Abstract
PURPOSE
NOD1 plays an important role in host defense and recognizes the minimal component of bacterial cell walls, meso-diaminopimelic acid (iE-DAP). Polymorphisms in NOD1 are associated with autoinflammatory diseases characterized by uveitis such as Crohn’s disease and sarcoidosis. NOD1 is homologous to NOD2, which is responsible for an autosomal dominant form of uveitis. Nonetheless, the role of NOD1 in intraocular inflammation has not been explored. The induction of uveitis by iE-DAP in mice and the potential contribution of interleukin (IL)-1β were investigated.
METHODS
BALB/c mice or mice deficient in caspase-1 or IL-1R1 and their congenic controls were injected intravitreally with iE-DAP or saline. The time course, dose response, and contribution of IL-1β to ocular inflammation were quantified by intravital video microscopy, histology, and immunohistochemistry. NOD1 and IL-1β were measured in eye tissue by immunoblotting and ELISA.
RESULTS
NOD1 protein is expressed in the eye and promotes ocular inflammation in a dose- and time-dependent fashion. The authors previously defined the role of IL-1β in NOD2 uveitis and tested whether NOD1 and NOD2 used similar mechanisms. Treatment with iE-DAP significantly increased IL-1β, which was caspase-1 dependent. However, in contrast to NOD2, caspase-1 and IL-1R1 were essential mediators of iEDAP– induced uveitis, suggesting that NOD1 and NOD2 induce ocular inflammation by distinct mechanisms involving IL-1β.
CONCLUSIONS
These findings demonstrate that NOD1 is expressed within the eye and that its activation results in uveitis in an IL-1β–dependent mechanism. Characterizing the differences between NOD1 and NOD2 responses may provide insight into the pathogenesis of uveitis.
Uveitis is a complex, immune-mediated set of diseases whose underlying mechanisms have yet to be clearly defined. It is becoming increasingly apparent that innate immunity plays a determinant role in previously considered autoimmune diseases. However, its role with respect to mechanisms of uveitis has been underappreciated. Pattern recognition receptors (PRRs), namely Toll-like receptors (TLRs) and nucleotide oligomerization domain (NOD)-like receptors (NLRs), are central to the induction of innate immunity through their capacity to detect pathogen-associated molecular patterns (PAMPs). PAMPs that trigger innate responses have also been implicated in the induction of autoinflammatory responses in several diseases associated with uveitis, such as sarcoidosis,1 Behçet’s disease,2 reactive arthritis,3 ankylosing spondylitis,4 and inflammatory bowel disease. Consistent with this, TLRs are expressed within the eye,5 and uveitis resulting from treatment with the TLR4 agonist lipopolysaccharide has been well characterized.6,7
NLRs are just as likely as TLRs to be crucial participants in the basic immunologic mechanisms involved in uveitis. Indeed, NLR family members have been identified as the genetic link between diverse autoinflammatory diseases,8,9 many of which are associated with ocular inflammation such as Muckle-Wells syndrome, neonatal onset multisystem inflammatory disease, familial cold urticaria, vitiligo, and Crohn’s disease. One NLR member in particular, NOD2, has been identified as the cause of the inflammatory disorder Blau syndrome, which is an autosomal dominant form of uveitis and granulomatous inflammation of the synovium and skin.10–14 Consistent with this, we have demonstrated that NOD2 is expressed in eye tissue15 and that its activation triggers uveitis in mice.16 Clearly, investigating the function of other NLR family members is important for understanding ocular inflammatory responses.
NOD2 is highly homologous with the NLR family member NOD1 in its structural domains and function. NOD1 and NOD2 share a C-terminal leucine-rich repeat (LRR) domain that is involved in PAMP recognition, a centrally located NOD domain, and an N-terminally located caspase recruitment domain (CARD) involved in mediating downstream signal transduction events.17 NOD1 and NOD2 play roles in host defense against invading bacterial pathogens through their ability to sense muropeptides derived from the bacterial cell wall component peptidoglycan (PGN). NOD1 recognizes molecules containing the minimal dipeptide meso-diaminopimelic acid (iE-DAP) produced by most Gram-negative bacteria.18 This is in contrast to NOD2, which senses muramyl dipeptide (MDP) and muramyl tripeptide (MTP) prevalent in Gram-positive and Gram-negative bacteria.19,20 In addition to pathogenic-derived ligands, some NLRs are thought to respond to danger signals derived from endogenous ligands.21,22 However, this has yet to be demonstrated for NOD1 or NOD2.
On recognition of its agonist, NOD1 appears to exert its inflammatory effects through the induction of signaling pathways similar to those of NOD2. As such, we hypothesized that, as with NOD2, the activation of NOD1 could elicit ocular inflammatory responses predisposing to uveitis. Both NOD1 and NOD2 require the kinase RIP2/RICK, which contains a CARD domain, for downstream signaling events leading to activation of the transcription factor NF-κB.23–25 Additional signaling pathways downstream of NOD1 have not been fully characterized. However, the enzyme caspase-1 has been implicated as a downstream effector of NOD1 in IL-1β production in vitro.26,27 Caspase-1 is the crucial enzymatic component of the “inflammasome,” a multiprotein complex responsible for the proteolytic cleavage of the proforms of IL-1β and other IL-1 family members to their secreted and active forms.28 Elucidation of how the inflammasome is regulated by other NLR family members (e.g., NALP3) has expanded our understanding of the molecular processes involved in autoinflammatory diseases such as Muckle-Wells syndrome and gout.29,30 We have demonstrated that IL-1β production in the eye induced by NOD2 activation by MDP requires caspase-1,31 suggesting that like NOD2, NOD1 may have a similar capacity to regulate IL-1β production within the eye.
In addition to its role in bacterial infection, it is plausible that NOD1 within the eye plays a role in the pathogenesis of inflammatory diseases with coincident uveitis. This postulate is based on the observations that polymorphisms in NOD1 have been associated with increased risk for diseases manifesting with uveitis, such as inflammatory bowel disease32 and sarcoidosis.33 However, the mechanisms through which NOD1 is involved in the pathogenesis of these diseases are still unclear. Undoubtedly, a better understanding of the intracellular events induced by NOD1 is crucial for our understanding not only of how the eye responds to infection but also for clarifying the pathogenesis of uveitis.
