Abstract
Background:
Despite the renewed interest in metal-on-metal implants in the past two decades, the underlying wear mechanisms and biological effects are still not fully understood.
Methods:
This paper first reviews the tribology of metal-on-metal bearings, bringing new insights into the interaction of wear and corrosion, and putting the characteristics and the potential origin of wear particles in perspective with the proposed wear mechanisms. It then summarizes the current knowledge on the biological effects of particles and metal ions in relation to these wear mechanisms.
Results:
Tribochemical reactions play an important role in the wear of metal-on-metal joints. The generated tribomaterial, which progressively forms by mechanical mixing of the uppermost nanocrystalline zone of the metal surface with proteins from the synovial fluid, governs the wear rate and influences the corrosive behavior of the bearing. Nanometer-sized wear particles may initially originate from the passivation layer covering the implant surface and then detach from this tribolayer. The inflammatory response observed surrounding metal-on-metal implants appears to be lower than that around metal-on-polyethylene implants. However, metallic byproducts, which can complex with proteins, may lead to a T lymphocyte-mediated hypersensitivity response.
Conclusions:
The tribolayer appears to have beneficial effects on the wear rate. Much information has been gained on wear particle characteristics, but the exact mechanisms of particle detachment remain to be further elucidated. Excessive wear along with a hypersensitivity response may be at the origin of the early adverse tissue reactions that have been recently reported in some patients with metal-on-metal implants.
Clinical Relevance:
Future development of new methods to improve the tribolayer retention and optimize the tribocorrosive properties of the implant may minimize the clinical impact of implant wear and immune responses.
Since the introduction of the first generation of metal-on-metal bearings in the early 1960s, progress has been made in understanding and controlling manufacturing variables. There has been a renewed interest in metal-on-metal implants in the past two decades because of the high wear performance compared with conventional metal-on-polyethylene bearings. Also, large femoral heads can be used with metal-on-metal implants, allowing hip resurfacing designs that preserve the proximal femoral bone stock. Progress has been largely based on empirical information, and the underlying wear mechanisms are not well understood. The release of metal ions, which can complex with proteins1-3, and the formation of nanometer-sized wear particles4-6 have raised concerns regarding metal hypersensitivity and potential genotoxicity.
Histological studies of the tissues surrounding second-generation metal-on-metal hip replacements generally support the primary rationale for the reintroduction of metal-on-metal bearings. Indeed, the extent of the inflammatory reaction and the presence of foreign-body giant cells appear to be much less marked than in tissues from conventional metal-on-polyethylene components7. However, a major cause for concern with metal-on-metal hip replacements has recently emerged because of increasing reports of unexpected early adverse local tissue reactions8-13, which compromise the functionality and survivorship of these implants. In some cases, even severe systemic complications have been associated with metal-on-metal implants14. These adverse reactions are of concern because metal-on-metal bearings currently constitute approximately 35% of the >300,000 primary hip replacements performed annually in the U.S.15.
In the present study, the current knowledge about metal-on-metal wear mechanisms, including new findings regarding the interaction of wear and corrosion, is reviewed first. Wear particle characteristics and their potential origin are then discussed. Finally, these new findings are placed in context with observed biological reactions.
Wear, Tribochemical Reactions, and Metal-on-Metal Tribolayers
Wear is defined as loss of material from a tribological system as a result of energy dissipation. Typically, the energy is introduced by friction. Wear is not a material property (like strength, ductility, or hardness) but depends on the structure of the tribological system. This means that counterbody, interfacial material (e.g., lubricant and intrinsic or extrinsic particles) and the environment (e.g., the temperature or pH of the surrounding media) are similarly important in the generation of wear. In fact, the specific conditions often define the acting wear mechanism, which itself defines how many particles per stroke and/or time increment are generated. Currently, there are four major known wear mechanisms, which are either purely mechanically dominated (abrasion and surface fatigue) or chemically and/or mechanically mixed (adhesion and tribochemical reactions). All of them can be subdivided into several submechanisms, depending on the nature of the specific contact situation and their interaction. Details have been summarized elsewhere16.
Studies have suggested that the governing wear mechanisms at the metal-on-metal interface are not adhesion and/or abrasion as in conventional hip bearing types, but predominantly tribochemical reactions and surface fatigue17,18. These tribochemical reactions result in the reaction of the metal surface with the synovial fluid and form a tribomaterial at the surface, also referred to as a tribolayer because it is generated at the surface. The term tribomaterial was introduced by Rigney et al.19 to point to the fact that this material is quite different from the base material. In the case of metal-on-metal joints, the tribomaterial shows a nanocrystalline structure with a grain size between 30 and 70 nm (note the size similarity to wear particles as shown in Figure 1, C and reviewed later) and consists of a mixture of organic, ceramic, and metallic constituents17,18.
Fig. 1.
A: Scanning electron micrograph of a tribolayer on a large-diameter cup. B: Transmission electron micrograph of a cross-sectional cut through a tribolayer. Note the carbon layer and the embedded nanocrystals. C: Transmission electron micrograph of wear particles generated by hip simulator testing of a metal-on-metal implant (cast alloy) in 95% bovine serum for 0 to 0.25 million cycles, showing an example of a particle containing Cr and O (1), an example of a particle containing Co and Cr with Cr>Co (2), and an example of a particle containing Cr and Co with Co>Cr (3). (Figure 1, C reprinted, with permission, from: Catelas I, Medley JB, Campbell PA, Huk OL, Bobyn JD. Comparison of in vitro with in vivo characteristics of wear particles from metal-metal hip implants. J Biomed Mater Res B Appl Biomater. 2004;70:167-78.)
