Abstract
Testing whether venoms may aid in digestion of the prey, eleven snake venoms were compared for the presence of proteases and endopeptidases that function in alkaline pH conditions. In vitro experiments examined the relative protease and endopeptidase activity of the venoms, which involved combining bovine muscle and snake venom in a buffered solution, encased within dialysis tubing. This mixture was then incubated at room temperature (∼20°C) for 24hr, with constant shaking. Bicinchoninic acid (BCA) assay and ninhydrin assay were used to determine peptide and amino acid concentrations. Histological and immunohistochemical investigations using N. kaouthia venom confirmed in vitro findings. Results show that B. arietans venom generated the highest amount of protein/peptides and amino acids in the dialysates, while O. scutellatus, N. ater niger and P. textilis venom did not show any significant protein degradation under alkaline conditions. Histological examination revealed varying degrees of muscle cell damage for each of the venom investigated, and the immunohistochemical study on N. kaouthia venom showed that the venom penetrated the muscle tissue to a significant degree. In vitro assays and histological results indicate that particular venoms may possess the ability to enhance digestion of bovine muscle tissue.
Keywords: Proteolytic activity, digestion, alkaline protease, elapid, viperid, venom
INTRODUCTION
Snake venoms are a complex mixture of proteins, peptides, carbohydrates, lipids, metal ions and organic compounds (Eggertsen et al, 1980), which primarily act to incapacitate and perhaps aid in the digestion of prey. The major physiological effects of envenomation include neurotoxication that results in general paralysis, haemotoxicity which includes toxins that cause coagulation disturbances (including hemorrhagic and haemolytic toxins), myolytic activity resulting in muscle breakdown and subsequent renal failure, and a direct nephrotoxic effect (Stewart, 2003).
The suggestion that snake venom plays more of a role than just subduing prey has often been raised (Deutsch and Diniz, 1955; Flachsenberger, 1995; Urdaneta et al, 2004; Nicholson et al, 2006), one suggestion being that venom actually aids digestion. Nicholson and colleagues (2006) undertook a study on the digestive properties of the Australian Coastal Taipan, Oxyranus scutellatus. The study found that the snake's venom increased the rate at which soluble proteins were released from a dismembered mouse hind leg. Although the mechanism was not studied, it was suggested that phospholipase A2 (PLA2) may play a role in aiding digestion by disrupting cell membranes, increasing the release of free proteins (Nicholson et al, 2006). This study only examined the acidic (peptic) digestive properties of the venom.
Interestingly, Van Der Walt and Joubert (1971) found that the venom of Bitis arietans exerted purely a proteolytic effect on casein and denatured haemoglobin. Blaylock, (2002) also investigated the venom of Bitis arietans and its local necrotic effect on live mouse hind legs. This study found that the venom caused much more significant necrosis of the local tissue when the hind leg was kept immobilized compared to those mice that were ambulatory, which could be partly due to a proteolytic action of the venom.
Thomas and Pough (1979) found that injecting live mice with Crotalus atrox (Western Diamondback Rattlesnake) venom, before ingestion by non-venomous snakes, increased the rate of digestion. They found that the venom's proteolytic activity weakened the internal organs of the prey and loosened hair which resulted in the more rapid rupturing of the visceral cavity. This provided the natural digestive secretions of the snake's stomach a larger surface area to act on, resulting in faster digestion of the prey. Not all studies, however, have agreed that venoms facilitate digestion. Marshall (2007) also performed a study on Crotalus atrox, a species of snakes known for its relatively high proteolytic activity, and found no statistically significant evidence that digestion was made more efficient by envenomation of prey. Contradicting studies such as these have fuelled debate on whether venoms in fact do or do not aid in prey digestion.
Other studies on various snake venoms have also come to different conclusions on the digestive role of venom. Reichert (1936) found that envenomated prey ingested by Bothrops jaracussu was digested over 4-5 days but when envenomation was withheld the digestion process lasted 12-14 days. Similar results were found Vipera aspis venom also, where normal prey digestion lasted 3 days, but when venom was withheld it lasted 5-8 days (Zeller, 1948). In contrast to these, a study by Urdaneta et al (2004) found that there was no significant proteolytic action in the venom of Micrurus nigrocinctus, as did a recent study on the Taiwanese pit vipers Trimeresurus gracilis and T. stejnegeri (Chu et al, 2009). The differing conclusions on the role of venoms in digestion may be due to variation in the components of these venoms.
The internal physiological pH of many small organisms is slightly alkaline, around 7.4 (Spigelman et al, 2002), and if a venom has the ability to completely or partially hydrolyse proteins in alkaline conditions, this suggests the venom may aid in the initial digestion of the prey. This digestion would occur inside of the prey as the venom was injected, and work its way outwards as the animal passes along the gastrointestinal tract (Thomas and Pough, 1979). Although many studies discuss the ability of snake venoms to aid digestion of prey, none have directly investigated the extent to which particular venoms may be able to digest proteins under alkaline conditions.
Of the many venom components, there are only a few that are of interest to this study. Among these are Lipase enzymes, such as phospholipases, which act to cleave phospholipids. The most common Phospholipases found in snake venoms include Phospholipase As and Phospholipase B, with a single report of Phospholipase C being found in Bothrops alternates venom (Bernheimer, 1986). Some small molecular weight myotoxins, which primarily act on paralysing the prey have also shown profound skeletal muscle degeneration which ultimately contributes to prey digestion. Prey digestion commences upon envenomation and continues beyond the prey's death until venom constituents are inactivated by prey protease inhibitors or proteases, or by the snakes digestive enzymes (Aird, 2002). It has been found that some venom constituents have digestive functions which are secondary to their immobilisation activities, while others apparently only serve to break down prey tissues. Hemorrhagic toxins (such as some fibrinogenases and metalloproteases) destroy the integrity of the prey vascular system and (probably) the lymphatic system as well, permitting the movement of catabolic endogenous and exogenous enzymes into the tissues. Interestingly, some have also been shown to induce apoptosis of muscle cells (Aird, 2002). Hyaluronidase has been known as a venom spreading factor for some time, and has been found in a variety of snake venoms. It acts to hydrolyse hyaluronan, therefore, degrading the integrity of tissues which facilitates penetration by microorganisms, parasites and toxins. It also expedites diffusion of released endogenous catabolic enzymes, acting in conjunction with venom hemorrhagic proteases. Therefore, venom hyaluronidase should also be viewed as playing a digestive role in venoms (Aird, 2002).