Given that the protein expression and function of NOD1 within the eye have yet to be explored, we sought to examine NOD1 expression in the eye and to test whether the activation of NOD1 results in ocular inflammation in mice. We defined the dose and kinetics of uveitis induced by the NOD1 agonist iE-DAP. We further explored the mechanism by which iE-DAP triggers uveitis to discern whether it is similar to that activated by NOD2 on MDP stimulation. As might be hypothesized, NOD1 functions like NOD2 to induce IL-1β production in the eye in a caspase-1–dependent mechanism. Deficiency in caspase-1 or IL-1R1 abrogated uveitis, identifying the IL-1 signaling pathway as an essential downstream mediator of NOD1-triggered ocular inflammation. This is in contrast to NOD2, which promotes IL-1β synthesis through caspase-1 but does not require IL-1 signaling events for the development of uveitis. Taken together, our findings establish a novel role for NOD1 in ocular inflammation and discern mechanisms distinguishing NOD1 from NOD2.
METHODS
Reagents
Synthetic iE-DAP and iE-Lys (an inactive derivative of iE-DAP) were purchased from Invivogen (San Diego, CA); MDP was purchased from Bachem (Torrance, CA). Peptides were dissolved in sterile saline for injections. All three reagents tested below the lower limit of detection of endotoxin activity by limulus amebocyte lysate assay. Mice were given intravitreal injections (2 µL volume) via a Hamilton syringe with a 30-gauge, half-inch needle.
Mice
Age-matched (8- to 10-week-old) female BALB/c mice or mice deficient in caspase-1 or IL-1R1 and their appropriate strain controls (nonobese diabetic [NOD] and C57BL/6, respectively) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were housed in a facility approved by the Association of Assessment and Accreditation of Laboratory Animal Care International. Procedures were carried out in accordance with National Institutes of Health and ARVO guidelines and Oregon Health and Science University Institutional Animal Care and Use policies.
Intravital Microscopy
Leukocyte responses within the vasculature and the extravascular tissue of the iris were assessed by intravital microscopy according to a previously established method.16 Briefly, at the time of imaging, animals were injected intraperitoneally with 35 mg/kg rhodamine 6G (Sigma, St. Louis, MO) and were anesthetized with 1.7% isoflurane. Digital images of the iris vasculature were captured with a black-and-white video camera (Kappa Scientific, Gleichen, Germany) on an epifluorescence intravital microscope (modified Orthoplan; Leica, Wetzlar, Germany) in three independent regions. Diameter and length of each vessel segment or iris tissue and leukocyte phenotype (rolling, adhering, infiltrating) were quantified off-line with Image J analysis software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html.), as previously described.16
Histology and Immunohistochemistry
Mouse eyes were prepared for histologic assessment as previously described.16 Briefly, whole eyes were dissected, fixed, and embedded in paraffin for sectioning. Seven-micrometer tissue sections were stained with hematoxylin and eosin. An observer masked to treatment groups quantified the number of leukocytes within the anterior chamber of seven sections for each eye (approximately every tenth slide of a whole eye sectioned completely). The mean number of leukocytes per section was then calculated for each mouse eye. For immunohistochemistry, tissue sections were deparaffinized, as discussed, incubated with 2% goat serum in PBS for 1 hour at room temperature, and incubated with 2.5 µg/mL rat anti–mouse Ly6G/GR1 antibody (R&D Systems, Minneapolis, MN) in a humidified atmosphere overnight at 4°C. Sections were washed and incubated in 1:500 dilution of goat anti–rat IgG antibody (eBioscience, San Diego, CA) overnight at 4°C, followed by extensive washing in PBS. Antibody-antigen complexes were visualized with the substrate diaminobenzidine (DAB) according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) and were counterstained with methyl green (Sigma). Slides were photographed at 400× with a microscope (DM500B; Leica, Wetzlar, Germany) and a digital camera (DC500; Leica).
Protein Analysis
Protein was extracted from whole eye tissue in the presence of protease inhibitors, as previously described.31 Protein concentrations were determined using a kit (BCA; Pierce-Endogen, Rockford, IL), and equal amounts of protein for each sample were measured for IL-1β by ELISA (R&D Systems). Expression of NOD1 was assessed by immunoblotting according to a modified protocol34 with the use of an anti–mouse/human NOD1 goat antibody (Santa Cruz Biotechnology, Santa Cruz, CA), which was detected with near infrared-fluorescence-labeled secondary antibody (IRDye-698; Licor-Odyssey, Lincoln, NE). Images were captured using commercially available software (Licor- Odyssey), and the fluorescence intensity of the bands was measured. Data were analyzed as a ratio of NOD1 expression to β-actin and expressed relative to saline controls.
Statistical Analysis
Data are represented as mean ± SEM. Mean differences between treatment and genotype were analyzed using two-way and one-way analyses of variance with Bonferroni test or t-test post hoc analyses. Differences were considered statistically significant when P < 0.05.
RESULTS
Expression of NOD1 in Murine Ocular Tissue
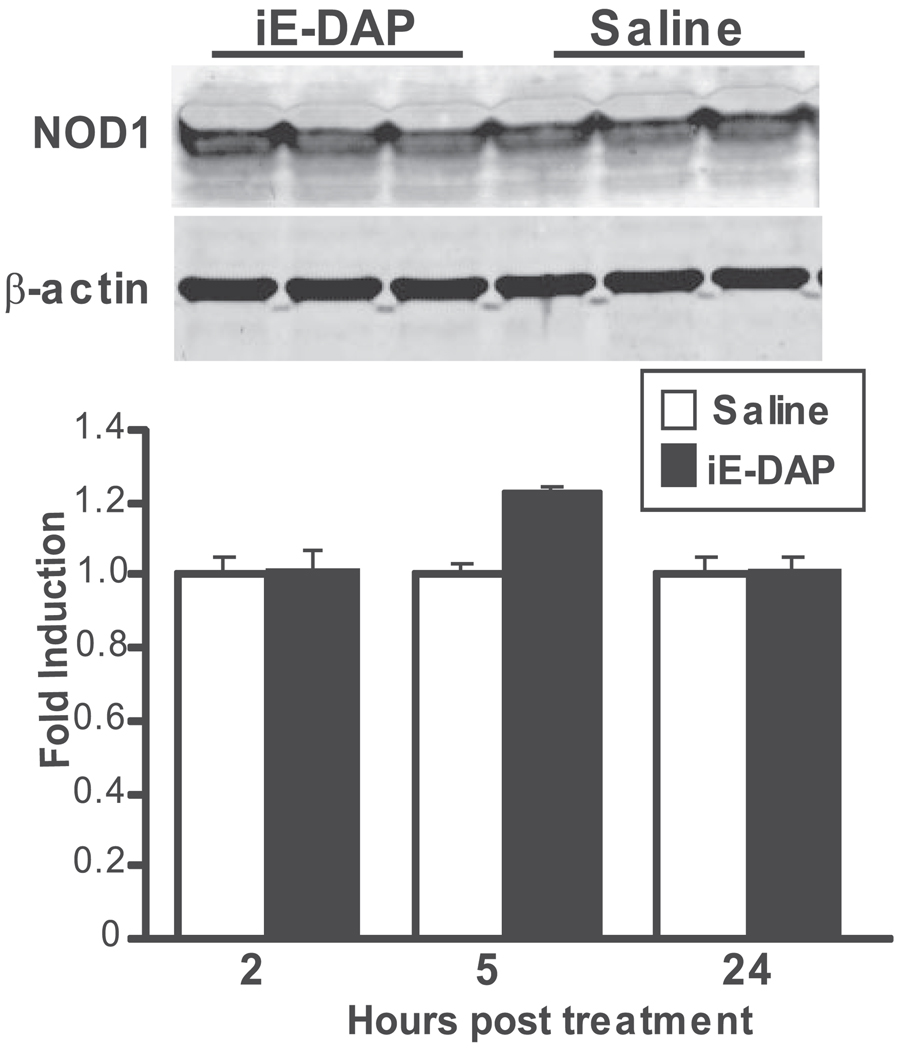
NOD1 has been considered primarily expressed within immune cells and lymphoid tissues. Although its mRNA expression has been documented in murine eye tissue,5 its protein expression in mouse eye tissues has yet to be determined. As shown in Figure 1, NOD1 is constitutively expressed within murine eye tissue. We did not observe any change in protein levels in response to local treatment with the NOD1-specific agonist iE-DAP at a dose and time that induced inflammation.
