Abstract
Present study shows that non-covalent interaction of kaouthiotoxin (KTX) with their respective pohospholipase A2 (PLA2) from the venom of N. kaouthia displayed marked synergism to exert cytotoxicity without altering the biochemical properties of PLA2. For example, although NK-PLA2 or KTX alone did not induce appreciable hemolysis of washed human erythrocytes; however, the hemolytic potency of NK-PLA2: KTX complex was significantly higher. Identically, selective lysis of virus infected Sf9 and normal Tn insect cells was further enhanced by the cognate NK-PLA2: KTX complex as compared to individual components of the complex. Gas-chromatographic analysis of fatty acids released from intact erythrocytes by cytotoxic action of individual NK-PLA2 and NK-PLA2: KTX complex demonstrated that ratio between saturated fatty acids (SFA) and unsaturated FA (UFA) was increasing with time of hydrolysis of RBC either in the case of NK-PLA2 or NK-PLA2-KTX complex suggesting NK-PLA2-KTX complex apparently displayed the more preference for glycerophospholipids with SFAs on the sn-2 position. Therefore, it may be suggested that KTX first destabilize the target cell membrane followed by higher enzymatic activity of PLA2 on dislocated and disorganized phospholipid bilayers resulting in a significantly higher (p < 0.05) membrane damage by NK-PLA2-KTX complex compared to individual components of the complex.
Keywords: Cytotoxicity, cobra venom, kaouthiotoxins, Naja kaouthia, phospholipase A2, protein-protein interaction
INTRODUCTION
Cobras are responsible for a large number of snakebite mortalities and morbidities particularly in Asian countries. The venom of this group of snakes contain many enzymes and non-enzyme proteins (toxins) that act individually or often with coordinated synergism to induce various toxic effects in experimental animals and victims (Harvey et al, 1983; Stocker, 1990; Mukherjee and Maity, 1998; Doley and Mukherjee, 2003; Mukherjee, 2007). Among these toxins, phospholipase A2 (PLA2), neurotoxins (NTXs) and cardiotoxins (CTXs) are the major classes of cobra-venom polypeptides involved in the toxicity and pharmacology of bite by these snakes (Harvey et al, 1983; Stocker, 1990; Mukherjee and Maity, 1998; Mukherjee and Maity, 2002; Doley and Mukherjee, 2003; Doley et al, 2004).
In many instances, venom protein complexes furnish stringent examples of protein complementation, which has been defined as the restoration of a biological activity by non-covalent interaction of different proteins/polypeptides of venom (Wang et al, 1999). For example, the presence of 3-5% (w/w) phospholipase from Naja naja siamensis caused a 20 to 30-fold increase in the hemolytic activity of the two cardiotoxins from the same venom without affecting the ability of these cardiotoxins to cause contracture of chick biventer cervicis (Harvey et al, 1983). Venom PLA2 enzymes contribute towards procurement of foods by paralyzing the prey through their inherent ability to induce various pharmacological effects in the victim (Stocker, 1990). There are probably many ways in which PLA2 enzymes induce these pharmacological effects.
Our previous study demonstrated that phospholipase A2 and low molecular weight (7–8 kDa) weak neurotoxins like molecules exhibiting cytotoxicity (kaouthiotoxins, KTXs) from Naja kaouthia venom form a complex by noncovalent interactions, although the exact pharmacological effect of this interaction on the pathophysiology of cobra bite remains unknown (Mukherjee, 2007; Mukherjee, 2008).The present study shows that NK-PLA2: KTX complex serves as an example of heteromeric complex and two components of the complex act synergistically to exhibit potent biological activity that may play a crucial role in the pathophysiology of cobra bite.
MATERIALS AND METHODS
Enzymatic assays
Procedures for the isolation and purification of PLA2 (NK-PLA2-A and NK-PLA2-B), and kaouthiotoxins (KTX-A and KTX-B) from N. kaouthia venom were described elsewhere (Mukherjee, 2007; Mukherjee, 2008). PLA2 activity was determined as described (Doley and Mukherjee, 2003).