Cobalt-chromium-molybdenum (CoCrMo) alloy recrystallizes under the surface because of the influence of shear from a face-centered cubic to a hexagonally closed, packed crystal lattice, which results in the nanometer-sized grains20-22. The small grains can rotate to accommodate the frictional shear stresses introduced by the counterbody, and thus dissipate energy without particle detachment. This is one of the reasons why CoCrMo can articulate in the ultramild sliding wear regime with a material loss of <1 nm/hr. Also, the rotation of grains results in the incorporation of the pseudosynovia, a process that has been termed mechanical mixing23. It can be attributed as a submechanism to tribochemical reactions because the chemical composition and properties of the uppermost material have been changed. In the case of metal-on-metal joints, an organometallic composite is generated on the basis of the remains of the lubricant (presumably denatured proteins) and metallic nanoparticles of the upper surface layer22. The thickness of the entire nanocrystalline zone ranges from 100 to 400 nm, while the tribomaterial itself is the near-surface fraction of it with a thickness between 0 and 300 nm22,24.
It is important to understand that a metal-on-metal bearing can only stabilize in the ultramild wear regime in the presence of adhering tribomaterial. While such tribomaterial has been observed on the surface of current metal-on-metal implants24,25 and is shown on a large-diameter ASR cup (articular surface replacement; DePuy, Warsaw, Indiana) and a 28-mm Ultima head (DePuy) in Figure 1, A and B, respectively, these tribolayers seem to be absent and/or worn off under high-stress conditions. For example, Pourzal et al.24 demonstrated that the tribolayer is absent in the stripe wear region of metal-on-metal joints (the stripe wear region is the area that interacts with the acetabular rim because of microseparation)26. It is also speculated that the implantation of the acetabular component in excessive anteversion and/or abduction may destabilize the layer and lead to excessive wear. This agrees with clinical observation27 and experimental hip wear testing28. Hence, a better understanding of the kinetics of the tribomaterial formation and removal at the hip joint could be crucial for improved clinical outcome. In particular, it is necessary to define the mechanical boundary conditions for optimal bearing performance. This may lead to alloy formulations and implant designs that exhibit a more robust tribomaterial and are less susceptible to wear.
Tribocorrosion Behavior of the Nanocrystalline and Mechanically Mixed Zone
While the tribomaterial should have advantageous effects on metal-on-metal wear, the corrosive and tribocorrosive behavior has yet to be evaluated. In order to report a positive effect, the corrosion behavior of an implant with a tribolayer must be superior compared with a (worn) CoCrMo surface without a tribolayer. Such questions are best addressed with use of an electrochemical approach with a standard three-electrode corrosion cell and an attached potentiostat29.
Muñoz and Mischler30 investigated the interaction of albumin and phosphates present in the body fluids on the passivation behavior of CoCrMo alloys. The study revealed that phosphates and proteins played an important role in the electrochemical properties of the metal-oxide-electrolyte interface. Mathew et al.31 reported that physiological conditions (simulated with bovine calf serum), and in particular the presence of proteins, improved the passivation kinetics of the CoCrMo alloy. However, at the same time, the pitting potential decreased, suggesting that pitting corrosion is more likely in a protein-containing solution. The presence of pits has been reported on the articulation of retrieved hip implants32, and thus pitting corrosion is of concern as a potential source of metal ions.
These studies do not take into account the tribolayer that forms during articulation. When using samples with artificially prepared surfaces (nanocrystalline without proteins and nanocrystalline with tribomaterial), Mathew et al. recently reported a higher anodic potential of the metal-on-metal heads with tribomaterial, suggesting a more noble behavior with better corrosion resistance than heads without tribomaterial coverage33. The variation in potential is, however, only an indicator of corrosion tendency, and further investigations are necessary to fully understand the interaction of the tribomaterial on the corrosive behavior in metal-on-metal joints. In particular, it is unclear how the corrosive properties of the tribomaterial change in the presence of contact movement, as is the case during articulation of the hip joint implants.
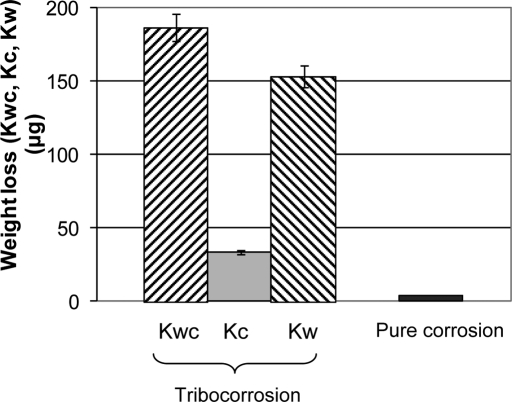
To better understand the synergism between wear and corrosion34, the following study was conducted: a 28-mm-diameter alumina ball was articulated against cylindrical pins of high-carbon CoCrMo (0.24% carbon, 12-mm diameter; ATI Allvac, Monroe, North Carolina) in a specifically designed test rig incorporated in an electrochemical cell. The flat surfaces of the pins were polished to a roughness Ra better than 10 nm with a nanocrystalline microstructure at the surface. Under potentiostatic conditions at a fixed potential close to the free potential, the wear tests were performed with use of a normal load of 16 N with ball rotation of ±15° at 1 Hz for 100,000 cycles. Diluted bovine calf serum (30 g/L protein) was used as the lubricant and electrolyte. Electrochemical impedance spectroscopy measurements were taken before and after the test. The total material loss was determined by three-dimensional profilometry, and the loss due to corrosion was calculated with Faraday's law. It was found that the wear scars on the pins showed the formation of a tribolayer. The weight loss due to wear (Kw) accounted for the majority of the total loss (Kwc), but the loss due to corrosion (Kc) was non-negligible, as shown in Figure 2 (where Kwc = Kw + Kc). The synergy ratio Kc/Kw of 0.26 indicates that corrosion plays an important role in the overall degradation process35, which is, a priori, not expected from corrosion experiments without mechanical activation of the surface. Therefore, decreasing or eliminating the synergistic corrosion component would lead to considerable reductions in the material loss of metal-on-metal bearings. Methods to accomplish such reductions could entail alloy formulations and/or surface textures that exhibit a more robust tribolayer. For example, a microtopological surface could anchor the tribolayer and thus help to better withstand shear forces. At the same time, the valleys could serve to capture particles during the initial wear cycles (referred to as the run-in period) of the bearing.