FIGURE 1.
NOD1 is expressed in murine eye tissue. Mice were treated with intravitreal injections of saline or 100 µg iE-DAP. NOD1 protein expression was measured by immunoblotting. Top: representative immunoblot at 5 hours after treatment. Bottom: densitometric quantification of NOD1 protein expression relative to β-actin as a function of time after treatment. Values are expressed as mean average of the fold induction relative to saline controls. Data are mean ± SEM (n = 3 pools of 3 mice/treatment).
Ocular Inflammation in Mice Resulting from Injection with the NOD1 Agonist iE-DAP
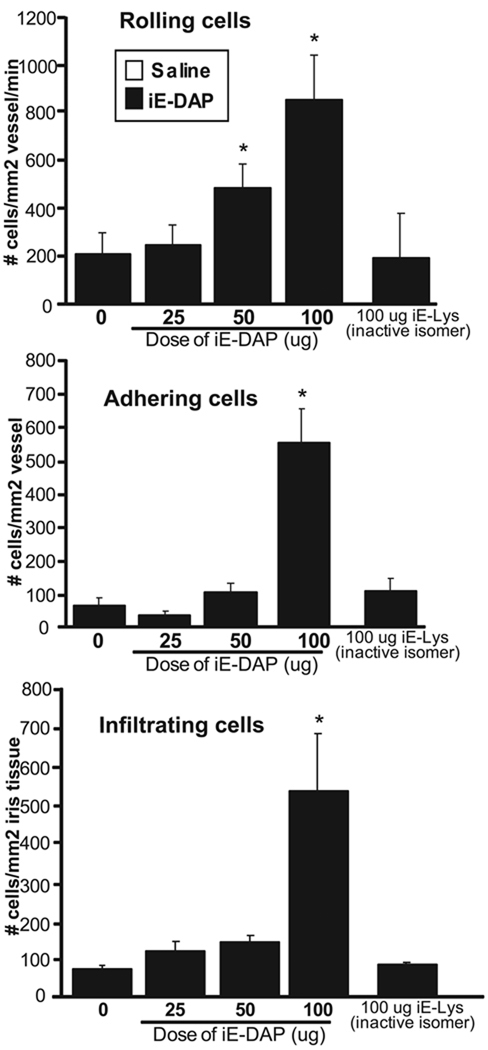
Our finding that NOD1 is present in eye tissue prompted us to further hypothesize that it could contribute to uveitis. To explore the functional significance of NOD1, we used chemically synthesized dipeptide, ie-DAP, which is devoid of contamination of other bacterial products. ie-DAP is considered the minimal motif of PGN responsible for the activation of NOD1.18 We examined the effects of increasing doses of locally administered ie-DAP on the ocular inflammatory response within the microvasculature of iris tissue by intravital microscopy (Fig. 2). We observed a dose-dependent effect of iE-DAP treatment with maximal inflammation achieved at a dose of 100 µg. Treatment with iE-Lys, which is an inactive derivative of iE-DAP that does not activate NOD1, did not cause any inflammation, indicating the specificity of iE-DAP. We were unable to test greater doses of iE-DAP because of limitations of the solubility of iE-DAP and the volume we could inject intravitreally in mice. Notably, we did not observe any influence of strain on the ocular inflammatory response to 100 µg iE-DAP when comparing BALB/c and C57BL/6tyr−/− mice, which lack pigment as a consequence of tyrosinase mutation (data not shown). The albino C57BL/6 mice were used in these studies because pigment interfered with detection of fluorescence by intravital microscopy.
FIGURE 2.
Treatment with the NOD1 agonist iE-DAP results in ocular inflammation in a dose-dependent manner. Mice received intravitreal injections of saline (0) or increasing doses of iE-DAP (25–100 µg). The ocular inflammatory response was assessed by intravital microscopy at 5 hours after treatment, and the numbers of rolling, adhering, and infiltrating leukocytes were determined. As an additional control, mice were treated with iE-Lys, an inactive derivative of iE-DAP. Data are mean ± SEM (n = 8–10 mice/treatment). *P < 0.05 (comparison between saline and iE-DAP treatments).