MATERIALS AND METHODS
Materials
All venoms were supplied by Venom Supplies Pty Ltd, Tanunda, South Australia, in lyophilised form. Horse radish peroxidise (HRP) conjugated anti-cobra antibodies used for the immunohistochemical study were made at the University of South Australia. Bovine muscle tissue (neck strap) was obtained from Strath Pastoral Pty Ltd, Strathalbyn, South Australia. Subtilisin A (7.1units/mg), Endoproteinase Glu-C (100units/mg), Proteinase K (39units/mg), Trypsin (10,000 BAEE units/mg) were obtained from Sigma-Aldrich, Inc. Liquid DAB+ Substrate Chromagen System (DakoCytomation), Bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Inc), Micro-BCA protein assay kit (Thermo Fisher Scientific, Inc), Commonwealth Serum Laboratories (CSL) Black snake antivenom (Batch No 0543-07001; expiry 11/04), Antivipmyn antivenom for C. vegrandis (Batch No B2J-03, expiry 09/09/2006, Bioclon), Dilaysis tubing (12kDa (Biolabs) and 1kDa (Spectra/Por) molecular weight cut off (MWCO)), Haematoxylin and Eosin staining reagents (Woods and Ellis, 1994) and Ninhydrin Reagent 2% (v/v) solution (Sigma Aldrich) were all of analytical grade.
Venoms
The lyophilised venoms were reconstituted to 100mg/ml protein concentration. Eleven venoms were tested in total, consisting of eight elapid species including the Red-bellied Black Snake (Pseudechis porphyriacus), Mulga/King Brown Snake (Pseudechis australis - QLD locality), Coastal Taipan (Oxyuranus scutellatus), Tiger Snake (Notechis ater niger - Kangaroo Island locality), Common/Eastern Brown Snake (Pseudonaja textilis - QLD locality), Common Death Adder (Acanthophis antarcticus - S.A. locality), Thai/Monocled Cobra (Naja kaouthia) and the King Cobra (Ophiophagus hannah). The three Viperid venoms tested include Eastern diamond back rattle snake (Crotalus adamanteus), Uracoan rattlesnake (Crotalus vegrandis), and Puff adder (Bitis arietans). Reconstituted venoms were stored at -80°C until being used in the experiment.
Muscle preparation
Bovine muscle tissue was used as the source of protein for the experiments. Tissue were collected immediately after slaughter and immersed in a sodium acetate buffer, pH 5.5 (0.05M CH3COONa.3H20, 0.15M NaCl and 0.015M NaN3 as an antibacterial) to prevent any digestion by endogenous enzymes during transport (O'Halloran et al, 1997). Samples were immediately taken to the laboratory, and sectioned in a UV sterilised laminar flow work station. Muscle ‘cubes’ (∼ 0.5mm2) were then washed in 2ml of sodium acetate buffer, and centrifuged at 985xg for 4min at 4ºC to remove any excess blood and endogenous enzymes that could potentially interfere with the experiments. The rinse was repeated until the filtrate collected in the centrifuge tube had a 280nm (UV) absorbance of ≤ 0.1. A final 4min “dry spin” at 985xg was done to dry the tissue which was then stored in 1.5ml microcentrifuge tubes at -80°C for future use. This procedure ensured a consistent source of protein for all the digestion experiments.
Digestion method
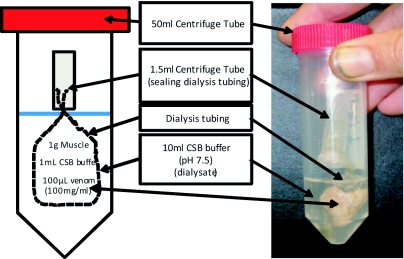
All experiments were set as detailed in Figure 1. Ten millilitres of Complete Sodium Bicarbonate buffer (CSB) (0.05M NaHCO3, 0.009M CaCl2, 0.0021M MgCl2, 0.15M NaCl and 0.015M NaN3), pH 7.5, was placed into each individual 50ml centrifuge tube, followed by sealed dialysis tubing (12kDa molecular weight cut of (MWCO) for protein-peptide experiments, and 1kDa MWCO for peptide-amino acid experiments) containing the correct components for each individual test (1gm of muscle + 1ml CSB buffer + 0.1ml of venom (100mg/ml). Tubes were gently agitated for 24hr in a shaking water bath (Ratek Instruments) at 20°C. After 24hr the CSB buffer (dialysate) was removed and quantitatively assayed for either peptide or amino acid concentration. Dialysates were assayed for protein using a Pierce BCA protein assay kit. A ninhydrin assay was used to measure amino acid concentration in the peptide-amino acid experiments (Moore and Stein, 1948).
Figure 1.
Apparatus in which the digestion experiments were performed. Dialysis tubing MWCO sizes vary between protein-peptide digestion experiment (12kDa) and amino acid (1kDa). After 24hr the 10ml of CSB buffer is termed the “Dialysate”.
Digestion positive controls
Positive controls for the experiments contained a mixture of protein digesting enzymes (in place of the venom). The protein-peptide experiments tubes contained Trypsin and Proteinase K (100µl of Trypsin (1000 BAEE units), 50µl of Proteinase K (0.35units)) with the bovine muscle and CSB buffer (within the dialysis tubing). The positive control for the amino acid experiments contained equal amounts (30µl each) of; Trypsin (330 units), Subtilisin A (0.11units), Endoproteinase Glu-C (3units) and Proteinase K (1.3units).
Digestion muscle negative control
Negative controls were made containing only the CSB buffer (1ml) and the muscle sample (1gm)*. This allowed any protein or peptide released from the muscle samples to be measured. The proteins in the negative controls were assayed with a micro-BCA kit to obtain accurate measurements at low concentrations.
Venom negative controls
Negative controls that contained only the CSB buffer (1ml), and venom (100µl, 100mg/ml, and no muscle sample). This negative control was done for all the separate venoms used in the digestion experiments.
Digestion tests
Test samples were set up identically to the positvie controls, except a specific venom was used in place of the enzymes. 100µl of venom (100mg/ml), 1gm of bovine muscle, and 1ml of CSB were placed within the dialysis tubing.
