Abstract
Symmetrical and unsymmetrical bithiophene-substituted heterocycles bearing carbonitriles including imidazo[1,2-a]pyridine, benzimidazole, and pyridine derivatives have been synthesized via different synthetic protocols. The bithiophene bis-imidazo[1,2-a]pyridine derivatives 3a,b were achieved in three steps starting from 2-acetyl-5-bromothiophene. Suzuki coupling reaction of 2a with 5-formylthiophen-2-ylboronic acid forms the formyl derivative 5, which by condensation with 3,4-diaminobenzonitrile in the presence of sodium bisulfite furnishes the unsymmetrical bithiophene derivative 6. The bis-benzimidazole derivative 8 was obtained via hexabutylditin-mediated homocoupling of 5-bromothiophene-2-carboxaldehyde, while the benzimidazole derivatives 12a,b were prepared via the formyl derivatives 11a,b, a product of Velsmier formylation reaction of 10a,b. Two synthetic protocols for the aryl/hetaryl-2,2′-bithiophene derivative 14 have also been presented. In addition, the guanyl hydrazones of bithiophenes, 16 and 17, were prepared from bis(tri-n-butylstannyl)-2,2′-bithiophene through a Stille coupling reaction followed by a condensation step.
Keywords: Bithiophene, formylation, Heck coupling, Stille coupling, Suzuki coupling
INTRODUCTION
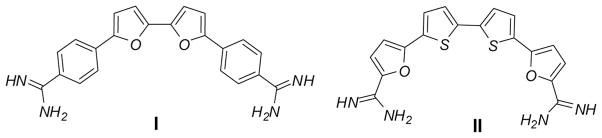
Bi- and oligothiophenes have recently received increased attention because of their wide applications as advanced materials.[1] They have been heavily investigated as organic semiconductors, with particular application to thin-film transistors (TFTs)[1b,c] and light-emitting devices (LEDs).[1d] Bithiophenes and their derivatives are also important synthetic precursors for biologically active materials.[2] Nitrile-containing compounds serve as precursors of diamidine-containing molecules, which exhibit broad-spectrum antimicrobial activity, including effectiveness against different protozoan diseases.[3–6] Moreover, bifuran diamidines I (Fig. 1) and analogs showed specific recognition of G-quadruplex DNA.[7] Very recently, the bithiophene diamidines of type II (Fig. 1) showed good activity against Trypanosoma cruzi.[8] In this context and in continuation of our interest in preparing biologically active diamidines, we recently reported an efficient homocoupling approach to 5,5′-diaryl-2,2′-bichalcophenes of type I.[9] This route allows only the synthesis of symmetrical analogs. Different triaryl/hetaryl systems of nitrile-containing bichalcophenes also have been reported.[10] Given the promising properties of pyridines,[11] imidazo[1,2-a]pyridines,[12] and benzimidazoles,[13] we decided to synthesize nitrile-containing bithiophenes of the aforementioned heterocycles. Because of their activity against trypanosomes,[14] this report also described the synthesis of two examples of guanyl hydrazones of a bithiophene derivative.
Figure 1.
Bithiophene diamidines of type II.
RESULTS AND DISCUSSION
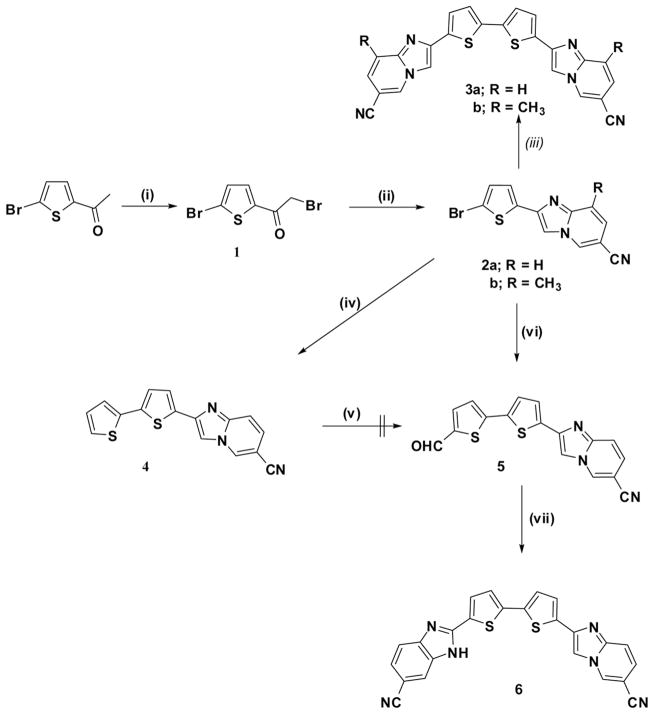
A three-step preparation of the symmetrical imidazo[1,2-a]pyridyl bithiophene derivatives 3a,b starting from 2-acetyl-5-bromothiophene is described as outlined in Scheme 1; thus, 2-acetyl-5-bromothiophene was brominated by using bromine in a mixed solvent of dioxane/ether (1:2) to give compound 1 in 71% yield. A condensation reaction of 6-aminonicotinonitrile or its methyl analog with 1 yielded the bromothiophen-2-yl-imidazo[1,2-a]pyridine-6-carbonitriles 2a,b, which underwent a Stille-type homocoupling reaction in the presence of hexabutylditin with a catalytic amount of Pd(PPh3)4 (~2 mol%), using refluxing toluene as solvent to afford the anticipated bithiophene derivatives 3a,b in 72 and 75% yields, respectively. On the other hand, the unsymmetrical imidazo[1,2-a]pyridyl-benzimidazolyl bithiophene derivative 6 was obtained in two steps utilizing Suzuki coupling reaction of 2a with 5-formylthiophen-2-ylboronic acid to form the anticipated 2-(5′-formyl-2,2′-bithiophen-5-yl)imidazo[1,2-a]pyridine-6-carbonitrile (5), which subsequently condensed with 3,4-diaminobenzonitrile in the presence of an equimolar ratio of sodium bisulfite to give the desired dinitrile 6. An alternative synthesis of the formyl bithiophene derivative 5 was tried through a Stille coupling reaction of 2 with 2-tributyltin thiophene, forming the product 4, followed by a Vilsmeier formylation reaction. However, the last step gives an inseparable mixture that may be attributed to the nucleophilic reactivity at C-3 of the imidazo[1,2-a]pyridine part of compound 4. This is consistent with the reported literature.[15]
Scheme 1.