Fig. 2.
Total weight loss (Kwc) of high-carbon wrought CoCrMo in bovine calf serum after tribocorrosive attack (100,000 cycles) at a fixed potential close to the free corrosion potential (Ecorr). The total weight loss is a combined effect of corrosion and wear, and its respective parts Kc and Kw are shown accordingly. Note that the weight loss due to corrosion (Kc) is much higher than the weight loss during pure corrosion at similar Ecorr conditions.
Wear Particle Characteristics
Multiple studies have described metal-on-metal particle characteristics, but they remain difficult to compare because of differences in the metallurgy of the prosthetic component alloys and differences in the isolation and characterization protocols that can affect the particles as demonstrated by Catelas et al.36,37. Despite that, there is now a general consensus that the average particle size ranges from about 30 to 100 nm4-6. Particle shape and composition, however, vary more from study to study. To understand the potential effects of the alloy and the number of loading cycles in a hip simulator (i.e., in vitro), as well as implant design and implantation time in vivo, Catelas et al. conducted several studies5,38,39. The same particle isolation and characterization protocol (developed by those authors and recently incorporated in International Organization for Standardization [ISO] 17853)40 was used for the serum in a hip simulator and for the tissue samples to avoid confounding factors inherent to the use of different protocols.
In a first study comparing the effects of alloys and the number of loading cycles in a hip simulator, results showed that, for all three CoCrMo alloys tested (high-carbon wrought, low-carbon wrought, and cast), the majority of the particles were round to oval and averaged about 52 nm in length with only small differences due to the alloy5. Energy-dispersive x-ray analysis revealed that these particles (which appeared lightly contrasted under transmission electron microscopy) contained Cr and O, but no Co, and were therefore likely chromium oxide particles, as they appeared lightly contrasted under transmission electron microscopy. However, some CoCrMo particles (darker under transmission electron microscopy) were also found, depicting varying ratios of Co and Cr, and were either small round or larger elongated particles. (Figure 1, C illustrates particles generated with the cast alloy.) The small round CoCrMo also usually depicted a peak of carbon. The proportion of these CoCrMo particles appeared to be associated with the number of loading cycles in the hip simulator, with more CoCrMo particles during the run-in than the steady-state wear period. Finally, more CoCrMo particles were found with the low-carbon wrought alloy than with the high-carbon wrought and cast alloys, although the majority of the particles remained chromium oxides.
When the effect of implant design and implantation time were analyzed, the results showed that, with the exception of hip replacements implanted for less than fifteen months that depicted almost exclusively chromium oxide particles, all other implants showed the presence of some CoCrMo particles, mainly needle-shaped. The percentage of these needle-shaped particles was the highest with hip replacements implanted for more than twenty years38. The average particle lengths (49 to 57 nm) were not markedly different between the different designs and implantation times except for the current design implants retrieved within fifteen months, which had a significantly smaller average length of 39 nm. No significant differences in particle characteristics were detected between hip resurfacing and stem-type devices. Overall, that study showed that implantation time appeared to affect particle characteristics (mainly the amount of CoCrMo particles), but implant design did not have a significant influence. Many manufacturers treat the surfaces before packaging (e.g., with nitric acid to increase the thickness of the protecting chromium-oxide layer). Hence, differences observed in particle characteristics between the very short (less than fifteen months) and longer-term implants might have been related to such chemical treatments. Once the passivation layer is worn away, CoCrMo particles are generated as well. It should, however, also be noted that the very short-term implants were revised for early clinical complications (e.g., dislocation or heterotopic ossification). The wear mechanisms and the wear rates might thus have been different and possibly contributed to the differences observed in the particle characteristics, especially considering that the volumetric wear of retrievals reported in the literature has suggested that the initial wear rates should be high41,42. As such, it can be concluded that any differences between original and more current metal-on-metal implant designs would probably be determined by the overall implant survival time rather than by differences in particle characteristics themselves38.
Finally, a study compared particles generated in the orbital bearing simulator (up to two million cycles) or isolated from tissues retrieved after one to forty-three months39. The results showed that the particles isolated from two patients at twenty-three and forty-three months had an average length, shape, and composition that were most comparable with those of the particles generated in the hip simulator during the run-in wear period (particles analyzed were generated between 0 and 0.25 million cycles). In this study, implants made of the same cast alloy to the same specifications and by the same manufacturer (Wright Medical Technology, Arlington, Tennessee) were selected to avoid confounding factors inherent to the metallurgy of the component alloys. Overall, this study supported the results of a large clinical retrieval study, suggesting that a run-in wear regime might occur in vivo for some six to thirty-six months41.