Gel filteration
In in vitro condition, interaction of kaouthiotoxins with NK-PLA2s as well as the stability of complex was checked by gel-filtration of individual PLA2, KTX and reconstituted complex on a Sephadex G-50 (1x64cm2) gel filtration column. The reconstituted NK-PLA2: KTX complex was loaded on the gel-filtration column equilibrated with 20mM K-phosphate buffer, pH 7.2. Elution was carried out with the same buffer at room temperature (∼23oC) at a flow rate of 24ml/hr and 1.0ml fraction was collected in each tube. Protein content of individual tube was determined spectrophotometrically at 660nm (Lowry et al, 1951). The gel-filtration column was calibrated with the following molecular weight marker proteins-aprotinin (6,500), cytochrome C (12,400), carbonic anhydrase (29,000), bovine serum albumin (66,000) and blue dextran (200000).
SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
The reconstituted NK-PLA2: KTX complex (gel-filtration fraction) was also analyzed by SDS-PAGE under both reducing and non-reducing conditions (Laemmli, 1970). For determining the stoichiometry of the binding between NK-PLA2 and KTX, the relative intensities of protein bands corresponding to NK-PLA2: KTX complexes, PLA2s and KTXs were quantified by densitometry scanning of the Commassie blue stained gel.
Fluorescence spectrometery
Measurement of interaction of PLA2 with respective kaouthiotoxin was also performed by using a Fluorescence spectrometer (LS55, Perkin Elmer). Briefly, a known amount of PLA2 (dissolved in 20 mM Tris-HCl, pH 8.0) was mixed with a fixed concentration of KTX in a fluorescence cuvette and fluorescence spectra were obtained at an excitation wavelength 270 nm, excitation and emission slits 5 nm, temperature 30oC. Wavelength shifts were measured by taking the midpoint at two third height of the spectrum. The maximum fluorescence of free protein (I0) was also measured.
Cytotoxicity assays and antibacterial activity
Cytotoxicity was assessed on four cell lines: Sf9 insect ovarian cells, Sf9 cells infected with wild-type (WT) baculovirus [sf9(i)], Tn cells and VERO cells, as described previously (Mukherjee, 2007). It is to be noted that phospatidylcholine (PC) content of the membrane of these cells decreases in the following order Sf9 (i) ≈ Tn(n) > Sf9 (n) > VERO ≈ human RBC (see Mukherjee, 2007). The Escherichia coli cell lacks PC in its membrane. Controls were also run in parallel where the cell cultures were treated with toxin-free growth medium (negative control) and medium without cells (blank). The anticoagulant activity of purified individual proteins or NK-PLA2: KTX complex on platelet-poor plasma (PPP) was assayed as described earlier (Doley and Mukherjee, 2003). Antibacterial activity on Escherichia coli cells was determined by our previously elucidated procedure (Mukherjee, 2007).
Statistical analysis
The procedures for isolating erythrocytes from volunteers, the assay of hemolysis and erythrocyte phospholipids hydrolysis have been described elsewhere (Doley et al, 2004). Total lipid released from the erythrocytes supernatant post treatment with 250nM purified NK-PLA2 / KTX / PLA2-KTX complex was extracted by the method of Folch et al (1957) and quantitated by evaporating a measured amount of extract. Fatty acid methyl esters were analyzed on a Varian GC-MS 3800, Saturn 2000 system (Doley et al, 2004). Results are represented as mean ±SD. Statistical analysis was done by Student's “t” test.