Reagents and conditions: (i) Br2; (ii) EtOH, reflux; (iii) (Bu3Sn)2, Pd(PPh3)4; (iv) 2-tributyltin thiophene, Pd(PPh3)4; (v) DMF-POCl3; (vi) 5-formylthiophen-2-ylboronic acid; (vii) 3,4-diaminobenzonitrile, sodium bisulfite, DMF, reflux.
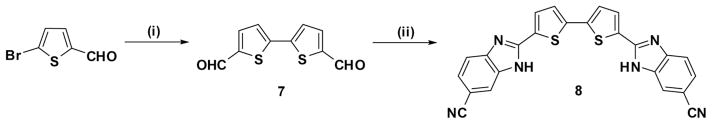
Scheme 2 outlines the preparation of 2-(2,2′-bithiophene-5,5′-diyl)-bis(1H-benzo[d]imidazole-6-carbonitrile) (8) via hexabutylditin-mediated homocoupling of 5-bromothiophene-2-carboxaldehyde in the presence of catalytic Pd(PPh3)4 using refluxing toluene as solvent at 90 °C to form 5,5′-diformyl-2,2′-bithiophene (7). Subsequent condensation of the diformyl 7 with 2 equivalents of 3,4-diaminobenzonitrile in the presence of sodium bisulfite gave the desired bithiophene-substituted benzimidazole 8.
Scheme 2.
Reagents and conditions: (i) toluene, (Bu3Sn)2, Pd(PPh3)4; (ii) 3,4-diaminobenzonitrile, sodium bisulfite, DMF, reflux.
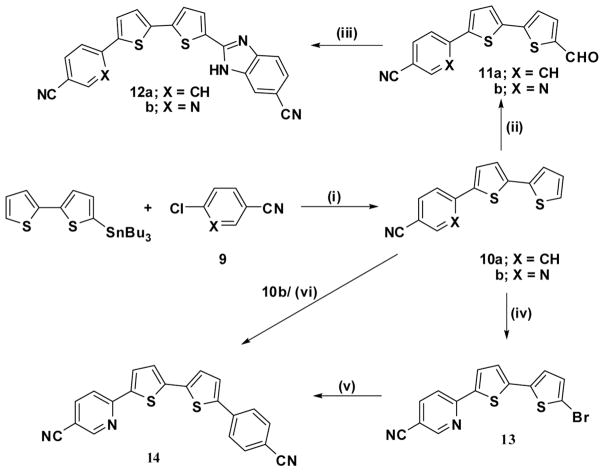
The preparation of bithiophene-substituted benzimidazole derivatives 12a,b is presented in Scheme 3. Thus, compound 12b was obtained in three steps starting with the Stille coupling reaction of 5-(tri-n-butylstannyl)-2,2′-bithiophene with 6-chloronicotinonitrile to form the anticipated 6-(2,2′-bithiophen-5-yl)nicotinonitrile (10b). A Vilsmeier formylation reaction of 10b furnished the formyl derivative 11b, which, upon condensation with 3,4-diaminobenzonitrile in the presence of sodium bisulfite, gave the desired dinitrile 12b. Two synthetic approaches for the synthesis of 6-[5′-(4-cyanophenyl)-2,2′-bithiophen-5-yl]nicotinonitrile (14) are also presented in Scheme 3. The first approach uses bromination of 10b with N-bromosuccinimide in dimethylformamide (DMF) to furnish 6-(5′-bromo-2,2′-bithiophen-5-yl)nicotinonitrile (13) in 93% yield followed by Suzuki coupling of 13 with 4-cyanophenylboronic acid to afford 14 in 69% yield. The second approach employs a Heck coupling reaction directly from the reaction of compound 10b with 4-bromobenzonitrile to afford 14 in 28% yield.
Scheme 3.
Reagents and conditions: (i) toluene, Pd(PPh3)4; (ii) DMF-POCl3; (iii) 3,4-diaminobenzonitrile, sodium bisulfite, DMF, reflux; (iv) NBS, DMF; (v) 4-cyanophenylboronic acid, Pd(PPh3)4; (vi) 4-bromobenzonitrile, KOAc, Pd(PPh3)4, 125–135 °C.
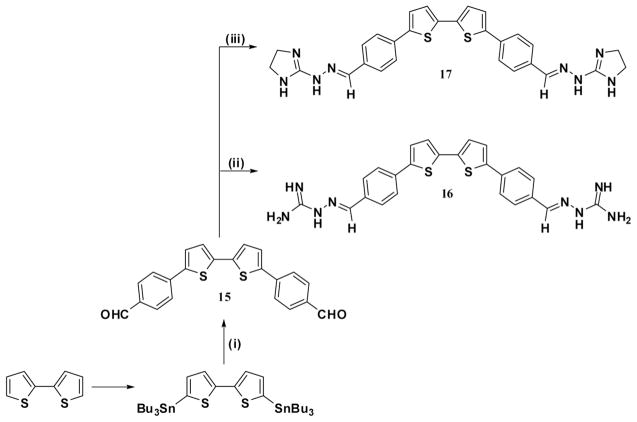
Guanylhydrazones are normally included in the DNA minor-groove binding category and have frequently been found to have activity against trypanosomes.[14] Thus, the preparation of the guanylhydrazone derivative 16 involved the condensation reaction between diformyl derivative 15, a Stille product of bis(tri-n-butylstannyl)-2,2′-bithiophene with 4-bromobenzaldehyde, and aminoguanidine hydrochloride in the presence of Et3N. In a similar manner, compound 17 was synthesized from the corresponding starting material. It should be noted that 4 equivalents of aminoguanidine or 2-hydrazino-2-imidazoline are necessary to drive the reaction to completion to form guanylhydrazones.
