Abstract
Venom from male and female specimens of the medically important Venezuelan scorpion Tityus nororientalis have been compared. Males showed a significantly higher venom yield (2.39mg/individual) compared to female scorpions (0.98mg/individual). Female venom was significantly more toxic than that of males, with a median lethal dose (LD50) in C57BL/6 mice of 9.46 μg venom protein/gm body weight [95% confidence interval (8.91-9.94)] whereas LD50 for males was 13.36(12.58-14.03) μg/gm. Mass spectral analyses by MALDI-TOF revealed differences in venom composition between males and females. From a clinical standpoint, the time course of toxicity course indicated a tendency, in the case of the female venom, to elicit the earlier occurrence of severe signs such as sialorrhea, dyspnea (bradypnea/apnea) and exophthalmus particularly in the late toxicity phase. Female venom was significantly less efficient than male venom to inhibit the binding of anti-T. discrepans antibodies to immobilized T. discrepans venom in ELISA assays, suggesting sex-related differences in the bioactive surfaces of T. nororientalis toxins. These results indicate that males and females of T. nororientalis produce venoms with different composition and activity which may have epidemiological implications.
Keywords: Intersexual variation, scorpion, Tityus nororientalis, Venezuela
INTRODUCTION
Envenoming by species belonging to the scorpion genus Tityus is a pediatric emergency in several countries in South America (Biondi-Queiroz et al, 1996; Otero et al, 2004; De Sousa et al, 2007; Chippaux and Goyffon, 2008; Lira-da-Silva et al, 2009; Gómez et al, 2010), and also Panamá (Coronado et al, 2008), and the Caribbean (Daisley et al, 1999). Clinical manifestations of scorpionism are dependent on the scorpion species, amount of venom injected, and also the age and venom sensitivity of the victim, which is significantly higher in children (< 8 year-old) and the elderly (Borges, 1996; Borges and De Sousa, 2006). Venezuela is one of the South American countries with highest incidence of scorpionism, mainly due to stings by the genus Tityus (Borges and De Sousa, 2009; De Sousa and Borges, 2009; Borges et al, 2010a).
Of the seven endemic macroregions of scorpionism identified in Venezuela (De Sousa et al, 2000; Borges and De Sousa, 2006), the Andean and Northeastern regions have the highest mortality rates (Borges and De Sousa, 2006). In Sucre state, northeastern Venezuela, total deaths for the period 1996-2000 were 7, with a rate of 1.73 deaths per million inhabitants for the period 1996-2000; national mortality rate for the same period was 0.42 (Borges and De Sousa, 2006). The most amply distributed Tityus species in Sucre is T. nororientalis, responsible for the majority of scorpion envenomation cases in this area (Borges and De Sousa, 2006). This species was originally described by González-Sponga from Catuaro, Ribero municipality, central Sucre state (González-Sponga, 1996; Rojas-Runjaic and De Sousa, 2007). As well as other Tityus species in the Venezuelan northeast (González-Sponga, 1996, 2001; Quiroga et al, 2000; Quiroga et al, 2004; De Sousa et al, 2006; De Sousa et al, 2008), T. nororientalis displays a marked sexual dimorphism, with males exhibiting larger length of metasomal segments compared to females (González-Sponga, 1996).
While intersexual differences in morphology (e.g., size and color) are common in a number of arachnid species, including scorpions, only a few studies have examined such differences in regard to venom yield, composition or bioactivity. Thus far, intersexual differences have been found in theraphosid (Celerier et al, 1993) and araneomorph spider venoms (Herzig et al, 2002; Herzig and Hodgson, 2009). For scorpions, only in the case of Opisthacanthus madagascariensis a male specific venom component has been identified (Yamaji et al, 2004). Intersexual differences in toxicity and antigenicity in scorpion venoms may have clinical, therapeutical, as well as epidemiological implications considering the 2:1 female-to-male ratio in house dwellings, at least in the Venezuelan northeast, for several species of the medically important genus Tityus (De Sousa et al, 2009a). In this regard, the present report is the first to document intersexual variations in venom composition and activity in Tityus species, reporting a reduced venom production and higher lethality for female T. nororientalis specimens compared to male individuals. Differences in toxicity correlate well with proteomic differences evaluated by mass spectrometry and reactivity of female venom towards the commercial antivenom is significantly reduced compared to male individuals.
MATERIALS AND METHODS
All animal experiments reported in this article were performed according to protocols approved by the Department of Physiological Sciences, School of Health Sciences, Universidad de Oriente, Anzoátegui Campus (for details see De Sousa et al, 2009b). The ethical procedures recommended by the Fondo Nacional de Ciencia, Tecnología e Innovación (Ministry of Science and Technology, Venezuela) were strictly followed during the research.
Scorpion collection
Tityus nororientalis specimens were collected at night from its type locality in Catuaro (10°23´59.1´´N, 63°30´00.6´´W, 455 meters above sea level), Ribero municipality, Sucre state (González-Sponga, 1996). Scorpions were found under the bark of fallen, decomposing trees, and also in the base of coffee plants (Coffea arabica) using ultraviolet lamps (Stachel et al, 1999). Geographical coordinates were determined using a Global Positional System device, model eTrex® H (Garmin). After completion of the experimental procedures, scorpions used during this work were deposited at the Scorpion Collection (CELT), Escuela de Ciencias de la Salud, Universidad de Oriente, where they were given the following catalog numbers: ♀♀, CELT-1142 to CELT-1155; ♂♂, CELT-1156 to CELT-1162. T. discrepans specimens were collected in San Antonio de Los Altos, Miranda state, Venezuela (10°23´01´´N, 66°56´58´´W) and venom extracted as described above.