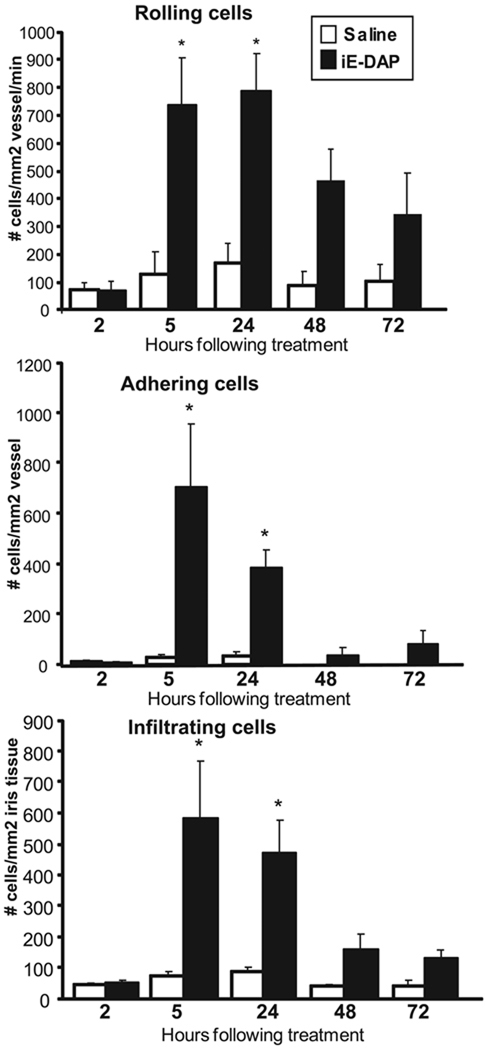
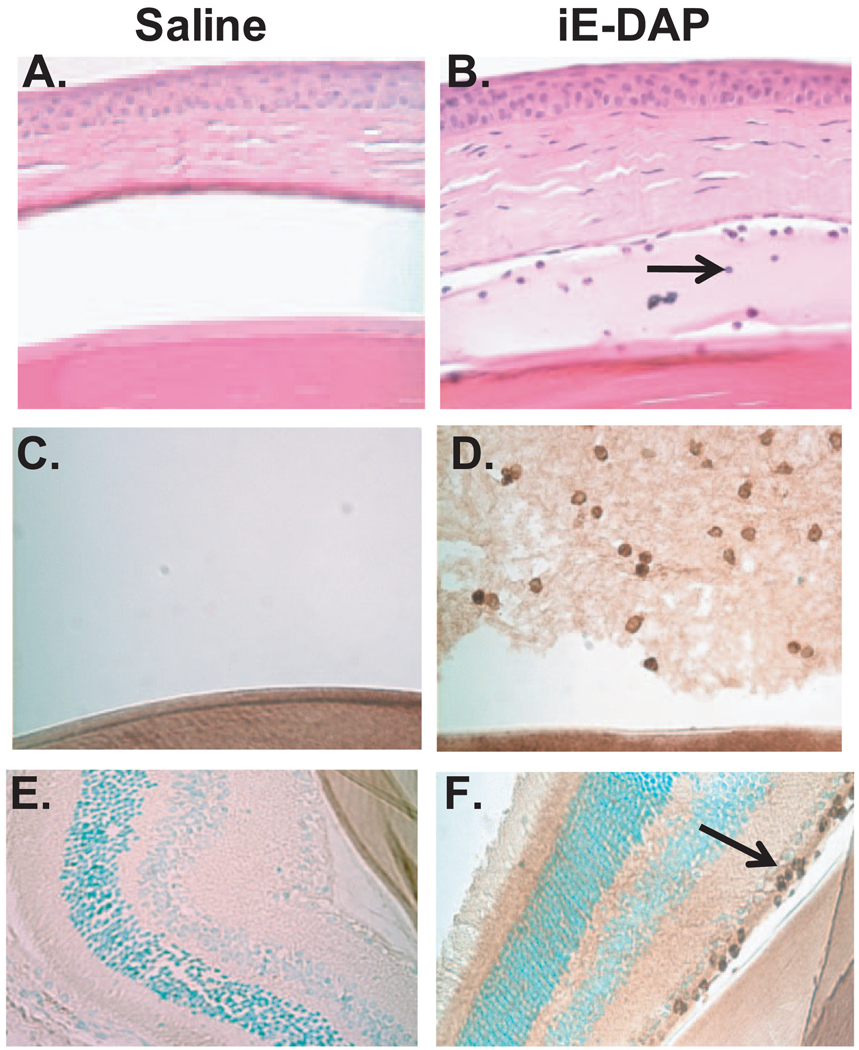
To determine the duration of the inflammatory response induced by iE-DAP, mice were treated with intravitreal injections of 100 µg iE-DAP or saline, and ocular inflammation was assessed by intravital microscopy as a function of time (Fig. 3). Inflammation was detectable as early as 5 hours after treatment and was still present at 24 hours, as indicated by the significant increase in the number of rolling, adhering, and infiltrating leukocytes. Interestingly, the inflammation induced by iE-DAP was more sustained than what we have reported for MDP-induced uveitis, wherein the ocular inflammatory response was completely resolved by 24 hours.16 We also observed a significant increase in leukocyte presence within the aqueous of the anterior chamber in mice treated with iE-DAP and increased fibrin deposition (Fig. 4B). At 24 hours after treatment, iE-DAP resulted in a significant increase in the number of leukocytes/tissue section (45 ± 2.9) within the aqueous of the anterior chamber of the eye compared with 3.5 ± 1.2 for saline (P < 0.05). Based on their characteristic nuclear morphology, most leukocytes appeared to be neutrophils. Indeed, immunohistochemistry confirmed the presence of neutrophils in the aqueous (Figs. 4C, 4D). We also observed a minor neutrophil infiltrate within the nerve fiber layer of the retina (Figs. 4E, 4F) and to some extent within the choroid (data not shown). We observed limited inflammation within the vitreous humor. Control sections stained with an isotype control antibody showed no positive staining (data not shown). These new data support the role for neutrophils in iE-DAP–induced uveitis, which would be consistent with our previous report of MDP induced uveitis16 and endotoxin-induced uveitis.35
FIGURE 3.
Treatment with the NOD1 agonist iE-DAP results in ocular inflammation within a specific time frame. Mice were treated with intravitreal injections of saline or 100 µg iE-DAP. The ocular inflammatory response was assessed by intravital microscopy at different times after treatment, and the numbers of rolling, adhering, and infiltrating leukocytes were determined. Data are mean ± SEM (n = 8–10 mice/treatment/time). *P < 0.05 (comparison between saline and iE-DAP treatments).
FIGURE 4.
iE-DAP treatment results in increased leukocytes within the anterior chamber of the eye. Mice were treated with intravitreal injections of saline or 100 µg iE-DAP. (A, B) The leukocyte infiltrate within the anterior chamber of the eye was assessed histologically with hematoxylin and eosin staining at 24 hours after treatment. Arrow: infiltrating leukocyte. (C–F) Neutrophil infiltrate was assessed by immunohistochemistry. (C, D) Immunoreactivity (brown) of leukocytes within the vitreous to Ly6G at 24 hours after treatment. (C) Saline control; (D) IE-DAP treated. (E, F) Immunoreactivity to Ly6G within the retina. (E) Saline control; (F) iE-DAP treated. n = 5–6 mice/treatment.