In conclusion, we have described concise synthetic approaches for novel bithiophene-substituted benzimidazole, imidazo[1,2-a]pyridine, and pyridine derivatives. The use of these nitrile-containing heterocycles in the synthesis of diamidines for biological evaluation will be reported in due course.
EXPERIMENTAL
General
Melting points were recorded using a Thomas-Hoover (Uni-Melt) capillary melting-point apparatus and are uncorrected. Thin-layer chromatography (TLC) analysis was carried out on silica-gel 60 F254 precoated aluminum sheets and detected under ultraviolet (UV) light. Infrared (IR) spectra were recorded on a Mattson 5000 Fourier transform FT–IR spectrometer. 1H and 13C NMR spectra were recorded employing a Varian Unity Plus 300 spectrometer at Georgia State University, and chemical shifts (δ) are in parts per million (ppm) relative to tetramethylsilane (TMS) as internal standard. Mass spectra were recorded on a VG analytical 70-SE spectrometer. Elemental analyses were obtained from Atlantic Microlab Inc. (Norcross, GA) and are within ±0.4 of the theoretical values. All chemicals and solvents were purchased from Aldrich Co., Fisher Scientific, Frontier and Lancaster. N-Bromosuccinimide (NBS) was recrystallized from nitromethane prior to use. All solvents were reagent grade. 4-(2,2′-Bithiophen-5-yl)benzonitrile[10] (10a), 5-(tri-n-butylstannyl)-2,2′-bithiophene[16], and bis(tri-n-butylstannyl)-2,2′-bithiophene[17] were prepared according to the reported literature.
2-Bromo-1-(5-bromothiophen-2-yl)-ethanone (1)
Bromine (2.08 mL, 40 mmol) was added portionwise to a solution of 2-acetyl-5-bromothiophene (8.20 g, 40 mmol) in 48 mL dioxane/ether (1:2) with cooling at 0–5 °C and stirring over 1 h. The reaction mixture was further stirred with cooling. After TLC indicated complete bromination, the reaction mixture was diluted with ether (200 mL) and water (200 mL). The ethereal layer was separated, washed with 1 M sodium bicarbonate aqueous solution, and dried over Na2SO4. The ether extract was distilled off to afford 1 in 71% yield as a colorless sheet, mp 89–90 °C (hexanes/ether) (lit.[18] mp 90–91 °C); IR (KBr) ν 3083, 3000, 2952, 1670 (CO) cm−1. 1H NMR (CDCl3): δ 4.28 (s, 2H), 7.14 (d, J =4.2 Hz, 1H), 7.54 (d, J =4.2 Hz, 1H). 13C NMR (CDCl3): δ 183.4, 142.1, 133.7, 131.5, 124.5, 29.6. MS (EI) m/e (rel. int.): 284, 286 (M+, 14, 7 bromine isotopes), 204 (2), 191 (100), 189 (92), 175 (7), 123 (4).
2-(5-Bromothiophen-2-yl)-imidazo[1,2-a]pyridine-6-carbonitrile (2a)
A mixture of 6-aminonicotinonitrile (1.19 g, 10 mmol) and 2-bromo-1-(5-bromothiophen-2-yl)-ethanone (1) (2.84 g, 10 mmol) in ethanol (50 mL) was heated at reflux for 24 h. The precipitated salt was filtered, suspended in water, and neutralized with aqueous NaHCO3 solution. The free base precipitate was filtered and dried to furnish 2a in 79% yield as an off-white solid, mp 249–250.5 °C (EtOH). IR (KBr) ν 3089, 3048, 2225 (CN), 1625, 1573, 1529 cm−1. 1H NMR (DMSO-d6): δ 7.28 (d, J =4.2 Hz, 1H), 7.49–7.55 (m, 2H), 7.72 (d, J =9.6 Hz, 1H), 8.42 (s, 1H), 9.33 (s, 1H). 13C NMR (DMSO-d6): δ 143.8, 140.5, 138.2, 133.9, 131.4, 125.2, 125.1, 117.0, 116.7, 111.6, 109.4, 97.3. MS (EI) m/e (rel. int.): 303, 305 (M+, 54, 52 bromine isotopes), 224 (100), 180 (30). Anal. calcd. for C12H6BrN3S: C, 47.38; H, 1.99; N, 13.81. Found. C, 47.61; H, 2.10; N, 13.64.
2-(5-Bromothiophen-2-yl)-8-methylimidazo[1,2-a]pyridine-6-carbonitrile (2b)
The same procedure described for preparation of 2a was used starting with 6-amino-5-methylnicotinonitrile instead of 6-aminonicotinonitrile. Yield 68% as off-white needles, mp 213–214.5 °C. IR (KBr) ν 3100, 3083, 2925, 2223 (CN), 1643, 1617 cm−1. 1H NMR (DMSO-d6): δ 2.46 (s, 3H), 7.21 (d, J =3.6 Hz, 1H), 7.28 (s, 1H), 7.40 (d, J =3.6 Hz, 1H), 8.31 (s, 1H), 9.09 (s, 1H). 13C NMR (DMSO-d6): δ 144.4, 139.8, 138.3, 131.4, 131.1, 127.1, 124.8, 122.9, 116.7, 111.2, 109.8, 97.1, 15.9. MS (ESI) m/e (rel. int.): δ 318, 320 (M+ + 1, 100, 96 bromine isotopes). Anal. calcd. for C13H8BrN3S: C, 49.07; H, 2.53; N, 13.21. Found. C, 49.40; H, 2.69; N, 13.02.