RESULTS AND DISCUSSION
Our previous study has shown that lytic activity of KTXs might be associated with their specific binding to the target site(s) on the membrane. To unravel the effect of non-covalent interaction of KTXs with PLA2s from the same venom, we investigated the biochemical properties and cytotoxicity of PLA2: KTX complex. In the present study, neither of the KTXs was found to influence the biochemical properties (enzyme activity, substrate specificity, thermostability, optimum pH) of the PLA2 enzymes in NK-PLA2: KTX complexes, as compared to these properties exhibited by native PLA2 enzymes (Mukherjee, 2007); therefore, the PLA2 activity shown by NK-PLA2: KTX complex was solely due to PLA2 component of the complex. Conformational analysis using CD spectroscopy indicated that the overall conformation of the reconstituted complex of NK-PLA2: KTX was similar to that of native PLA2: KTX complex (data not shown). The interaction of KTX with NK-PLA2 did not appear to influence the predominantly α-helical secondary structure of the PLA2 enzyme.
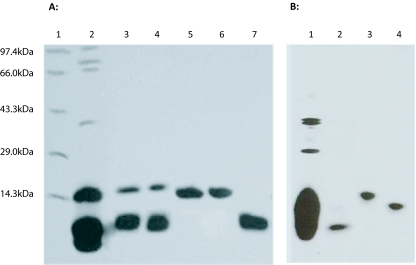
Gel-filtration patterns of individual NK-PLA2, KTX and reconstituted NK-PLA2: KTX showed appearance of a big peak at around fraction 30 of gel-filtration indicated the formation of NK-PLA2: KTX complex (data not shown) which was re-confirmed by SDS-PAGE analysis of this peak where it showed two bands corresponding to KTX and PLA2 (Figure 1A). However, by native-PAGE, proteins of this fraction migrated as a single band with molecular weight higher than molecular weight of individual KTX and PLA2 reinforcing the formation of KTX-PLA2 complex by non-covalent interaction (Figure 1B). The excitation of fluorescence of free PLA2 and KTX (0.4μM) was done at 270nm and emission maximum were observed at 340nm and 338nm, respectively (data not shown). A large increase in fluorescence intensity (about 2.8 to 3.1-fold increase) of PLA2 post mixing with respective KTX was observed (data not shown). The data presented in the present study vouch for a non-covalent NK-PLA2: KTX interaction, an example of the protein-protein interaction of the two components of snake venom.
Figure 1.
15% (w/v) SDS-PAGE gel of crude N. kaouthia venom, reconstituted (gel filtration fraction) NK-PLA2: KTX-A complex, and purified venom proteins (KTX-A and KTX-B) under reduced (A) and non-reduced (B) conditions. A. Lane 1, molecular weight markers: phosphorylase b (97,400Da), bovine serum albumin (66,000 Da), ovalbumin (43,000Da), carbonic anhydrase (29,000Da), and lysozyme (14,300Da); Lane 2, reduced crude N. kaouthia venom (50μg); Lane 3, reduced fraction of reconstituted NK-PLA2A: KTX-A complex (30μg); Lane 4, reduced fraction of reconstituted NK-PLA2B: KTX-B complex (30μg); Lane 5, reduced NK-PLA2 A (30μg); Lane 6, reduced NK-PLA2 B (30μg); Lane 7, reduced KTX-A (30μg). B. Lane 1, non-reduced crude N. kaouthia venom (50μg); Lane 2, non-reduced KTX-A (10μg); Lane 3, non-reduced reconstituted NK-PLA2 A:KTX-A complex (10μg); Lane 4, non-reduced NK-PLA2 A (10μg).