Antivenom experiments
Two antivenom/venom mixes were used to test the specific antivenoms efficacy at inhibiting the venom's protease activity. CSL Blacksnake antivenom was used against P. porphyriacus and P. australis venom, while polyclonal Antivipmyn antivenom was used to test C. vegrandis venom. Both antivenoms were dialysed to remove any small molecular weight proteins (50kDa MWCO dialysis tubing, and 2x1l (0.15M NaCl)). After dialysing, the Blacksnake antivenom was centrifuged in a sterile 3kDa MWCO MACROSEP Centrifugal Concentrator 5000xg for 10-12hr, thereby removing excess water, and concentrating the antivenom.
The dialysed antivenom was tested for total protein concentration (using 280nm UV absorbance), and for its immunological activity on the venoms using an immunoelectrophoresis assay. For the Blacksnake antivenom test a stock solution venom-antivenom mix was made by incubating the mixture (400µl antivenom (50mg/ml) + 100µl venom (100mg/ml)) for 1hr at 37°C. The Crotalus vegrandis antivenom mixture was made by mixing 550µl of Crotalus vegrandis venom with 1650µl of the Antivipmyn antivenom and incubating for 1hr at 37°C. Samples were then cooled to 4°C, and centrifuged at 16,100xg for 5min. The clear supernatant was retained for use and any precipitates (i.e., bound venom/antivenom aggregates) were discarded. The Blacksnake antivenom tests were conducted in an identical manner to the protein-peptide digestion experiments, except the ‘venom’ within the dialysis tubing was replaced with the venom-antivenom supernatant (100µl venom/antivenom supernatant, 1gm muscle, 10ml CSB buffer). Tests performed using the Crotalus vegrandis antivenom/venom mix each contained 1gm muscle, 400µl venom-antivenom mix (100µl Crotalus vegrandis venom + 300µl Antivipmyn antivenom) and 1ml CSB buffer.
EDTA digestion inhibition experiments
A stock solution of the venom-EDTA mixture was made containing 250µl (100mg/ml) of venom with 25µl (0.20M) EDTA solution. This was incubated at room temperature for 10min before centrifuging at 16,100xg for 5min. The clear venom-EDTA supernatant was removed and used in the digestion experiment (i.e., replaced the ‘venom’ sample within the dialysis tubing). The dialysis bag (12kDa MWCO) for this experiment contained 1gm muscle, 110µl of venom-EDTA mix (100µl of venom (100mg/ml) + 10µl of 0.20M EDTA) and 1ml of CSB buffer. The experimental methodology was the same as described in “Digestion method”.
Study of muscle tissue penetration by the venom of Naja kaouthia during the digestion experiment
An immunohistochemical study was performed using N. kaouthia venom and a horse radish peroxidise (HRP) conjugated anti-cobra venom antibodies, made at the University of South Australia. The HRP conjugate was tested prior to its use in this study to ensure that it had significant biological activity (i.e., the ability to bind to the cobra venom antigens). Paraffin sections were cut from muscle samples from the initial N. kaouthia digestion experiment. Sections initially had a 5min peroxidise block (using peroxidise quenching solution), and were permeabilised with Triton-X100 (0.1%, v/v) for 10min before being blocked with horse serum (30min). The HRP conjugate was then added and was left to incubate for 30min, rinsed, and a Dako diaminobenzidine (DAB) Plus Kit was used to visualise the degree of binding. DAB forms a very stable, brown end-product at the site of the target antigen or nucleic acid. The sections were counterstained with Ehrlich's Haematoxylin (Woods and Ellis, 1994), for 20sec and differentiated in 1% (w/v) acid/alcohol if necessary. Finally, the sections/slides were dehydrated, cleared (in Xylene) and mounted. Slides were analysed using an Olympus BX40 microscope, fitted with an Olympus DP70 camera. Results from this process showed DAB deposition as brown colour, and nuclei as purple-blue.
Histological analysis of muscle tissue
Haematoxylin and eosin (H&E) stains were performed on 4µm paraffin sections of the muscle tissue which had been collected and fixed in 10% (w/v) formal buffered saline throughout the digestion experiments. Histological analysis provided visual clarification on the effects of the individual venoms had on the muscle structure. The stains were performed using methodology by Woods and Ellis (1994).
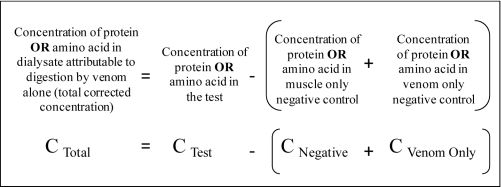
Procedure used for calculating the results
The total corrected concentration of either peptides or amino acids in the dialysate were calculated using the following equation detailed in Figure 2, which takes into account the universal ‘muscle only’ negative control and the ‘venom only’ negative controls of the individual venoms. The ‘positive control’ corrected value used the same equation but without a venom only control.
Figure 2.
Calculation for the total (corrected) protein/amino acid concentration in dialysate (the concentration in dialysate attributable to digestion of the muscle by the venom alone).
Statistical analysis
A clustered regression analysis was used to test significance (P ≤0.05) using ‘Stata 10’, for all protein and amino acid comparisons. Students T-test's were used (P≤0.05) to test significance of the Antivenom and EDTA experiments.
RESULTS
Protein Assay
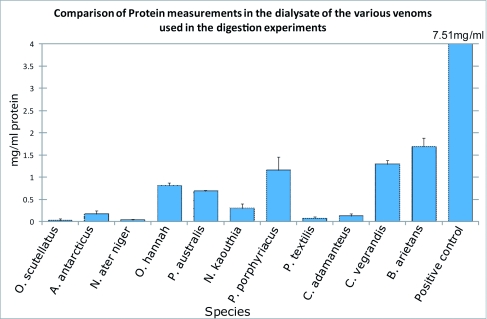
Testing for protease activity in the venoms show that 8 out of the 11 venoms investigated showed a statistically significant increase in protein-peptide accumulation in the dialysate when compared to the negative control (see Table 1 and Figure 3).
Table 1.