2,2′-(2,2′-Bithiophene-5,5′-diyl)-bis(imidazo[1,2-a]pyridine-6-carbonitrile) (3a)
Hexa-n-butylditin (2.9 g, 5 mmol) was added to a solution of 2a (1.52 g, 5 mmol) and tetrakis(triphenylphosphine) palladium (100 mg, 0.085 mmol) in toluene (40 mL). The reaction mixture was heated under N2 at 120 °C for 6 h, then cooled, and the precipitate was filtered and washed with hexanes. Recrystallization from DMF gave compound 3a in 72% as a dark-green powder, mp > 320 °C. IR (KBr) ν 3083, 3050, 2225 (CN), 1629, 1567 cm−1. 1H NMR (DMSO-d6): δ 7.36–7.69 (m, 8H), 8.37 (s, 2H), 9.23 (s, 2H). MS (ESI) m/e (rel. int.): 448 (M+, 13), 449 (M+ + 1, 87), 409 (24), 320 (100). High-resolution mass calcd. for C24H13N6S2: 449.0643. Observed: 449.0641. Anal. calcd. for C24H12N6S2: C, 64.27; H, 2.70. Found. C, 63.94; H, 2.81.
2,2′-(2,2′-Bithiophene-5,5′-diyl)-bis(8-methylimidazo[1,2-a]pyridine-6-carbonitrile) (3b)
The same procedure described for preparation of 3a was used starting with 2b. Yield 75% as a dark-green powder, mp 304–306 °C. IR (KBr) ν 3072, 2954, 2921, 2225 (CN), 1623, 1573 cm−1. 1H NMR (DMSO-d6): δ 2.54 (s, 6H, 2 × CH3), 7.27–7.54 (m, 6H), 8.33 (s, 2H), 9.07 (s, 2H). MS (ESI) m/e (rel. int.): 477 (M+ + 1, 33), 478 (M+ + 2, 10), 452 (22), 409 (58), 331 (100). High-resolution mass calcd. for C26H17N6S2: 477.0956. Observed: 477.0967. Anal. calcd. for C26H16N6S2: C, 65.53; H, 3.38. Found. C, 65.22; H, 3.19.
2-(2,2′-Bithiophen-5-yl)imidazo[1,2-a]pyridine-6-carbonitrile (4)
A mixture of 2a (3.03 g, 10 mmol), 2-tri-n-butyltin thiophene (3.72 g, 10 mmol), and tetrakis(triphenylphosphine) palladium (200 mg) in dry dioxane (50 mL) was heated under nitrogen at 100–110 °C for 24 h. The solvent was evaporated under reduced pressure, and the resulting residue was dissolved in ethyl acetate. This solution was passed through celite to remove Pd. The solution was evaporated, and the residue was chromatographed on silica gel using hexanes/EtOAc (3:7) as an eluent to furnish compound 4 in 76% yield as a pale yellow solid, mp 219–220.5 °C. 1H NMR (DMSO-d6): δ 7.08–7.11 (m, 1H), 7.27–7.34 (m, 2H), 7.43–7.54 (m, 3H), 7.68 (d, J =9.6 Hz, 1H), 8.35 (s, 1H), 9.24 (s, 1H). 13C NMR (DMSO-d6): δ 143.9, 141.1, 136.6, 136.2, 135.1, 133.8, 128.3, 125.7, 125.5, 125.1, 124.8, 124.1, 116.9, 109.5, 97.1. MS (EI) m/e (rel. int.): 307 (M+, 100), 308 (M++1, 23), 274 (5), 262 (5), 131 (13). High-resolution mass calcd. for C16H9N3S2: 307.0237. Observed: 307.0234. Anal. calcd. for C16H9N3S2: C, 62.52; H, 2.95. Found. C, 62.60; H, 3.01.
2-(5′-Formyl-2,2′-bithiophen-5-yl)imidazo[1,2-a]pyridine-6-carbonitrile (5)
To a stirred solution of 2a (1.51 g, 5 mmol), and tetrakis(triphenylphosphine) palladium (200 mg) in toluene (10 mL) under a nitrogen atmosphere, 10 mL of a 1 M aqueous solution of NaHCO3 were added, followed by 5-formylthiophen-2-ylboronic acid (936 mg, 6 mmol) in 5 mL of methanol. The vigorously stirred mixture was warmed to 80 °C for 16 h. The solvent was evaporated, and the precipitate was partitioned between methylene chloride (300 mL) and aqueous solution containing 5 mL of concentrated ammonia. The organic layer was dried (Na2SO4) and then concentrated to dryness under reduced pressure to afford 5 in 61% yield as a brown-yellow solid, mp 268–270 °C (DMF). IR (KBr) ν 3137, 3081, 2227 (CN), 1650 (CO), 1573 cm−1. 1H NMR (DMSO-d6): δ 7.46–7.69 (m, 6H), 8.43 (s, 1H), 9.26 (s, 1H), 9.87 (s, 1H). 13C NMR (DMSO-d6): δ 183.2, 145.1, 143.8, 141.2, 140.5, 138.5, 138.0, 134.9, 133.7, 127.5, 125.8, 125.1, 124.9, 116.9, 116.5, 110.0, 97.2. MS (EI) m/e (rel. int.): 335 (M+, 100), 307 (12), 262 (28). Anal. calcd. for C17H9N3OS2: C, 60.88; H, 2.70; N, 12.53. Found. C, 60.75; H, 2.73; N, 12.67.
