Abstract
Chen et al. demonstrate endothelial expression of Toll-like receptor 4 (TLR4) in the outer medulla of the kidney early in the course of ischemic acute kidney injury. Furthermore, they provide data that support the hypothesis that activation of the endothelial TLR4 receptor in the early extension phase of AKI by damage associated molecular pattern molecules released from injured tubules results in endothelial activation. This activation can serve to amplify inflammation and tubular damage.
There is a long-standing appreciation that altered vascular function contributes to decreasing glomerular filtration rate (GFR) during acute kidney injury (AKI). Although overall renal blood flow is only transiently diminished following an inciting insult to the kidney, there is a more persistent and profound (relative to the cortex) decrease in outer medullary blood flow that is implicitly linked to the complex interplay between tubular injury, inflammation, and endothelial alteration 1. This interplay not only serves to adversely impact GFR but can also promote further tubular cell injury beyond the initial insult by extending tissue hypoperfusion. Activation of inflammation is an important component of both the initiation and extension of injury in ischemic AKI and enhancement of leukocyte-endothelial interactions is a salient feature of this process. Interruption of the leukocyte-endothelial interaction has been an attractive target for therapeutic intervention; however, the mechanisms of endothelial activation in AKI are poorly defined. The manuscript by Chen et al.2 in this issue of Kidney International sheds light on this area by providing evidence that incriminates endothelial Toll-like receptor 4 (TLR4) in the initiation of endothelial cell activation and further implicates the innate immune system in the pathophysiology of ischemic AKI 3.
The recognition of TLR4 as the receptor for endotoxin over a decade ago revolutionized the fields of innate immunity and infectious diseases. TLR4 is one of over a dozen receptors belonging to the TLR superfamily which are now known to play a central role in mammalian innate immunity. These receptors detect their respective microbial ligands through molecular pattern recognition. This mechanism lacks high specificity but increases the repertoire of each receptor to multiple ligands with frequent overlap. The inflammatory response initiated upon receptor-ligand interaction serves as the first line defense against a large spectrum of bacteria, fungi and viruses and is essential for microbial clearance and the subsequent activation of the adaptive immune system. However, it is also recognized that tissue damage can be a byproduct of this defensive inflammatory response when severe and prolonged. For example, the sepsis syndrome is now understood to be in part a manifestation of the destructive inflammatory arm of the innate immune response 4.
Recently, the field of TLRs and their ligands has witnessed an expansion both in location and function. It is now known that TLRs are expressed on cells other than the traditional members of the innate immune system. They have been detected in epithelial, endothelial, muscular and neuronal cells to cite just a few and may serve functions in these cells similar to those they perform in innate immune cells. Furthermore, the realization that endogenous non-microbial ligands can also activate these receptors has immensely broadened the field beyond infectious diseases. Substances released during tissue injury such as heat shock proteins, high-mobility group box 1 (HMGB1), and fragments of the extracellular matrix can activate inflammatory responses through TLRs. Consequently, TLRs may serve as important monitors of tissue damage and modulators of disease in a variety of conditions like atherosclerosis, autoimmune diseases and ischemia-reperfusion injury (IRI).
TLR4 is known to be expressed on renal tubular epithelial cells and respond robustly to local and systemic infections5. More recently, it has been demonstrated that TLR4 is an important player in ischemic AKI. Elegant studies by Wu et al. 6 using chimeric mice specifically pointed to tubular epithelial TLR4 as an important modulator of ischemic AKI. The manuscript by Chen and et al. now extends this role of TLR4 into the endothelium of the kidney and furthers our understanding of the complex role these receptors play in the kidney. In this study, Chen and coworkers utilize multiple approaches to demonstrate that expression of TLR4 increases in the endothelium of the outer medullary peritubular microvasculature following IRI. This increase in the endothelial TLR4 expression has an apparent peak at 4 hours of reperfusion – much earlier than the increase observed at 24 hours in tubular epithelial cells. They complement this observation with in vitro studies demonstrating that endothelial stressors likely to occur during IRI, including ROS production, can increase expression of TLR4 mRNA in isolated microvascular endothelial cells. They further build on this observation with studies demonstrating that the increase in mRNA for a variety of adhesion molecules in microvascular endothelial cells isolated from kidneys four hours after IRI is abrogated in endothelial cells isolated from TLR4 (−/−) mice. Finally, they demonstrate that HMGB1 stimulates an increase in mRNA for a variety of adhesion molecules in microvascular endothelial cells isolated from kidneys of wild type mice but not TLR4 (−/−) mice. In total, these studies provide evidence for the hypothesis that increased expression of TLR4 on endothelial cells early in the course of ischemic AKI provides fertile ground for the activation of endothelial cells by endogenous molecules released from injured tubules - thus fueling the inflammatory fire of AKI and further extending tubular injury.