Protein assay results.
| Species | Protein (mg/ml) |
|---|---|
| Pseudechis porphyriacus | 1.16 ± 0.30 |
| Pseudechis australis | 0.68 ± 0.03 |
| Acanthophis antarcticus | 0.17 ± 0.08 |
| Ophiophagus Hannah | 0.81 ± 0.06 |
| Naja kaouthia | 0.29 ± 0.11 |
| Crotalus adamanteus | 0.12 ± 0.06 |
| Crotalus vegrandis | 1.29 ± 0.09 |
| Bitis arietans | 1.69 ± 0.19 |
| Oxyuranus scutellatus | 0.02 ± 0.05* |
| Notechis ater niger | 0.03 ± 0.02* |
| Pseudonaja textilis | 0.07 ± 0.04* |
*The three venoms that showed no statistically significant protein-peptide release included Oxyuranus scutellatus, Notechis ater niger, and Pseudonaja textilis.
Figure 3.
Protein comparison.
Amino acid assay (endopeptidase effect)
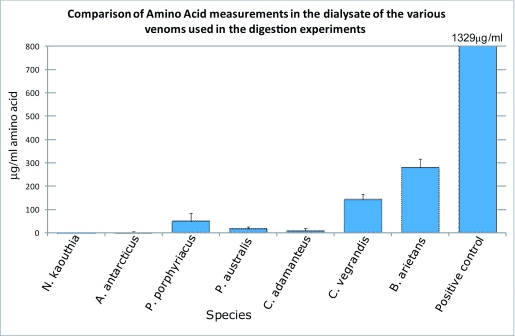
Peptide-Amino acid assay results show that only 2 out of the 7 venoms investigated generated a statistically significantly higher amount of amino acids in the dialysate compared to the negative control. The results of this assay are given in Table 2 and Figure 4.
Table 2.
Amino acid assay results.
| Species | Amino acid (µg/ml) |
|---|---|
| Crotalus vegrandis | 142.58 ± 22.60 |
| Bitis arietans | 279.31 ± 36.62 |
| Naja kaouthia | 0.00 ± 11.69* |
| Acanthophis antarcticus | 0.02 ± 8.85* |
| Pseudechis porphyriacus | 48.69 ± 34.99* |
| Pseudechis australis | 16.14 ± 10.63* |
| Crotalus adamanteus | 7.34 ± 11.70* |
*Venoms that did not show a statistically significant increase when compared to the negative control.
Figure 4.
Amino acid comparison.
Antivenom inhibition
After observing the alkaline protease action of C. vegrandis, P. porphyriacus and P. australis venom on digestion of muscle proteins, experiments were conducted to test whether specific antivenoms were able to inhibit the proteolytic activity observed in these venoms. Immuno-electrophoresis assays were performed to ensure the antivenom's antibodies reacted with the venoms antigens (toxins) and results showed that both antivenoms had strong immunological activity against their target venoms. This was done because both antivenoms used were past their expiry dates.
Results show (see Table 3) that the 50mg/ml antivenom concentration totally inhibited all the proteolytic activity in P. australis venom. The P. porphyriacus venom proteolytic activity was also significantly inhibited by the antivenom. The Antivipmyn antivenom inhibited C. vegrandis venoms proteolytic activity from 1.29 ±0.09mg/ml to 0.80 ±0.23mg/ml.
Table 3.
Antivenom inhibition assay results.
| Antivenom concentration used | Protein concentration (mg/ml)* | Protein concentration (mg/ml)** |
|---|---|---|
| P. porphyriacus (50mg/ml antivenom) | 1.16 ± 0.30 | 0.16 ± 0.08 |
| P. australis (50mg/ml antivenom) | 0.68 ± 0.03 | 0.05 ± 0.08 |
| Crotalus vegrandis (30mg/ml antivenom) | 1.29 ± 0.09 | 0.80 ± 0.23 |
*Before inhibition by Antivenom
**After inhibition by Antivenom
EDTA Inhibition
Results show (see Table 4) that there was a significant decrease of proteolytic activity when the 200mM concentration of EDTA was used on P. porphyriacus venom. The P. porphyriacus venom-EDTA experiment shows a significant reduction in protein release from 1.16 ±0.30mg/ml (P≤0.05) to 0.09 ±0.04mg/ml. Therefore, the alkaline protease activity can be attributed to one or more metalloproteins present in P. porphyriacus venom. C. vegrandis venom exhibited similar results, where protease activity was significantly inhibited by EDTA.
Table 4.
EDTA inhibition assay results.
| EDTA concentration | Protein concentration (mg/ml)* | Protein concentration (mg/ml)** |
|---|---|---|
| P. porphyriacus (200mM/ml EDTA) | 1.16 ± 0.30 | 0.09 ± 0.04 |
| Crotalus vegrandis (200mM/ml EDTA) | 1.29 ± 0.09 | 0.28 ± 0.10 |
*Before inhibition by EDTA
**After inhibition by EDTA
Immunohistochemistry
In this study we also investigated the degree of muscle tissue penetration of N. kaouthia venom after 24hr using an immunohistochemical study as digestion rates are related to food surface area to volume ratios. The following histological findings (see Figure 5) show the degree of penetration of N. kaouthia venom on a piece of bovine tissue after 24hr.
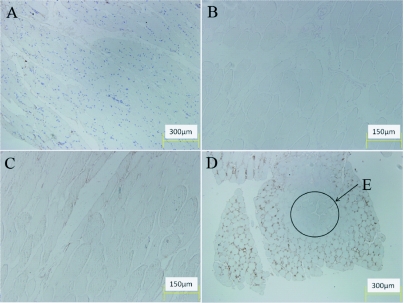
Figure 5.
Immunohistological findings. (A) Negative Control, (B) Control for non-specific DAB binding, (C) Background binding of DAB, (D) N. kaouthia test exhibiting brown DAB deposition formed when the DAB reacted with the HRP- anti cobra venom conjugate, (E) Area of muscle tissue without DAB precipitate present.
Histology
Histological analysis throughout this study was used to visually confirm the in vitro findings. The venom studies showed various degrees of tissue damage, ranging from O. scutellatus, which showed limited damage (if any), to B. arietans and P. porphyriacus, which exhibited marked muscle cell destruction, as shown in Figure 6.
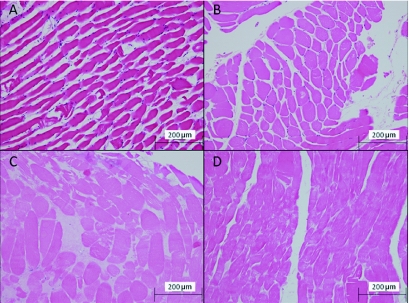
Figure 6.