Most of the toxic effects of snake venom PLA2 appear to result from their promotion of membrane dysfunction by hydrolyzing phospholipids of cellular and/or subcellular membranes (Doley et al, 2004; Mukherjee, 2007). Similarly, KTXs also display cell specific cytotoxicity however, at a significantly lower magnitude (p< 0.05) than the cytotoxic potency of N. kaouthia venom PLA2 enzymes (Table 1). Due to limiting amounts of pure NK-PLA2/KTX molecules, a complete dose-response curve for estimation of LC50 for culture cells could not be obtained. Study showed that cytotoxicity of individual PLA2/ KTX molecule on target cells was further enhanced by the cognate NK-PLA2-KTX complex (Table 1). Interaction of NK-PLA2-A with KTX-A, as well as interaction of NK-PLA2-B with KTX-B, synergistically potentiates the cytotoxicity of the individual PLA2, or KTX. For example, although NK-PLA2 or KTX alone did not induce appreciable hemolysis of washed erythrocytes, the hemolytic effect of reconstituted NK-PLA2: KTX complex was significantly higher. However, both KTXs failed to influence the anticoagulant activity of NK-PLA2 and like individual PLA2 / KTX molecule, NK-PLA2: KTX complex did not show antibacterial activity against E. coli cells (Table 1). These results lead us to conclude that binding of KTXs with PLA2s from venom of N. kaouthia enhances their cytotoxicity but does not alter their target cell specificity. Furthermore, nanomolar concentration of PLA2/KTX/PLA2: KTX complex used in the present study ruled out the non-specific binding and subsequent hydrolysis of the target membrane.
Table 1.
Pharmacological properties of NK-PLA2-A, NK-PLA2-B, KTX-A, KTX-B and PLA2-KTX complexes. Values are mean ± SD of four experiments. Values in the same row within each experiment followed by different superscripts are significantly different (P<0.05).
| Properties | Control | PLA2-A | KTX-A | PLA2-A: KTX-A | PLA2-B | KTX-B | PLA2-B: KTX-B |
|---|---|---|---|---|---|---|---|
| Anticoagulant activity1 | 87 ± 3.0a | 99 ± 4.0b | 89 ± 3.0a | 99 ± 3.0b | 100 ± 5.0b | 90 ± 3.0a | 99 ± 4.0b |
| Antibacterial activity2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cytotoxicity3 | |||||||
| (a) VERO | 0.3 ± 0.08a | 1.3 ± 0.2b | 0.8 ± 0.1c | 8.2 ± 0.3d | 1.0 ± 0.1b | 0.6 ± 0.1c | 6.8 ± 0.6d |
| (b) Sf9 (n) | 0.8 ± 0.1a | 4.9 ± 0.5b | 1.1 ± 0.1c | 12.2 ± 1.3d | 3.8 ± 0.4b | 0.9 ± 0.1c | 10.2 ± 1.1d |
| (c) SF9 (in) | 2.5 ± 0.1a | 8.1 ± 0.6b | 3.3 ± 0.4c | 20.1 ± 0.8d | 6.9 ± 0.4b | 3.9 ± 0.4c | 18.5 ± 1.3d |
| (d) Tn (n) | 1.8 ± 0.2a | 7.9 ± 0.5b | 4.8 ± 0.3c | 21.0 ± 1.1d | 6.6 ± 0.5b | 4.1 ± 0.3c | 20.2 ± 1.2d |
| Direct hemolysis4 (time in min) | |||||||
| 30 | 0a | 0a | 0a | 1.5 ± 0.5b | 0a | Oa | 1.0 ± 0.04b |
| 60 | 0a | 0.21 ± 0.02b | 0.11 ± 0.01c | 6.3 ± 1.1d | 0a | 0.1 ± 0.01c | 4.1 ± 0.1 |
| 90 | 0.1a | 0.73 ± 0.01b | 0.45 ± 0.02c | 14.3 ± 2.1d | 0a | 0.41 ± 0.02c | 13.2 ± 1.3d |
| 120 | 0.2a | 3.3 ± 0.2b | 1.5 ± 0.1c | 32.0 ± 3.4d | 0.4 ± 0.03e | 1.4 ± 0.1c | 29.1 ± 2.1d |
aPlasma Ca-clotting time in seconds caused by 4.0μM PLA2/KTX/PLA2-KTX complex.
bPost 12hr incubation with NK-PLA2 / KTX / PLA2-KTX at a concentration of 500μM
cPercent of cell death post 3hr treatment with NK-PLA2/KTX/PLA2-KTX at a concentration of 250nM.
dPercent of hemoglobin released from 5% (v/v) RBC suspension post incubation for indicated time period by 250nM PLA2/KTX/PLA2-KTX complex post 60min incubation at 37°C. Cent-percent hemolysis was achieved by adding 1% (v/v) Triton X-100 to RBC suspension.