IL-1β Role in Ocular Inflammation Induced by iE-DAP
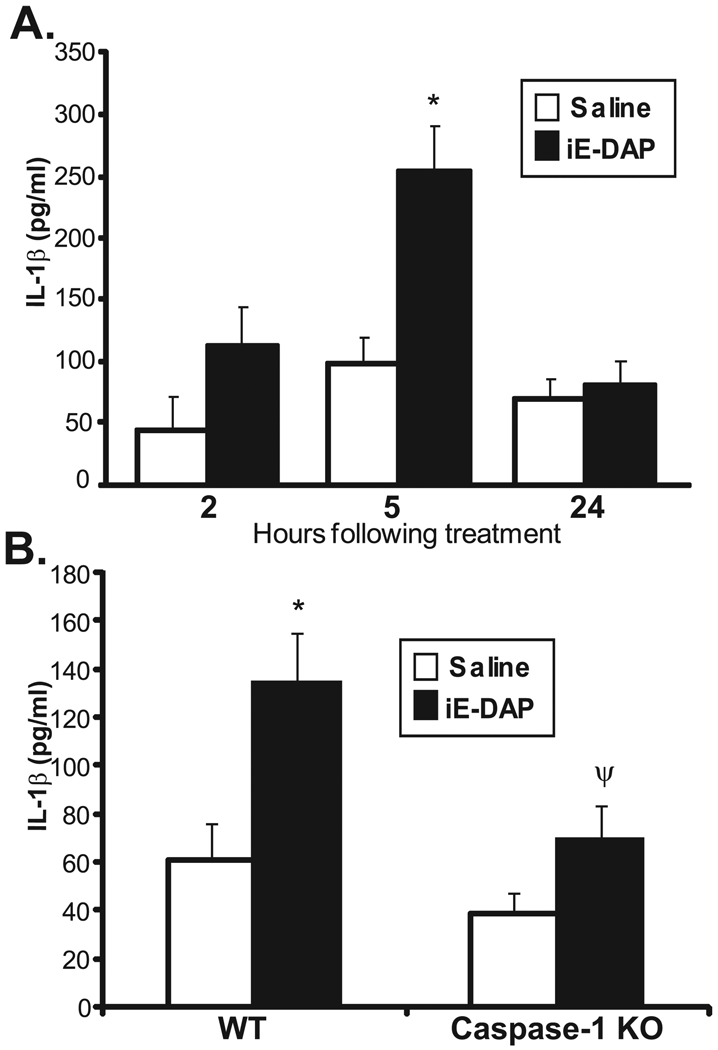
NOD1 and NOD2 share common structural domains and functions in that they both activate downstream signaling pathways by way of their interactions with RIP2 and their CARD domains. Therefore, we hypothesized that NOD1 and NOD2 might use similar mediators to trigger ocular inflammation. To test this hypothesis, we chose to focus on the roles of IL-1β and caspase-1, which we have defined in NOD2-driven ocular inflammation.31 We demonstrated that NOD2 activation induces IL-1β expression in the eye and that caspase-1 plays an essential role in MDP-induced IL-1β production.31 As such, we tested whether iE-DAP induced IL-1β production in the eye and whether its proteolytic processing was dependent on caspase-1. As shown in Figure 5A, we found that protein levels of IL-1β within iE-DAP–injected eyes were significantly increased at 5 hours after treatment. Moreover, mice deficient in caspase-1 showed a significant reduction in IL-1β production (Fig. 5B). These data indicate that NOD1 activation results in increased IL-1β and that IL-1β production depends on caspase-1. This finding is consistent with what we have shown for the effect of NOD2 on IL-1β production in the eye.
FIGURE 5.
Activation of NOD1 by iE-DAP results in increased IL-1β in the mouse eye by caspase-1. Mice were treated with intravitreal injection of saline or 100 µg iE-DAP. Cytokine production of IL-1β in eye tissue homogenates was measured by ELISA as a function of time after treatment (A) or in caspase-1 knockout (KO) mice (B) 5 hours after treatment. Data are mean ± SEM (n = 6 mice/treatment/time/genotype). *P < 0.05 comparison between saline and iE-DAP treatments within a genotype. ψP < 0.05 (comparison between iE-DAP–treated wild-type and caspase-1 KO mice).
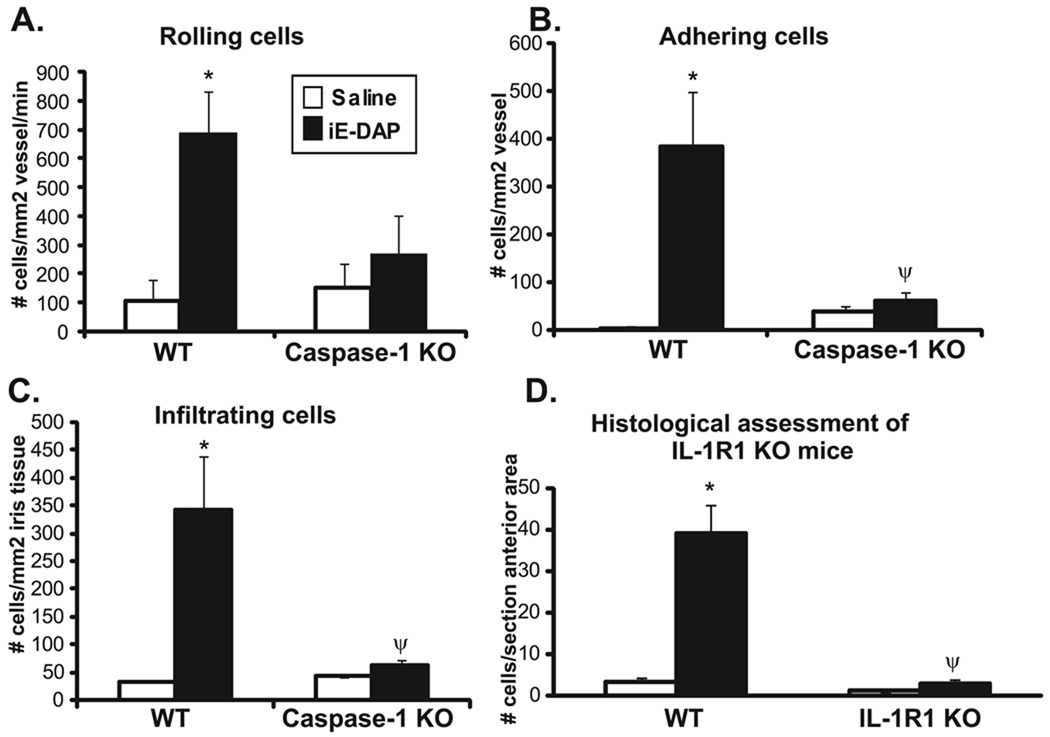
We then assessed the functional role of IL-1 processing and signaling events in the ocular inflammatory response elicited by iE-DAP (Fig. 6). Caspase-1 KO mice and their congenic controls were treated with iE-DAP, and the peak ocular inflammatory response was assessed by intravital microscopy at 5 hours (Figs. 6A–6C). Caspase-1 deficiency significantly diminished the development of ocular inflammation in response to iE-DAP. The number of rolling leukocytes tended to be decreased, and the numbers of adhering and infiltrating leukocytes were significantly reduced compared with congenic controls. We did not observe any significant difference between genotypes at the 24-hour time period (data not shown). Because caspase-1 is responsible for the proteolytic cleavage of pro–IL-1β to its mature form, in addition to other cytokines such as IL-18, we used IL-1R1 KO mice to assess the specific contribution of IL-1β signaling events. The IL-1R1 KO mice are on a C57BL/6 background with iris pigmentation, which obscures the detection of fluorescent leukocytes through intravital microscopy. Thus, we assessed the number of leukocytes within the anterior chamber by histology (Fig. 6D). Deficiency in IL-1R1 completely abolished the iE-DAP–induced leukocyte response within the anterior chamber, indicating an essential role for IL-1 in ocular inflammation downstream of NOD1. Surprisingly, this finding is in contrast to that of NOD2, wherein MDP-induced uveitis occurred independently of IL- 1β.31 Taken together, these findings indicate that IL-1β signaling events are crucial in NOD1-driven ocular inflammation, thereby distinguishing mechanisms of NOD1 from NOD2-uveitis.