Histological findings. (A) Negative Control, (B) O. scutellatus test, (C) B. arietans test, (D) P. porphyriacus test.
DISCUSSION
Venoms used in this investigation were chosen due to their availability, previous data, and to provide a broad spectrum of venoms that show various envenomation characteristics. In particular, elapid venoms were included in this study because they are known to exhibit some necrotic/proteolytic effects (e.g., P. australis), and others that are thought to be void of this activity (e.g., P. textilis and A. antarcticus) (Wickramaratna et al, 2003b). There have been numerous studies investigating the digestive properties of venoms which have utilised either whole mice (Flachsenberger and Mirtschin, 1995; Marshall, 2005), or parts of the mice, such as hind legs (Nicholson et al, 2006), as a protein source. Although, bovine muscle is not the typical food source for the studied snakes, whereas mice/rats may be, it provided a single piece of consistent muscle tissue for the experiments, and a reasonable source of mammalian protein to test the digestive action of the venoms. The main proteins in the muscle cells are actin and myosin (and myoglobin to a lesser extent) (Vandekerckhove and Weber, 1978; Sellers, 2000), and these proteins have molecular sizes that would prevent them passing through the dialysis membrane pores, unless they were digested by proteolytic enzymes. The vast majority of studies investigating digestive properties have investigated the venoms role in acidic environments, whilst there has not been a study directed to the venoms action in alkaline conditions. The alkaline conditions were designed to mimic the slightly alkaline conditions in prey and the small intestine of a snake, and internal pH of a prey item (such as a mice/rat) (Spigelman et al, 2002). Sodium bicarbonate was used to mimic small intestinal conditions and the ions of sodium, calcium and magnesium were also incorporated to ensure their presence as cofactors for any digestive enzymes (such as, metalloproteases). Finally, sodium azide was present as an antibacterial to prevent any bacterial growth, causing breakdown of the muscle over the 24hr time period of each experiment (Lichstein and Soule, 1944). Another aspect for choosing the 24hr time period relates to the digestive processes of a snake. When prey is envenomed and ingested, the strong acidic environment of the snakes stomach (i.e., digestion occurring externally), and digestive enzymes would reach and effectively denature any venom within the first few days. So, at least initially, the venom may be able to survive in the stomach (inside the ingested prey) and contribute to digestion.
Careful selection and documentation of venom parameters (e.g., time of milking, yield, storage conditions) was another important aspect that is commonly overlooked in venom studies. This study used pooled samples (multiple snakes) of venom from distinct geographical areas, which decreased the chances in having venom variation (e.g., geographic (Williams et al, 1988; Chippaux et al, 1991; Yang et al, 1991; Flight et al, 2006) from individual snakes. Factors such as geographic location of the snakes, different venom batches, and date of milking (seasonal variation could be a factor), snake age, snake health may influence results. Some of these were controlled.
Results of the protein-peptide digestion experiments revealed elapid venoms, which are generally known for their limited necrotic effects, showed a broad spectrum of activity. The Australian Red Bellied Black snake, P. porphyriacus, and Mugla snake, P. australis (both from the Pseudechis genus or ‘black snake’ group), showed higher protein digestion indicating that there is at least one active protease in their venoms, which can function under alkaline conditions. Other elapid species that did not demonstrate a significant degree of protein digestion included, P. textilis, A. antarcticus, O. scutellatus and N. ater niger, and whether these very low levels (if any) are of any significant use in protein digestion within living systems requires further investigation. Study conducted by Flachsenberger and Mirtschin (1995) found that at 18°C O. scutellatus venom delayed decay onset, therefore producing a ‘lack of digestion’, as seen in our study. The findings of the present study do not imply that O. scutellatus venom does not contain any digestive capabilities as a whole, but perhaps lacks the proteolytic enzymes which can function in an alkaline environment or function efficiently at the temperatures used in this study. This requires further investigation.
Histological analysis of the bovine muscle samples that were exposed to P. textilis venom showed that the tissue was seemingly unaffected when compared to a control, therefore confirming the histological observations seen by Harris and Maltin (1981) where no cell damage was evident. On the other hand, other venoms, such as P. porphyriacus and P. australis, showed marked cell destruction. These results confirm the histological observations seen of Hodgson and Wickramaratna (2006) on the effect of P. australis venom, probably due to PLA2 and myotoxic venom components, such as PA-myotoxin (Geh et al, 1992; Ponraj and Gopalakrishnakone, 1995; Fry, 1999). The low concentration of peptide formation obtained from N. ater niger is surprising, because majority of the literature discusses muscle damage, collagen destruction and possible renal failure associated with envenomation of the common tiger snake (Soto et al, 1988; Jolles et al, 1998). Perhaps the alkaline protease activity of N. ater niger is somewhat lower compared to common tiger snakes (N. scutatus) due to the high variation in venom proteins that has been documented between isolated populations of tiger snakes (Williams et al, 1988; Yang et al, 1991). It is also possible that whilst the PLA2 myotoxins present in the venom do damage muscle cells, the muscle proteins released into the dialysis bag are too large to go through the pores of the dialysis membrane (MWCO ∼12kDa).
Low protein-peptide results found in A. antarcticus venom compliment much of the literature that has been published, where the venom has been described as lacking necrotic and myotoxic activity (Mebs and Samejima, 1980; Sutherland et al, 1981; Wickramaratna and Hodgson 2001; Wickramaratna et al. 2003b). However, recent reports have described venom components that show myotoxic activity in some Acanthophis species including A. rugosus and A. sp. seram (Wickramaratna et al, 2003a; Wickramaratna et al. 2003b; Hart et al, 2005). Naja kaouthia results were as expected, showing some degree of protease activity, because this species has been documented as commonly causing necrotic activity (Reali et al, 2003; Wongtongkam et al, 2005). Out of all of the venoms studied, it was expected that O. hannah would show the greatest alkaline protease activity because the venom has been documented as being enzymatically active and exceptionally high in alkaline phosphomonoesterase activity (Tan and Hj, 1989). This study agrees with findings of Kocholaty et al, (1971) as O. hannah venom showed proteolytic effects (but not at extremely high levels, as we were expecting). Reasoning behind this reduction in proteolytic activity could be because O. hannah typically injects a high volume of venom into its prey, and the present study controlled the amount of venom used (using much less than O. hannah could inject into a prey item).
