Abstract
Wasp venoms contain a number of pharmacologically active biomolecules, undertaking a wide range of functions necessary for the wasp's survival. We purified and characterized a novel bioactive peptide (vespin) from the venoms of Vespa magnifica (Smith) wasps with unique primary structure. Its amino acid sequence was determined to be CYQRRVAITAGGLKHRLMSSLIIIIIIRINYLRDNSVIILESSY. It has 44 residues including 15 leucines or isoleucines (32%) in the sequence. Vespin showed contractile activity on isolated ileum smooth muscle. The cDNA encoding vespin precursor was cloned from the cDNA library of the venomous glands. The precursor consists of 67 amino acid residues including the predicted signal peptide and mature vespin. A di-basic enzymatic processing site (-KR-) is located between the signal peptide and the mature peptide. Vespin did not show similarity with any known proteins or peptides by BLAST search, suggesting it is a novel bioactive peptide from wasp venoms.
Keywords: Wasp venom, Vespa magnifica, smooth muscle, contraction, novel peptide
INTRODUCTION
Over the past few decades the venoms of arthropods, especially the wasp venom, have attracted considerable interest as a potential source of bioactive substances. The members of the Vespidae family include hornets (genera Vespa and Dolichovespula), yellow jackets (genus Vespula) and paper wasps (genus Polistes). They all possess highly toxic venoms, which are a complex mixture of amines, small peptides and high molecular weight proteins, such as enzymes, allergens and toxins (Habermann, 1972; Nakajima, 1984; Yang et al, 2008; de Graaf et al, 2009). As a predator, the venoms from these stinging wasps are important weapons both in the defense of the colony and the capture of the prey. The wasp inserts and withdraws its sting much easier than that of a bee because of its smooth outer lining, while a bee's sting has a barbed outer sheath appearing like a fish hook, which is easy to insert but difficult to extract. Thus, the multi-sting capacity of the wasp bestows it a more aggressive nature.
A single wasp sting could kill or paralyze their pray insects instantly. The clinical symptoms induced in humans include local reactions (such as pain, wheal, edema and swelling), immunological reactions usually leading to anaphylaxis with subsequent anaphylactic shock, and systemic toxic reactions caused by large doses of venom, resulting in hemolysis, coagulopathy, rhabdomyolysis, acute renal failure, hepatotoxicity, aortic thrombosis and cerebral infarction (Evans and Summer, 1986; Sakhuja et al, 1988; Korman et al, 1990; Watemberg et al, 1995; Chao and Lee, 1999; Chen et al, 2004). Several families of peptides or proteins from wasp venoms have been proven to contribute to some of these clinical symptoms, such as phospholipase A1 platelet activator (Yang et al, 2008), serine proteinases, antigen 5-related (Ag 5) proteins and hyaluronidases (Nakajima, 1984). Most of the molecular mechanisms of clinical symptoms induced by wasp venoms are unknown. Thus it is critical to identify more bioactive components from wasp venoms and to understand their mechanisms of action. In this study, we purified and characterized a novel peptide from the wasp venom, which is able to induce contraction of smooth muscle.
MATERIALS AND METHODS
Wasp venom
Wasps (V. magnifica) were collected in Yunnan Province, China, and the venom was extracted according to our previously reported methods (Xu et al, 2006a; Yu et al, 2007). Briefly, wasps were stimulated by alternative current (6V) for 6-10sec. This led to the secretion of venom, which was collected onto a clean glass plate (50x50cm). The venom was immediately collected and stored at -20oC until further use.
Peptide purification
The wasp venom was dissolved in 0.1M phosphate buffered saline, pH 6.0 (PBS). The sample was applied to a Sephadex G-50 (Superfine, Amersham Biosciences, 2.6x100cm) gel filtration column equilibrated with 0.1M PBS as previously reported (Xu et al, 2006a; Yu et al, 2007). Elution was performed with the same buffer; 3.0ml fractions were collected and the elutent was monitored at 280nm. The peaks that demonstrated activity on our smooth muscle assay were further subjected to a CM-Sephadex C-25 ion exchange column (Amersham Biosciences, 2.6x50cm), equilibrated with 0.1M PBS. Elution was achieved with a linear NaCl gradient in 0.1M PBS, and the elutent was monitored at 215nm. The final purification of peptide was performed using C18 reverse phase high-performance liquid chromatography (RP-HPLC, Hypersil BDS C18, 30x0.46cm), and the elutent was monitored at 215nm.