Venom extraction and protein determination
Venom was milked by electrical stimulation of the telson (the last caudal segment of the scorpion metasoma) according to the method of Quiroga et al (1982) using a neurostimulator Phipps-Bird (Richmond, Virginia, USA). Fourteen adult females and 7 adult males were milked according to this procedure. Venom was kept at -20°C until further use. Protein content was determined by measuring absorbance of venom solutions at 280nm using a 6405 Jenway UV/vis spectrophotometer (Staffordshire, UK) considering one unit of absorbance equivalent to 1mg/ml (Possani et al, 1977). Previous to mass spectrometry and electrophoretic analyses venom was lyophilized at 50mBar of pressure at -50oC.
Mice
17-23 g female mice of the strain C57BL/6 (Bioterium, Venezuelan Institute for Science Research, IVIC) were used throughout. Animals were maintained using natural light cycles, room temperature, feeding and water ad libitum as described by De Sousa et al (2009b).
Signs of acute toxicity and LD50 evaluation
Fifty lethal doses (LD50) for female and male T. nororientalis venoms were calculated as described by Dixon and Mood (1948) (with the modifications presented by Sevcik, 1987) for a one-hour period of experimentation as presented by De Sousa et al (2009b) for scorpion venom lethality titration. Venom was injected intraperitoneally (ip) with a Hamilton 505l microsyringe. Doses administered were calculated based on the weight of individual mice. Clinical signs elicited by scorpion venom toxicity were chronologically recorded during the observation period. Briefly, LD50s were calculated as follows: The first mouse injected ip received an initial dose, X1 = antiLog 1 = 10 μg venom protein/g of body weight. If the first animal dies, a second mouse will receive a dose X2 = antiLog (X1) - d, where d is a constant factor corresponding to 0.05 (De Sousa et al, 2009b). If the first animal survives, a second mouse will receive a dose X2 = antilog (X1) + d. The protocol is continued thereby increasing or reducing the dose depending on the outcome (death or survival) of the mouse previously injected until completing four cycles of increasing/decreasing doses, i.e., † 0 † 0 † 0 † 0 † ⊗ († = dead mouse, 0 = surviving mouse, ⊗ = end point) or 0 † 0 † 0 † 0 † 0 ⊗. The LD50 value was determined from the median of the doses starting from the first inflexion point in the lethality curve, which comprise the valid data sequence.
Statistical analysis
The median of the doses taken from the first inflexion point in the lethality curves was calculated according to Hodges and Lehmann (1963) together with the confidence intervals for the LD50 values and for the occurrence times of the acute toxicity signs. Differences between medians were tested using the Kruskal-Wallis analysis of variance (Kruskal and Wallis, 1952) considering a level of significance of p < 0.05 (De Sousa et al, 2009b). For data processing, the program V-8.34 was utilized, which was developed by Dr Carlos Sevcik, Venezuelan Institute for Science Research. To assess the significance of the difference in venom production between female and male T. nororientalis specimens, difference of proportions were calculated with independent sampling (Z value) and considering a p < 0.05. Variability index (VI) was evaluated as VI = [( LD50 top limit - LD50 bottom limit )/Median of LD50] x100.
Mass spectrometry
Mass spectra of positively charged ions from female and male T. nororientalis venoms were analyzed by MALDI-TOF MS in a Biflex III MALDI-TOF MS (Bruker, FRG) basically as described by Borges et al (2006a,b). Briefly, samples for analyses (200-500 μg) were lyophilized, resuspended in 100 μl of MilliQ water and diluted 10-fold with 0.1% (v/v) trifluoroacetic acid (TFA). One μl of the diluted sample was mixed with 5 μl of matrix solution (10mg/ml of 3,5-dimethoxy-4-hydroxycinnamic acid in a 1:1 mixture of acetonitrile and 0.1% (v/v) TFA. One μl from this mixture was spotted on the target plate. Mass spectra of positively charged ions were recorded on the Biflex III instrument operated in the linear mode. The total acceleration voltage and the detector voltage were 19kV and 0.55kV, respectively. A total of 100 to 150 single shots were accumulated for each sample. Molecular masses were calculated from at least three independent analyses.
Inhibition of anti-Tityus discrepans antivenom binding
An ELISA assay was used to determine the ability of T. nororientalis venoms (female and male) to inhibit binding of horse anti-T. discrepans antivenom antibodies to T. discrepans venon adbsorbed onto microtitration plates according to the method of King et al (1985). Competing venom samples were serially diluted starting from 10 μg/ml and incubated for 1hr at 37oC with the antivenom (Biotecfar, Universidad Central de Venezuela, Lot No. 049) at a dilution corresponding to 50% of the maximum antibody binding to immobilized T. discrepans venom. The latter venom was a mixture of equal proportions of female and male T. discrepans venoms. One hundred μl of the competing venom: antivenom mixtures were added onto the plates previously coated with T. discrepans venom (100ng/well) and incubated for 1hr at 37°C. Plates were then washed and horse radish peroxidase-labeled anti-horse IgG antibodies (Sigma) added as described by Borges et al (2008). The antigen:antibodies complexes were recorded by measuring color formation after the peroxide-driven reaction at 492nm. Data was plotted in a semilogarithmic scale and used to determine the percentage of inhibition of antivenom binding to immobilized T. discrepans venom as a result of preincubation with T. nororientalis female and male venoms.
Acetic acid-urea gel electrophoresis
Electrophoresis of protein samples in acid-urea gels was carried out according to Wang et al (1997) in 12.5% (w/v) polyacrylamide gels, using 5% (v/v) acetic acid as running buffer and pyronine Y as tracking dye.