2-{5′-(6-Cyanoimidazo[1,2-a]pyridin-2-yl)-2,2′-bithiophen-5-yl}-1H-benzimidazole-6-carbonitrile (6)
A solution of 5 (670 mg, 2 mmol), 3,4-diaminobenzonitrile (267 mg, 2 mmol), and sodium bisulfite (261 mg, 2.5 mmol) in 10 mL DMF was allowed to reflux overnight. After cooling, the reaction mixture was poured onto water. The solid was collected by filtration and washed with aqueous sodium bicarbonate (2.5%) and water to furnish 3a in 77% yield as a brown solid, mp > 320 °C (DMF). IR (KBr) ν 3353 (NH), 3083, 2962, 2221 (CN), 1619, 1567 cm−1. 1H NMR (DMSO-d6): δ 7.45–7.85 (m, 8H), 8.04 (s, 1H), 8.41 (s, 1H), 9.26 (s, 1H), 13.30 (s, 1H, NH). MS (ESI) m/e (rel. int.); 449 (M+ + 1, 60), 413 (30), 365 (32), 331 (100). High-resolution mass calcd. for C24H13N6S2: 449.0643. Observed: 449.0641. Anal. calcd. for C24H12N6S2: C, 64.27; H, 2.70. Found. C, 64.05; H, 2.79.
2,2′-Bithiophene-5,5′-dicarboxaldehyde (7)
The same procedure described for preparation of 3a was used starting with 5-bromothiophene-2-carboxaldehyde (the temperature of the reaction maintained at 90 °C). Yield 70% as a golden solid, mp 212.5–214 °C (lit.[19] mp 215–217 °C; lit.[20] mp 185–195 °C). IR (KBr) ν 3100, 1654 (CO), 1538, 1436 cm−1. 1H NMR (DMSO-d6): δ 7.73 (d, J =3.9 Hz, 2H), 8.02 (d, J =3.9 Hz, 2H), 9.93 (s, 2H). 13C NMR (DMSO-d6): δ 184.3, 143.5, 143.4, 138.9, 127.9. MS (EI) m/e (rel. int.): 222 (M+, 100), 193 (18), 149 (32).
2,2′-(2,2′-Bithiophen-5,5′-diyl)-bis(1H-benzimidazole-6-carbonitrile) (8)
The same procedure described for the preparation of 6 was used, employing dialdehyde derivative 7 (1 equiv) and 3,4-diaminobenzonitrile (2 equiv). Yield 75% as a brown powder, mp > 320 °C (DMF). IR (KBr) ν 3455 (NH), 3089, 2215 (CN), 1614, 1563 cm−1. 1H NMR (DMSO-d6): δ 7.31–7.71 (m, 6H), 7.88 (d, J =3.9 Hz, 2H), 8.08 (s, 2H), 13.70 (br s, 2H, 2NH). MS (EI) m/e (rel. int.): 448 (M+, 100), 321 (38). Anal. calcd. for C24H12N6S2: C, 64.27; H, 2.70; N, 18.74. Found. C, 63.90; H, 2.85; N, 18.46.
6-(2,2′-Bithiophen-5-yl)nicotinonitrile (10b)
Adopting the same procedure used for the preparation of 4, a Stille coupling reaction was performed using 6-chloronicotinonitrile and 5-(tri-n-butylstannyl)-2,2′-bithiophene to yield the cyano derivative 10b in 85% yield as a yellow solid; mp 176–177 °C (EtOH). IR (KBr) ν 2217 (CN), 1639, 1583, 1513 cm−1. 1H NMR (DMSO-d6): δ 7.10–7.14 (m, 1H), 7.40 (d, J =3.9 Hz, 1H), 7.45 (d, J =3.9 Hz, 1H), 7.59 (dd, J =5.1, 1.2 Hz, 1H), 7.95 (d, J =3.9 Hz, 1H), 8.11 (d, J =8.4 Hz, 1H), 8.28 (dd, J =8.4, 2.1 Hz, 1H), 8.92 (d, J =2.1 Hz, 1H). 13C NMR (DMSO-d6): δ 154.3, 152.6, 141.13, 141.08, 140.5, 136.0, 129.4, 128.6, 126.7, 125.4, 125.3, 118.4, 117.3, 106.4. Anal. calcd. for C14H8N2S2: C, 62.66; H, 3.00; N, 10.44. Found. C, 62.57; H, 2.97; N, 10.45.
4-(5′-Formyl-2,2′-bithiophen-5-yl)benzonitrile (11a)
Freshly distilled DMF (4.2 mL) was stirred in an ice bath with dropwise addition of POCl3 (14 mL), followed by a suspension of compound 10a (1.86 g, 7 mmol) in methylene chloride (12 mL). The reaction mixture was stirred under heating at 85–95 °C for 6 h. The solvent was distilled off under reduced pressure, then poured onto ice water, and the solid was collected by filtration and washed with aqueous sodium bicarbonate (2.5%) and water to furnish 11a in 81% yield as a golden solid, mp 197–198 °C (EtOH). IR (KBr) ν 3095, 2921, 2223 (CN), 1654 (CO), 1600 cm−1. 1H NMR (DMSO-d6): δ 7.59 (d, J =3.3 Hz, 1H), 7.66 (d, J =3.9 Hz, 1H), 7.79 (d, J =3.9 Hz, 1H), 7.87 (s, 4H), 8.00 (d, J =3.3 Hz, 1H), 9.88 (s, 1H). 13C NMR (DMSO-d6): δ 184.0, 144.7, 142.5, 141.8, 139.2, 137.1, 136.5, 133.2, 128.5, 127.8, 126.0, 125.8, 110.2, 99.5. MS (EI) m/e (rel. int.): 295 (M+, 100), 267 (8), 222 (29). High-resolution mass calcd. for C16H9NOS2: 295.0125. Observed: 295.0126. Anal. Calcd for C16H9NOS2: C, 65.06; H, 3.07. Found. C, 65.15; H, 3.13.