Structural analysis
The eluted fraction containing smooth muscle contractile activity from RP-HPLC was subjected to mass analysis by a matrix-assisted laser desorption ionization time-of-flight mass spectrometer (MALDI-TOF-MS) AXIMA CFR (Kratos Analytical) in positive ion and linear mode. The operation parameters were as follows: The ion acceleration voltage was 20kV, the accumulating time of a single scanning was 50sec, and the polypeptide mass standard (Kratos Analytical) served as external controls. The accuracy of mass determinations was within 0.1%. The complete amino acid sequences of antimicrobial peptides were determined by Edman degradation in an Applied Biosystems pulsed liquid-phase sequencer (Model 491).
Smooth muscle contraction assay
As previously described (Liang et al, 2006), the contractile activity of isolated guinea pig ileum was tested. About 10cm of the distal ileum of male or female guinea pigs (150-250gm body weight) was isolated immediately after death and kept in Tyrode solution (137mM NaCl, 2.7mM KCl, 1.36mM CaCl2, 0.49mM MgCl2, 0.36mM NaH2PO4, 11.9mM NaHCO3, 5.04mM D-glucose). Cut segments with 2 cm length of the isolated ileum were mounted isotonically, under 1-g load, in a 5ml muscle bath containing Tyrode solution maintained at 37oC and bubbled with air. Different concentrations of test sample were added into the organ bath. Tension was recorded and analyzed by PcLab software package (Beijing Microsignalstar Technology Development Co Ltd, Beijing, China). Each dosage of the tested sample was repeated five times. All animal experiments were conducted in line with the local ethical guidelines of working with animals.
SMART cDNA synthesis
This was done as previously described, using a SMARTTM PCR cDNA synthesis kit (Clontech, Palo Alto, CA) (Xu et al, 2006a; Yu et al, 2007). Venomous glands (n=30) were dissected from V. magnifica. Total RNA was extracted using TRIzol (Life Technologies, Ltd). The first strand was synthesized with the cDNA 3’ SMART CDS Primer II A, 5’AAGCAGTGGTATCAACGCAGAGTACT (30) YZ3’ (Y=A, G or C; Z=A, C, G or T), and SMART II A oligonucleotide, 5’AAGCAGTGGTATCAACGCAGAGTACGCGGG3’. The second strand was amplified by using Advantage polymerase and 5’ PCR primer II A, 5’AAGCAGTGGTATCAACGCAGAGT3’. Finally, the PCR products were cloned into pGEM®-T Easy vector (Promega, Madison, WI).
cDNA cloning
The cDNA synthesized by SMARTTM techniques was used as template library to screen cDNA clones. Two oligonucleotide primers, S1 (5’TG(T/C)TA(T/C)GA(A/G)AG(A/G)AG(A/G)GT(A/T/C/G)GC(A/T/C/G)AT3’) (in the sense direction), a degenerate primer designed according to the sequence determined by Edman degradation, and primer II A (in the anti-sense direction) used in the previous step were used in PCR, which was conducted using the Advantage polymerase from Clontech (Palo Alto, CA) as follows: 2min at 94oC, then 30 cycles of 10sec at 92oC, 30sec at 50oC and 40sec at 72oC. DNA sequencing was performed in an Applied Biosystems DNA sequencer (Model ABI PRISM 377).
Synthetic peptide
The peptide used for bioassays was synthesized by GL Biochem (Shanghai) Ltd (Shanghai, China) and analyzed by HPLC and mass spectrometry to confirm purity which was greater than 98%.
RESULTS
Peptide Purification
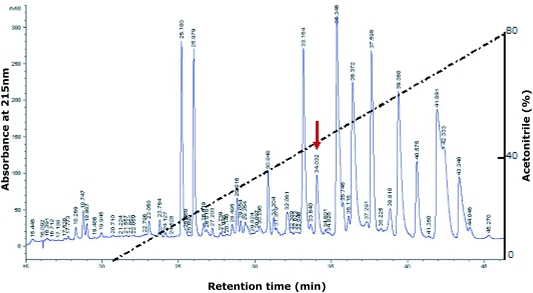
As reported in our previous work, the wasp venom was fractionated into five peaks by Sephadex G-50 gel filtration. Fraction III contains activity to contract isolated smooth muscle. Fraction III was applied to a CM-Sephadex C-25 ion- exchange column and 12 further fractions were eluted (Xu et al, 2006a; Yu et al, 2007). The smooth muscle contractile activity was found in fraction XII. After final purification by C18 RP-HPLC, the fraction XII was subdivided into more than 20 fractions. The eluted peak at 34min with an acetonitrile concentration of 45% (labeled with an arrow in Figure 1) contained the activity producing contraction of the isolated smooth muscles.
Figure 1.
Purification of vespin from V. magnifica venoms. After Sephadex G-50 gel filtration and CM-Sephadex C-25 ion-exchange column (Xu et al, 2006a; Yu et al, 2007), fraction XII from CM-Sephadex C-25 column was found to exert contractile activity on smooth muscle. Fraction XII was applied to a Hypersil BDS C18 RP-HPLC column (30x0.46cm) equilibrated with 0.1% (v/v) trifluoroacetic acid/water. The elution was performed with the indicated gradient of acetonitrile at a flow rate of 0.7ml/min, and fractions were tested for smooth muscle contractile activity. The purified vespin is labeled with an arrow.