RESULTS
Venom production
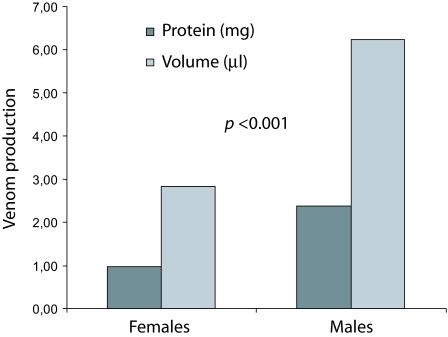
Figure 1 shows venom production by female and male T. nororientalis obtained by electrical stimulation. A significantly lesser amount of venom (Z = 2.42; p < 0.001) in terms of protein and volume was produced by females (n = 14) compared to males (n = 7). In total females produced 13.77mg of venom whereas males produced 16.73mg. The venom yield per individual was 0.98mg in 2.343l for females, and 2.39mg and 6.252l for males.
Figure 1.
Comparison of venom production between female and male Tityus nororientalis.
Venom lethality and signs of toxicity
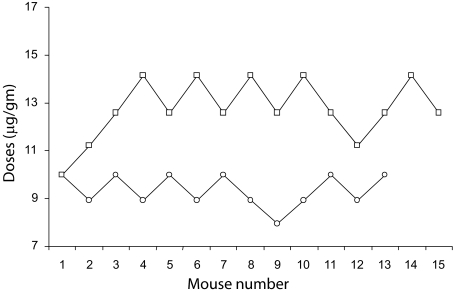
Figure 2 compares the lethality of female and male venoms through the determination of their 1-h LD50 values in C57BL/6 mice. Female venom LD50 was 9.46(8.91-9.46) μg/gm, significantly (Kruskal-Wallis = 18.95; p = 0.00001) lower than the male venom LD50, 13.36(12.58-13.36) μg/gm. Variability indexes were 5.8% for both LD50 determinations.
Figure 2.
Medium lethal dose determination for female and male T. nororientalis venom in C57BL/6 mice. The inflexion point (arrow) (death/survival or survival/death events) is placed in mouse #2 for the female venom (open circles) and in mouse #4 for male venom (open squares). Mice considered for female LD50 calculation were from mouse #2 until mouse #12 + end point (n = 12). To evaluate the toxicity signs elicited by female venom, animals included mouse #2 until mouse #12 (n = 11). Mice considered for male LD50 calculation were from mouse #4 until mouse #14 + end point (n = 12). To evaluate the toxicity signs elicited by male venom, animals included were from mouse #4 until mouse #14 (n = 11). Total female venom utilized in the test was 2.16mg and 3.60mg in the case of males. A control mouse was used for each determination which did not develop any toxicity signs after being injected with 20 μl of saline (0.90% (w/v) NaCl).
Table 1 presents the mortality frequency obtained in the valid data sequences for LD50 determination for female and male T. nororientalis venoms in mice. For females, the dose corresponding to the highest rate was 10.00 μg/gm (n = 4, 80.0%). At 8.91 μg/gm the frequency was low (n = 1, 20.0%) and with 7.94 μg/gm no deaths were recorded. For males, the dose corresponding to the highest rate was 14.13 μg/gm (n = 5, 83.3%). At 12.58 μg/gm the frequency was low (n = 1, 16.7%) whereas at 11.22 μg/gm no deaths were recorded.
Table 1.
Mortality frequency in C57BL/6 mice injected with female or male Tityus nororientali s venom.
| VENOM | Death Rate | |||||
|---|---|---|---|---|---|---|
| Female n = 5 (100%) |
Male n = 6 (100%) |
|||||
| Doses (Anti Log) Doses (μg/gm) |
0.90 7.94 |
0.95 8.91 |
1.00 10.00 |
1.05 11.22 |
1.10 12.58 |
1.15 14.13 |
| Ratio Percentage (%) |
0/5 0.0 |
1/5 20.0 |
4/5 80.0 |
0/6 0.0 |
1/6 16.7 |
5/6 83.3 |
Table 2 compares the signs of toxicity occurring after injection of female and male venoms into mice. With the female venom, 24 signs of toxicity were recorded. Hyperactivity, tachypnea, sialorrhea, piloerection, and deglutatory movements were observed in all mice corresponding to the valid data sequence. 80-90% of the mice developed mouth and nose cleaning, ataxic movements, Straub sign, convulsions, abundant sialorrhea, and dyspnea characterized by forced abdominal breathing. 72.7% of the injected mice presented bradypnea/apnea, ocular secretion, pasty defecation, abdominal distension, and generalized tremors, whereas 63.6% developed exophthalmus and hypotonic rear limbs. Spastic paralysis was observed in 45.6% of the injected animals. Signs associated with high mortality were (in n/n dead mice and n/n surviving mice): bradypnea/apnea (5/5 and 3/6), pasty defecation (5/5 and 3/6), exophthalmus (5/5 and 2/6), hypotonic rear limbs (5/5 and 2/6) and spastic paralysis (5/5 and 0/6).
Table 2.