Wear Particle Origin
Some of the previous studies that have identified chromium oxide particles in vivo had hypothesized that Co may have been dissolved in the tissue environment, leaving Cr and O4. Other studies that have described particles with varying proportions of cobalt, ranging from approximately that of the original alloy material (about 57%) to zero, had interpreted this reduction as an indication of in vivo corrosion43. Another possibility that has been reported is that chromium oxide particles may be produced by wear of the passivation layer covering the implant surface, or may originate from some oxidized chromium carbides5. However, after run-in of the implant, the oxide film likely exhibits a thickness of 2 nm to a maximum of 10 nm only44, which would be too thin to be the origin of the wear-generated chromium oxide particles45. It is, however, possible during the run-in period of the implant. Catelas et al. further suggested that small CoCrMo particles (depicting a peak of carbon and a higher peak of Cr than Co) may originate from carbides, and larger ones (depicting a higher peak of Co than Cr) may come from the bulk material. Energy-dispersive x-ray analysis peaks were considered to be likely proportional to the Co and Cr elements, considering the very similar atomic number of Co and Cr elements5. However, further analytic work was still required to determine with more certainty the origin of these particles.
In a recent study, Pourzal et al. used energy-filtered transmission electron microscopy and diffraction pattern analysis to gain further knowledge on the chemical composition and the crystalline structure of the particles45. Results showed that the smallest chromium oxide particles generated from a high-carbon wrought alloy cycled in an EndoLab hip simulator had the lattice structure of Cr2O3 and depicted lattice defects, suggesting that these particles had been likely subjected to high mechanical and chemical reactions. These small (5 to 15-nm) Cr2O3 particles appeared to be flaking off larger particles that were depicting a very high intensity of oxygen, as well as chromium, and only background of Co. Other larger particles depicted Co locally. It is possible that these particles may have been generated from the tribomaterial as described earlier in the present paper and in the literature17,20-22. Previous studies have noted that in tribosystems operating in the ultra-low wear regime, particles are generated from tribolayers formed in situ rather than in the underlying deformed subsurface zones46,47. Nevertheless, the exact mechanism of particle detachment and the absence of Co in most of the particles are still not fully understood, since particles should exhibit Co if they are originating from the nanocrystalline zone. It is possible that particles become oxidized by mechanical mixing within the tribolayer or in the contact area between the femoral head and the cup after their detachment45. Through oxidation, these metallic particles could be a source of metal ions.
Biological Effects
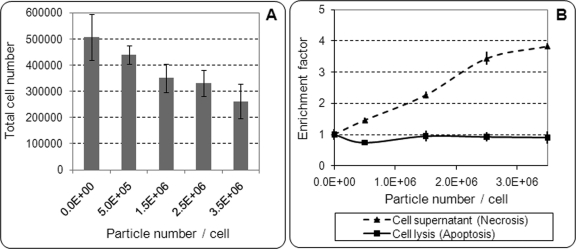
Most of the cell culture studies on the biological effects of metal-on-metal particles have been conducted with use of CoCrMo particles, mainly in the micrometer range48-51. Few studies have used CoCrMo particles in the nanometer range52,53, but the biological effects of chromium oxide particles have not been described specifically. This is likely related to the lack of knowledge about the precise characteristics of the metal-on-metal particles until recently, as well as the difficulty of generating chromium oxide particles in the nanometer range for cell culture studies. In a recent study analyzing the macrophage response to commercial nanometer-sized Cr2O3 particles, VanOs et al. demonstrated that 60-nm-sized Cr2O3 particles, when present in very large concentrations (up to 3.5 million particles per cell), could induce a significant decrease in the total cell number (Fig. 3) and a significant increase in cell necrosis54. However, tumor necrosis factor-α release remained at low levels, similar to those in control samples (i.e., cells with no particles). This study suggested that overall, nanometer-sized Cr2O3 particles, a stable form of chromium oxide ceramic, induced a lower inflammatory response compared with previously reported micrometer-sized ultra-high molecular weight polyethylene particles at lower concentrations55.
Fig. 3.
Effect of 60-nm Cr2O3 particle number (per cell) on total J774 mouse macrophage number after twenty-four-hour incubation. A half-million macrophages were initially incubated for each condition, in tubes containing 1 mL of culture medium. Tubes were put in rotation to allow constant resuspension of cells and particles. After twenty-four-hour incubation, total cell numbers (viable and dead) were measured with use of a hemocytometer. Results are given as the mean and standard deviation of three experiments performed in triplicates.
In vivo studies have noted the presence of cytokines associated with osteolysis in tissues surrounding metal-on-metal implants, demonstrating the presence of a nonspecific inflammatory response as previously described with metal-on-polyethylene implants, but the extent of such response and the presence of foreign body-type giant cells appeared to be less substantial in tissues surrounding metal-on-metal implants7,56,57. In addition to this nonspecific inflammatory response, the presence of lymphocytic infiltrations around metal-on-metal implants has generated a cause for concern56,58-61, suggesting a potential hypersensitivity response to metal wear products. The mechanisms and causes of this hypersensitivity remain largely unknown. They may be due to metal degradation products that can complex with serum proteins1-3 to form haptens62, to which individuals may have a different response threshold63. It is also possible that proteins complexed with the particles detaching from the tribolayers, as described earlier, form haptens, especially considering that these proteins are likely denatured from exposure to high shear rates and elevated temperatures. Implant-related hypersensitivity response has been thought to be a delayed type-IV reaction62. However, in a study on failed modern metal-on-metal total hip replacements, Willert et al. reported the presence of plasma cells, B lymphocytes, and massive fibrin exudation that is not characteristic of a delayed type-IV hypersensitivity reaction61. The authors described this reaction histologically as an aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) or as a lymphocyte-dominated immunological answer (LYDIA).