FIGURE 6.
iE-DAP–induced ocular inflammation is dependent on caspase-1 and IL-1 signaling. Mice were treated with intravitreal injections of 100 µg iE-DAP or saline. The ocular inflammatory response was assessed by intravital microscopy 5 hours after treatment in caspase-1 KO mice and their controls (wild-type [WT]), and the numbers of rolling, adhering, and infiltrating leukocytes were determined (A–C). Histologic assessment was performed 24 hours after treatment in IL-1R1 KO mice and their controls (WT), and the number of leukocytes within the anterior chamber was determined (D). Data are mean ± SEM (n = 5–6 mice/treatment/time/genotype). *P < 0.05 (comparison between saline and iE-DAP treatments within a genotype). ψP < 0.05 (comparison between iE-DAP–treated WT and caspase-1 KO mice or IL-1R1 KO mice).
DISCUSSION
Elucidation of the role of different pattern recognition receptors in the eye could lead to new perspectives on the ocular inflammatory processes underlying uveitis. The connection between NLR family members, such as NOD2, and autoinflammatory diseases manifesting with uveitis undoubtedly warrants further investigation of NLR family members in ocular inflammatory responses. Here, we demonstrate that another NLR family member, NOD1, is present in murine eye tissue and is functionally active as local treatment with its agonist results in ocular inflammation in a dose- and time-dependent fashion. Like that of NOD2, NOD1 activation resulted in the production of IL-1β in the eye in a caspase-1–dependent mechanism. Yet, to our surprise, NOD1 and NOD2 differed with respect to the role of IL-1 signaling events in the development of ocular inflammation.
Although NOD1 was originally considered to be expressed predominantly in lymphoid tissue, its expression has more recently been documented in nonlymphoid tissue.36–38 We demonstrate here that NOD1 protein is expressed in eye tissue. Our finding is consistent with an earlier report of Nod1 mRNA expression in murine eye tissue5 and in human corneal epithelial cells.39 Thus, it is plausible that NOD1 could trigger uveitis by acting locally within the eye, especially considering that local injection of its agonist results in ocular inflammation. It is also possible that leukocytes expressing NOD1 and recruited to the eye could contribute to the propagation of the inflammatory response in the setting of the appropriate ligand. Indeed, we found an increased presence of neutrophils, which are known to express NOD1, within the aqueous, vitreous, and the nerve fiber layer of the retina. The location of the ocular inflammation resulting from an intravitreal injection of iE-DAP may be indicative of the location of NOD1 within the eye. Our data suggest a role for NOD1 within the anterior and posterior segments of the eye. Future investigation of the specific cell types within the eye that express NOD1 and their responsiveness to iE-DAP should be informative. We did not observe any variation in NOD1 protein levels in response to stimulation with its ligand at the peak of inflammation (5 hours) or at 2 or 24 hours after treatment. This may indicate that its activity was determined by its homodimerization and interactions with RIP2 rather than by its regulation at the protein expression level.
The mechanisms by which NOD1 could trigger ocular inflammation have not been explored until now. Our studies here identify IL-1β processing through caspase-1 and IL-1R1 signaling as contributing events. IL-1β mRNA expression is regulated by NF-κB, but its biological activity is further regulated by its proteolytic processing and secretion, which are mediated by caspase-1 in the inflammasome. Unlike TLRs, which activate only the NF-κB pathway and require a second signal for activation of the caspase-1 pathway, NLRs appear to have the capacity to directly regulate caspase-1 activity.28 Indeed, we have previously demonstrated that NOD2 activation results in IL-1β production in a caspase-1–dependent fashion in the eye.31 We report here the novel finding that NOD1 has a similar capacity in vivo, which places importance on the previously underexplored caspase-1 or inflammasome pathway in ocular inflammatory responses. Although caspase-1 has been identified as a downstream mediator of NOD1 with respect to IL-1β production in vitro,26,27 our findings here are the first demonstration of this capacity in vivo. Because of the described ability of NOD1 to activate NF-κB, we might also speculate that NOD1, like NOD2, is able to initiate signals to NF-κB and to caspase-1 pathways, thereby resulting in the production of IL-1β independently of other TLR signals. Increased NF-κB–regulated expression of cytokines and chemokines, which promote leukocyte-endothelium interactions and infiltration, may also influence the ocular inflammatory response downstream of NOD1. Our data suggest the involvement of IL-1 signaling events in leukocyte adherence and infiltration because these processes were affected the greatest in caspase-1 KO mice. Alternatively, IL-1 could be involved in crucial early events, such that in its absence the development of subsequent inflammatory events is impeded. The inflammatory effects of IL-1R1 activation—including the upregulation of numerous inflammatory mediators transcribed by NF-κB, such as cytokines and chemokines that could contribute to inflammation at later times—have been described.
Interestingly, our finding that IL-1β processing and signaling events are essential in NOD1-mediated uveitis is in contrast to what we demonstrated for NOD2. In NOD2-mediated uveitis, IL-1β is produced within the eye in response to MDP in a caspase-1–dependent manner, similar to what we found after iE-DAP activation of NOD1. However, caspase-1 and IL-1β were not essential for ocular inflammation mediated by NOD2, though they were involved in systemic inflammatory responses triggered by MDP.31 The difference between NOD1 and NOD2 mechanisms of uveitis was surprising given the homology in protein structure and downstream signaling events involving RIP2, NF-κB, and caspase-1. However, it has been suggested that distinct signaling mediators, such as TRAF6 in NOD2, could be involved rather than TRAF2 and TRAF5 in NOD1 signaling.40 Consistent with our findings, a recent report described differential functional roles for NOD1 and NOD2 in regulating acute joint inflammation in a mouse model of arthritis, 41 supporting the idea of distinct inflammatory events that distinguish NOD1 and NOD2 and warrant further investigation in other inflammatory diseases.