Frequency of clinical manifestations induced by Tityus nororientalis female and male venom in C57BL/6 mice.
| Toxicity sign | Female venom | Male venom | ||||
|---|---|---|---|---|---|---|
| Survived n/6 |
Died n/5 |
Total % (n/11) |
Survived n/5 |
Died n/6 |
Total % (n/11) |
|
| Hiperactivity | 6/6 | 5/5 | 100.0 | 5/5 | 6/6 | 100.0 |
| Dyspnea: Tachypnea | 6/6 | 5/5 | 100.0 | 5/5 | 6/6 | 100.0 |
| Sialorrhea | 6/6 | 5/5 | 100.0 | 5/5 | 6/6 | 100.0 |
| Piloerection | 6/6 | 5/5 | 100.0 | 5/5 | 6/6 | 100.0 |
| Deglutory movements | 6/6 | 5/5 | 100.0 | 5/5 | 6/6 | 100.0 |
| Mouth and nose cleaning | 6/6 | 4/5 | 90.9 | 4/5 | 6/6 | 90.9 |
| Ataxic movements | 6/6 | 4/5 | 90.9 | 4/5 | 6/6 | 90.9 |
| Straub sing | 5/6 | 5/5 | 90.9 | 3/5 | 6/6 | 81.8 |
| Convulsions | 5/6 | 5/5 | 90.9 | 4/5 | 5/6 | 81.8 |
| Abundant sialorrhea | 4/6 | 5/5 | 81.8 | 3/5 | 6/6 | 81.8 |
| Dyspnea: Forced abdominal breathing | 4/6 | 5/5 | 81.8 | 3/5 | 6/6 | 81.8 |
| Dyspnea: Bradypnea/Apnea | 3/6 | 5/5 | 72,7 | 2/5 | 6/6 | 72.7 |
| Ocular secretion | 5/6 | 3/5 | 72.7 | 4/5 | 4/6 | 72.7 |
| Pasty defecation | 3/6 | 5/5 | 72.7 | 4/5 | 4/6 | 72.7 |
| Abdominal distension | 4/6 | 4/5 | 72.7 | 3/5 | 5/6 | 72.7 |
| Tremors | 4/6 | 4/5 | 72.7 | 2/5 | 5/6 | 63.6 |
| Bilateral exophthalmus | 2/6 | 5/5 | 63.6 | 1/5 | 6/6 | 63.6 |
| Hypotonic rear limbs | 2/6 | 5/5 | 63.6 | 1/5 | 5/6 | 54.5 |
| Spastic paralysis | 0/6 | 5/5 | 45.6 | 0/5 | 6/6 | 54.5 |
| Dehydration | 3/6 | 1/5 | 36.4 | 1/5 | 2/6 | 27.3 |
| Liquid defecation | 2/6 | 1/5 | 27.3 | 0/5 | 2/6 | 18.2 |
| Hypotonic forelimbs | 1/6 | 1/5 | 18.2 | 0/5 | 2/6 | 18.2 |
| Miction | 1/6 | 1/5 | 18.2 | 1/5 | 0/6 | 9.1 |
| Relaxation of sphincters | 1/6 | 1/5 | 18.2 | 1/5 | 0/6 | 9.1 |
Similarly, the male venom elicited occurrence of 24 signs of toxicity with frequencies equivalent to those developed by the female venom. Signs associated with high mortality were as follows (mice with the sign/total mice, dead and surviving mice respectively): bradypnea/apnea (6/6 and 2/5), exophthalmus (6/6 and 1/5), hypotonic rear limbs (5/6 and 1/5) and spastic paralysis (6/6 and 0/5).
Time course of toxicity signs
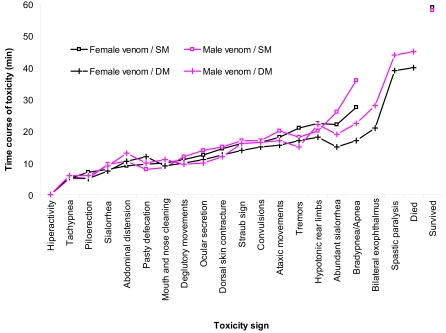
Figure 3 presents the chronology of signs of acute toxicity developed in mice after injection of female and male scorpion venoms during a 1hr period. Type of sign and occurrence time were similar for female and male venoms during the first 20min of observation. Signs of acute toxicity manifested within 10min of venom injection started with hyperactivity and then followed by tachypnea, piloerection, sialorrhea, abdominal distension, pasty defecation, mouth/nose cleaning, and deglutatory movements. Between 10 and 20min, ocular secretion, dorsal skin contracture, Straub sign, convulsions, and ataxic movements were observed. Beyond the 20min time point, which corresponds to the late phase of toxicity, differences were noted in the chronology of signs in mice injected with female and male scorpion venoms. Occurrence time for toxicity signs were as follows (in min, females/males): tremors and muscle fasciculations (21/18), hypotonic rear limbs (22.5/20), abundant sialorrhea (22/26), dyspnea with alternate episodes of bradypnea and apnea (27.5/36); animals presenting bilateral exophthalmus (21/28) and spastic paralysis (39/44) always died (40/45), whereas those not presenting these signs survived. Although there is tendency in female-envenomed mice to manifest earlier signs of toxicity compared to male-envenomed animals, no statistically significant difference was found between their times of occurrence. In general, sialorrhea and dyspnea (bradypnea/apnea) occurred significantly (Kruskal-Wallis = 6.22; p = 0.04) earlier in those mice which died after injection of either female or male venoms, compared with surviving mice.
Figure 3.
Time course for the occurrence of toxicity signs elicited after female and male scorpion venom injection in C57BL/6 mice. Black line corresponds to mice injected with female venom; red line corresponds to male venom-injected mice. Open squares indicate surviving mice (SM) whereas crosses correspond to dead mice (DM) as a result of venom toxicity.
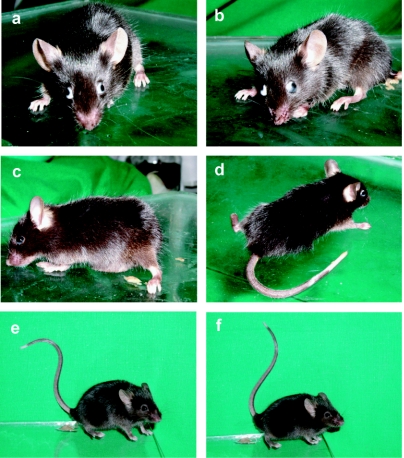
Figure 4 Presents photographs recording toxicity signs in envenomed mice such as toxic facies, sialorrhea, ocular secretion, pasty defecation, Straub sign, convulsions, and hypotonic rear limbs.
