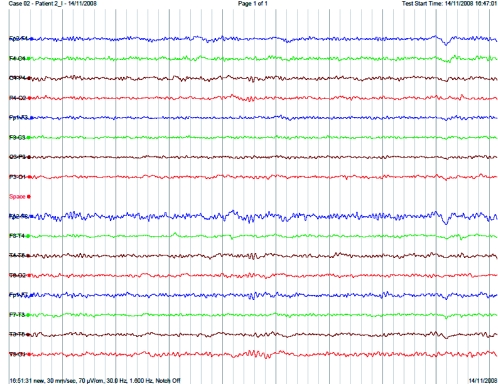
EEG (Figure 3) revealed absent alpha and delta activity and dominant theta activity (4-5Hz) on the day of the bite. Serial daily EEGs revealed that alpha activity and delta did not appear while theta activity dominated for 7 days after the bite.
Figure 3.
EEG of patient 1 done on the day of the bite demonstrating absent alpha and delta activity with dominant theta activity.
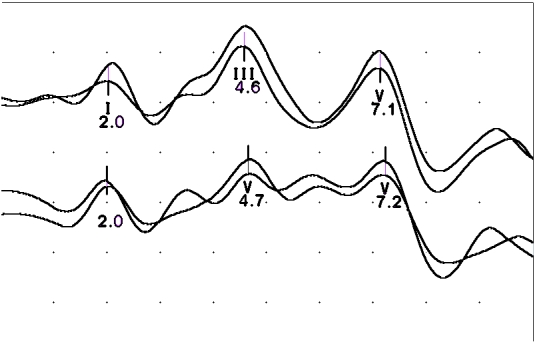
VEP waves were absent. BAEP study (Figure 4) revealed grossly delayed brainstem waves. Common peroneal and RNS studies were normal.
Figure 4.
Delayed brainstem auditory evoked potentials to click stimulus in Patient 1. Top trace recorded from A2-Fz and bottom trace from A1-Fz.
Patient 2
A 32-year-old male was admitted to the same local hospital as in Case 1 following a Bungarus caeruleus bite. He developed respiratory paralysis 10hr after the bite and was intubated. He had normal cardiovascular functions throughout the illness. He was transferred to the study hospital with a GCS of 3/15 with no brain stem and spinal reflexes. On the 3rd day after the bite, he regained consciousness and was extubated on the 4th day and discharged with no residual neurological complications. He had no memory of events that occurred after the bite until the third day.
His serum potassium was low (2.9mmol/L) on the day of the admission and improved (3.8mmol/L)on day 3. His urinary potassium excretion was normal (i.e., 1-1.5mmol/kg/day) on day 1 but reduced on days 2 and 3 (1.16, 0.2 and 0.22 mmol/kg/day on admission, day 2 and day 3, respectively) (Figure 1). EEG on day 1 (Figure 5) revealed theta activity with absent alpha and delta activity for 2 days. Alpha activity returned on day 3.
Figure 5.
EEG of patient 2 done on the day of the bite demonstrating theta activity with absent alpha and delta activity.
DISCUSSION
These two cases demonstrate clearly that severely envenomed patients with Bungarus caeruleus have depressed cortical activity and brain stem functions. In addition to deep coma, both patients had identical EEG activity of absent alpha and delta waves and dominant theta waves after the bite. The survivor had restored alpha activity on the third day while in the other, alpha activity did not return. Both patients demonstrated hypokalaemia with reduced excretion of urinary potassium. Replacement of potassium was unable to improve serum potassium or urinary potassium excretion. The cause of death in the Patient 1 was a combination of disseminated intravascular coagulopathy and adult respiratory distress syndrome (ARDS). The latter complication has been reported previously by Kularatne (Kularatne, 2002) in 5 patients who died while being in a deep coma. Necrosis of diaphragmatic muscles following Bungarus caeruleus has not been reported previously and may have contributed to the respiratory failure.