The differential cytotoxicity of PLA2s on various cells was suggested on the basis of specific activity, phospholipids head-group specificity of phospholipases and membrane domain hypothesis (Diaz et al, 2001; Doley et al, 2004; Mukherjee, 2007). The presence of different amounts of PC in the outer membranes of tested cells supports this hypothesis (Doley et al, 2004; Mukherjee, 2007). Interestingly, although KTXs are devoid of any enzymatic activity but they demonstrated cytotoxicity which may presumably by binding of highly basic, water soluble KTXs at the lipid bilayer surface to exert their cytotoxicity (Mukherjee, 2008). It is worthy to mention that this binding is preferential because KTXs could not disrupt the bacterial cell membrane (Table 1) thus excluding its affinity for the E. coli membrane. The mechanism of action of KTXs thus resembles other cytolytic toxins, such as Streptolysis S (Dunkan and Buckingham, 1981), Staphylococcus α-toxin (Durkin and Shier, 1981) and Bacillus thuringenesis var israelensis delta-endotoxin (Thomas and Ellar, 1983), which bind preferentially to membrane phospholipids and cause lysis by perturbing and rearranging the lipid bilayer, leading to disruption of membrane integrity. The non-enzymatic mechanism of membrane disruption is also shown by netexin, a presynaptic basic phospholipase A2 neurotoxin purified from venom of Notechis scutatus scutatus (Kaoa et al, 2007). However, unlike bee venom melittin, KTXs were unable to activate the endogenous PLA2 of RBC membrane because synthesis and release of arachidonic acid from RBC membrane post treatment with KTXs could not be observed (Mukherjee AK and Bordoloi NK, unpublished data). Therefore, the possibility of activation of some indigenous phospholipase A2 of RBC by KTX which in turn disrupt the erythrocyte membrane may be ruled out (Shier, 1979).
To further investigate into the mode of attack, hydrolysis pattern of erythrocyte membrane phospholipids by individual NK-PLA2, KTX, or NK-PLA2-KTX complex was examined (Table 2). RBC was chosen to study the phospholipids hydrolysis pattern of these toxins or their complexes, because RBC is a unique model system and practically all the lipids are contained in the plasma membrane. Gas-chromatographic (GC) analysis of fatty acids release patterns from intact erythrocyte by individual NK-PLA2 or NK-PLA2:KTX complex revealed that with an increase in the incubation time, there was a progressive increase in the release of free fatty acids from the RBC membrane; however, the membrane hydrolysis by NK-PLA2-I was more pronounced than NK-PLA2-B (Figure 2). This substrate preference study demonstrated that catalytic activity of NK-PLA2-A towards PC was significantly higher than NK-PLA2-B (Mukherjee, 2007) which may explain the differential membrane hydrolysis by these two PLA2 molecules. On the other hand, release of FFAs from RBC membranes was observed post 60min of incubation with either NK-PLA2 or KTX (Table 2). Furthermore, the quantity of FFAs released from RBC membrane post treatment with KTXs was significantly lower compared to the FFAs released after treatment with PLA2 enzymes (Table 2).
Table 2.
Percent of erythrocyte phospholipids hydrolysis by individual NK-PLA2s, KTXs and NK-PLA2-KTX complexes at a final concentration of 250nM. Values are mean ± SD of four experiments. Values in the same row within each experiment followed by different superscripts are significantly different (P<0.05).