Figure 4.
Signs of autonomic toxicity in C57BL/6 mice injected with female T. nororientalis venom. (a and b) Bilateral exophthalmus with evident ocular secretion and including piloerection. (c) Hypotonic rear limbs, pasty defecation and abdominal distension. (d) Muscular weakness of rear limbs. (e and f) Straub sign.
Mass spectrometry and electrophoretic analyses of scorpion venoms
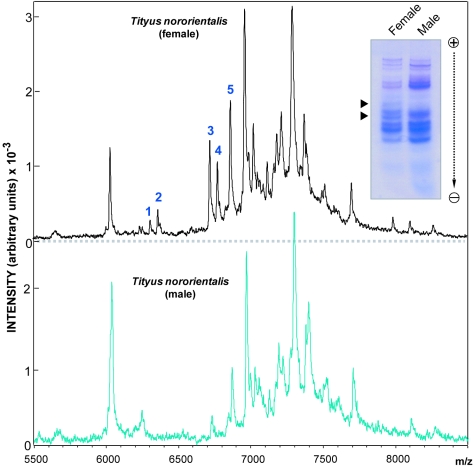
Figure 5 shows the results of compositional analysis of female and male T. nororientalis venoms using mass spectrometry via MALDI-TOF in the 5500-8000 Da range, which corresponds to the molecular mass range of long-chain neurotoxins responsible for the lethality to vertebrates and insects of buthid scorpion venoms (Rodríguez de la Vega and Possani, 2005). Overall, mass spectra are similar in females and males although differences are apparent in the lower mass range. Particularly, components (in Da) 1 (6100.00), 2 (6362.403), 3 (6502.00), and 4 (6710.00) are present almost exclusively in female venom. Component corresponding to peak 5 (6863.74) is present in both sexes but it is approximately 2-fold more abundant in the female venom. Inset of Figure 5 presents a representative polyacrylamide gel electrophoresis performed in the presence of acetic acid and urea (Wang et al, 1997) which has been shown to improve resolution of cationic components in scorpion venoms which bear similar masses (Borges et al, 2006a). Two components out of 14 bands stained with Coomassie Blue are shown to be female-specific components.
Figure 5.
Compositional analyses of female and male Tityus nororientalis venoms. Top pannel, Mass spectral (MALDI-TOF) analysis of female venom; numbers indicate female-specific venom components or components present at higher abundance in females. Y-axis corresponds to Arbitrary Intensity Units (x10-3); X axis corresponds to molecular mass (m/z, in Da). Bottom pannel, Mass spectral analysis of male venom. Inset, Urea-acetic acid gel electrophoresis (12.5%, w/v, gel) of 15 μg venom protein from female and male T. nororientalis specimens. Arrows indicate female-specific venom components.
Venom immunological reactivity
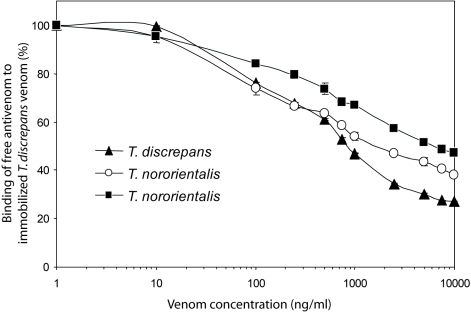
A comparison of the ability of female and male T. nororientalis venoms to inhibit binding of anti-T. discrepans antibodies to immobilized T. discrepans venom as a measure of their cross-reactivity is presented in Figure 6. Horse anti-T. discrepans antivenom is the only antiscorpion antivenom commercially available in Venezuela to treat accidents in the country (Borges et al, 2008). By increasing protein concentration from 1 to 10 000ng/ml both female and male venoms were able to reduce the amount of free antibodies available for binding to T. discrepans venom immobilized onto ELISA plates although they did not overlap the curve obtained with the control, T. discrepans venom. The venom doses (in ng/ml) producing 50% inhibition of antibody binding were as follows: 862.9 (T. discrepans), 1678.6 (male, T. nororientalis), and 6098.7 (female, T. nororientalis).
Figure 6.
Differences in immunological reactivity between Tityus nororientalis female and male venoms. Microplates were coated with T. discrepans venom (100ng/well) and binding inhibition of anti-Tityus discrepans antibodies was measured after incubation with T. nororientalis venoms as described in Materials and Methods.
DISCUSSION
The results obtained in this work demonstrate intersexual differences in composition, toxicity and antigenicity in the venom produced by T. nororientalis, a scorpion species endemic to the Venezuelan northeast and responsible annually for severe envenoming cases in the area (Borges and De Sousa, 2006; De Sousa and Borges, 2009; Borges et al, 2010b). This species exhibits a marked sexual dimorphism, particularly in the length and shape of metasomal segments I to V (González-Sponga, 1996). These morphological differences are not sufficient to explain the toxinological variations reported in this work, with females producing significantly less venom than males. Such variation in T. nororientalis is opposite to that found in the South American spider Phoneutria nigriventer where the greater venom production by females is attributed to their larger body size (Herzig et al, 2002). Venom toxicity is, however, significantly higher in T. nororientalis females compared to males, similarly to what has been observed in P. nigriventer by Herzig et al (2002).