Similar to MDP, iE-DAP in itself is not a highly potent inflammatory agent.18 In models of inflammation in other organ systems, comparable doses of iE-DAP and MDP were used in vivo.16,42–44 NLRs, which are located within the cytoplasm, may require greater doses of ligand for their activation than are needed for the activation of cell-surface receptors. However, the sensitivity of the receptor or the tissue type may also influence responses because comparable amounts of the TLR2 agonist PGN are used to induce local knee inflammation in mice.45 Exactly how the NODs are activated in response to their agonist is not understood, but several theories have been proposed. Active phagocytosis seems reasonable in bacterial infection or in response to PGN, which could subsequently be broken down to peptides activating NOD1 or NOD2 within the cytosol. In addition, direct bacterial infection could occur. The agonist of NOD2, MDP, is directly internalized in cells,46 and an active intracellular muropeptide transport system involving PepT1 has been described in epithelial cells.47 There is yet to be any evidence demonstrating a direct interaction between NOD1 and NOD2 and their agonists, suggesting that they might even require some additional factors in the cytosol or plasma membrane for recognition of their agonists. As such, it is possible that the NODs act as downstream signaling transducers. Nonetheless, we emphasize that despite the dose, iE-DAP did elicit a specific response because the same dose of the inactive form of the peptide did not cause ocular inflammation.
Even though the inflammatory effects of NOD1 and NOD2 agonists are lower than those of TLR agonists, a synergistic effect between the two systems appears to occur, indicating an additional regulatory function of the NODs. Simultaneous stimulation of macrophages with iE-DAP and LPS results in the synergistic production of cytokines18,48,49 indicating that NOD1 may serve to amplify TLR responsiveness. The synergistic effect of MDP and TLR agonists in vitro and in vivo has been more widely reported.48–52 The cross-talk between the NLR and TLR systems has been proposed as an important regulatory function in immune homeostasis. This could make sense in situations in which TLR responsiveness might be compromised or suppressed, as in the intestine, where TLRs are thought to be tolerant or refractory to activation as a consequence of continual exposure to bacteria in the intestinal tract. Indeed, a recent report indicates that NOD1 and NOD2 are important components in LPS tolerance.53 The role for NOD1 and NOD2 in promoting inflammatory responses is contradictory to other findings of NOD2 in which NOD2 functions to negatively regulate TLR2-inflammatory responses. Mice deficient in NOD2 are more susceptible to intestinal inflammation54,55 and produce greater amounts of IFN-γ and T-helper 1 T cells, which are considered part of the pathologic mechanisms of Crohn’s disease and colitis. This function may explain, in part, how NOD2 polymorphisms predispose to Crohn’s disease because the common polymorphism (Leu1007fsinsC) results in a truncated form of NOD2 that is unresponsive to MDP.51,56 Such a loss of function may result in unregulated TLR2 responsiveness, thereby rendering the intestine prone to hyperinflammation in the face of continual bacterial exposure. Whether NOD1 performs similar regulatory functions within the intestine has not been explored to the same extent. Bouskra et al.57 published findings on the importance of NOD1 in regulating intestinal homeostasis through the maintenance of lymphoid follicles. Thus, although intracellular Gram-negative infections are relatively rare within the eye, NOD1 is nonetheless likely to be an important mediator of intraocular inflammation directly or perhaps through its regulatory effects on TLR responses. Future studies to investigate the possible interplay of NOD1 activation with TLR responses or even NOD2 responses in vivo should be informative.
In conclusion, the NLR family plays an important role in immune surveillance and just as likely plays an important role in regulating ocular inflammatory responses. Our findings here are the first steps toward understanding how direct activation of NOD1 triggers ocular inflammatory events that might predispose a person to uveitis.
Acknowledgments
The authors thank Monica Jann, Julie Ji, and Tessa Diebel for their technical contributions.
Supported by National Eye Institute Grants F32-EY017254 and EY01309; Research to Prevent Blindness; and the Stan and Madelle Rosenfeld Family Trust.
Footnotes
Disclosure: H.L. Rosenzweig, None; K.T. Galster, None; S.R. Planck, None; J.T. Rosenbaum, None
References
- 1.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J. 2007;30:508–516. doi: 10.1183/09031936.00002607. [DOI] [PubMed] [Google Scholar]
- 2.Yanagihori H, Oyama N, Nakamura K, Mizuki N, Oguma K, Kaneko F. Role of IL-12B promoter polymorphism in Adamantiades-Behçet’s disease susceptibility: an involvement of Th1 immunoreactivity against Streptococcus sanguinis antigen. J Invest Dermatol. 2006;126:1534–1540. doi: 10.1038/sj.jid.5700203. [DOI] [PubMed] [Google Scholar]
- 3.Saari KM, Vilppula A, Lassus A, Leirisalo M, Saari R. Ocular inflammation in Reiter’s disease after Salmonella enteritis. Am J Ophthalmol. 1980;90:63–68. doi: 10.1016/s0002-9394(14)75077-9. [DOI] [PubMed] [Google Scholar]
- 4.Taurog JD, Richardson JA, Croft JT, et al. The germ-free state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Martinez S, Cancino-Diaz ME, Jimenez-Zamudio L, Garcia-Latorre E, Cancino-Diaz JC. TLRs and NODs mRNA expression pattern in healthy mouse eye. Br J Ophthalmol. 2005;89:904–910. doi: 10.1136/bjo.2004.056218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–108. doi: 10.1136/bjo.2005.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum JT, Rosenzweig HL, Smith JR, Martin TM, Planck SR. Uveitis secondary to bacterial products. Ophthalmic Res. 2008;40:165–168. doi: 10.1159/000119870. [DOI] [PubMed] [Google Scholar]
- 8.McGonagle D, Savic S, McDermott MF. The NLR network and the immunological disease continuum of adaptive and innate immunemediated inflammation against self. Semin Immunopathol. 2007;29:303–313. doi: 10.1007/s00281-007-0084-1. [DOI] [PubMed] [Google Scholar]
- 9.Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17:586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 10.Blau EB. Familial granulomatous arthritis, iritis, and rash. J Pediatr. 1985;107:689–693. doi: 10.1016/s0022-3476(85)80394-2. [DOI] [PubMed] [Google Scholar]
- 11.Jabs DA, Houk JL, Bias WB, Arnett FC. Familial granulomatous synovitis, uveitis, and cranial neuropathies. Am J Med. 1985;78:801–804. doi: 10.1016/0002-9343(85)90286-4. [DOI] [PubMed] [Google Scholar]
- 12.Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Kuivaniemi H, Bonavita G, et al. CARD15 mutations in familial granulomatosis syndromes: a study of the original Blau syndrome kindred and other families with large-vessel arteritis and cranial neuropathy. Arthritis Rheum. 2002;46:3041–3045. doi: 10.1002/art.10618. [DOI] [PubMed] [Google Scholar]