| Incubation time (min) | PLA2-A | KTX-A | PLA2-A: KTX-A | PLA2-B | KTX-B | PLA2-B: KTX-B |
|---|---|---|---|---|---|---|
| 30 | 0a | 0a | 3.8 ± 0.3b | 0a | 0a | 1.3 ± 0.1c |
| 60 | 0.34 ± 0.01a | 0.12 ± 0.01b | 7.3 ± 1.2c | 0.11 ± 0.01b | 0a | 5.2 ± 0.3e |
| 90 | 0.91 ± 0.01a | 0.15 ± 0.02b | 21.0 ± 1.9c | 0.34 ± 0.02b | 0.15 ± 0.01d | 15.1 ± 1.0e |
| 120 | 4.8 ± 0.3a | 0.32 ± 0.1b | 35.2 ± 2.1c | 1.0 ± 0.01b | 0.22 ± 0.02d | 31.2 ± 1.8c |
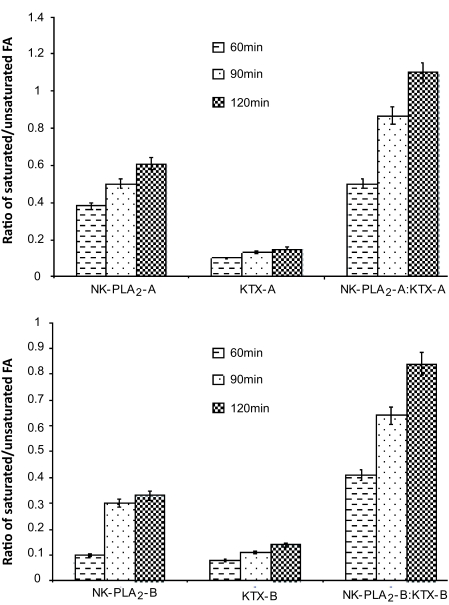
Figure 2.
Analysis of free fatty acid (FFA) released pattern from intact human erythrocytes by individual NK-PLA2, KTX or NK-PLA2: KTX complex. Released FFAs were separated by thin layer chromatography and their methylated esters were analyzed by GC-MS. Values are mean ±SD of three individual experiments.
From Figure 2 It is evident that the ratio between saturated fatty acids (SFA) and unsaturated FA (UFA) was progressively increasing with increasing the time of hydrolysis of RBC with either NK-PLA2 or NK-PLA2-KTX complex suggesting NK-PLA2-KTX complex apparently displayed the more preference for glycerophospholipids with SFAs on the sn-2 position. However, such a linear increase in the ratio between SFA and UFA from RBC membrane could not be observed for either KTX-A or KTX-B. One possible explanation for the synergistic membrane damaging activity of NK-PLA2-KTX complex is first destabilization of the membrane by KTX followed by higher enzymatic activity of PLA2 component of the complex on dislocated and disorganized phospholipid bilayers that resulted in significantly higher (P < 0.01) toxicity of the NK-PLA2-KTX complex compared to the individual component of the complex.
CONCLUSIONS
KTXs exhibited cell specific synergism with their respective PLA2 from venom of N. kaouthia in lysing the tested cells and thereby greatly influence the cytotoxicity of the associated proteins. It may be concluded that NK-PLA2-KTX complex furnishes an example of protein complementation for restoration of the biological activity by non-covalent interaction of two polypeptides of cobra venom which may play an important role in the pathophysiology of cobra bite.
Acknowledgments
Author expresses his sincere thanks to Dr. Glenn F King, University of Connecticut Health Center, Farmington, CT, USA for providing laboratory facility for doing some of the experiments. Author is grateful to Mr R Doley and Mr NK Bordoloi, Tezpur University, for their technical assistance. Author was recipient of BOYSCAST fellowship from DST, New Delhi. A part of this project was supported by the financial grant to AKM from the University Grants Commission, New Delhi.
STATEMENT OF COMPETING INTERESTS
None declared.