Inspection of female and male venom composition using MALDI-TOF also indicated differences in abundance as well as the presence of female-specific venom components. Such differences may account, at least in part, for the higher toxicity of female T. nororientalis venom in C57BL/6 mice, although this requires further investigation. Similar results have been obtained with other arachnids, showing intersexual differences in the abundance and specificity of venom components (Binford, 2001; Herzig and Hodgson, 2009). In the case of scorpions, Abdel-Rahman et al (2009) have already shown that female specimens of the Egyptian Scorpio maurus palmatus, a species of poor medical importance, produce a significantly more complex venom than males based on electrophoretic (SDS-PAGE) analysis although this was restricted to differences in the abundance of high molecular mass (> 16 kDa) components. Our work concentrated on the most lethal, low molecular mass (6-8 kDa) components using for the first time mass spectral analyses to investigate venom intersexual differences in a species of the medically important genus Tityus. Such low molecular mass components within Tityus venoms are α- and β-toxins which affect the gating mechanism of vertebrate and invertebrate voltage-sensitive sodium channels (Borges et al, 2006b). Mass spectral analyses by MALDI-TOF confirmed that component 5 (6863.74 Da) is more abundant in female venom whereas components 1-4 (from 6100.00 to 6710.00 Da) appear to be female-specific components. Acetic acid-urea gel electrophoresis, used before to study toxin composition in Tityus venoms (Borges et al, 2006a), also demonstrated the presence of female-specific components. On-going chromatographic procedures in our laboratory are aimed at isolating and characterizing these components and elucidate their mode of action.
The main effect of these gating-modifying toxins is the exacerbated discharge of catecholamines and acetylcholine as a result of the sustained depolarization of pre- and postganglionic nerve terminals of the autonomic nervous system (Borges and De Sousa, 2006). The acute neurotoxicity signs elicited after injection of female and male T. nororientalis venom were very similar and attest to the presence of components with related activity in the venom of both sexes, resembling the toxic syndrome elicited by other Venezuelan Tityus species responsible of severe envenoming (Borges et al, 2004; Borges and De Sousa, 2006; De Sousa et al, 2009b). Notwithstanding such similarity, there is a tendency, in the case of the female venom, to elicit the earlier occurrence of severe signs such as sialorrhea, dyspnea (bradypnea/apnea) and exophthalmus particularly in the late toxicity phase, i.e., from 20 min of evolution onwards. In spite of the above tendency, there is no significant difference in the time of occurrence of these signs between female and male venoms indicating that the mouse model probably does not offer enough resolution to distinguish between the neurotoxic syndromes elicited by these venoms. As pointed out by Abdel-Rahman et al (2009), the sex with more effective venom differs across venomous animal species, and is a function of the target animal and/or the specific neurophysiological target. In this sense, the possibility exists that T. nororientalis sex-related toxic components might be targeting arthropod sodium channels, the usual prey of scorpions, instead of those of vertebrates, and that an arthropod lethality bioassay may be more useful to resolve sex-related differences in toxicity.
Other arachnids, such as Coremiocnemis tropix and Phoneutria nigriventer, demonstrate intersexual toxinological differences but the potency of their venoms is not only related to protein content but also to the species affected and the victim´s physiological condition. Herzig and Hodgson (2009) have shown that venom from C. tropix males contains components more potent on insects when compared with females. Vertebrate active toxins were, on the contrary, more common in C. tropix female venom. Similar results were obtained when testing venom from P. nigriventer females on insects (Herzig et al, 2002). An explanation for the presence of female-specific toxins in T. nororientalis could be related to the fact that females with young react aggressively to any disturbance (LDS, personal observation) and that maternal care could involve venom with higher toxicity to defend offspring and to provide nutrients during embryonic development.
Interestingly, female T. nororientalis venom was significantly less reactive towards anti-T. discrepans antibodies compared to male venom. In females, 7-fold more venom protein (compared to T. discrepans) was needed to produce 50% inhibition of antibody binding to immobilized T. discrepans compared to 2-fold in the case of males. This can be taken to indicate differences in the bioactive surface of female T. nororientalis toxins involved in antibody binding and/or target receptors. It would be of interest to determine whether these differences in toxicity and antigenicity can also be found in other Tityus venoms, particularly T. discrepans. Antivenom preparation could benefit from these findings as to compensate for such intersexual variations when milking scorpion venom for immunization.
CONCLUSIONS
Intersexual variations were found in venom production, toxicity and antigenicity in the Venezuelan scorpion species, T. norientalis, suggesting the existence of sex-related differences in venom composition and activity.
Acknowledgments
Financial support was from Proyecto Consejo de Investigación, Universidad de Oriente, CI-3-040602-1342/07 (to LDS), Fonacit S1-2001000674 (to AB) and from FONACIT, Red Nacional de Producción de Antivenenos (N° 2007000672): Subproject 1: “Caracterización epidemiológica de los envenenamientos ofídicos y escorpiónicos en Venezuela, con taxonomía de las especies asociadas y bancos de venenos” and Subproject 2: “Bioensayos para el estudio de la diversidad de toxinas de la fauna venenosa del país.”
LIST OF ABBREVIATIONS
- ♀
female
- ♂
male
- Da
Daltons
- ELISA
Enzyme-linked immunosorbent assay
- MALDI-TOF
Matrix-Assisted laser desorption time-of-flight mass spectrometry
- LD50
Medium lethal dose
STATEMENT OF COMPETING INTERESTS
None declared.