The previous work by Marshall (2007), on the Western Diamond back rattlesnake (Crotalus atrox) concluded that this venom had no significant digestive properties. This snake is a close relative of the Eastern Diamond back rattlesnake (Crotalus adamanteus) whose venom was investigated in this study. The venom of Crotalus adamanteus did not show any significant proteolytic activity in this study, therefore, supporting the work of Marshall (2007) in stating that the venom of these two closely related species of snakes has no significant (alkaline) protein digestive activity. However, the venom of Crotalus vegrandis and Bitis arietans did show significant proteolytic activity. The Crotalus vegrandis venom results support previous work by Aguilar et al (2001), who observed the presence of fibrinolytic and proteolytic enzymes in the venom of this species. Similarly, our Bitis arietans venom results support the work of Van Der Walt and Joubert (1971), who were able to purify an alkaline protease from the venom of Bitis arietans. The venom from both Crotalus vegrandis and Bitis arietans, therefore, were found to contain compounds that aid in digestion of proteins into smaller peptides.
Of the seven venoms that were investigated tested for peptidase activity, only two venoms were found to have the ability to generate elevated levels of amino acids in the dialysate, when compared to their respective venom negative control. These findings support the study conducted by Tu and Toom (1967), where they found that snake venoms do contain components that can hydrolyse peptides. However, as compared to the positive controls, the values are very low so it can be assumed that the total efficacy of the venom to fully hydrolyse protein into amino acids is limited, and has an overall negligible effect in amino acid production for digestive purposes (at the tested temperature). Further research into these enzymes would be required to indicate any digestive advantage in vivo. It is also possible that there are endoproteinases in the venom. These enzymes would hydrolyse only a specific C terminal amino acid (i.e., glutamine), unlike endopeptidases which will hydrolyse the complete protein into individual amino acids. This could only be proven by identifying the individual amino acids released into the dialysate. There are many variables that need to be considered including the mass of venom injected into prey items (i.e., greater mass of venom may increase the overall peptide and amino acid formation from venom), the size of the prey, prey type, and its internal body temperature and pH. The nature of this study cannot conclude which amino acids were produced.
Antivenom inhibition experiments
Gel immunoelectrophoretic assays were used to test the activity of antivenom antibody binding to the crude venom. Precipitation lines showed a high degree of antibody binding to the various venom antigens.
The Blacksnake antivenom experiments showed that P. porphyriacus venom proteolytic activity was reduced by > 80% compared with the initial digestion experiment for this venom, and P. australis activity was reduced by more than 90%, thereby showing that the antivenom contains the appropriate antibodies to inhibit venom alkaline protease activity.
Although Antivipmyn is not the antivenom of choice for C. vegrandis venom neutralisation, results indicate that the antivenom had cross reactivity and could successfully inhibit some of the enzyme(s) responsible for alkaline protease activity. Therefore, it may be able to reduce necrotic activity (muscle/protein destruction) of these venoms. More specific antivenom may have a greater inhibition potential, but Antivipmyn antivenom was used in this study because of its availability.
EDTA inhibition experiment on P. porphyriacus
EDTA is a well known metal ion complexing agent and has the ability of inhibiting ions from taking part in reactions (Vassil et al, 1998; Jones and Atkins, 1999). Inhibition of digestion by EDTA could therefore indicate that some or all of the digestive enzymes in the venom are metalloproteinases. Only protein-peptide digestion was investigated using 12kDa dialysis tubing. The peptide concentration in the dialysate from P. porphyriacus venom-EDTA mixture was compared to that of the P. porphyriacus peptide digestion experiment, and showed that the enzyme(s) responsible for the alkaline protein digestion are probably all metalloproteinases. Crotalus vegrandis venom also exhibited a large decrease in activity, indicating one or more metalloproteins are primarily responsible for the alkaline protease activity.
Immunohistochemical study on N. kaouthia
Due to the rate of protein digestion being largely dependent on food volume to area ratios, we investigated if the venom may penetrate or only work on the surface of muscle cells, and to explore the degree of absorption (or diffusion) of venom into a piece of muscle tissue after 24hr. Due to the muscle's previous treatment, it is difficult to comment in specific detail on cell structures, or detailed morphology. However, results clearly show that after 24hr N. kaouthia venom had penetrated approximately 300µm into the piece of beef muscle tissue, and caused the loss of cell nuclei. This observation enables highlighting of several important factors underlying the digestion experiments that were conducted. It shows that results for the protein-peptide and peptide-amino acid digestion experiments not only depends on the mass of protein within the dialysis tubing (i.e., 1gm), but also on the relative surface area to volume ratio of these samples. This would imply that one would expect higher protein digestion results for muscle samples that had greater surface area. Although, care was taken to try and standardise the size of muscle tissue samples that were used throughout the digestion experiments, there would have been slight differences in the surface area of each digestion experiment that was done. It cannot be concluded that all of the other venoms act in this way with ‘partial-penetration’ into muscle tissue due to the varying makeup and compositions of snake venoms, but may be an explanation for the variance seen in some test results.
CONCLUSIONS
Seven of the eight elapid venoms and two of the three viperid venoms contain alkaline proteases. The species that showed the highest activity was B. arietans followed by C. vegrandis, P. porphyriacus, O. hannah, P. australis and N. kaouthia. Several other venoms, including P. textilis, A. antarcticus, and N. ater niger. O. scutellatus showed no significant protease activity under alkaline conditions.
Two of the seven venoms that were tested for endopeptidase activity can hydrolyse protein down to individual amino acids, including P. porphyriacus and P. australis. However, these results need to be confirmed by identifying the individual amino acids, and ultimately purifying and characterising the enzymes responsible.
All histological findings complemented in vitro findings, with venoms such as P. porphyriacus and P. australis showing marked cell destruction, in accordance with the higher peptide concentrations found in the digestion assays.
Histological analysis confirmed the low peptide readings (such as, O. scutellatus, N ater niger, C. adamanteus and P. textilis) with little (if any) cell damage compared to the negative control.
Further investigation into the digestive role of venom revealed that one or more of the enzymes responsible for hydrolysis of protein into peptides are metalloproteinases, due to its inhibition by EDTA.
Black snake antivenom significantly inhibited these proteolytic enzymes in P. porphyriacus and P. australis venoms. Antivipmyn antivenom also showed some inhibition of alkaline proteases in C. vegrandis venom.