Venom of Bungarus caeruleus contains a mixture of alpha- and beta-bungarotoxin and a caerulotoxin. Beta-bungarotoxin acts on the presynaptic terminal while alpha-bungarotoxin and caerulotoxin act on the post synaptic membrane (Abe et al, 1977; Bon and Changeux, 1977). Beta-bungarotoxin of Bungarus multicinctus causes rapid release of acetyl choline (ACh) from the pre-synaptic membrane, but rapidly causes inhibition of choline re-uptake into the pre-synaptic membrane thus depleting choline available for recycling of ACh. Therefore, ACh levels are decreased leading to an inhibition of neurotransmission (Sen et al, 1976), which causes peripheral neuromuscular paralysis. In addition to its effects on the peripheral nervous system, beta bungarotoxin has been shown to possess the ability to act on the central cholinergic systems of the cortex, cerebellum and hippocampus in rats (Gulya et al, 1984). Inhibition of central cholinergic neurotransmission may theoretically cause deep coma with representative EEG abnormalities. In the rat, the alpha waves are generated from the thalamocortical pathways, predominantly in the sensory motor cortex (Semba and Komisaruk, 1984). In the Rhesus, monkey, alpha-bungarotoxin binds to the cortex (Han et al, 2003). Both patients presented in this report had absent alpha activity, which gives indirect evidence of inhibition of cortical neurotransmission. Theta activity is generated from the hippocampal area in animals. Alpha-bungarotoxin binds extensively throughout the hippocampi of both the rat and the Rhesus monkey (Freedman et al, 1993; Han et al, 2003). In the rat, binding is particularly heavy with GABAergic interneurones in the dentate gyrus and the Ammon of the hippocampus (Freedman et al, 1993). Theta activity is believed to be generated in the hippocampus in both animals and man (Miura et al, 1985). This is further strengthened by the fact that addition of scopolamine, a acetylcholine inhibitor, inhibits the generation of theta activity in the rat hippocampus (Brazhnik et al, 1993). Excess theta activity observed in both patients perhaps indicates that bungarotoxin does not bind, or does so less avidly, in the humans. In patients with other forms of coma, such as cerebral malaria, delta activity, a waveform believed to be generated from the thalamus in association of the reticular activating system (RAS), is the predominant wave form observed in the EEG (Thumasupapong et al, 1995). Neither of the patients showed delta activity indicating that a mechanism different to that observed in metabolic coma is in operation in patients with Bungarus caeruleus bite-induced coma.
Further, beta-bungarotoxin from Bungarus multicinctus has been shown to cause release of the inhibitory neurotransmitter, GABA, in experimental models (Wernicke et al, 1975), which may also contribute to deep coma. Patient 2 and all the severely envenomed patients reported by Kularatne (Kularatne, 2002) had anterograde memory loss, a phenomenon that further supports reversible inhibition of these structures.
There may be dose-dependent structural damage to the synapse membranes, which may explain the lack of recovery in one of the patients presented (Ueno and Rosenberg, 1996; Herkert et al, 2001). Preferential binding of bungarotoxin to the brain stem structures is the most likely explanation of absent brain stem reflexes and VEP and grossly delayed BAEP. Involvement of brain stem should be a key contributory factor in the rapid the development of respiratory failure.
Ramachandran reported EEG abnormalities of a heterogeneous group of patients with snake bites in Sri Lanka (Ramachandran et al, 1995). None of the 26 cases in this report included a Bungarus caeruleus bite and therefore a comparison cannot be made.
Daily renal potassium excretion is proportional to the dietary intake and an average adult looses about 80-120mmol of potassium each day (1-1.5mmol/kg/day) (Giebisch and Wang, 1996; Rabinowitz, 1996). Urinary potassium excretion was extremely low in both patients, and neither patient excreted the 1-1.5mmol/kg/day (70 to 90mmol) of potassium given to them each day. This indicates near maximum renal conservation of potassium. Further, daily replacement of 1-1.5mmol/Kg of potassium did not normalize serum potassium in the deceased patient while it took three days in the survivor. This suggests that, extracellular potassium was either being lost to the gastrointestinal tract or being shifted intracellularly. The former is unlikely as none of the two patients had ileus or diarrhoea. Furthermore, the contribution of gastrointestinal loss in normal potassium homeostasis is minimal (Giebisch and Wang, 1996). It could be argued that there was a significant intracellular shift of potassium in both patients similar to that seen in patients with barium poisoning (Bradberry and Vale, 1995). As only 2% of total body potassium is extracellular and contributes to maintenance of resting membrane potential, shifting of potassium into the intracellular compartment should contribute significantly to neuromuscular weakness of these patients. The mechanism of this shift in Bungarus caeruleus is unclear and warrants further study.
CONCLUSIONS
The clinical features, EEG, VEP and BAEP, suggest that bungarotoxin induced deep and reversible coma is due to its effects on the cortical and brain stem structures. Hypokalaemia is most likely due to intracellular shifting of potassium. Knowledge of the potential underlying mechanisms of these phenomena could be used in the clinical management and would stimulate further research.
Acknowledgments
We thank the South Asian Clinical Toxicology Research Collaboration (SACTRC) for funding the laboratory studies and providing the digital EEG facility. SACTRC is funded by Wellcome Trust & Australian National Health and Medical Research Council International Collaborative Capacity Building Research Grant (GR071669MA ). IG is funded by AusAID grant no ALA 000379.