6-(5′-Formyl-2,2′-bithiophen-5-yl)nicotinonitrile (11b)
The same procedure described for preparation of 11a was used starting with 10b. Yield 77% as a golden solid, mp 258–259.5 °C. IR (KBr) ν 3085, 3050, 2794, 2223 (CN), 1656 (CO), 1587, 1515 cm−1. 1H NMR (DMSO-d6): δ 7.65 (d, J =3.9 Hz, Hz, 1H), 7.68 (d, J =3.9 Hz, 1H), 8.00 (d, J =3.9 Hz, 1H), 8.03 (d, J =3.9 Hz, 1H), 8.16 (d, J =8.4 Hz, 1H), 8.31 (dd, J =8.4, 2.1 Hz, 1H), 8.95 (d, J =2.1 Hz, 1H), 9.90 (s, 1H). Anal. calcd. for C15H8N2OS2: C, 60.79; H, 2.72; N, 9.45. Found. C, 60.60; H, 2.82; N, 9.23.
2-[5′-(4-Cyanophenyl)-2,2′-bithiophen-5-yl]-1H-benzimidazole-6-carbonitrile (12a)
The same procedure described for preparation of 6 was used starting with 11a. Yield 74% as a brown powder, mp 320–322 °C (DMF). IR (KBr) ν 3415 (NH), 3075, 2221 (CN), 1596, 1577, 1536 cm−1. 1H NMR (DMSO-d6): δ 7.52–7.61 (m, 3H), 7.70–7.88 (m, 6H), 7.97–8.08 (m, 2H), 13.40 (s, 1H, NH). 13C NMR (DMSO-d6): δ 149.0, 141.1, 139.5, 138.8, 137.2, 136.9, 133.1, 130.7, 130.2, 130.0, 127.6, 126.9, 126.2, 125.8, 125.2, 120.0, 119.8, 118.7, 115.4, 109.9, 104.4. MS (EI) m/e (rel. int.): 408 (M+, 100). High-resolution mass calcd. for C23H12N4S2: 408.0503. Observed: 408.0519. Anal. calcd. for C23H12N4S2: C, 67.62; H, 2.96. Found. C, 67.30; H, 3.07.
2-[5′-(5-Cyanopyridin-2-yl)-2,2′-bithiophen-5-yl]-1H-benzimidazole-6-carbonitrile (12b)
The same procedure described for preparation of 6 was used starting with 11b. Yield 70% as a brown powder, mp > 320 °C (DMF). IR (KBr) ν 3097, 2223 (CN), 1658, 1621, 1587 cm−1. 1H NMR (DMSO-d6): δ 7.50–7.57 (m, 3H), 7.71 (d, J =8.1 Hz, 1H), 7.89 (d, J =3.9 Hz, 1H), 7.97 (d, J =3.9 Hz, 1H), 8.04 (s, 1H), 8.10 (d, J =8.4 Hz, 1H), 8.27 (dd, J =8.4, 2.1 Hz, 1H), 8.93 (d, J =2.1 Hz, 1H) 13.65 (s, 1H, NH). MS (EI) m/e (rel. int.): 409 (M+, 100), 376 (4), 320 (10). High-resolution mass calcd. for C22H11N5S2: 409.0456. Observed: 409.0464. Anal. calcd. for C22H11N5S2: C, 64.53; H, 2.71. Found: C, 64.21; H, 2.86.
6-(5′-Bromo-2,2′-bithiophen-5-yl)nicotinonitrile (13)
NBS (1.78 g, 10 mmol) was added portionwise to a solution of 10b (2.67 g, 10 mmol) in DMF (30 mL) with stirring. The reaction mixture was stirred overnight, then poured onto cold water. The precipitate that formed was collected, washed with water, and dried to give 13 in 93% yield as a yellow solid, mp 209–210 °C. IR (KBr) ν 3077, 3045, 2229 (CN), 1641, 1585, 1550 cm−1. 1H NMR (DMSO-d6): δ 7.26–7.33 (m, 2H), 7.43 (d, J =4.2 Hz, 1H), 7.98 (d, J =4.2 Hz, 1H), 8.15 (d, J =8.4 Hz, 1H), 8.31 (dd, J =8.4, 2.1 Hz, 1H), 8.93 (d, J =2.1 Hz, 1H). 13C NMR (DMSO-d6): δ 150.1, 148.6, 137.8, 136.6, 135.6, 133.7, 127.9, 125.3, 122.0, 121.8, 114.5, 113.2, 107.5, 102.6. MS (EI) m/e (rel. int.): 346, 348 (M+, 100, 95 bromine isotopes), 267 (13), 223 (40). High-resolution mass calcd. for C14H7BrN2S2: 345.9234. Observed: 345.9234. Anal. calcd. for C14H7BrN2S2: C, 48.42; H, 2.03. Found. C, 48.29; H, 2.01.
6-[5′-(4-Cyanophenyl)-2,2′-bithiophen-5-yl]nicotinonitrile (14)
Method A
Adopting the same procedure used for the preparation of 5, a Suzuki coupling reaction was performed using 13 and 4-cyanophenylboronic acid to yield 14 in 69% yield as a brick-red solid, mp 307–309.5 °C (DMF). IR (KBr) ν 3062, 2225 (CN), 1635, 1587 cm−1. 1H NMR (DMSO-d6): δ 7.45–7.57 (m, 3H), 7.70–7.93 (m, 5H), 8.08–8.25 (m, 2H), 8.91 (s, 1H). MS (EI) m/e (rel. int.): 369 (M+, 100). High-resolution mass calcd. for C21H11N3S2: 369.0394. Observed: 369.0382. Anal. calcd. for C21H11N3S2: C, 68.27; H, 3.00. Found. C, 67.88; H, 3.12.