Immunohistochemical study found that N. kaouthia venom substantially penetrated the bovine muscle tissue after 24hr.
Acknowledgments
Funding was supplied by Venom Supplies Pty Ltd, and the University of South Australia. We would also like to thank Dr Julian White (Women's and Children's Hospital, Adelaide, South Australia) for the donation of the Blacksnake antivenom used in this study.
STATEMENT OF COMPETING INTERESTS
None declared.
[*This negative control was called; “Universal muscle negative” and this value was estimated by performing 20 separate estimations, and the (mean) value obtained was used in all subsequent calculations (see Figure 2).]
REFERENCES
- Aguilar I, Girón ME, Rodríguez-Acosta A. Purification and characterisation of a haemorrhagic fraction from the venom of the Uracoan rattlesnake Crotalus vegrandis. Biochim Biophys Acta (BBA)-Protein Structure and Molecular Enzymology. 2001;1548:57–65. doi: 10.1016/s0167-4838(01)00217-5. [DOI] [PubMed] [Google Scholar]
- Aird SD. Ophidian envenomation strategies and the role of purines. Toxicon. 2002;40:335–393. doi: 10.1016/s0041-0101(01)00232-x. [DOI] [PubMed] [Google Scholar]
- Bernheimer AW, Weinstein SA, Linder R. Isoelectric analysis of some Australian elapid snake venoms with special reference to phospholipase B and hemolysis. Toxicon. 1986;24:841–849. doi: 10.1016/0041-0101(86)90109-1. [DOI] [PubMed] [Google Scholar]
- Blaylock RSM. Immediate active movement following Bitis arietans or Naja mossambica venom injection diminishes or prevents necrosis in mice. Toxicon. 2002;40:1675–1677. doi: 10.1016/s0041-0101(02)00182-4. [DOI] [PubMed] [Google Scholar]
- Chippaux JP, Williams V, White J. Snake venom variability: methods of study, results and interpretation. Toxicon. 1991;29:1279–1303. doi: 10.1016/0041-0101(91)90116-9. [DOI] [PubMed] [Google Scholar]
- Chu CW, Tsai TS, Tsai IH, Lin YS, Tu MC. Prey envenomation does not improve digestive performance in Taiwanese pit vipers (Trimeresurus gracilis and T. stejnegeri stejnegeri) Comp Biochem Physiol-Part A: Molecular & Integrative Physiology. 2009;152:579–585. doi: 10.1016/j.cbpa.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Deutsch HF, Diniz CR. Some Proteolytic Activities of Snake Venoms. J Biol Chem. 1955;216:17–26. [PubMed] [Google Scholar]
- Eggertsen G, Fohlman J, Sjöquist J. In vitro studies on complement inactivation by snake venoms. Toxicon. 1980;18:87–96. doi: 10.1016/0041-0101(80)90034-3. [DOI] [PubMed] [Google Scholar]
- Flachsenberger W. Digestive Properties of Snake Venom. Herpetofauna. 1995;25:28–31. [Google Scholar]
- Flachsenberger W, Mirtschin P. Digestive properties of snake venoms. Herpetofauna. 1995;25:28–31. [Google Scholar]
- Flight S, Mirtschin P, Masci PP. Comparison of active venom components between Eastern brown snakes collected from South Australia and Queensland. Ecotoxicology. 2006;15:133–141. doi: 10.1007/s10646-005-0047-z. [DOI] [PubMed] [Google Scholar]
- Fry BG. Structure-function properties of venom components from Australian elapids. Toxicon. 1999;37:11–32. doi: 10.1016/s0041-0101(98)00125-1. [DOI] [PubMed] [Google Scholar]
- Geh SL, Rowan EG, Harvey AL. Neuromuscular effects of four phospholipases A2 from the venom of Pseudechis australis, the Australian king brown snake. Toxicon. 1992;30:1051–1057. doi: 10.1016/0041-0101(92)90050-f. [DOI] [PubMed] [Google Scholar]
- Harris JB, Maltin CA. The effects of the subcutaneous injection of the crude venom of the Australian common brown snake, Pseudonaja textilis on the skeletal neuromuscular system. Br J Pharmacol. 1981;73:157–163. doi: 10.1111/j.1476-5381.1981.tb16785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AJ, Smith AI, Reeve S, Hodgson WC. Isolation and characterisation of acanmyotoxin-2 and acanmyotoxin-3, myotoxins from the venom of the death adder Acanthophis sp. Seram. Biochem Pharmacol. 2005;70:1807–1813. doi: 10.1016/j.bcp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Hodgson WC, Wickramaratna JC. Snake venoms and their toxins: An Australian perspective. Toxicon. 2006;48:931–940. doi: 10.1016/j.toxicon.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Jolles S, Lawrence S, Gallagher J, Fulcher D. Severe rhabdomyolysis after tiger snake bite. J R Soc Med. 1998;91:267–268. doi: 10.1177/014107689809100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Atkins P. Chemistry: Molecules, Matter and Change. W.H. Freeman Company; New York, USA: 1999. [Google Scholar]
- Kocholaty WF, Ledford EB, Daly JG, Billings TA. Toxicity and some enzymatic properties and activities in the venoms of Crotalidae, Elapidae and Viperidae. Toxicon. 1971;9:131–138. doi: 10.1016/0041-0101(71)90006-7. [DOI] [PubMed] [Google Scholar]
- Lichstein HC, Soule MH. Studies of the Effect of Sodium Azide on Microbic Growth and Respiration: I. The Action of Sodium Azide on Microbic Growth. J Bacteriol. 1944;47:221–230. doi: 10.1128/jb.47.3.221-230.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DM. Enzyme activities and biological functions of snake venoms. Appl Herpetol. 2005;2:109–123. [Google Scholar]
- Marshall DM. Prey envenomation does not improve digestive performance in western diamondback rattlesnakes (Crotalus atrox) J Experiment Zool-Part A: Ecological Genetics and Physiology. 2007;307A:568–577. doi: 10.1002/jez.411. [DOI] [PubMed] [Google Scholar]
- Mebs D, Samejima Y. Purification, from Australian elapid venoms, and properties of phospholipases A which cause myoglobinuria in mice. Toxicon. 1980;18:443–454. doi: 10.1016/0041-0101(80)90052-5. [DOI] [PubMed] [Google Scholar]