More recently, some studies have described early adverse tissue reactions, including pseudotumors (described as soft-tissue masses or fluid-filled bursae)8,9 that can lead to devastating short-term implant failures. Some studies noted revision rates of up to 3.2% for hip resurfacing arthroplasty at thirty-five months and 6% for femoral stem-type devices at forty-one months10. These alarming reactions have led to growing concerns within the orthopaedic community during the last year. The causes of these reactions, however, are not well understood. Some studies have described an association between these reactions and greater wear of the retrieved components10-12. The importance of implant size and implant design (clearance and cover [arc angle]) has been noted. Also, acetabular component positioning and femoral head-neck offset influence the risk of impingement and edge-loading that are usually associated with high wear rates. On the other hand, Campbell et al. reported that, of thirty-two hip replacements revised because of pseudotumor, some patients had a high ALVAL score in the absence of high wear, suggesting a hypersensitivity origin13. Hence, the origin of these early adverse reactions is likely multifactorial and may be due to excessive wear, but also possibly due to metal hypersensitivity, a combination of the two, or even a yet unknown cause13. Pseudotumor-like reactions have also been reported in non-metal-on-metal bearings64-66. In these cases, the histological findings showed accumulations of macrophages and giant cells, suggesting an excessive wear origin.
Finally, metal soluble and particulate products have generated concerns about potential genotoxicity, especially considering their propensity to disseminate systemically, as depicted by elevated levels of metal ions in serum, urine, whole blood, and erythrocytes of patients with metal-on-metal implants67-70, and particles observed in the liver and the spleen71. Multiple studies have shown the potential toxicity of Co and Cr ions, with Co2+ being more toxic than Cr3+ in several investigations72-75 and Cr6+ being rapidly soluble and able to induce both mutagenic and replication-blocking DNA lesions in cultured cells76. Milosev and Remskar identified small amounts of Cr6+ in the surface layer of retrieved implants77. In addition, some studies have described an increase of aneuploidy, chromosome translocation, and DNA damage in cultured cells exposed to CoCrMo particles in vitro, or a higher chromosomal aberration rate in cells adjacent to the prosthesis at revision surgery78,79. Overall, those studies showed potential genotoxicity of metal ions and wear particles. However, the clinical consequences of the potential DNA and chromosome damages, if any, remain unknown, and future epidemiologic studies should include comparison between patients with implants of different composition and at long postoperative survivals63.
Conclusion
Tribochemical reactions play an important role in the wear of metal-on-metal joints. The generated tribomaterial, which progressively forms by mechanical mixing of the upmost, nanocrystalline zone of the metal surface with proteins from the synovial fluid, governs the wear rate and influences the corrosive behavior of the bearing. Initial studies have suggested that such a tribolayer has beneficial effects on the wear rate; however, a stronger retention may be warranted to better withstand high-stress situations. The influence of the tribolayer on the corrosion behavior needs to be further evaluated. Much information has been gained on wear particles, which may initially originate from the passivation layer covering the implant surface, and then detach from the tribolayer. However, the exact mechanism of particle detachment and the absence of Co in most of the particles remain to be further elucidated.
The inflammatory response observed surrounding metal-on-metal implants appears to be lower than that around conventional metal-on-polyethylene implants. However, in addition to this nonspecific inflammatory response, metallic by-products complexed with serum or synovial fluid proteins or denatured proteins possibly originating from the tribolayer covering the implant may lead to a T lymphocyte-mediated immune response. Such response along with excessive wear, combined or separately, may be at the origin of the early adverse tissue reactions that have been recently reported in some patients with metal-on-metal implants. Unfortunately, as of now, there is no generally accepted screening method to assess the patient's risk of developing such reactions. However, future development of new methods to improve the tribolayer retention and minimize pitting corrosion may minimize the clinical impact of implant wear and immune responses.
Acknowledgments
Note: The authors thank Drs. Alfons Fischer, Joshua Jacobs, Michel Laurent, Mathew T. Mathew, Robin Pourzal, and Robilyn VanOs for valuable discussion and access to investigation material. The ASR implant was provided by Dr. Morlock.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the Natural Sciences and Engineering Research Council of Canada, the Canada Research Chairs Program, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (NIH RC2 AR058993). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Merritt K, Brown SA, Sharkey NA. Blood distribution of nickel, cobalt, and chromium following intramuscular injection into hamsters. J Biomed Mater Res. 1984;18:991-1004 [DOI] [PubMed] [Google Scholar]