Structural Characterization
Purified peptide, named vespin, was subjected to amino acid sequence analysis by automated Edman degradation, which produced the following amino acid sequence: CYQRRVAITAGGLKHRLMSSLIIIIIIRINYLRDNSVIILESSY. The mass spectrometry gave an observed mass of 5077.3 that matches well with the predicted mass of 5077.1. Vespin is composed of 44 amino acid residues including 6 basic (K or R) and 2 acidic (D or E) amino acid residues. This peptide is characterized by a single half-cysteine positioned at the N-terminus and 15 leucines or isoleucines.
cDNA cloning
Upon screening of a venomous gland cDNA library, several clones containing inserts of around 520 base pairs were identified and isolated. Both strands of these clones were sequenced. The complete nucleotide sequence and deduced amino acid sequence of the representative clone is presented in Figure 2. It was found to contain a coding region of 201 nucleotides, which encodes the precursor of vespin. The encoded amino acid sequence corresponds to a polypeptide precursor of 57 amino acid residues, which included the predicted signal peptide and a mature vespin. The deduced amino acid sequence was identical to the amino acid sequence determined by Edman degradation sequencing. BLAST searches did not return any peptides or proteins similar to vespin.
Figure 2.
The nucleotide sequences encoding vespin from V. magnifica and the deduced amino acid sequence of the precursor polypeptide. The sequence of mature vespin peptide is underlined. The bar (-) indicates the stop condon.
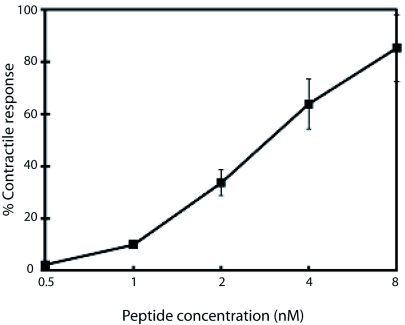
Myotropic effects on isolated guinea pig ileum
The contractile activity of the vespin was evaluated in isolated guinea pig ileum in aerated Tyrode's solution at 37oC. 15nM vespin was found to induce maximal ileum contraction (Data not shown). 0.5, 1.0, 2.0, 4.0, and 8.0nM vespin was added to a 5ml organ bath at 10min intervals serially. As shown in Figure 3, vespin was found to elicit concentration-dependent contractile effects on isolated guinea pig ileum.
Figure 3.
Concentration-response curves of vespin on isolated guinea pig ileum. The ileum was stimulated with increasing concentrations of vespin, previously incubated for 10min, at 30oC, with 1x Tyrode solution. Each point represents the mean ± S.E.M. of five different experiments. The 100% contractile activity was the contractile ability of the ileum induced by 15nM vespin.
DISCUSSION
Several families of bioactive peptides have been identified in wasp venoms. These peptides are bradykinin-like peptides, chemotactic peptides and mastoparans (Higashijima et al, 1979; Piek, 1984). Local or systemic reactions may come from these biologically active peptides. These major actions include, limited in vitro antimicrobial effects (Xu et al, 2006b) and inflammation induction by lysing cell membrane or stimulating mast cell degranulation, histamine release and consequent vasodilatation, and increasing neutrophils and T helper cells chemotaxis (Argiolas and Pisano, 1985; Nakajima et al, 1986; Hancock et al, 1995; Wu and Hancock, 1999). All of these effects are related with cardiovascular system, nervous system and immunological system of mammals, including humans.
In the current study we purified and characterized a novel bioactive peptide (vespin) containing 44 amino acid residues. It exerts contractile function on isolated ileum smooth muscle. Bradykinin-like peptides in wasp venoms have also function to induce contraction of ileum smooth muscle. They exert their bioactivity by interacting with bradykinin receptors. All members of bradykinin-like peptides share conserved –PPGF(T/S)P(F/L)- structure at their C-terminus (Liu et al, 2008). No such structure was found in the sequence of vespin, suggesting that vespin does not belong to the bradykinin-like peptide family.
CONCLUSIONS
The primary focus of the current work was the purification and characterization of this peptide with unique primary structure from the wasp venoms, which was able to induce contraction of isolated ileum smooth muscles. Further work is ongoing in our laboratory to identify the possible bioactivities of vespin and the corresponding molecular mechanisms.
Acknowledgments
This work was supported by the Chinese Academy of Sciences (KSCX2-YW-G-024), the Ministry of Science and Technology (2010CB529800) and the Ministry of Agriculture (2009ZX08009-159B).