- Moore S, Stein WH. Photometric Ninhydrin Method for use in the Chromatography of Amino Acids. J Biol Chem. 1948;176:367–388. [PubMed] [Google Scholar]
- Nicholson J, Mirtschin P, Madaras F, Venning M, Kokkinn M. Digestive properties of the venom of the Australian Coastal Taipan, Oxyuranus scutellatus (Peters, 1867) Toxicon. 2006;48:422–428. doi: 10.1016/j.toxicon.2006.06.007. [DOI] [PubMed] [Google Scholar]
- O'Halloran GR, Troy DJ, Buckley DJ, Reville WJ. The role of endogenous proteases in the tenderisation of fast glycolysing muscle. Meat Sci. 1997;47:187–210. doi: 10.1016/s0309-1740(97)00046-6. [DOI] [PubMed] [Google Scholar]
- Ponraj D, Gopalakrishnakone P. Morphological changes induced by a generalized myotoxin (myoglobinuria-inducing toxin) from the venom of Pseudechis australis (king brown snake) in skeletal muscle and kidney of mice. Toxicon. 1995;33:1453–1467. doi: 10.1016/0041-0101(95)00091-y. [DOI] [PubMed] [Google Scholar]
- Reali M, Serafim FG, da Cruz-Hofling MA, Fontana MD. Neurotoxic and myotoxic actions of Naja naja kaouthia venom on skeletal muscle in vitro. Toxicon. 2003;41:657–665. doi: 10.1016/s0041-0101(03)00005-9. [DOI] [PubMed] [Google Scholar]
- Reichert E. Bothrops jararacussu. 1936:228–231. Bl Aquar. K47, pp. [Google Scholar]
- Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta (BBA)-Molecular Cell Research. 2000;1496:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- Soto JG, Perez JC, Minton SA. Proteolytic, hemorrhagic and hemolytic activities of snake venoms. Toxicon. 1988;26:875–882. doi: 10.1016/0041-0101(88)90328-5. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Zhiwei L, Banerjee P, Mihalek R, Homanics G, Olsen R. Behavior and Physiology of Mice Lacking the GABA-A-Receptor 3B4 Subunit. Epilepsia. 2002;43:3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Stewart CJ. Snake bite in Australia: first aid and envenomation management. Accident Emergency Nursing. 2003;11:106–111. doi: 10.1016/s0965-2302(02)00189-3. [DOI] [PubMed] [Google Scholar]
- Sutherland SK, Campbell DG, Stubbs AE. A study of the major Australian snake venoms in the monkey (Macaca fascicularis) Pathology. 1981;13:705–713. doi: 10.3109/00313028109086644. [DOI] [PubMed] [Google Scholar]
- Tan NH, Hj MN. Enzymatic and toxic properties of Ophiophagus hannah (king cobra) venom and venom fractions. Toxicon. 1989;27:689–695. doi: 10.1016/0041-0101(89)90020-2. [DOI] [PubMed] [Google Scholar]
- Thomas RG, Pough FH. The effect of rattlesnake venom on digestion of prey. Toxicon. 1979;17:221–228. doi: 10.1016/0041-0101(79)90211-3. [DOI] [PubMed] [Google Scholar]
- Tu AT, Toom PM. Hydrolysis of peptides by snake venoms of Australia and New Guinea. Aust J Exp Biol Med Sci. 1967;45:561–567. doi: 10.1038/icb.1967.55. [DOI] [PubMed] [Google Scholar]
- Urdaneta AH, Bolanos F, Gutierrez JM. Feeding behavior and venom toxicity of coral snake Micrurus nigrocinctus (Serpentes: Elapidae) on its natural prey in captivity. Comp Biochem Physiol-Part C: Toxicology and Pharmacology. 2004;138:485–492. doi: 10.1016/j.cca.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Van Der Walt SJ, Joubert FJ. Studies on puff adder (Bitis arietans) venom--I. Purification and properties of protease A. Toxicon. 1971;9:153–161. doi: 10.1016/0041-0101(71)90009-2. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J, Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978;126:783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Vassil AD, Kapulnik Y, Raskin I, Salt DE. The Role of EDTA in Lead Transport and Accumulation by Indian Mustard. Plant Physiol. 1998;117:447–453. doi: 10.1104/pp.117.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramaratna JC, Hodgson WC. A pharmacological examination of venoms from three species of death adder (Acanthophis antarcticus, Acanthophis praelongus and Acanthophis pyrrhus) Toxicon. 2001;39:209–216. doi: 10.1016/s0041-0101(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Wickramaratna JC, Fry BG, Aguilar MI, Kini RM, Hodgson WC. Isolation and pharmacological characterization of a phospholipase A2 myotoxin from the venom of the Irian Jayan death adder (Acanthophis rugosus) Br J Pharmacol. 2003a;138:333–342. doi: 10.1038/sj.bjp.0705046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramaratna JC, Fry BG, Hodgson WC. Species-Dependent Variations in the in Vitro Myotoxicity of Death Adder (Acanthophis) Venoms. Toxicol Sci. 2003b;74:352–360. doi: 10.1093/toxsci/kfg144. [DOI] [PubMed] [Google Scholar]
- Williams V, White J, Schwaner TD, Sparrow A. Variation in venom proteins from isolated populations of tiger snakes (Notechis ater niger, N. scutatus) in South Australia. Toxicon. 1988;26:1067–1075. doi: 10.1016/0041-0101(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Wongtongkam N, Wilde H, Sitthi-Amorn C, Ratanabanangkoon K. A study of Thai cobra (Naja kaouthia) bites in Thailand. Mil Med. 2005;170:336–341. doi: 10.7205/milmed.170.4.336. [DOI] [PubMed] [Google Scholar]
- Woods A, Ellis R. Laboratory Histopathology- A Complete Reference. Churchill Livingstone; New York, USA: 1994. [Google Scholar]
- Yang CC, Chang LS, Wu FS. Venom constituents of Notechis scutatus scutatus (Australian tiger snake) from differing geographic regions. Toxicon. 1991;29:1337–1344. doi: 10.1016/0041-0101(91)90120-g. [DOI] [PubMed] [Google Scholar]
- Zeller EA. Enzymes of Snake Venoms and their Biological Significance. Adv Enzymol Related Areas Mol Biol. 1948:459–495. [Google Scholar]