While this study by Chen et al. brings new focus to the role of endothelial toll signaling in the pathophysiology of AKI, it also raises some questions. In particular what is the contribution of endothelial TLR signaling vs. tubular epithelial TLR signaling in the final outcome? The temporal difference in expression during the course of ischemic AKI demonstrated by this study suggest that TLR signaling through endothelial cells and tubular epithelial cells may be complimentary and additive in promoting injury. In support of this, studies of endothelial TLR4 in other organ systems suggest a critical role for endothelial TLR4 in activating endothelium and recruiting leukocytes to areas of injury 7-8. Further studies in models of AKI utilizing mice expressing TLR4 exclusively in the endothelium and mice with TLR4 specifically knocked out of the endothelium should provide further clarification of the role of endothelial TLR4 in AKI.
Another issue raised is the nature of the endogenous ligands that stimulate TLR4 during AKI. As mentioned above, ischemic AKI has the potential to produce a variety of potential TLR4 ligands. Wu et al. identified increased expression of HMGB1, hyaluronan, and biglycan 24 hours after ischemic injury 6 and postulated these as potential TLR4 ligands following injury. While Chen et al. utilized HMGB1 as an activating ligand for TLR4 for their in vitro studies of isolated endothelial cells, the important activating ligands, released in the early phases of ischemic AKI have not been clarified. The observation that the TLR4 −/− mouse appears to be more protected in models of ischemic AKI than the MyD88−/− mouse 6,9 suggests that both MyD88-dependent and MyD88-independent pathways are activated by TLR4 activation. This may also suggest different ligand-TLR4 receptor complexes coordinating unique signaling cascades10. This is of potential importance because, as outlined by the authors, “each ligand may activate its own TLR4-receptor complex and intracellular signaling pathways” and thus produce different physiological results. Nonetheless, targeting the ligand-TLR4 interaction may be a more fruitful therapeutic endeavor than targeting the downstream pathways in ischemic AKI.
In conclusion, this manuscript by Chen et al. has again brought the endothelium into the conversation as an important therapeutic target in AKI. The overall understanding of non-glomerular endothelial cell biology and how it relates to kidney disease is still in a relative early stage as compared to other organs. While studies like the one highlighted here continue to make strides in our knowledge, there is still considerable progress to be made before this knowledge is translated into meaningful therapeutic advances for AKI.
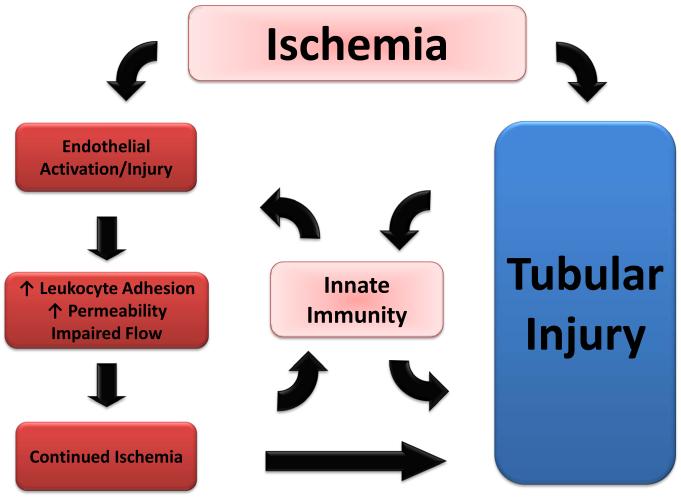
Figure 1.
Schematic representation of the interaction between endothelial alterations, innate immunity (canonical and non-canonical) and tubular injury in ischemic AKI. This interaction serves to initiate and extend injury resulting in diminished GFR.
References
- 1.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, John R, Richardson, et al. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 2011;79:288–299. doi: 10.1038/ki.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Achkar TM, Dagher PC. Renal Toll-like receptors: recent advances and implications for disease. Nat Clin Pract Nephrol. 2006;2:568–581. doi: 10.1038/ncpneph0300. [DOI] [PubMed] [Google Scholar]
- 5.El-Achkar TM, Huang X, Plotkin Z, et al. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol. 2006;290:F1034–1043. doi: 10.1152/ajprenal.00414.2005. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andonegui G, Bonder CS, Green F, et al. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, Andonegui G, Wong CH, et al. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J Immunol. 2009;183:5244–5250. doi: 10.4049/jimmunol.0901309. [DOI] [PubMed] [Google Scholar]
- 9.Pulskens WP, Teske GJ, Butter LM, et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zughaier SM, Zimmer SM, Datta A, et al. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun. 2005;73:2940–2950. doi: 10.1128/IAI.73.5.2940-2950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]