Method B
A mixture of compound 10b (1.34 g, 5 mmol), 4-bromobenzonitrile (925 mg, 5 mmol), tetrakis(triphenylphosphine) palladium (200 mg), and potassium acetate (2.5 g, 25 mmol) in dry DMF (20 mL) was heated under nitrogen at 130–135 °C overnight. The reaction mixture then poured onto cold water. The precipitate that formed was collected and recrystallized from DMF to afford compound 14 in 28% yield.
4,4′-(2,2′-Bithiophen-5,5′-diyl)dibenzaldehyde (15)
Adopting the same procedure used for the preparation of 4, a Stille coupling reaction was performed using 4-bromobenzaldehyde (2 equiv) and bis(tri-n-butylstannyl)-2,2′-bithiophene (1 equiv) to yield 15 in 82% yield as a green-yellow solid; mp 244–246 °C (DMF). IR (KBr) ν 3100, 3062, 2861, 1694 (CHO), 1598, 1563 cm−1. 1H NMR (DMSO-d6): δ 7.47 (d, J =3.6 Hz, 2H), 7.71 (d, J =3.6 Hz, 2H), 7.89 (d, J =7.5 Hz, 4H), 7.95 (d, J =7.5 Hz, 4H), 10.01 (s, 2H). MS (EI) m/e (rel. int.): 374 (M+, 100), 346 (87). High-resolution mass calcd. for C22H14O2S2: 374.0435. Observed: 374.0412. Anal. calcd. for C22H14O2S2: C, 70.56; H, 3.77. Found. C, 70.75; H, 3.63.
N-[4,4′-(2,2′-Bithiophen-5,5′-diyl)dibenzylidene]-N′-amidino Hydrazine (16)
A mixture of 15 (374 mg, 1 mmol), aminoguanidine hydrochloride (440 mg, 4 mmol), and triethylamine (4 mmol) in absolute ethanol (40 mL) was heated at reflux overnight. The formed precipitate was filtered, washed with water, and dried to give 16 in 65% yield as a brown-yellow solid, mp > 320 °C (DMF/EtOH). IR (KBr) ν 3432, 3365, 3342 (NH, NH2), 3070, 1687, 1598 cm−1. 1H NMR (DMSO-d6): δ 5.53 (br s, 4H), 5.95 (br s, 4H), 7.37 (d, J =4.2 Hz, 2H), 7.53 (d, J = 4.2 Hz, 2H), 7.62 (d, J =8.4 Hz, 4H), 7.71 (d, J =8.4 Hz, 4H), 7.98 (s, 2H). Anal. calcd. for C24H22N8S2: C, 59.24; H, 4.56; N, 23.03. Found: C, 59.50; H, 4.60; N, 22.73.
N-[4,4′-(2,2′-Bithiophen-5,5′-diyl)dibenzylidene]-N′-(4,5-dihydro-1H-imidazol-2-yl) Hydrazine (17)
The same procedure described for 16 was used, employing 2-hydrazino-2-imidazoline hydrobromide instead of aminoguanidine hydrochloride. Yield 69% as an orange solid, mp > 320 °C. IR (KBr) ν 3430 (NH), 3143, 3066, 2923, 2883, 2829, 1635, 1573, 1544 cm−1. 1H NMR (DMSO-d6): δ 3.44 (s, 8H), 6.23 (br s, 2H), 6.61 (br s, 2H), 7.34 (d, J =3.9 Hz, 2H), 7.49 (d, J =3.9 Hz, 2H), 7.63 (d, J =8.4 Hz, 4H), 7.71 (d, J =8.4 Hz, 4H), 8.00 (s, 2H). Anal. calcd. for C28H26N8S2: C, 62.43; H, 4.86; N, 20.80. Found: C, 62.09; H, 4.87; N, 20.94.
Scheme 4.
Reagents and conditions: (i) 4-bromobenzaldehyde, toluene, Pd(PPh3)4; (ii) aminoguanidine hydrochloride/Et3N, EtOH; (iii) 2-hydrazino-2-imidazoline hydrobromide/Et3N, EtOH.
Acknowledgments
This work was supported by the Bill and Melinda Gates Foundation and by National Institute of Health Grant AI46365.
References
- 1.(a) Fichou D. Handbook of Oligo- and Polythiophenes. Wiley-VCH; Weinheim, Germany: 1999. [Google Scholar]; (b) Ong BS, Wu Y, Liu P, Gardner S. High-performance semiconducting polythiophenes for organic thin-film transistors. J Am Chem Soc. 2004;126:3378–3379. doi: 10.1021/ja039772w. [DOI] [PubMed] [Google Scholar]; (c) Chang PC, Lee J, Huang D, Subramanian V, Murphy AR, Frechet JMJ. Film morphology and thin film transistor performance of solution processed oligothiophenes. Chem Mater. 2004;16:4783–4789. [Google Scholar]; (d) Fichou D. Structural order in conjugated oligothiophenes and its implications on optoelectronic devices. J Mater Chem. 2000;10:571–588. [Google Scholar]; (e) Negishi N, Takimiya K, Otsubo T, Harima Y, Aso Y. Synthesis and photovoltaic effects of oligothiophenes incorporated with two [60]fullerenes. Chem Lett. 2004;33:654–655. [Google Scholar]
- 2.Taylor R. In: Thiophene and Its Derivatives. part 1. Gronowitz S, editor. Vol. 44. Wiley; New York: 1985. ch. 3. [Google Scholar]
- 3.Tidwell RR, Boykin DW. Dicationic DNA minor groove binders as antimicrobial agents. In: Demeunynck M, Bailly C, Wilson WD, editors. Small Molecule DNA and RNA Binders: From Synthesis to Nucleic Acid Complexes. Vol. 2. Wiley-VCH; New York: 2003. pp. 416–460. [Google Scholar]
- 4.Wilson WD, Nguyen B, Tanious FA, Mathis A, Hall JE, Stephens CE, Boykin DW. Dications that target the DNA minor groove: Compound design and preparation, DNA interactions, cellular distribution and biological activity. Curr Med Chem-Anti-Cancer Agents. 2005;5:389–408. doi: 10.2174/1568011054222319. [DOI] [PubMed] [Google Scholar]