- 2.Hallab NJ, Mikecz K, Vermes C, Skipor A, Jacobs JJ. Orthopaedic implant related metal toxicity in terms of human lymphocyte reactivity to metal-protein complexes produced from cobalt-base and titanium-base implant alloy degradation. Mol Cell Biochem. 2001;222:127-36 [PubMed] [Google Scholar]
- 3.Tkaczyk C, Huk OL, Mwale F, Antoniou J, Zukor DJ, Petit A, Tabrizian M. Investigation of the binding of Cr(III) complexes to bovine and human serum proteins: a proteomic approach. J Biomed Mater Res A. 2010;94:214-22 [DOI] [PubMed] [Google Scholar]
- 4.Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA, Amstutz HC. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res. 1998;42:103-11 [DOI] [PubMed] [Google Scholar]
- 5.Catelas I, Bobyn JD, Medley JB, Krygier JJ, Zukor DJ, Huk OL. Size, shape, and composition of wear particles from metal-metal hip simulator testing: effects of alloy and number of loading cycles. J Biomed Mater Res A. 2003;67:312-27 [DOI] [PubMed] [Google Scholar]
- 6.Brown C, Williams S, Tipper JL, Fisher J, Ingham E. Characterisation of wear particles produced by metal on metal and ceramic on metal hip prostheses under standard and microseparation simulation. J Mater Sci Mater Med. 2007;18:819-27 [DOI] [PubMed] [Google Scholar]
- 7.Doorn PF, Mirra JM, Campbell PA, Amstutz HC. Tissue reaction to metal on metal total hip prostheses. Clin Orthop Relat Res. 1996;329 Suppl:S187-205 [DOI] [PubMed] [Google Scholar]
- 8.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847-51 [DOI] [PubMed] [Google Scholar]
- 9.Clayton RA, Beggs I, Salter DM, Grant MH, Patton JT, Porter DE. Inflammatory pseudotumor associated with femoral nerve palsy following metal-on-metal resurfacing of the hip. A case report. J Bone Joint Surg Am. 2008;90:1988-93 [DOI] [PubMed] [Google Scholar]
- 10.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92:38-46 [DOI] [PubMed] [Google Scholar]
- 11.Kwon YM, Glyn-Jones S, Simpson DJ, Kamali A, McLardy-Smith P, Gill HS, Murray DW. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J Bone Joint Surg Br. 2010;92:356-61 [DOI] [PubMed] [Google Scholar]
- 12.De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291-7 [DOI] [PubMed] [Google Scholar]
- 13.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:2847-51 [DOI] [PubMed] [Google Scholar]
- 15.Kurtz SM, Röder C, Lau E, Ong K, Widmer M, Maravic M, Gomez-Barrena E, Pina F, Manno V, Geesink R. International Survey of Primary and Revision Total Hip Replacement. : Transactions Volume 35, 56 th Annual Meeting of the Orthopaedic Research Society; 2010 Mar 6-9. New Orleans: Orthopaedic Research Society; Paper no. 214 [Google Scholar]
- 16.Wimmer MA, Fischer A. Tribology. : Callaghan JJ, Rosenberg AG, Rubash HE, editors. The adult hip. 2nd ed, vol 1 Philadelphia: Lippincott Williams & Wilkins; 2007. p 215-26 [Google Scholar]
- 17.Wimmer MA, Loos J, Nassutt R, Heitkemper M, Fischer A. The acting wear mechanisms on metal-on-metal hip joint bearings: in vitro results. Wear. 2001;250:129-39 [Google Scholar]
- 18.Wimmer MA, Sprecher C, Hauert R, Täger G, Fischer A. Tribochemical reaction on metal-on-metal hip joint bearings: a comparison between in-vitro and in-vivo results. Wear. 2003;255:1007-14 [Google Scholar]
- 19.Rigney DA, Chen LH, Naylor MGS, Rosenfield AR. Wear processes in sliding systems. Wear. 1984;100:195-219 [Google Scholar]
- 20.Büscher R, Täger G, Dudzinski W, Gleising B, Wimmer MA, Fischer A. Subsurface microstructure of metal-on-metal hip joints and its relationship to wear particle generation. J Biomed Mater Res B Appl Biomater. 2005;72:206-14 [DOI] [PubMed] [Google Scholar]
- 21.Büscher R, Fischer A. The pathways of dynamic recrystallization in all-metal hip joints. Wear. 2005;259:887-97 [Google Scholar]
- 22.Wimmer MA, Fischer A, Büscher R, Pourzal R, Sprecher C, Hauert R, Jacobs JJ. Wear mechanisms in metal-on-metal bearings: the importance of tribochemical reaction layers. J Orthop Res. 2010;28:436-43 [DOI] [PubMed] [Google Scholar]
- 23.Rigney DA. Transfer, mixing and associated chemical and mechanical processes during the sliding of ductile materials. Wear. 2000;245:1-9 [Google Scholar]
- 24.Pourzal R, Theissmann R, Williams S, Gleising B, Fisher J, Fischer A. Subsurface changes of a MoM hip implant below different contact zones. J Mech Behav Biomed Mater. 2009;2:186-91 [DOI] [PubMed] [Google Scholar]
- 25.Pourzal R, Theissmann R, Morlock M, Fischer A. Micro-structural alterations within different areas of articulating surfaces of a metal-on-metal hip resurfacing system. Wear. 2009;267:689-94 [Google Scholar]
- 26.Williams S, Stewart TD, Ingham E, Stone MH, Fisher J. Metal-on-metal bearing wear with different swing phase loads. J Biomed Mater Res B Appl Biomater. 2004;70:233-9 [DOI] [PubMed] [Google Scholar]
- 27.Morlock MM, Bishop N, Zustin J, Hahn M, Rüther W, Amling M. Modes of implant failure after hip resurfacing: morphological and wear analysis of 267 retrieval specimens. J Bone Joint Surg Am. 2008;90 Suppl 3:89-95 [DOI] [PubMed] [Google Scholar]