REFERENCES
- Abdel-Rahman MA, Omran MA, Abdel-Nabi IM, Ueda H, McVean A. Intraspecific variation in the Egyptian scorpion Scorpio maurus palmatus venom collected from different biotopes. Toxicon. 2009;53:349–359. doi: 10.1016/j.toxicon.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Binford GJ. An analysis of geographic and intersexual variation in venoms of the spider Tegenaria agrestis (Agelenidae) Toxicon. 2001;39:955–968. doi: 10.1016/s0041-0101(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Biondi-Queiroz I, García-Santana V, Rodrigues D. Estudo retrospectivo do escorpionismo na Região Metropolitana de Salvador (RSM)-Bahia, Brasil. Sitientibus. 1996;15:273–285. [Google Scholar]
- Borges A. Escorpionismo en Venezuela. Acta Biol Venez. 1996;16:65–75. [Google Scholar]
- Borges A, De Sousa L. Escorpionismo en Venezuela: una aproximación molecular, inmunológica y epidemiológica para su estudio. Rev Fac Farm (UCV) 2006;69:15–27. [Google Scholar]
- Borges A, De Sousa L. Una aproximación multidisciplinaria para el estudio del envenenamiento por arácnidos en Venezuela. In: Arrivillaga J, El Souki M, Herrera B, editors. Enfoques y Temáticas en Entomología. Ediciones Astrodata; Caracas: 2009. pp. 137–153. (eds) [Google Scholar]
- Borges A, Trejo E, Vargas A, CÉspedes G, Hernández A, Alfonzo M. Pancreatic toxicity in mice elicited by Tityus zulianus and Tityus discrepans scorpion venom. Inv Clín. 2004;45:269–276. [PubMed] [Google Scholar]
- Borges A, García C, Lugo E, Alfonzo M, Jowers M, Op den Camp H. Diversity of long-chain toxins in Tityus zulianus and Tityus discrepans venoms (Scorpiones: Buthidae): Molecular, immunological, and mass spectral analyses. Comp Biochem Physiol C Toxicol Pharmacol. 2006a;142:240–252. doi: 10.1016/j.cbpc.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Borges A, De Sousa L, Manzanilla J. Description of a New Tityus species (Scorpiones: Buthidae) from Sierra de Portuguesa, western Venezuela, based on morphological and mitochondrial DNA evidence. Zootaxa. 2006b;1107:49–68. [Google Scholar]
- Borges A, De Sousa L, Espinoza J, et al. Characterization of Tityus scorpion venoms using synaptosome binding assays and reactivity towards Venezuelan and Brazilian antivenoms. Toxicon. 2008;51:66–79. doi: 10.1016/j.toxicon.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Borges A, Bermingham E, Herrera N, Alfonzo M, Sanjur O. Molecular systematics of the neotropical scorpion genus Tityus (Buthidae): The historical biogeography and venom antigenis diversity of toxic Venezuela species. Toxicon. 2010a;55:436–454. doi: 10.1016/j.toxicon.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Borges A, Rojas-Runjaic FJM, Diez N, Faks J, De Sousa L. Envenoming by the scorpion Tityus breweri González-Sponga in the Guayana Shield, Southeast Venezuela: Report of a case, reactivity towards antivenom and proposal for a toxinological partitioning of the Venezuelan scorpion fauna. Wilderness Environ Med. 2010b. in press. [DOI] [PubMed]
- Celerier ML, Paris C, Lange C. Venom of an aggressive African Theraphosidae (Scodra griseipes): milking the venom, a study of its toxicity and its characterization. Toxicon. 1993;31:577–590. doi: 10.1016/0041-0101(93)90113-w. [DOI] [PubMed] [Google Scholar]
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008;107:71–79. doi: 10.1016/j.actatropica.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Coronado L, Alvarado M, Dunari JE. Características clínicas y epidemiológicas del alacranismo. Período 2002-2007. Hospital del Niño. Panamá. Pediátr Panamá. 2008;37:36–46. [Google Scholar]
- Daisley H, Alexander D, Pitt-Miller P. Acute myocarditis following Tityus trinitatis envenoming: morphological and pathophysiological characteristcs. Toxicon. 1999;37:159–165. doi: 10.1016/s0041-0101(98)00174-3. [DOI] [PubMed] [Google Scholar]
- De Sousa L, Borges A. Escorpiones y escorpionismo en Venezuela. In: Arrivillaga J, El Souki M, Herrera B, editors. Enfoques y Temáticas en Entomología. Ediciones Astrodata; Caracas: 2009. pp. 154–165. (eds) [Google Scholar]
- De Sousa L, Parrilla-Álvarez P, Quiroga M. An epidemiological review of scorpion sting in Venezuela. The northeastern region. J Venom Anim Toxins. 2000;6:127–165. [Google Scholar]
- De Sousa L, Manzanilla J, Parrilla-Álvarez P. Nueva especie de Tityus (Scorpiones: Buthidae) del Turimiquire, Venezuela. Rev Biol Trop. 2006;54:489–504. [PubMed] [Google Scholar]
- De Sousa L, Boadas J, Kiriakos D, et al. Scorpionism due to Tityus neoespartanus (Scorpiones, Buthidae) in Margarita Island, northeastern Venezuela. Rev Soc Bras Med Trop. 2007;40:681–685. doi: 10.1590/s0037-86822007000600017. [DOI] [PubMed] [Google Scholar]
- De Sousa L, Manzanilla J, Borges A, Cornejo-Escobar P, Gregoriani T. Discovery and description of the male of Tityus uquirensis (Scorpiones: Buthidae) from Paria Peninsula, northeastern Venezuela. Zootaxa. 2008;1828:57–68. [Google Scholar]