REFERENCES
- Argiolas A, Pisano JJ. Bombolitins, a new class of mast cell degranulating peptides from the venom of the bumblebee Megabombus pennsylvanicus. J Biol Chem. 1985;260:1437–1444. [PubMed] [Google Scholar]
- Chao SC, Lee YY. Acute rhabdomyolysis and intravascular hymolysis following extensive wasp stings. Int J Dermatol. 1999;38:135–137. doi: 10.1046/j.1365-4362.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- Chen DM, Lee PT, Chou KJ et al. Descending aortic thrombosis and cerebral infarction after massive wasp stings. Am J Med. 2004;116:567–569. doi: 10.1016/j.amjmed.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Evans R, Summers S. Clinical aspects of Hymenoptera sensitivity. In: Levine MI, Lockey RF, editors. American Academy of Allergy and Immunology, Monography on insect allergy. Lambert Associates; Pittsburgh, USA: 1986. pp. 23–28. (Eds) [Google Scholar]
- De Graaf DC, Alerts M, Danneels E, Devreese B. Bee, wasp and ant venomics pave the way for a component-resolved diagnosis of sting allergy. J Proteomics. 2009;72:145–154. doi: 10.1016/j.jprot.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Falla T, Brown M. Cationic bactericidal peptides. Adv Microb Physiol. 1995;37:135–175. doi: 10.1016/s0065-2911(08)60145-9. [DOI] [PubMed] [Google Scholar]
- Higashijima T, Inubushi T, Ueno T, Miyazawa T. NMR saturation transfer and line shape analyses of cyclic tetradepsipeptide AM toxin II: conformational equilibrium with very unequal populations. FEBS Lett. 1979;105:337–340. doi: 10.1016/0014-5793(79)80643-2. [DOI] [PubMed] [Google Scholar]
- Korman SH, Jabbour S, Harari MD. Mutiple hornet (Vespa orientalis) stings with fatal outcome in a child. J Paediatr Child Health. 1990;26:283–285. doi: 10.1111/j.1440-1754.1990.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Han Y, Li J, Xu X, Rees HH, Lai R. A novel bradykinin-like peptide from skin secretions of rufous-spotted torrent frog, Amolops loloensis. Peptides. 2006;27:2683–2687. doi: 10.1016/j.peptides.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Liu X, You D, Chen L, Wang X, Zhang K, Lai R. A novel bradykinin-like peptide from skin secretions of the frog, Rana nigrovittata. J Pept Sci. 2008;14:626–630. doi: 10.1002/psc.958. [DOI] [PubMed] [Google Scholar]
- Nakajima T. Biochemistry of vespid venoms. In: Tu AT, editor. Handbook of Natural Toxins. Marcel Dekker; New York, USA: 1984. pp. 109–133. (Ed) [Google Scholar]
- Nakajima T, Uzu S, Wakamatsu K et al. Amphiphilic peptides in wasp venom. Biopolymers. 1986;25((Suppl)):S115–121. [PubMed] [Google Scholar]
- Piek T. Pharmacology of hymenoptera venom. In: Tu AD, editor. Handbook of Natural Toxins. Vol 2. Marcel Dekker; New York, USA: 1984. pp. 135–185. (Ed) [Google Scholar]
- Sakhuja V, Bhalla A, Pereira BJ, Kapoor MM, Bhusnurmath SR, Chugh KS. Acute renal failure following multiple hornet stings. Nephron. 1988;49:319–321. doi: 10.1159/000185083. [DOI] [PubMed] [Google Scholar]
- Watemberg N, Weizman Z, Shahak E, Aviram M, Maor E. Fatal multiple organ failure following massive hornet stings. J Toxicol Clin Toxicol. 1995;33:471–474. doi: 10.3109/15563659509013757. [DOI] [PubMed] [Google Scholar]
- Wu M, Hancock RE. Interaction of the cyclic antimicrobial cationic peptide bectenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- Xu X, Li J, Lu Q, Yang H, Zhang Y, Lai R. Two families of antimicrobial peptides from wasp (Vespa magnifica) venom. Toxicon. 2006a;47:249–253. doi: 10.1016/j.toxicon.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Xu X, Yang H, Yu H, Li J, Lai R. The mastoparanogen from wasp. Peptides. 2006b;27:3053–3057. doi: 10.1016/j.peptides.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Yang H, Xu X, Ma D, Zhang K, Lai R. A phospholipase A1 platelet activator from the wasp venom of Vespa magnifica (Smith) Toxicon. 2008;51:289–296. doi: 10.1016/j.toxicon.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Yu H, Yang H, Ma D et al. Vespid chemotactic peptide precursor from the wasp, Vespa magnifica (Smith) Toxicon. 2007;50:377–382. doi: 10.1016/j.toxicon.2007.04.023. [DOI] [PubMed] [Google Scholar]