REFERENCES
- Diaz C, Leon G, Rucavado A, Rojas N, Schoroits AJ, Gutierrez MJ. Modulation of susceptibility of human erythrocytes to snake venom myotoxic phospholipase A2: Role of negatively charged phospholipids as potential membrane binding sites. Arch Biochem Biophys. 2001;391:56–64. doi: 10.1006/abbi.2001.2386. [DOI] [PubMed] [Google Scholar]
- Doley R, Mukherjee AK. Purification and characterization of an anticoagulant phospholipase A2 from Indian monocled cobra (Naja kaouthia) venom. Toxicon. 2003;41:81–89. doi: 10.1016/s0041-0101(02)00213-1. [DOI] [PubMed] [Google Scholar]
- Doley R, King GF, Mukherjee AK. Differential hydrolysis of erythrocyte and mitochondrial membrane phospholipids by two phospholipase A2 isoenzymes (NK-PLA2-I and NK-PLA2-II), from Indian monocled cobra Naja kaouthia venom. Arch Biochem Biophys. 2004;425:1–13. doi: 10.1016/j.abb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Duncan JL, Buckingham L. Effect of streptolysin S on liposomes Influence of membrane lipid composition on toxin action. Biochim Biophys Acta. 1981;648:6–12. doi: 10.1016/0005-2736(81)90119-x. [DOI] [PubMed] [Google Scholar]
- Durkin JP, Shier WT. Staphylococcal delta toxin stimulates endogenous phospholipase A2 activity and prostaglandin synthesis in fibroblasts. Biochim Biophys Acta. 1981;663:467–479. doi: 10.1016/0005-2760(81)90175-2. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–507. [PubMed] [Google Scholar]
- Harvey AL, Hider RC, Khader F. Effect of phospholipase A on action of cobra venom cardiotoxins on erythrocytes and skeletal muscles. Biochim Biophys Acta. 1983;728:215–221. doi: 10.1016/0005-2736(83)90474-1. [DOI] [PubMed] [Google Scholar]
- Kaoa PH, Linb SR, Chang LS. Phospholipase A2 activity-independent membrane-damaging effect of notexin. Toxicon. 2007;50:952–959. doi: 10.1016/j.toxicon.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry DH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mukherjee AK, Maity CR. The composition of Naja naja venom samples from three districts of West Bengal, eastern India. Comp Biochem Physiol. 1998;119A:621–627. doi: 10.1016/s1095-6433(97)00475-3. [DOI] [PubMed] [Google Scholar]
- Mukherjee AK, Maity CR. Biochemical composition, lethality and pathophysiology of venom from two cobras- Naja naja and Naja kaouthia. Comp Biochem Physiol. 2002;131(B):125–132. doi: 10.1016/s1096-4959(01)00473-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee AK. Correlation between phospholipids domain of the target cell membrane and the extent of Naja kaouthia PLA2 induced membrane damage: evidence of distinct catalytic and cytotoxic sites in PLA2 molecules. Biochem et Biophys Acta. 2007;1770:187–195. doi: 10.1016/j.bbagen.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Mukherjee AK. Phospholipase A2-interacting weak neurotoxins from venom of monocled cobra Naja kaouthia display cell specific cytotoxicity. Toxicon. 2008;51:1538–1543. doi: 10.1016/j.toxicon.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Shier WT. Activation of high levels of endogenous Phospholipase A2 in cultured cells. Proc Natl Acad Sci USA. 1979:195–199. doi: 10.1073/pnas.76.1.195. (Volume No missing; please complete this reference) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker KF. Composition of Snake Venom. In: Stocker KF, editor. Medical Use of Snake Venom Proteins. First edition. CRC Press, Florida; USA: 1990. p. 33. [Google Scholar]
- Thomas WE, Ellar DJ. Mechanism of action of Turingiensis var israelensis insecticidal ∂-endotoxin. FEBS Lett. 1983;154:362–368. doi: 10.1016/0014-5793(83)80183-5. [DOI] [PubMed] [Google Scholar]
- Wang R, Kini RM, Chung MCM. Rhodocetin, a novel platelet –aggregation inhibitor from the venom of Colloselasma rhodostoma (Malayan pit viper): synergistic and noncovalent interaction between its subunits. Biochemistry. 1999;38:7584–7593. doi: 10.1021/bi982132z. [DOI] [PubMed] [Google Scholar]