- De Sousa L, Rengifo C, Manzanilla J, et al. First technical report, subproject 1: “Epidemiological characterization of ophidian and scorpion envenomation in Venezuela, including taxonomy of associated species and venom production.” National Project on Antivenom Production, National Fund for Science, Technology and Innovation (Fonacit) Venezuela. 2009a;143 [Google Scholar]
- De Sousa L, Parrilla-Álvarez P, PÉrez Di Gaeta P, Romero L, Quiroga M. Evaluación de la actividad biológica del veneno de Tityus gonzalespongai (Scorpiones, Buthidae) en el modelo C57BL/6. Saber. 2009b;21:47–59. [Google Scholar]
- Dixon W, Mood A. A method for obtaining and analyzing sensitivity data. J Am Stat Assoc. 1948;43:109–126. [Google Scholar]
- Gómez JP, Quintana JC, Arbeláez P, et al. Picaduras por escorpión Tityus asthenes en Mutatá, Colombia: aspectos epidemiológicos, clínicos y toxinológicos. BiomÉdica. 2010;30:126–139. [PubMed] [Google Scholar]
- González-Sponga MA. Arácnidos de Venezuela: seis nuevas especies del gÉnero Tityus y redescripción de Tityus pococki Hirst, 1907, Tityus rugosus (Schenkel, 1932) n. comb. y Tityus nematochirus Mello-Leitão, 1940 (Scorpionida: Buthidae) Acta Biol Venez. 1996;16:1–38. [Google Scholar]
- González-Sponga MA. Arácnidos de Venezuela: cuatro especies nuevas del gÉnero Tityus (Scorpionida: Buthidae) Acta Biol Venez. 2001;21:69–83. [Google Scholar]
- Herzig V, Hodgson W. Intersexual variations in the pharmacological properties of Coremiocnemis tropix (Araneae, Theraphosidae) spider venom. Toxicon. 2009;53:196–205. doi: 10.1016/j.toxicon.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Herzig V, Ward RJ, Ferreira dos Santos W. Intersexual variations in the venom of the Brazilian “armed” pider Phoneutria nigriventer (Keyserling, 1891) Toxicon. 2002;40:1399–1406. doi: 10.1016/s0041-0101(02)00136-8. [DOI] [PubMed] [Google Scholar]
- Hodges JL, Lehmann EL. Estimation of location based on ranks. Ann Math Statist. 1963;34:598–611. [Google Scholar]
- King TP, Joslyn A, Kochoumian MS. Antigenic cross-reactivity of venom proteins from hornets, wasps, and yellow jackets. J Allergy Clin Immunol. 1985;75:621–628. doi: 10.1016/0091-6749(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- Lira-da-Silva RM, de Amorim AM, Carvalho FM, Brazil TK. Acidentes por escorpião na cidade de Salvador, Bahia, Brasil (1982-2000) Gaz MÉd Bahia. 2009;79:43–49. [Google Scholar]
- Otero R, Navio E, CÉspedes F, et al. Scorpion envenoming in two regions of Colombia: clinical, epidemiological and therapeutic aspects. Trans R Soc Trop Med Hyg. 2004;98:742–750. doi: 10.1016/j.trstmh.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Possani LD, Alagón A, Fletcher P, Jr, Erikson B. Purification and properties of mammalian toxins from venom of the Brazilian scorpion Tityus serrulatus Lutz and Mello. Arch Biochem Biophys. 1977;180:394–403. doi: 10.1016/0003-9861(77)90053-4. [DOI] [PubMed] [Google Scholar]
- Quiroga M, Illanes A, González A, Jaramillo E. Mantención de escorpiones en el laboratorio, Orden Scorpionida, Buthidae, Rhopalurus laticauda (Thorell, 1876). Influencia de la alimentación. Acta Cient Venez. 1982;33:502–508. [PubMed] [Google Scholar]
- Quiroga M, De Sousa L, Parrilla-Álvarez P. The description of Tityus caripitensis. A new Venezuelan scorpion (Scorpionida, Buthidae) J Venom Anim Toxins. 2000;6:99–117. [Google Scholar]
- Quiroga M, De Sousa L, Parrilla-Álvarez P, Manzanilla J. The first report of Tityus (Scorpiones: Buthidae) in Anzoátegui State, Venezuela. A new species. J Venom Anim Toxins incl Trop Dis. 2004;10:10–33. [Google Scholar]
- Rodríguez de la Vega RC, Possani LD. Overview of scorpion toxins specific for Na+ channels and related peptides: biodiversity, structure-function relationships and evolution. Toxicon. 2005;46:831–844. doi: 10.1016/j.toxicon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Rojas-Runjaic FJM, De Sousa L. Catálogo de los escorpiones de Venezuela (Arachnida: Scorpiones) Bol SEA. 2007;40:281–307. [Google Scholar]
- Sevcik C. DL50 determinations: Objections to the method of Beccari as modified by Molinengo. Toxicon. 1987;25:779–783. doi: 10.1016/0041-0101(87)90128-0. [DOI] [PubMed] [Google Scholar]
- Stachel SJ, Stockwell SA, Van Vranken DL. The fluorescence of scorpions and cataractogenesis. Chem Biol. 1999;6:531–539. doi: 10.1016/S1074-5521(99)80085-4. [DOI] [PubMed] [Google Scholar]
- Wang MC, Pang JS, Selsted ME. Semidry electroblotting of peptides and proteins from acid–urea polyacrylamide gels. Anal Biochem. 1997;253:225–230. doi: 10.1006/abio.1997.2347. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Dai L, Sugase K, Andriantsiferana M, Nakajima T, Iwashita T. Solution structure of IsTX, a male scorpion toxin from Opisthacanthus madagascariensis (Ischnuridae) Eur J Biochem. 2004;271:3855–3864. doi: 10.1111/j.1432-1033.2004.04322.x. [DOI] [PubMed] [Google Scholar]