- 28.Angadji A, Royle M, Collins SN, Shelton JC. Influence of cup orientation on the wear performance of metal-on-metal hip replacements. Proc Inst Mech Eng H. 2009;223:449-57 [DOI] [PubMed] [Google Scholar]
- 29.ASTM G61-86 (2009) Standard test method for conducting cyclic potentiodynamic polarization measurements for localized corrosion susceptibility of iron-, nickel-, or cobalt-based alloys. West Conshohocken, PA: ASTM International; 2009. DOI: 10.1520/G0061-86R09 [Google Scholar]
- 30.Muñoz AI, Mischler S. Interactive effects of albumin and phosphate ions on the corrosion of CoCrMo implant alloy. J Electrochem Soc. 2007;154:C562-70 [Google Scholar]
- 31.Mathew MT, Pourzal R, Fischer A, Hallab N, Jacobs J, Wimmer M. Electrochemical behavior of CoCrMo alloy: effect of protein on corrosion kinetics. Trans Orthop Res Soc. 2010;35:2269 [Google Scholar]
- 32.Hallam P, Haddad F, Cobb J. Pain in the well-fixed, aseptic titanium hip replacement. The role of corrosion. J Bone Joint Surg Br. 2004;86:27-30 [PubMed] [Google Scholar]
- 33.Mathew MT, Nagelli CV, Runa MJ, Pourzal R, Fischer A, Laurent M, Jacobs JJ, Wimmer MA. Does the tribolayer on a Metal-on-Metal (MoM) hip joint exhibit superior corrosion behavior? Trans Orthop Res Soc. 2011;36:384 [Google Scholar]
- 34.Mathew MT, Pai PS, Pourzal R, Fischer A, Wimmer MA. Significance of tribocorrosion in biomedical applications: overview and current status. Adv Tribol. 2009;ID250986 [Google Scholar]
- 35.Stack MM, Jawan H, Mathew MT. On the construction of micro-abrasion maps for a steel/polymer couple in corrosive environments. Tribol Int. 2005;38:848-56 [Google Scholar]
- 36.Catelas I, Bobyn JD, Medley JB, Krygier JJ, Zukor DJ, Petit A, Huk OL. Effects of digestion protocols on the isolation and characterization of metal-metal wear particles. I. Analysis of particle size and shape. J Biomed Mater Res. 2001;55:320-9 [DOI] [PubMed] [Google Scholar]
- 37.Catelas I, Bobyn JD, Medley JJ, Zukor DJ, Petit A, Huk OL. Effects of digestion protocols on the isolation and characterization of metal-metal wear particles. II. Analysis of ion release and particle composition. J Biomed Mater Res. 2001;55:330-7 [DOI] [PubMed] [Google Scholar]
- 38.Catelas I, Campbell PA, Bobyn JD, Medley JB, Huk OL. Wear particles from metal-on-metal total hip replacements: effects of implant design and implantation time. Proc Inst Mech Eng H. 2006;220:195-208 [DOI] [PubMed] [Google Scholar]
- 39.Catelas I, Medley JB, Campbell PA, Huk OL, Bobyn JD. Comparison of in vitro with in vivo characteristics of wear particles from metal-metal hip implants. J Biomed Mater Res B Appl Biomater. 2004;70:167-78 [DOI] [PubMed] [Google Scholar]
- 40.ISO17853: 2010 Wear of implant materials- polymer and metal wear particles- isolation and characterization. TC 150/SC4. Geneva: International Organization for Standardization; 2010 [Google Scholar]
- 41.Rieker C, Shen M, Köttig P. In vivo tribological performance of 177 metal on metal hip articulations. : Rieker C, Oberholzer S, Wyss U, editors. World Tribology Forum in Arthroplasty. Bern: Hans Huber; 2001. p 135-40 [Google Scholar]
- 42.Rieker CB, Köttig P. In vivo tribological performance of 231 metal-on-metal hip articulations. Hip Int. 2002;12:73-6 [DOI] [PubMed] [Google Scholar]
- 43.Shahgaldi BF, Heatley FW, Dewar A, Corrin N. In vivo corrosion of cobalt-chromium and titanium wear particles. J Bone Joint Surg Br. 1995;77:962-6 [PubMed] [Google Scholar]
- 44.Gilbert JL. Basic science: metals. : Callaghan JJ, Rosenberg AG, Rubash HE, editors. The adult hip. 2nd ed, vol 1 Philadelphia: Lippencott-Raven Press; 2005. p 128-43 [Google Scholar]
- 45.Pourzal R, Catelas I, Theissmann R, Kaddick C, Fischer A. Characterization of wear particles generated from CoCrMo alloy under sliding wear conditions. Wear. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godet M. The third-body approach: a mechanical view of wear. Wear. 1984;100:437-52 [Google Scholar]
- 47.Scherge M, Martin JM, Pöhlmann K. Characterization of wear debris of systems operated under low wear-rate conditions. Wear. 2006;260:458-61 [Google Scholar]
- 48.Wooley PH, Petersen S, Song Z, Nasser S. Cellular immune responses to orthopaedic implant materials following cemented total joint replacement. J Orthop Res. 1997;15:874-80 [DOI] [PubMed] [Google Scholar]
- 49.Trindade MC, Lind M, Sun D, Schurman DJ, Goodman SB, Smith RL. In vitro reaction to orthopaedic biomaterials by macrophages and lymphocytes isolated from patients undergoing revision surgery. Biomaterials. 2001;22:253-9 [DOI] [PubMed] [Google Scholar]
- 50.Kanaji A, Caicedo MS, Virdi AS, Sumner DR, Hallab NJ, Sena K. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation. Bone. 2009;45:528-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mostardi RA, Kovacik MW, Ramsier RD, Bender ET, Finefrock JM, Bear TF, Askew MJ. A comparison of the effects of prosthetic and commercially pure metals on retrieved human fibroblasts: the role of surface elemental composition. Acta Biomater. 2010;6:702-7 [DOI] [PubMed] [Google Scholar]
- 52.Germain MA, Hatton A, Williams S, Matthews JB, Stone MH, Fisher J, Ingham E. Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro. Biomaterials. 2003;24:469-79 [DOI] [PubMed] [Google Scholar]
- 53.Brown C, Fisher J, Ingham E. Biological effects of clinically relevant wear particles from metal-on-metal hip prostheses. Proc Inst Mech Eng H. 2006;220:355-69 [DOI] [PubMed] [Google Scholar]