- 5.Soeiro MNC, de Souza EM, Stephens CE, Boykin DW. Aromatic diamidines as antiparasitic agents. Expert Opin Invest Drugs. 2005;14:957–972. doi: 10.1517/13543784.14.8.957. [DOI] [PubMed] [Google Scholar]
- 6.Wenzler T, Boykin DW, Ismail MA, Hall JE, Tidwell RR, Brun R. New treatment option for second-stage African sleeping sickness: In vitro and in vivo efficacy of aza analogs of DB289. Antimicrob Agents Chemother. 2009;53(10):4185–4192. doi: 10.1128/AAC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White EW, Tanious FA, Ismail MA, Reszka AP, Neidle S, Boykin DW, Wilson DW. Structure specific recognition of quadruplex DNA by organic cations: Influence of shape, substituents and charge. Biophys Chem. 2007;126:140–153. doi: 10.1016/j.bpc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Batista DG, Pacheco MG, Kumar A, Branowska D, Ismail MA, Hu L, Boykin DW, Soeiro MN. Biological, ultrastructural effect, and subcellular localization of aromatic diamidines in Trypanosoma cruzi . Parasitology. 2009;21:1–9. doi: 10.1017/S0031182009991223. [DOI] [PubMed] [Google Scholar]
- 9.Ismail MA, Boykin DW, Stephens CE. An efficient synthesis of 5,5′-diaryl-2,2′-bichalcophenes. Tetrahedron Lett. 2006;47:795–797. [Google Scholar]
- 10.Ismail MA. An efficient synthesis of 5′-(4-cyanophenyl)-2,2′-bifuran-5-carbonitrile and analogues. J Chem Res. 2006:733–737. [Google Scholar]
- 11.Ismail MA, Brun R, Easterbrook JD, Tanious FA, Wilson DW, Boykin DW. Synthesis and antiprotozoal activity of aza-analogues of furamidine. J Med Chem. 2003;46:4761–4769. doi: 10.1021/jm0302602. [DOI] [PubMed] [Google Scholar]
- 12.(a) Ismail MA, Arafa RK, Wenzler T, Brun R, Tanious FA, Wilson DW, Boykin DW. Synthesis and antiprotozoal activity of novel bis-benzamidino imidazo[1,2-a]pyridines and 5,6,7,8-tetrahydroimidazo[1,2-a]pyridines. Bioorg Med Chem. 2008;16:683–691. doi: 10.1016/j.bmc.2007.10.042. [DOI] [PubMed] [Google Scholar]; (b) Ismail MA, Brun R, Wenzler T, Tanious FA, Wilson WD, Boykin DW. Novel dicationic imidazo[1,2-a]pyridines and 5,6,7,8-tetrahydroimidazo[1,2-a]pyridines as antiprotozoal agents. J Med Chem. 2004;47:3658–3664. doi: 10.1021/jm0400092. [DOI] [PubMed] [Google Scholar]
- 13.(a) Ismail MA, Miao Y, Wilson DW, Wenzler T, Brun R, Boykin DW. Dicationic near-linear biphenyl benzimidazole derivatives as DNA-targeted antiprotozoal agents. Bioorg Med Chem. 2005;13:6718–6726. doi: 10.1016/j.bmc.2005.07.024. [DOI] [PubMed] [Google Scholar]; (b) Ismail MA, Brun R, Wenzler T, Tanious FA, Wilson DW, Boykin DW. Dicationic biphenyl benzimidazole derivatives as antiprotozoal agents. Bioorg Med Chem. 2004;12:5405–5413. doi: 10.1016/j.bmc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Wang CC. Molecular mechanisms and therapeutic approaches to the treatment of african trypanosomiasis. Annu Rev Pharmacol Toxicol. 1995;35:93–127. doi: 10.1146/annurev.pa.35.040195.000521. [DOI] [PubMed] [Google Scholar]
- 15.(a) El Kazzouli S, Berthault A, Berteina-Raboin S, Mouaddib A, Guillaumet G. Solution and solid-phase functionalization of imidazo[1,2-a]pyridines. Lett Org Chem. 2005;2:184–187. [Google Scholar]; (b) Salgado-Zamora H, Velazquez M, Mejia D, Campos-Aldrete ME, Jimenez R, Cervantes H. Influence of the 2-aryl group on the ipso electrophilic substitution process of 2-arylimidazo[1,2-a]pyridines. Heterocyclic Commun. 2008;14(1–2):27–32. [Google Scholar]
- 16.Zhu SS, Swager TM. Conducting polymetallorotaxanes: Metal ion–mediated enhancements in conductivity and charge localization. J Am Chem Soc. 1997;119:12568–12577. [Google Scholar]
- 17.Hucke A, Cava MP. Synthesis of mixed thiophene/furan oligomers by Stille coupling. J Org Chem. 1998;63:7413–7417. doi: 10.1021/jo981159l. [DOI] [PubMed] [Google Scholar]
- 18.Bagli JF, Ferdinandi E. Thiophene isosters of phenylethanolamines. Can J Chem. 1975;53:2598–2607. [Google Scholar]
- 19.Batista RMF, Costa SPG, Belsley M, Lodeiro C, Raposo MM. Synthesis and characterization of novel (oligo) thienyl-imidazo-phenanthrolines as versatile π-conjugated systems for several optical applications. Tetrahedron. 2008;64:9230–9238. [Google Scholar]
- 20.Mitsumori T, Inoue K, Koga N, Iwamura H. Exchange interactions between two nitronyl nitroxide or iminyl nitroxide radicals attached to thiophene and 2,2′-bithienyl rings. J Am Chem Soc. 1995;117:2467–2478. [Google Scholar]