- 14.Rose CD, Doyle TM, McIlvain-Simpson G, et al. Blau syndrome mutation of CARD15/NOD2 in sporadic early onset granulomatous arthritis. J Rheumatol. 2005;32:373–375. [PubMed] [Google Scholar]
- 15.Davey MP, Martin TM, Planck SR, Lee J, Zamora D, Rosenbaum JT. Human endothelial cells express NOD2/CARD15 and increase IL-6 secretion in response to muramyl dipeptide. Microvasc Res. 2006;71:103–107. doi: 10.1016/j.mvr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig HL, Martin TM, Jann MM, et al. NOD2, the gene responsible for familial granulomatous uveitis, is essential in a mouse model of muramyl dipeptide-induced uveitis. Invest Ophthalmol Vis Sci. 2008;49:1518–1524. doi: 10.1167/iovs.07-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inohara N, Ogura Y, Nunez G. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr Opin Microbiol. 2002;5:76–80. doi: 10.1016/s1369-5274(02)00289-8. [DOI] [PubMed] [Google Scholar]
- 18.Chamaillard M, Hashimoto M, Horie Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 19.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 20.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2: implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 21.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol. 2007;82:259–264. doi: 10.1189/jlb.1206755. [DOI] [PubMed] [Google Scholar]
- 22.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girardin SE, Tournebize R, Mavris M, et al. CARD4/Nod1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K, Inohara N, Hernandez LD, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 25.Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 26.Yoo NJ, Park WS, Kim SY, et al. Nod1, a CARD protein, enhances pro-interleukin-1β processing through the interaction with procaspase-1. Biochem Biophys Res Comm. 2002;299:652–658. doi: 10.1016/s0006-291x(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 27.Netea MG, Azam T, Ferwerda G, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1β and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Neven B, Callebaut I, Prieur AM, et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood. 2004;103:2809–2815. doi: 10.1182/blood-2003-07-2531. [DOI] [PubMed] [Google Scholar]
- 30.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 31.Rosenzweig HL, Martin TM, Planck SR, et al. Activation of NOD2 in vivo induces IL-1 beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J Leukoc Biol. 2008;84:529–536. doi: 10.1189/jlb.0108015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Bores L, Fonseca GC, Villeda MA, Yamamoto-Furusho JK. Novel genetic markers in inflammatory bowel disease. World J Gastroenterol. 2007;13:5560–5570. doi: 10.3748/wjg.v13.i42.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanabe T, Ishige I, Suzuki Y, et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim Biophys Acta. 2006;1762:794–801. doi: 10.1016/j.bbadis.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Rosenzweig HL, Minami M, Lessov NS, et al. Endotoxin preconditioning protects against the cytotoxic effects of IL-1α after stroke: a novel role for IL-1α in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007;27:1663–1674. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- 35.Planck SR, Becker MD, Crespo S, et al. Characterizing extravascular neutrophil migration in vivo in the iris. Inflammation. 2008;31:105–111. doi: 10.1007/s10753-007-9055-x. [DOI] [PubMed] [Google Scholar]
- 36.Barton JL, Berg T, Didon L, Nord M. The pattern recognition receptor Nod1 activates CCAAT/enhancer binding protein beta signalling in lung epithelial cells. Eur Respir J. 2007;30:214–222. doi: 10.1183/09031936.00143906. [DOI] [PubMed] [Google Scholar]
- 37.Park JH, Kim YG, Shaw M, et al. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol. 2007;179:514–521. doi: 10.4049/jimmunol.179.1.514. [DOI] [PubMed] [Google Scholar]
- 38.Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57:67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 39.Benko S, Tozser J, Miklossy G, et al. Constitutive and UV-B modulated transcription of Nod-like receptors and their functional partners in human corneal epithelial cells. Mol Vis. 2008;14:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw MH, Reimer T, Kim YG, Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joosten LA, Heinhuis B, Abdollahi-Roodsaz S, et al. Differential function of the NACHT-LRR (NLR) members Nod1 and Nod2 in arthritis. Proc Natl Acad Sci U S A. 2008;105:9017–9022. doi: 10.1073/pnas.0710445105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hitotsumatsu O, Ahmad RC, Tavares R, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe T, Asano N, Murray PJ, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masumoto J, Yang K, Varambally S, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joosten LA, Koenders MI, Smeets RL, et al. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- 46.Marina-Garcia N, Franchi L, Kim YG, et al. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 47.Vavricka SR, Musch MW, Chang JE, et al. hPepT1 transports muramyl dipeptide, activating NF-κB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127:1401–1409. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 48.Fritz JH, Girardin SE, Fitting C, et al. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol. 2005;35:2459–2470. doi: 10.1002/eji.200526286. [DOI] [PubMed] [Google Scholar]
- 49.van Heel DA, Ghosh S, Butler M, et al. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur J Immunol. 2005;35:2471–2476. doi: 10.1002/eji.200526296. [DOI] [PubMed] [Google Scholar]
- 50.Yang S, Tamai R, Akashi S, et al. Synergistic effect of muramyl-dipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun. 2001;69:2045–2053. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Netea MG, Ferwerda G, de Jong DJ, Girardin SE, Kullberg BJ, van der Meer JW. NOD2 3020insC mutation and the pathogenesis of Crohn’s disease: impaired IL-1β production points to a loss-of-function phenotype. Netherlands J Med. 2005;63:305–308. [PubMed] [Google Scholar]
- 52.Pauleau A-L, Murray PJ. Role of Nod2 in the response of macrophages to Toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim YG, Park JH, Daignault S, Fukase K, Nunez G. Cross-tolerization between Nod1 and Nod2 signaling results in reduced refractoriness to bacterial infection in Nod2-deficient macrophages. J Immunol. 2008;181:4340–4346. doi: 10.4049/jimmunol.181.6.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473–485. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Moran T, Swanson E, et al. Regulation of IL-8 and IL-1β expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–1725. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 57.Bouskra D, Brezillon C, Berard M, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]