REFERENCES
- Abe T, Alema S, Miledi R. Isolation and characterization of presynaptically acting neurotoxins from the venom of Bungarus snakes. Eur J Biochem. 1977;80:1–12. doi: 10.1111/j.1432-1033.1977.tb11849.x. [DOI] [PubMed] [Google Scholar]
- Ariaratnam CA, Sheriff MH, Theakston RD, Warrell DA. Distinctive epidemiologic and clinical features of common krait (Bungarus caeruleus) bites in Sri Lanka. Am J Trop Med Hyg. 2008;79:458–462. [PubMed] [Google Scholar]
- Bon C, Changeux JP. Chemical and pharmacological characterization of toxic polypeptides from the venom of Bungarus caeruleus. Eur J Biochem. 1977;74:31–42. doi: 10.1111/j.1432-1033.1977.tb11363.x. [DOI] [PubMed] [Google Scholar]
- Bradberry SM, Vale JA. Disturbances of potassium homeostasis in poisoning. J Toxicol Clin Toxicol. 1995;33:295–310. doi: 10.3109/15563659509028915. [DOI] [PubMed] [Google Scholar]
- Brazhnik ES, Vinogradova OS, Stafekhina VS, Kitchigina VF. Acetylcholine, theta-rhythm and activity of hippocampal neurons in the rabbit-I. Spontaneous activity. Neuroscience. 1993;53:961–970. doi: 10.1016/0306-4522(93)90481-t. [DOI] [PubMed] [Google Scholar]
- Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L. Alpha-bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebisch G, Wang W. Potassium transport: from clearance to channels and pumps. Kidney Int. 1996;49:1624–1631. doi: 10.1038/ki.1996.236. [DOI] [PubMed] [Google Scholar]
- Gulya K, Budai D, Kasa P, Rakonczay Z. In vivo effects of beta-bungarotoxin on the acetylcholine system in different brain areas of the rat. J Neurochem. 1984;43:112–119. doi: 10.1111/j.1471-4159.1984.tb06685.x. [DOI] [PubMed] [Google Scholar]
- HanZY, Zoli M, Cardona A, Bourgeois JP, Changeux JP, Le Novere N. Localization of [3H]nicotine, [3H]cytisine, [3H]epibatidine, and [125I]alpha-bungarotoxin binding sites in the brain of Macaca mulatta. J Comp Neurol. 2003;461:49–60. doi: 10.1002/cne.10659. [DOI] [PubMed] [Google Scholar]
- Herkert M, Shakhman O, Schweins E, Becker CM. Beta-bungarotoxin is a potent inducer of apoptosis in cultured rat neurons by receptor-mediated internalization. Eur J Neurosci. 2001;14:821–828. doi: 10.1046/j.0953-816x.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- Kularatne SA. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: a prospective clinical study, 1996-98. Postgrad Med J. 2002;78:276–280. doi: 10.1136/pmj.78.919.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Ito T, Kadokawa T. Acute EEG changes in rats by brainstem ischemia and its dopaminergic involvement. Jap J Pharmacol. 1985;39:443–451. doi: 10.1254/jjp.39.443. [DOI] [PubMed] [Google Scholar]
- Rabinowitz L. Aldosterone and potassium homeostasis. Kidney Int. 1996;49:1738–1742. doi: 10.1038/ki.1996.258. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Ganaikabahu B, Pushparajan K, Wijesekera J. Electroencephalographic abnormalities in patients with snake bites. Am J Trop Med Hyg. 1995;52:25–28. doi: 10.4269/ajtmh.1995.52.25. [DOI] [PubMed] [Google Scholar]
- Sellahewa K. Lessons from four studies on the management of snake bite in Sri Lanka. Ceylon Med J. 1997;42:8–15. [PubMed] [Google Scholar]
- Semba K, Komisaruk BR. Neural substrates of two different rhythmical vibrissal movements in the rat. Neuroscience. 1984;12:761–774. doi: 10.1016/0306-4522(84)90168-4. [DOI] [PubMed] [Google Scholar]
- Sen I. Grantham PA and Cooper JR. 1976. Mechanism of action of beta-bungarotoxin on synaptosomal preparations. Proc Natl Acad Sci USA. 73:2664–2668. doi: 10.1073/pnas.73.8.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumasupapong S, Tin T, Sukontason K, Sawaddichi C, Karbwang J. Electroencephalography in cerebral malaria. Southeast Asian J Trop Med Public Health. 1995;26:34–37. [PubMed] [Google Scholar]
- Ueno E, Rosenberg P. Mechanism of action of beta-bungarotoxin, a presynaptically acting phospholipase A2 neurotoxin: its effect on protein phosphorylation in rat brain synaptosomes. Toxicon. 1996;34:1219–1227. doi: 10.1016/s0041-0101(96)00113-4. [DOI] [PubMed] [Google Scholar]
- Wernicke J F, Vanker AD, Howard BD. The mechanism of action of beta-bungarotoxin. J Neurochem. 1975;25:483–496. doi: 10.1111/j.1471-4159.1975.tb04354.x. [DOI] [PubMed] [Google Scholar]