- 54.VanOs R, Beaulé PE, Catelas I. Macrophage response to nanometer-size chromium oxide particles. Trans Soc Biomat. 2010;32:642. [DOI] [PubMed] [Google Scholar]
- 55.Petit A, Catelas I, Antoniou J, Zukor DJ, Huk OL. Differential apoptotic response of J774 macrophages to alumina and ultra-high-molecular-weight polyethylene particles. J Orthop Res. 2002;20:9-15 [DOI] [PubMed] [Google Scholar]
- 56.Campbell PA, Wang M, Amstutz HC, Goodman SB. Positive cytokine production in failed metal-on-metal total hip replacements. Acta Orthop Scand. 2002;73:506-12 [DOI] [PubMed] [Google Scholar]
- 57.Catelas I, Campbell PA, Dorey F, Frausto A, Mills BG, Amstutz HC. Semi-quantitative analysis of cytokines in MM THR tissues and their relationship to metal particles. Biomaterials. 2003;24:4785-97 [DOI] [PubMed] [Google Scholar]
- 58.Al-Saffar N. Early clinical failure of total joint replacement in association with follicular proliferation of B-lymphocytes: a report of two cases. J Bone Joint Surg Am. 2002;84:2270-3 [DOI] [PubMed] [Google Scholar]
- 59.Böhler M, Kanz F, Schwarz B, Steffan I, Walter A, Plenk H, Jr., Knahr K. Adverse tissue reactions to wear particles from Co-alloy articulations, increased by alumina-blasting particle contamination from cementless Ti-based total hip implants. A report of seven revisions with early failure. J Bone Joint Surg Br. 2002;84:128-36 [DOI] [PubMed] [Google Scholar]
- 60.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18-27 [DOI] [PubMed] [Google Scholar]
- 61.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28-36 [DOI] [PubMed] [Google Scholar]
- 62.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28:5044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Catelas I. Biological consequences of wear particles. In: Streicher RM, editor. Tribology and bearing surfaces in total joint replacements. Kerala, India: Transworld Research Network; In press [Google Scholar]
- 64.Griffiths HJ, Burke J, Bonfiglio TA. Granulomatous pseudotumors in total joint replacement. Skeletal Radiol. 1987;16:146-52 [DOI] [PubMed] [Google Scholar]
- 65.Howie DW, Cain CM, Cornish BL. Pseudo-abscess of the psoas bursa in failed double-cup arthroplasty of the hip. J Bone Joint Surg Br. 1991;73:29-32 [DOI] [PubMed] [Google Scholar]
- 66.Leigh W, O'Grady P, Lawson EM, Hung NA, Theis JC, Matheson J. Pelvic pseudotumor: an unusual presentation of an extra-articular granuloma in a well-fixed total hip arthroplasty. J Arthroplasty. 2008;23:934-8 [DOI] [PubMed] [Google Scholar]
- 67.Skipor AK, Campbell PA, Patterson LM, Amstutz HC, Schmalzried TP, Jacobs JJ. Serum and urine metal levels in patients with metal-on-metal surface arthroplasty. J Mater Sci Mater Med. 2002;13:1227-34 [DOI] [PubMed] [Google Scholar]
- 68.MacDonald SJ, McCalden RW, Chess DG, Bourne RB, Rorabeck CH, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;406:282-96 [DOI] [PubMed] [Google Scholar]
- 69.Clarke MT, Lee PT, Arora A, Villar RN. Levels of metal ions after small- and large-diameter metal-on-metal hip arthroplasty. J Bone Joint Surg Br. 2003;85:913-7 [PubMed] [Google Scholar]
- 70.Jacobs JJ, Skipor AK, Campbell PA, Hallab NJ, Urban RM, Amstutz HC. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty. 2004;19(8 Suppl 3):59-65 [DOI] [PubMed] [Google Scholar]
- 71.Urban RM, Tomlinson MJ, Hall DJ, Jacobs JJ. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty. J Arthroplasty. 2004;19(8 Suppl 3):94-101 [DOI] [PubMed] [Google Scholar]
- 72.Sun ZL, Wataha JC, Hanks CT. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J Biomed Mater Res. 1997;34:29-37 [DOI] [PubMed] [Google Scholar]
- 73.Morais S, Sousa JP, Fernandes MH, Carvalho GS. In vitro biomineralization by osteoblast-like cells. I. Retardation of tissue mineralization by metal salts. Biomaterials. 1998;19:13-21 [DOI] [PubMed] [Google Scholar]
- 74.Catelas I, Petit A, Zukor DJ, Antoniou J, Huk OL. TNF-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro-qualitative analysis of apoptosis. Biomaterials. 2003;24:383-91 [DOI] [PubMed] [Google Scholar]
- 75.Hallab NJ, Anderson S, Caicedo M, Brasher A, Mikecz K, Jacobs JJ. Effects of soluble metals on human peri-implant cells. J Biomed Mater Res A. 2005;74:124-40 [DOI] [PubMed] [Google Scholar]
- 76.Quievryn G, Peterson E, Messer J, Zhitkovich A. Genotoxicity and mutagenicity of chromium(VI)/ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry. 2003;42:1062-70 [DOI] [PubMed] [Google Scholar]
- 77.Milosev I, Remskar M. In vivo production of nanosized metal wear debris formed by tribochemical reaction as confirmed by high-resolution TEM and XPS analyses. J Biomed Mater Res A. 2009;91:1100-10 [DOI] [PubMed] [Google Scholar]
- 78.Papageorgiou I, Yin Z, Ladon D, Baird D, Lewis AC, Sood A, Newson R, Learmonth ID, Case CP. Genotoxic effects of particles of surgical cobalt chrome alloy on human cells of different age in vitro. Mutat Res. 2007;619:45-58 [DOI] [PubMed] [Google Scholar]
- 79.Case CP, Langkamer VG, Howell RT, Webb J, Standen G, Palmer M, Kemp A, Learmonth ID. Preliminary observations on possible premalignant changes in bone marrow adjacent to worn total hip arthroplasty implants. Clin Orthop Relat Res. 1996;329 Suppl:S269-79 [DOI] [PubMed] [Google Scholar]